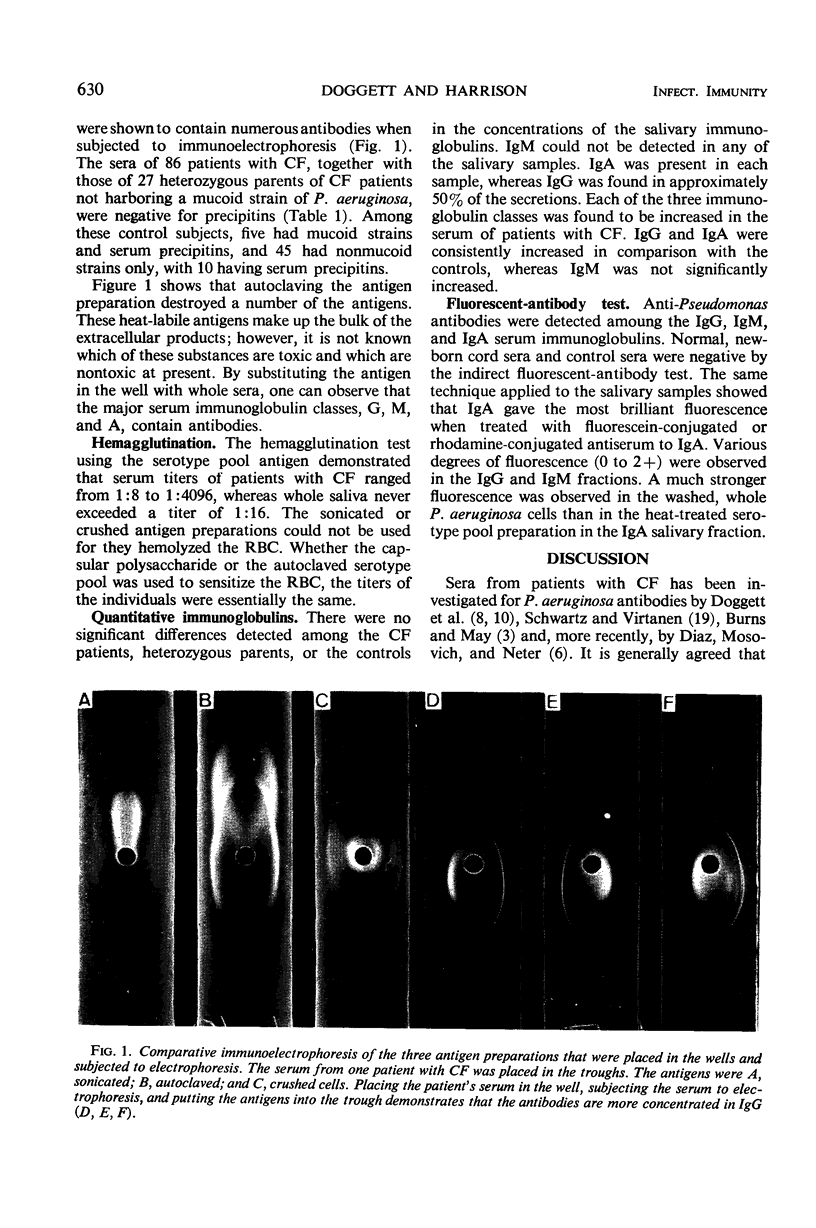
Abstract
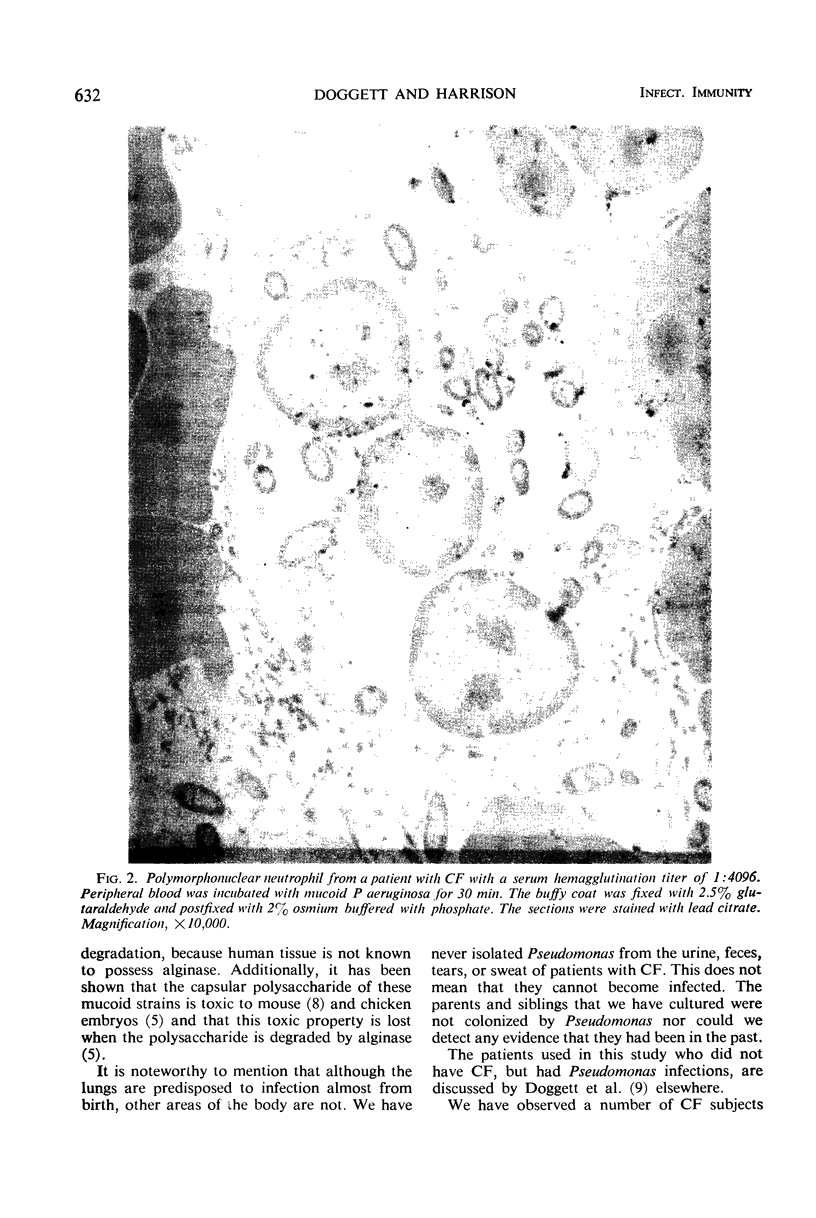
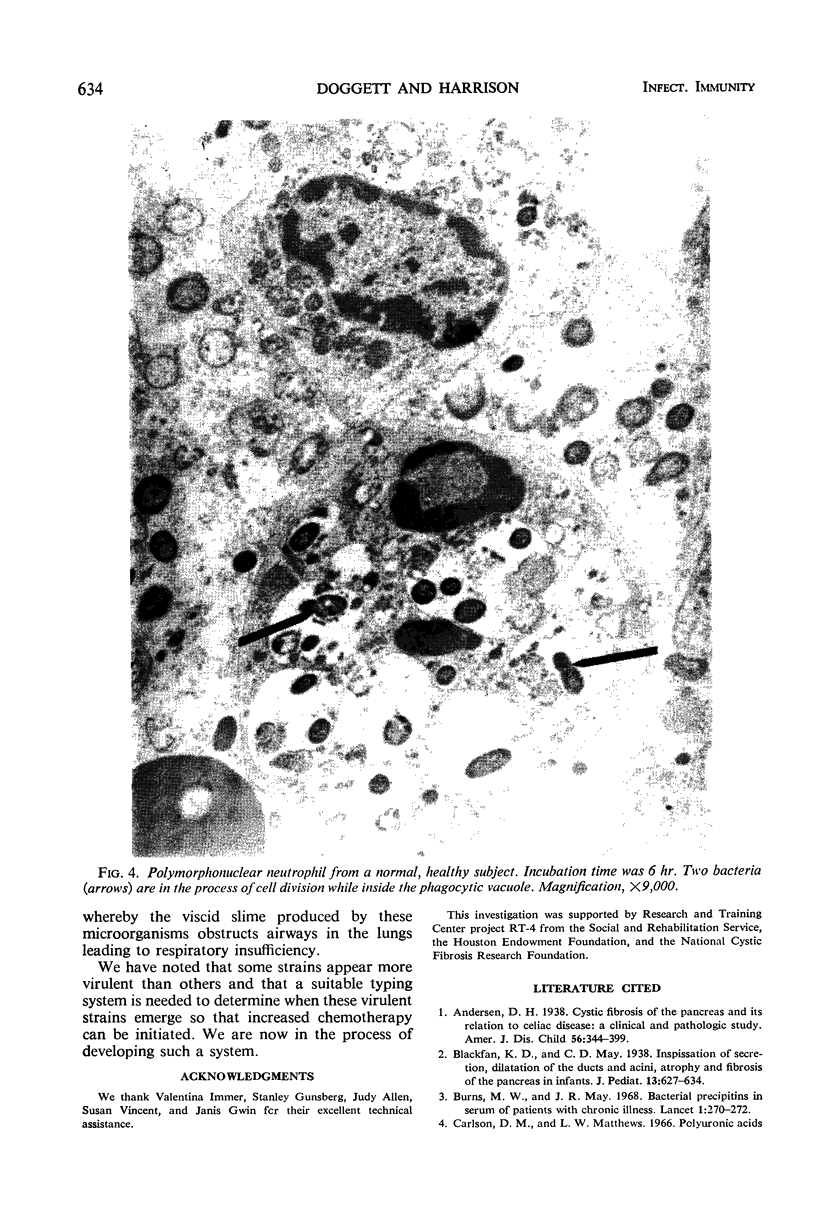
In order to have a better understanding of the clinical significance of Pseudomonas aeruginosa, circulating and secretory antibodies were measured. Of 100 patients diagnosed as having cystic fibrosis (CF) and an atypical mucoid P. aeruginosa cultured from their sputum, each possessed serum precipitins. These immunoprecipitates, however, were not detected in the sera of 40 CF patients, some of whom were chronically ill with pulmonary colonization by typically rough-smooth strains of P. aeruginosa. The sera of 46 CF patients and 27 CF patient parents not colonized by P. aeruginosa were negative for the precipitins. The sera from 15 of 45 chronically ill patients not having CF, however, but harboring P. aeruginosa, also possessed serum precipitins. The sera from 85 subjects not having CF and not clinically infected with P. aeruginosa were negative for precipitins. Serum hemagglutination titers as high as 1:4096 were measured in older CF patients having advanced pulmonary disease and who were infected with mucoid P. aeruginosa. Salivary titers ranged from 1:8 to 1:64. Increased levels of both circulating and secretory antibodies of the immunoglobulin A and G classes were demonstrated in patients with CF. Once a patient with CF becomes colonized with P. aeruginosa a process of conversion from the rough and smooth forms to the mucoid form is almost inevitable. Although the mucoid form predominates in the sputum, intermediates of the various colony types are often present. Serum precipitins were demonstrable only after the appearance of mucoid strains in the sputum of patients with CF. Although antibiotics tend to reduce the number of mucoid microorganisms, they are rarely, if ever, eradicated from these patients' lungs. Recurrent episodes of servere pulmonary infection and the evidence of increasing antibody formation to mucoid strains indicates the invasiveness of these particular strains.
Full text
PDF

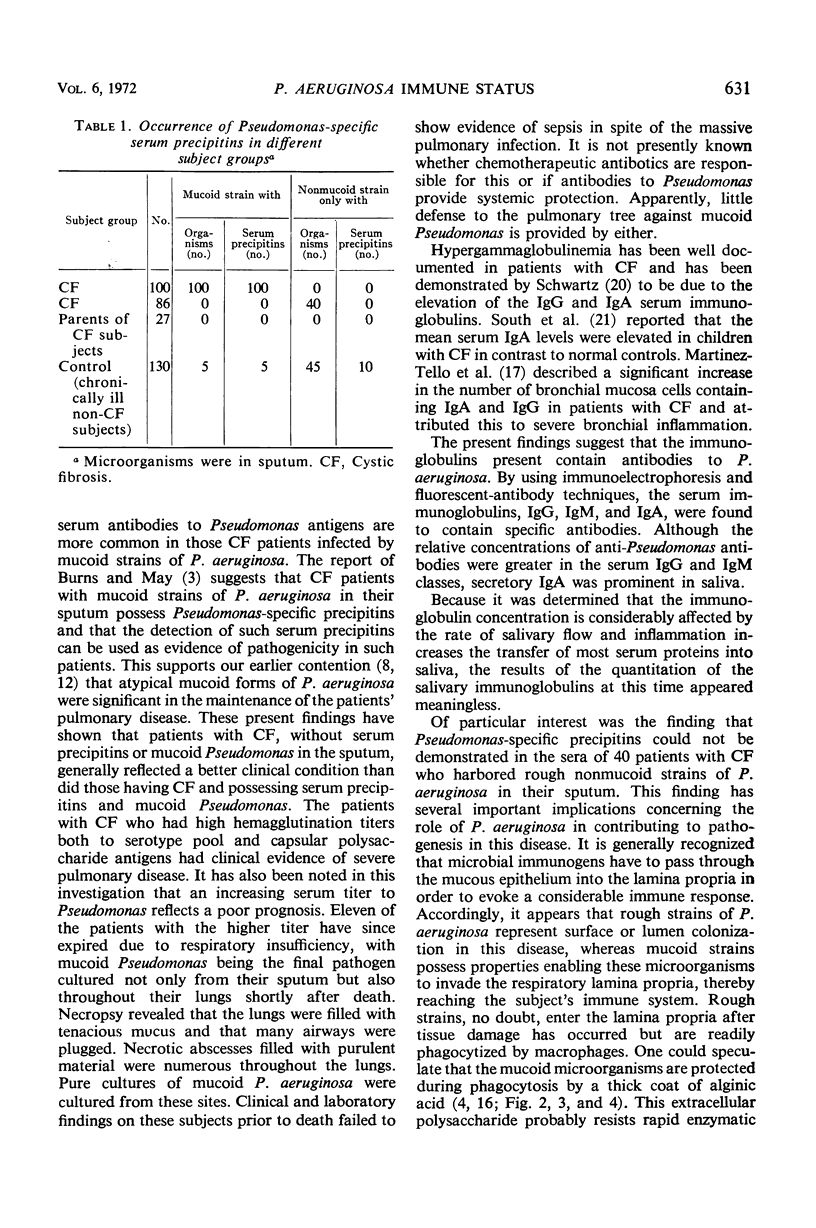


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burns M. W., May J. R. Bacterial precipitins in serum of patients with cystic fibrosis. Lancet. 1968 Feb 10;1(7537):270–272. doi: 10.1016/s0140-6736(68)90121-9. [DOI] [PubMed] [Google Scholar]
- Carlson D. M., Matthews L. W. Polyuronic acids produced by Pseudomonas aeruginosa. Biochemistry. 1966 Sep;5(9):2817–2822. doi: 10.1021/bi00873a006. [DOI] [PubMed] [Google Scholar]
- DOGGETT R. G., HARRISON G. M., STILLWELL R. N., WALLIS E. S. ENZYMATIC ACTION ON THE CAPSULAR MATERIAL PRODUCED BY PSEUDOMONAS AERUGINOSA OF CYSTIC FIBROSIS ORIGIN. J Bacteriol. 1965 Feb;89:476–480. doi: 10.1128/jb.89.2.476-480.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOGGETT R. G., HARRISON G. M., WALLIS E. S. COMPARISON OF SOME PROPERTIES OF PSEUDOMONAS AERUGINOSA ISOLATED FROM INFECTIONS IN PERSONS WITH AND WITHOUT CYSTIC FIBROSIS. J Bacteriol. 1964 Feb;87:427–431. doi: 10.1128/jb.87.2.427-431.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz E., Mosovich L. L., Neter E. Serogroups of Pseudomonas aeruginosa and the immune response of patients with cystic fibrosis. J Infect Dis. 1970 Mar;121(3):269–274. doi: 10.1093/infdis/121.3.269. [DOI] [PubMed] [Google Scholar]
- Doggett R. G., Harrison G. M., Carter R. E. Mucoid Pseudomonas aeruginosa in patients with chronic illnesses. Lancet. 1971 Jan 30;1(7692):236–237. doi: 10.1016/s0140-6736(71)90973-1. [DOI] [PubMed] [Google Scholar]
- Doggett R. G. Incidence of mucoid Pseudomonas aeruginosa from clinical sources. Appl Microbiol. 1969 Nov;18(5):936–937. doi: 10.1128/am.18.5.936-937.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAHEY J. L., MCKELVEY E. M. QUANTITATIVE DETERMINATION OF SERUM IMMUNOGLOBULINS IN ANTIBODY-AGAR PLATES. J Immunol. 1965 Jan;94:84–90. [PubMed] [Google Scholar]
- Fisher M. W., Devlin H. B., Gnabasik F. J. New immunotype schema for Pseudomonas aeruginosa based on protective antigens. J Bacteriol. 1969 May;98(2):835–836. doi: 10.1128/jb.98.2.835-836.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINKER A., JONES R. S. A POLYSACCHARIDE RESEMBLING ALGINIC ACID FROM A PSEUDOMONAS MICRO-ORGANISM. Nature. 1964 Oct 10;204:187–188. doi: 10.1038/204187a0. [DOI] [PubMed] [Google Scholar]
- Martinez-Tello F. J., Braun D. G., Blanc W. A. Immunoglobulin production in bronchial mucosa and bronchial lymph nodes, particularly in cystic fibrosis of the pancreas. J Immunol. 1968 Nov;101(5):989–1003. [PubMed] [Google Scholar]
- Schwartz R. H. Serum immunoglobulin levels in cystic fibrosis. Am J Dis Child. 1966 Apr;111(4):408–411. doi: 10.1001/archpedi.1966.02090070106015. [DOI] [PubMed] [Google Scholar]
- South M. A., Warwick W. J., Wolheim F. A., Good R. A. The IgA system. 3. IgA levels in the serum and saliva of pediatric patients--evidence for a local immunological system. J Pediatr. 1967 Nov;71(5):645–653. doi: 10.1016/s0022-3476(67)80199-9. [DOI] [PubMed] [Google Scholar]
- Tourville D., Bienenstock J., Tomasi T. B., Jr Natural antibodies of human serum, saliva, and urine reactive with Escherichia coli. Proc Soc Exp Biol Med. 1968 Jul;128(3):722–727. doi: 10.3181/00379727-128-33109. [DOI] [PubMed] [Google Scholar]