To the Editor:
Imai and colleagues reported that oxidized phospholipids generated during influenza A pneumonia induce Toll-like receptor 4 (TLR4)-dependent inflammation and contribute to the development of acute lung injury (1). In two subsequent papers, Vogel and colleagues showed that Tlr4−/− mice are resistant to influenza A–induced lung injury and mortality and that the administration of Eritoran, a potent TLR4 antagonist, blocked influenza A–induced lethality in wild-type mice (2, 3). In contrast, other investigators have reported that the genetic loss of TLR4 had either no effect or worsened influenza A–induced lung injury and mortality in mice (4, 5). These discrepancies may be important, as a clinical trial in which Eritoran was administered to patients with severe sepsis showed no benefit, even in a prespecified subgroup of patients in whom the lung was the primary site of infection (6).
We purchased Tlr4−/− mice from the Jackson Laboratory (Bar Harbor, ME). The TLR4-deficient strain commercially available from the Jackson Laboratory (C57BL/10ScN) is derived from a spontaneous mutation that was observed to confer hyporesponsiveness to LPS (7). To confirm LPS unresponsiveness, we injected wild-type and Tlr4−/− mice intraperitoneally with LPS (Escherichia coli O111:B4, 54 mg/kg; Sigma-Aldrich, St. Louis, MO) (8). We found that the Tlr4−/− mice were unresponsive to LPS whereas the wild-type mice underwent 66% mortality by Day 3 (data not shown). This shows that the strain has not regained an intact TLR4 gene from the C57BL/6 background.
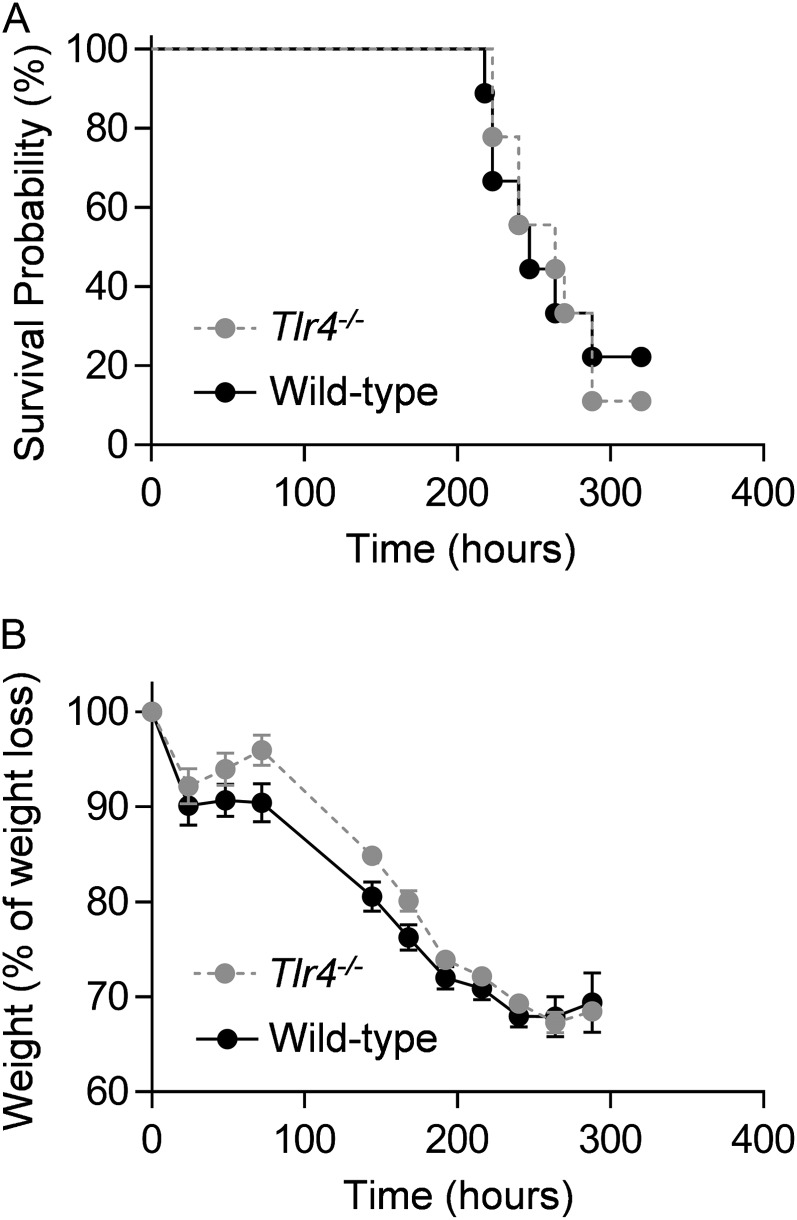
We then infected the Tlr4−/− and wild-type mice with a murine-adapted influenza A virus (A/WSN/33 [H1N1]) (WSN) (500 plaque-forming units per mouse). Weight loss and mortality were similar in Tlr4−/− mice and wild-type mice (Figures 1A and 1B). Our findings are similar to those of Abdul-Careem and colleagues, who administered another murine-adapted H1N1 influenza A virus (A/PR/8/34) (PR/8) to the same strain of mice, but contradict the results of Vogel and colleagues and call into question the conclusion that TLR4 signaling plays a role in the development of influenza A–induced lung injury (5).
Figure 1.
Influenza A infection reveals no significant difference in mortality between Tlr4−/− and wild-type mice. We treated wild-type and Tlr4−/− mice with influenza A virus (A/WSN/33 [H1N1]), using a dose predicted to kill approximately 90% of the mice (∼LD90) (500 plaque-forming units per mouse), and measured (A) mortality and (B) weight daily. n = 9 per group.
We speculate that differences in murine knockout strains might explain these discordant results. Hashimoto and colleagues reported their first results with a TLR4 knockout strain created in 1999 (4, 9). The reported phenotype of these animals after influenza infection is variable, with two papers reporting that the loss of TLR4 confers resistance to influenza A infection (2, 3) and one reporting sensitization (4). In contrast, the TLR4-deficient strain commercially available from the Jackson Laboratory (C57BL/10ScN) is derived from a spontaneous mutation that was observed to confer hyporesponsiveness to LPS (7). The mutant mice lack 74,723 bp of genomic DNA encompassing the Tlr4 gene without other identified mutations (7, 10, 11). Our results and those of Abdul-Careem and colleagues show that influenza A–induced mortality in these mice is similar to that of wild-type controls (5).
Furthermore, the differences between the studies are unlikely to be due to genetic variation in the influenza A virus. As circulating influenza A viruses show poor tropism for the murine lung epithelium, most laboratories use one of several murine-adapted strains. The mice generated by Hoshino and colleagues have been reported to be resistant to both the PR/8 and A/California/07/2009 strains; the C57BL/10ScN strain has been reported to show no resistance to infection with the PR/8 strain; and, in this letter, we report no protection against the WSN strain (2–5).
In summary, we independently confirmed the findings of Abdul-Careem and colleagues, using a different strain of influenza virus (5). The similarity of our findings using distinct influenza strains makes it unlikely that strain variations explain the differential sensitivity of these TLR4 knockout mice to influenza A pneumonia. Instead, we suggest that differences between TLR4 knockout strains might explain the reported resistance to influenza A pneumonia in some studies of TLR4 knockout mice. Collectively, our results and others suggest that more preclinical data are needed before TLR4 antagonists may be considered as therapy for patients with influenza A pneumonia, especially in light of negative clinical trials of Eritoran in patients with sepsis (6). It may be possible, however, to exploit these strain differences to identify genetic, epigenetic, or environmental factors that explain the dramatic difference in phenotype reported between these almost genetically identical mice. Such studies might identify biomarkers to identify individuals who would benefit from TLR4 antagonists.
Acknowledgments
Acknowledgment
The authors thank Robert Lamb, Ph.D., Sc.D. (Department of Molecular Biosciences, Northwestern University, Evanston, IL) for providing the influenza A virus.
Footnotes
Supported by the Parker B. Francis Fellowship, NRSA 1F32AI094976, T32HL076139, a Northwestern Memorial Foundation Dixon Young Investigator Grant, NIH ES015024, ES013995, HL071643, and a VA Merit Award.
Author Contributions: Conception and design: L.M.-N., G.M.M., G.R.S.B., and K.A.R.; analysis and interpretation: L.M.-N., G.M.M., G.R.S.B., and K.A.R.; drafting the manuscript for important intellectual content: L.M.-N., G.M.M., G.R.S.B., and K.A.R.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, Ermolaeva M, Veldhuizen R, Leung YH, Wang H, et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shirey KA, Lai W, Scott AJ, Lipsky M, Mistry P, Pletneva LM, Karp CL, McAlees J, Gioannini TL, Weiss J, et al. The TLR4 antagonist Eritoran protects mice from lethal influenza infection. Nature. 2013;497:498–502. doi: 10.1038/nature12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nhu QM, Shirey K, Teijaro JR, Farber DL, Netzel-Arnett S, Antalis TM, Fasano A, Vogel SN. Novel signaling interactions between proteinase-activated receptor 2 and Toll-like receptors in vitro and in vivo. Mucosal Immunol. 2010;3:29–39. doi: 10.1038/mi.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashimoto Y, Moki T, Takizawa T, Shiratsuchi A, Nakanishi Y. Evidence for phagocytosis of influenza virus-infected, apoptotic cells by neutrophils and macrophages in mice. J Immunol. 2007;178:2448–2457. doi: 10.4049/jimmunol.178.4.2448. [DOI] [PubMed] [Google Scholar]
- 5.Abdul-Careem MF, Firoz Mian M, Gillgrass AE, Chenoweth MJ, Barra NG, Chan T, Al-Garawi AA, Chew MV, Yue G, van Roojen N, et al. FimH, a TLR4 ligand, induces innate antiviral responses in the lung leading to protection against lethal influenza infection in mice. Antiviral Res. 2011;92:346–355. doi: 10.1016/j.antiviral.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Opal SM, Laterre PF, Francois B, LaRosa SP, Angus DC, Mira JP, Wittebole X, Dugernier T, Perrotin D, Tidswell M, et al. ACCESS Study Group. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: the ACCESS randomized trial. JAMA. 2013;309:1154–1162. doi: 10.1001/jama.2013.2194. [DOI] [PubMed] [Google Scholar]
- 7.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 8.Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 9.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 10.Vogel SN, Hansen CT, Rosenstreich DL. Characterization of a congenitally LPS-resistant, athymic mouse strain. J Immunol. 1979;122:619–622. [PubMed] [Google Scholar]
- 11.Poltorak A, Smirnova I, Clisch R, Beutler B. Limits of a deletion spanning Tlr4 in C57BL/10ScCr mice. J Endotoxin Res. 2000;6:51–56. doi: 10.1177/09680519000060010701. [DOI] [PubMed] [Google Scholar]