Abstract
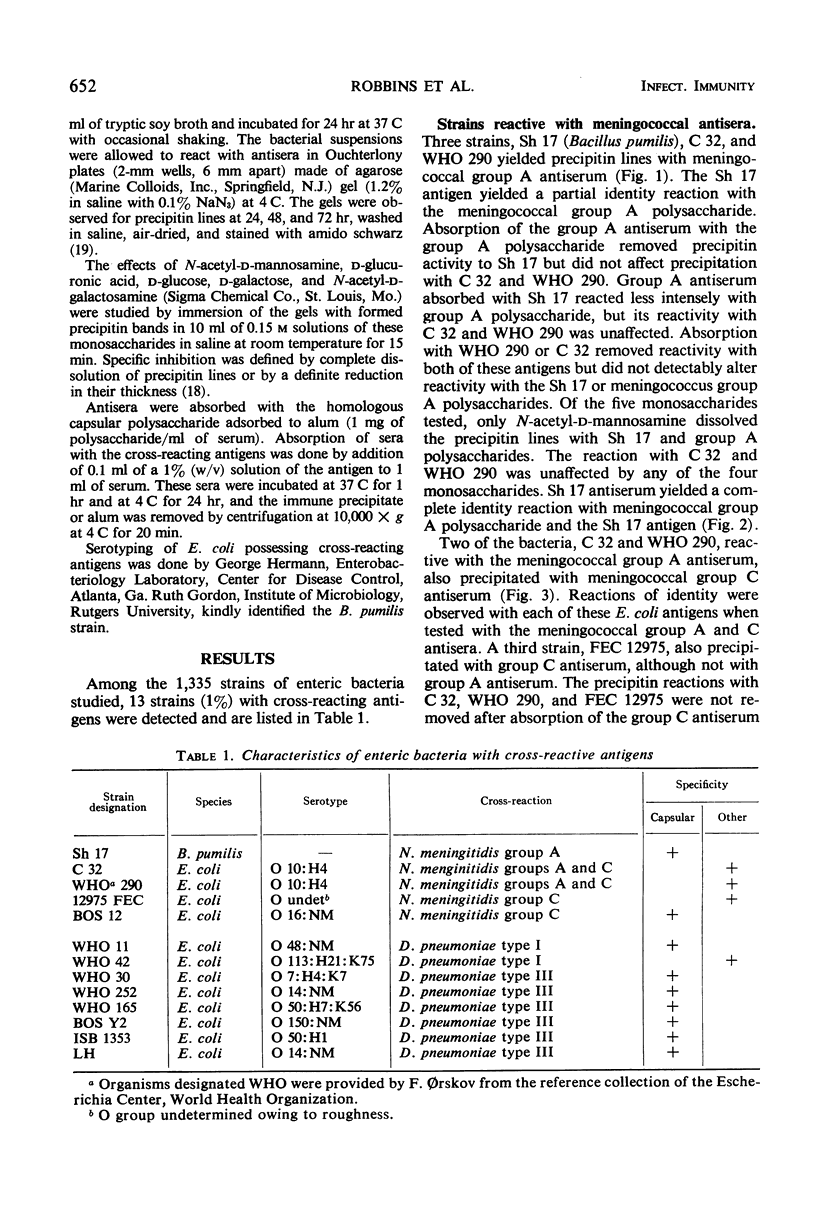
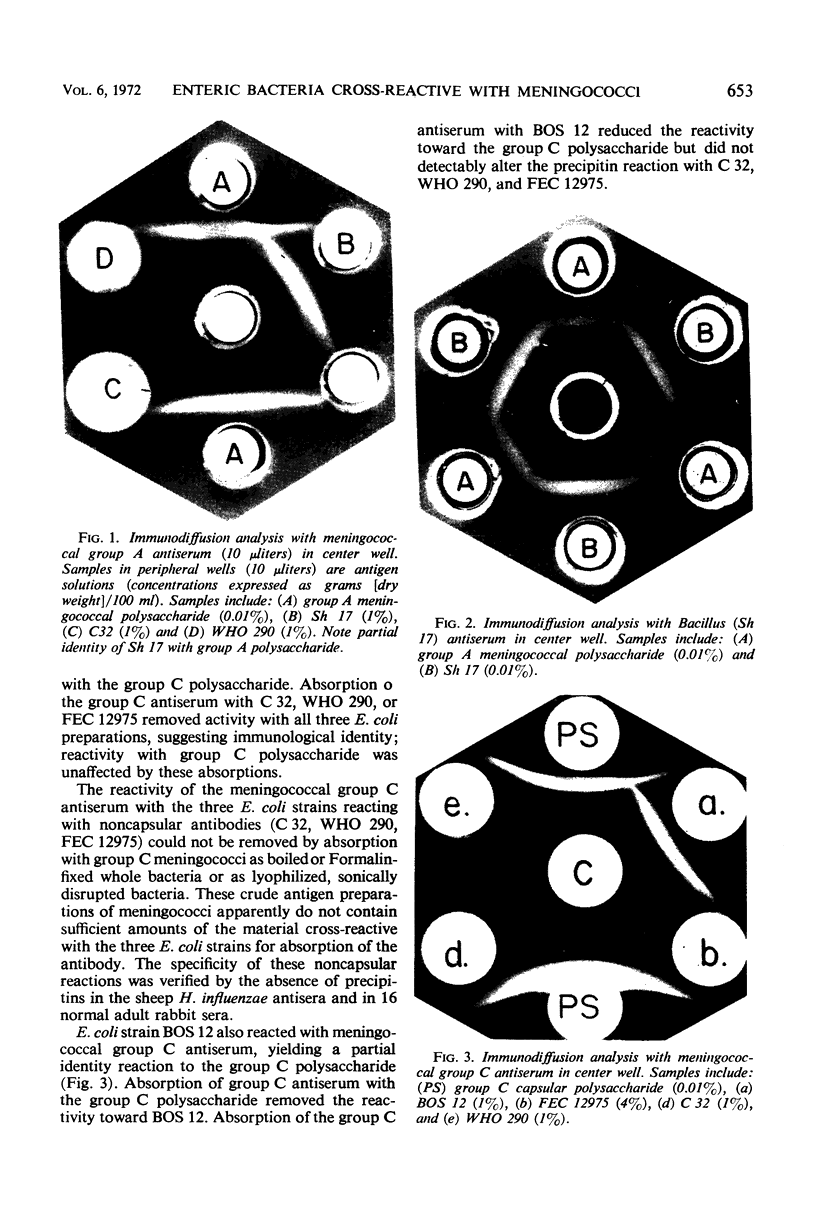
Enteric bacteria of 1,335 individual strains were studied for serological cross-reactions with Neisseria meningitidis groups A and C and Diplococcus pneumoniae types I and III. Enterobacterial antigens cross-reactive with the capsular polysaccharides of these four bacteria were found. Bacteria cross-reactive with noncapsular antigens of meningococci and pneumococci were also observed. Since some enteric bacteria possess antigens with serological specificities similar to those of meningococci, the possibility that enteric bacteria cross-reactive with meningococcal antigens provide an antigenic stimulus for the observed age-related “natural” immunity to this pathogen is discussed.
Full text
PDF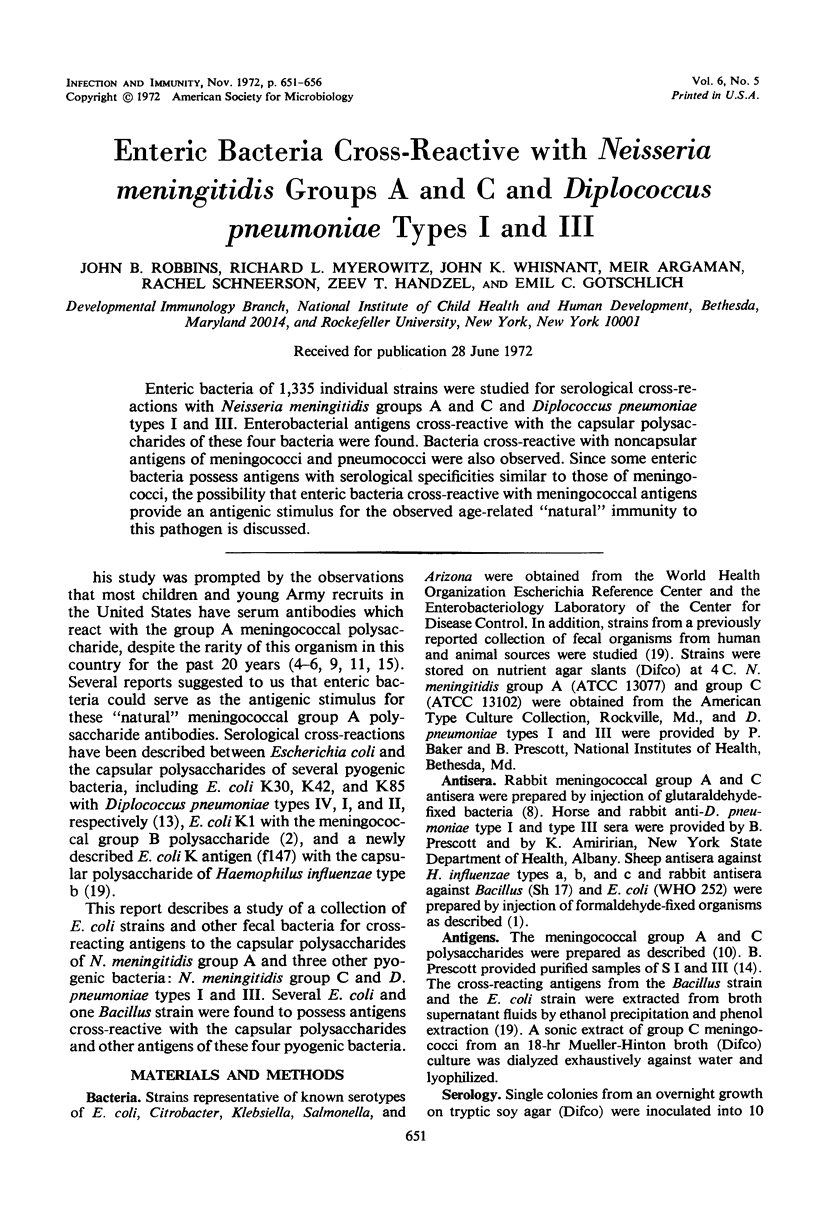



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AYCOCK W. L., MUELLER J. H. Meningococcus carrier rates and meningitis incidence. Bacteriol Rev. 1950 Jun;14(2):115–160. doi: 10.1128/br.14.2.115-160.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw M. W., Schneerson R., Parke J. C., Jr, Robbins J. B. Bacterial antigens cross-reactive with the capsular polysaccharide of Haemophilus influenzae type b. Lancet. 1971 May 29;1(7709):1095–1096. doi: 10.1016/s0140-6736(71)91837-x. [DOI] [PubMed] [Google Scholar]
- EICKHOFF T. C., FINLAND M. CHANGING SUSCEPTIBILITY OF MENINGOCOCCI TO ANTIMICROBIAL AGENTS. N Engl J Med. 1965 Feb 25;272:395–398. doi: 10.1056/NEJM196502252720804. [DOI] [PubMed] [Google Scholar]
- Evans J. R., Artenstein M. S., Hunter D. H. Prevalence of meningococcal serogroups and description of three new groups. Am J Epidemiol. 1968 May;87(3):643–646. doi: 10.1093/oxfordjournals.aje.a120854. [DOI] [PubMed] [Google Scholar]
- Goldschneider I., Gotschlich E. C., Artenstein M. S. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969 Jun 1;129(6):1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschneider I., Gotschlich E. C., Artenstein M. S. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969 Jun 1;129(6):1327–1348. doi: 10.1084/jem.129.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Rey M., Triau R., Sparks K. J. Quantitative determination of the human immune response to immunization with meningococcal vaccines. J Clin Invest. 1972 Jan;51(1):89–96. doi: 10.1172/JCI106801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grados O., Ewing W. H. Antigenic relationship between Escherichia coli and Neisseria meningitidis. J Infect Dis. 1970 Jul-Aug;122(1):100–103. doi: 10.1093/infdis/122.1-2.100. [DOI] [PubMed] [Google Scholar]
- Heidelberger M., Jann K., Jann B., Orskov F., Orskov I., Westphal O. Relations between structures of three K polysaccharides of Escherichia coli and cross-reactivity in antipneumococcal sera. J Bacteriol. 1968 Jun;95(6):2415–2417. doi: 10.1128/jb.95.6.2415-2417.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOVE B. D., Jr, FINLAND M. In vitro susceptibility of meningococci to eleven antibiotics and sulfadiazine. Am J Med Sci. 1954 Nov;228(5):534–539. doi: 10.1097/00000441-195411000-00006. [DOI] [PubMed] [Google Scholar]
- Leedom J. M., Ivler D., Mathies A. W., Jr, Thrupp L. D., Fremont J. C., Wehrle P. F., Portnoy B. The problem of sulfadiazine-resistant meningococci. Antimicrob Agents Chemother (Bethesda) 1966;6:281–292. [PubMed] [Google Scholar]
- Minden P., Farr R. S. Binding between components of the tubercle bacillus and humoral antibodies. J Exp Med. 1969 Nov 1;130(5):931–954. doi: 10.1084/jem.130.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter M., Leon M. A. Three IgA myeloma immunoglobulins from the BALB/ mouse: precipitation with pneumococcal C polysaccharide. Science. 1968 Oct 18;162(3851):369–371. doi: 10.1126/science.162.3851.369. [DOI] [PubMed] [Google Scholar]
- Schneerson R., Bradshaw M., Whisnant J. K., Myerowitz R. L., Parke J. C., Jr, Robbins J. B. An Escherichia coli antigen cross-reactive with the capsular polysaccharide of Haemophilus influenzae type b: occurrence among known serotypes, and immunochemical and biologic properties of E. coli antisera toward H. influenzae type b. J Immunol. 1972 Jun;108(6):1551–1562. [PubMed] [Google Scholar]
- Schneerson R., Robbins J. B. Age-related susceptibility to Haemophilus influenzae type b disease in rabbits. Infect Immun. 1971 Oct;4(4):397–401. doi: 10.1128/iai.4.4.397-401.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TURK D. C. Naso-pharyngeal carriage of Haemophilus influenza type B. J Hyg (Lond) 1963 Jun;61:247–256. doi: 10.1017/s0022172400020957. [DOI] [PMC free article] [PubMed] [Google Scholar]







