Abstract
Rationale: The pathogenesis of asthma in obesity is poorly understood, but may be related to breathing at low lung volumes.
Objectives: To determine if lung function in obese patients with asthma and control subjects would respond differently to weight loss.
Methods: Lung function was evaluated by conventional clinical tests and by impulse oscillometry in female late-onset, nonallergic patients with asthma and control subjects before, and 12 months after, bariatric surgery.
Measurements and Main Results: Patients with asthma (n = 10) had significantly lower FEV1 (79.8 ± 10.6 vs. 95.5 ± 7.0%) and FVC (82.4 ± 13.2 vs. 93.7 ± 8.9%) compared with control subjects (n = 13). There were no significant differences in FRC or TLC at baseline. Twelve months after surgery, control subjects had significant increases in FEV1 (95.5 ± 7.0 to 100.7 ± 5.9), FVC (93.6 ± 8.9 to 98.6 ± 8.3%), FRC (45.4 ± 18.5 to 62.1 ± 15.3%), and TLC (84.8 ± 15.0 to 103.1 ± 15.3%), whereas patients with asthma had improvement only in FEV1 (79.8 ± 10.6 to 87.2 ± 11.5). Control subjects and patients with asthma had a significantly different change in respiratory system resistance with weight loss: control subjects exhibited a uniform decrease in respiratory system resistance at all frequencies, whereas patients with asthma exhibited a decrease in frequency dependence of resistance. Fits of a mathematical model of lung mechanics to these impedance spectra suggest that the lung periphery was more collapsed by obesity in patients with asthma compared with control subjects.
Conclusions: Weight loss decompresses the lung in both obese control subjects and patients with asthma, but the more pronounced effects of weight loss on lung elastance suggest that the distal lung is inherently more collapsible in people with asthma.
Keywords: bariatric surgery, forced oscillation technique, impedance, lung volume
At a Glance Commentary
Scientific Knowledge on the Subject
Obesity is a major risk factor for asthma. It has been thought that this may be related to airway reactivity induced by breathing at low lung volumes.
What This Study Adds to the Field
Differences in lung volume do not distinguish between obese patients with and without asthma. Changes with weight loss suggest that obese patients with asthma have more collapsible peripheral airways than obese patients without asthma, suggesting that asthma in obesity is related to an abnormality in the lung periphery.
Obesity is an important risk factor for asthma (1–3), especially in women (1), and is associated with poor asthma control (4, 5) and resistance to standard controller therapies (6, 7). The mechanistic link between asthma and obesity remains unclear. A number of causative factors have been proposed, including systemic and/or airway inflammation, mechanical effects caused by chronic lung compression, and comorbidities of obesity (3). Asthma in obesity may arise for a variety of different reasons. Indeed, some obese patients with asthma have an early onset form of allergic disease that is complicated by the development of obesity, whereas others develop de novo asthma later in life as a consequence of obesity (8). These represent two distinct obese asthma phenotypes (9–11) that likely have distinctly different causes.
Here we focus on the late-onset nonallergic phenotype of asthma in obesity, because this form of disease seems to be a direct consequence of obesity. We have previously shown that these patients with asthma have minimal airway inflammation, which does not change with weight loss (11). This form of asthma has a potentially straightforward explanation: obese patients with asthma breathe at low lung volumes as a result of mass loading of their chest wall, leading to the kind of airways hyperresponsiveness that has been modeled in normal-weight volunteers via imposed reductions in lung volume (12). This explanation is supported by recent data showing that weight loss improves lung function and airway reactivity, particularly in those with late-onset low-IgE disease (9). However, only a subset of the obese population has asthma; most obese individuals have normal lung function (13) even though one would presume that all obese individuals are at risk for breathing at low lung volumes. The role of lung volume in the asthma of obesity thus remains an open question.
Prior studies suggest that obesity may cause abnormalities particularly in peripheral lung function (14–16). This is poorly measured by conventional lung function tests, which involve deep breaths and forced maneuvers that mask changes in the lung periphery, and so we used impulse oscillometry as an adjunctive measure of lung function before and after weight loss. Impulse oscillometry uses the dynamic relationship between an imposed flow signal and the resulting airway pressure signal to determine the mechanical impedance of the respiratory system over a range of frequencies from 5 to 35 Hz. Importantly, no large changes in lung volume are involved, so the measurements reflect lung function at volumes relevant to tidal breathing.
Current evidence suggests that not everyone is affected in the same way by obesity-related reductions in lung volume; some otherwise normal individuals develop nonatopic asthma when they become obese, whereas others remain nonasthmatic. This leads to the hypothesis that these two groups of obese individuals respond differently when their lung volumes are normalized through major weight loss. Verification of this hypothesis would provide important insights into the mechanisms of obese asthma. Accordingly, in the present study we compared lung function in two groups of obese female subjects, one with late-onset low-IgE asthma and the other without asthma, and then determined how lung function changed in both groups after major weight loss because of bariatric surgery. Some of these results have previously been published in abstract form (17), and data from these subjects pertaining to asthma control and inflammatory changes with weight loss have previously been published (9, 11).
Methods
Participants
Participants undergoing evaluation for bariatric surgery at the University of Vermont teaching hospital were invited to participate in this study. We studied female subjects because of their greater propensity to develop asthma when obese (1), compared with males. The study was reviewed by the local institutional review board, and written informed consent was obtained from all participants. Participants were evaluated before, and 12 months after, bariatric surgery. Late-onset nonallergic participants with asthma (n = 10) were initially diagnosed with asthma at more than 12 years of age and had physiologic evidence of asthma. This evidence was either provocative concentration of methacholine causing a 20% drop in FEV1 (PC20) less than 16 mg/ml (18), or improvement in FEV1, FVC, or both of greater than or equal to 12% and 200 ml with bronchodilator (19). All subjects also had IgE less than 100 IU/ml. Participants with no asthma (n = 13) had no diagnosis of asthma and did not respond in a clinically significant manner to either methacholine or bronchodilator (PC20 >16 mg/ml methacholine, response to bronchodilator <12%, and/or 200 ml in FEV1). Exclusion criteria included (1) smoking history more than 20 pack-years; (2) smoking within the prior 6 months; (3) FEV1 less than 60% predicted; (4) treatment with systemic steroids during the prior 6 weeks; (5) active pulmonary disease other than asthma (those with obstructive sleep apnea were not excluded); and (6) significant other disease that, in the opinion of the investigators, would interfere with study participation.
Study Design
We performed a cross-sectional comparison of asthmatic versus nonasthmatic to establish baseline differences between these two groups. We then performed a prospective observational study comparing the responses to weight loss. In all subjects we measured standard spirometric parameters, lung volume by gas dilution, and diffusing capacity of carbon monoxide according to American Thoracic Society guidelines (19–21). We also measured respiratory system impedance between 5 and 35 Hz during tidal breathing using impulse oscillometry (Jaeger, Wurzburg, Germany) during normal tidal breathing (details in online supplement).
Patients with asthma performed a methacholine challenge test using the five-breath dosimeter method according to American Thoracic Society guidelines (18) both before and 12 months after bariatric surgery.
Statistical Analyses
Data were summarized using descriptive statistics in terms of mean values and standard deviations. We used a Mann-Whitney test to compare differences between obese subjects with and without asthma. Paired t tests were used to compare changes in measures within subjects from baseline to 12 months after bariatric surgery. Mixed models repeated measures analysis of variance was used to compare changes within the asthmatic and control groups over time. Given the exploratory nature of this study, we did not attempt to control for multiple comparisons. Statistical tests were performed with STATA 11.0 (College Station, TX).
Mathematical Modeling of Impedance
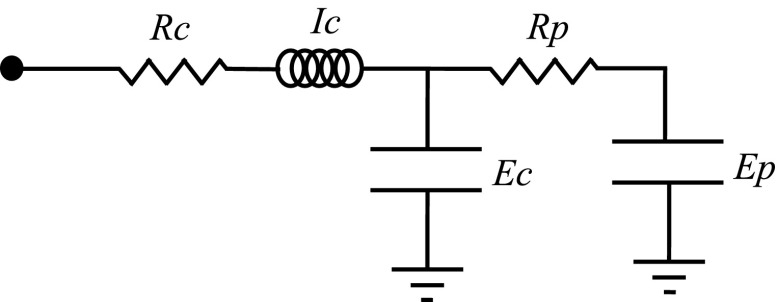
We fit the two-compartment model represented in Figure 1 to the measured impedance spectra. This model attempts to strike a balance between representing the likely most relevant physiologic features of the lung while remaining simple enough to provide a unique fit to a given set of impedance data. Model simulation and fitting details are provided in the online supplement.
Figure 1.
Electric circuit representation of lung mechanics in which the proximal airways (resistance [Rc]; gas inertance [Ic]) connect to a central elastic compartment representing the stiffness (Ec) of the conducting airways. The central compartment in turn connects to a distal compartment representing the elastance of the alveolar tissue (Ep) via a conduit representing the resistance (Rp) of the peripheral airways.
Results
Baseline Comparisons
At baseline, patients with asthma had significantly lower FEV1 and FVC, and tended to have a higher residual volume/TLC, compared with control subjects. There was no difference in FRC or TLC between patients with asthma and control subjects (Table 1). Patients with asthma tended to be slightly heavier at baseline, although this did not reach statistical significance (Table 1).
Table 1.
Baseline Demographics and Lung Function
| Control | Asthma | P Value | |
|---|---|---|---|
| Number | 13 | 10 | |
| Age, yr | 43.6 ± 7.1 | 47.8 ± 6.6 | 0.17 |
| BMI, preoperative | 43.1 ± 5.6 | 48.5 ± 10.0 | 0.12 |
| BMI, postoperative | 32.7 ± 4.0 | 38.6 ± 7.2 | 0.02 |
| FEV1 | 95.5 ± 7.0 | 79.8 ± 10.6 | <0.001 |
| FVC | 93.7 ± 8.9 | 82.4 ± 13.2 | 0.02 |
| FEV1/FVC | 102.2 ± 4.8 | 97.6 ± 9.4 | 0.09 |
| TLC | 84.8 ± 15.0 | 87.7 ± 11.9 | 0.63 |
| IC | 123.6 ± 16.3 | 117.4 ± 19.7 | 0.42 |
| SVC | 90.4 ± 9.0 | 83.1 ± 12.5 | 0.13 |
| FRC | 45.4 ± 18.5 | 45.5 ± 10.0 | 0.79 |
| ERV | 29.9 ± 21.5 | 21.1 ± 15.4 | 0.29 |
| RV | 81.7 ± 30.3 | 98.5 ± 23.5 | 0.17 |
| RV/TLC | 94.6 ± 28.3 | 112.3 ± 22.6 | 0.13 |
| DlCO | 93.0 ± 16.3 | 94.8 ± 14.0 | 0.78 |
Definition of abbreviations: BMI = body mass index; DlCO = diffusing capacity of carbon monoxide; ERV = expiratory reserve volume; IC = inspiratory capacity; RV = residual volume; SVC = slow vital capacity.
Values are expressed as mean and standard deviation % predicted. P values are for unpaired t tests for normally distributed data, and the Kruskal-Wallis test for nonnormally distributed data. Lung function values are shown as % predicted using Hankinson and coworkers (34) for spirometry, Goldman and Becklake (35) for lung volumes, and Gaensler and Wright (36) for DlCO.
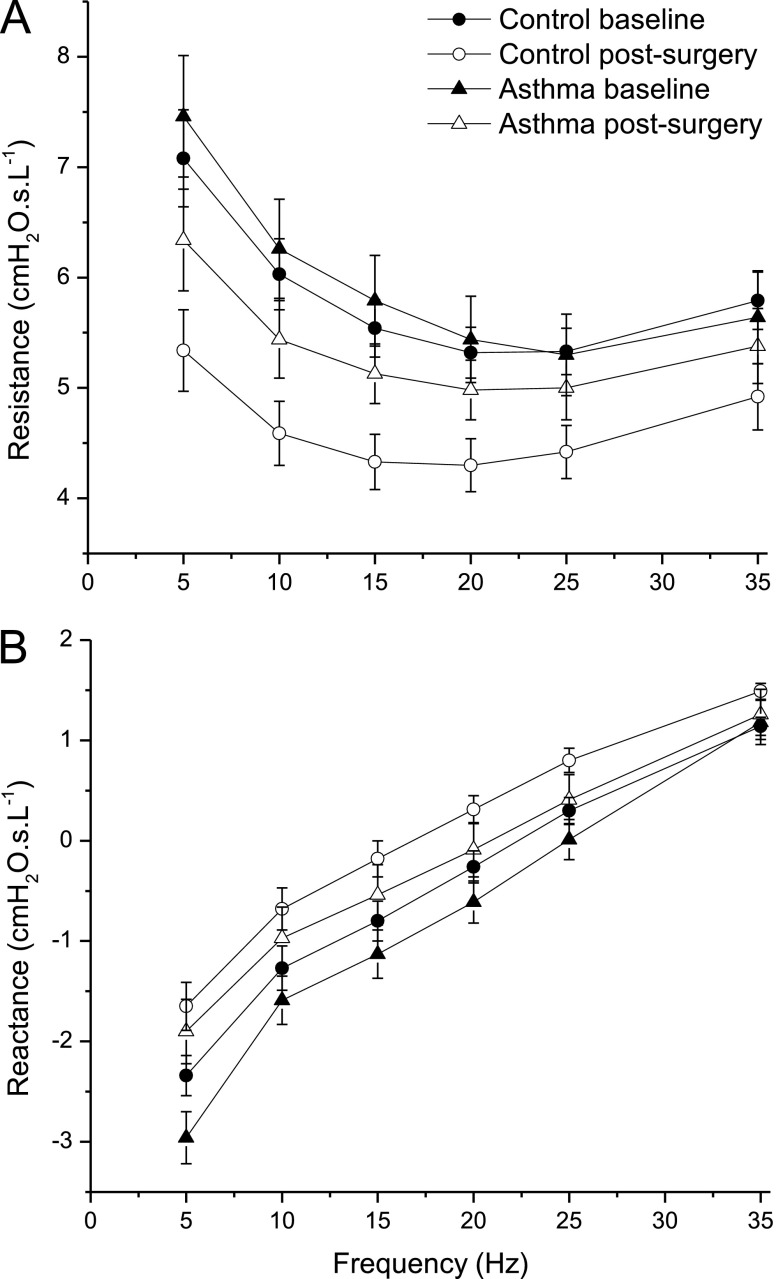
There were no significant differences in baseline impedance between patients with asthma and control subjects, although both resistance and reactance tended to be more dependent on frequency in the patients with asthma (Figures 2A and 2B, respectively).
Figure 2.
Respiratory system resistance (A) and reactance (B) measured (mean ± SE) in control subjects and patients with asthma both at baseline and at 12 months after bariatric surgery. Mixed models repeated measures analysis of variance showed a significant group × visit interaction for change in resistance in response to weight loss between patients with asthma and control subjects (P = 0.03 both unadjusted and adjusted for body mass index), but not for change in reactance (P = 0.68 unadjusted and P = 0.48 adjusted for body mass index).
Effects of Weight Loss on Lung Function Tests
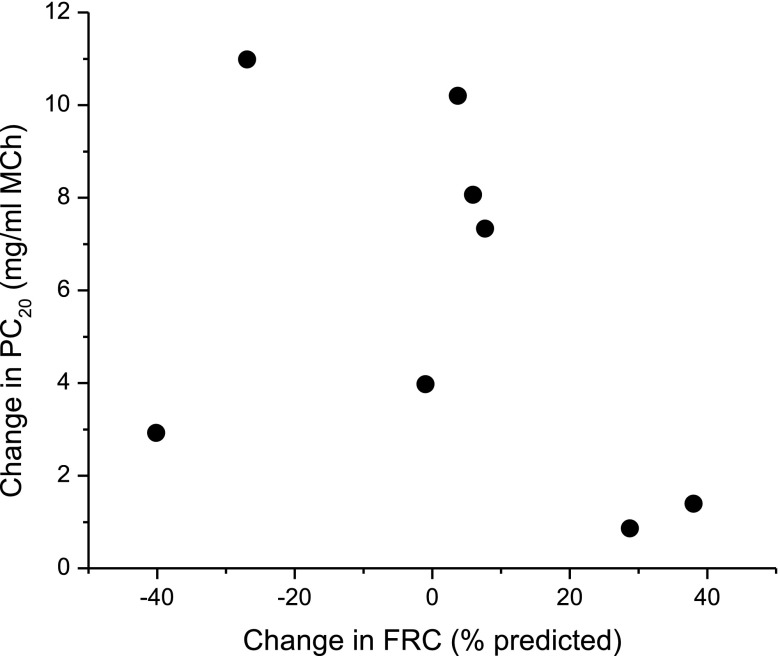
Twelve months after bariatric surgery, FEV1, FVC, FRC, and TLC increased significantly in control subjects, suggesting reduced lung restriction (Table 2). FEV1 and PC20 also improved significantly in patients with asthma after surgery (Table 3), but there was no change in either FRC or TLC in this group, and TLC and residual volume tended to increase with weight loss more in control subjects than in patients with asthma (Table 4). Bronchodilator responsiveness measured by FEV1 did not change with surgery in patients with asthma, although there was a trend toward less response in FVC (Table 3). This suggests unchanged airway tone, but perhaps less response in terms of airway closure, after weight loss and that the primary change with surgery was related to changes in airway closure and the lung periphery. There was no relationship between increase in FRC and improvement in PC20 in subjects with asthma (Figure 3; r = −0.4; P = 0.32). There was no change in diffusing capacity with weight loss. Patients with asthma and control subjects still tended to be obese after weight loss, at which point the patients with asthma were significantly heavier than the control subjects (Table 1).
Table 2.
Change in Pulmonary Function with Surgery in Control Subjects
| Baseline | 12 mo after Surgery | P Value | |
|---|---|---|---|
| FEV1 | 95.5 ± 7.0 | 100.7 ± 5.9 | <0.01 |
| FVC | 93.7 ± 8.9 | 98.6 ± 8.3 | <0.01 |
| FEV1/FVC | 102.2 ± 4.8 | 102.5 ± 6.3 | 0.84 |
| TLC | 84.8 ± 15.0 | 103.1 ± 15.3 | 0.01 |
| IC | 123.6 ± 16.3 | 122.0 ± 25.1 | 0.78 |
| SVC | 90.4 ± 9.0 | 95.3 ± 10.6 | 0.14 |
| FRC | 45.4 ± 18.5 | 62.1 ± 15.3 | 0.04 |
| ERV | 29.9 ± 21.5 | 49.8 ± 29.4 | <0.01 |
| RV | 81.7 ± 30.3 | 113.7 ± 38.4 | 0.07 |
| RV/TLC | 94.6 ± 28.3 | 108.1 ± 25.0 | 0.27 |
| DlCO | 93.0 ± 16.3 | 91.5 ± 14.1 | 0.74 |
Definition of abbreviations: DlCO = diffusing capacity of carbon monoxide; ERV = expiratory reserve volume; IC = inspiratory capacity; RV = residual volume; SVC = slow vital capacity.
Values are expressed as % predicted mean and standard deviation. P values are for paired t test. For all lung volume parameters and DlCO, n = 12 at baseline and n = 9 after surgery.
Table 3.
Change in Pulmonary Function with Surgery in Subjects with Asthma
| Baseline | 12 Months after Surgery | P Value | |
|---|---|---|---|
| FEV1 | 79.8 ± 10.6 | 87.2 ± 11.5 | 0.03 |
| FVC | 82.4 ± 13.2 | 87.5 ± 14.1 | 0.21 |
| FEV1 BD ∆* | 6.56 ± 3.94 | 6.55 ± 4.74 | 0.95 |
| FVC BD ∆* | 6.33 ± 4.56 | 3.22 ± 6.04 | 0.12 |
| FEV1/FVC | 97.6 ± 9.4 | 100.1 ± 6.5 | 0.50 |
| TLC | 87.7 ± 11.9 | 88.8 ± 21.2 | 0.87 |
| IC | 117.4 ± 19.7 | 103.3 ± 35.0 | 0.27 |
| SVC | 83.1 ± 12.5 | 85.6 ± 13.0 | 0.44 |
| FRC | 45.5 ± 10.0 | 46.7 ± 25.9 | 0.89 |
| RV | 98.5 ± 23.5 | 85.5 ± 52.8 | 0.47 |
| ERV | 21.1 ± 15.4 | 34.6 ± 25.8 | 0.20 |
| RV/TLC | 112.3 ± 22.6 | 90.2 ± 37.2 | 0.12 |
| DlCO | 94.8 ± 14.0 | 91.9 ± 11.5 | 0.56 |
| PC20 (mg/ml methacholine) | 4.7 ± 4.0 | 9.9 ± 6.4 | <0.001 |
Definition of abbreviations: BD = bronchodilator; DlCO = diffusing capacity of carbon monoxide; ERV = expiratory reserve volume; IC = inspiratory capacity; PC20 = provocative concentration of methacholine causing a 20% drop in FEV1; RV = residual volume; SVC = slow vital capacity.
Values are expressed as mean and standard deviation % predicted, except where indicated.
For all lung volume parameters and DlCO, n = 10 at baseline and n = 8 after surgery.
Values are % improvement with bronchodilator and standard deviation P values are for paired t test.
Table 4.
Difference in Changes over Time in Lung Function between Control Subjects and Patients with Asthma in Response to Bariatric Surgery
| Control Subjects Mean Difference (95% CI) | Patients with Asthma Mean Difference (95% CI) | P Value* | Adjusted P Value† | |
|---|---|---|---|---|
| FEV1 | 5.2 (0.9 to 9.4) | 7.4 (2.5 to 12.2) | 0.49 | 0.29 |
| FVC | 4.9 (−0.1 to 9.9) | 5.1 (−0.6 to 10.8) | 0.96 | 0.83 |
| FEV1/FVC | 0.3 (−4.3 to 4.8) | 2.5 (−2.7 to 7.7) | 0.50 | 0.51 |
| TLC | 17.1 (4.6 to 29.7) | 1.5 (−11.4 to 14.3) | 0.08 | 0.16 |
| IC | −2.7 (−20.6 to 15.2) | −13.2 (−31.6 to 5.1) | 0.4 | 0.64 |
| SVC | 3.1 (−2.9 to 9.1) | 3.0 (−3.0 to 9.0) | 0.98 | 0.95 |
| FRC | 15.8 (0.3 to 31.3) | 1.2 (−14.8 to 17.2) | 0.18 | 0.19 |
| ERV | 19.2 (3.2 to 35.2) | 12.6 (−3.7 to 29.0) | 0.55 | 0.37 |
| RV | 30.8 (−1.5 to 63.1) | −12.8 (−46.2 to 20.6) | 0.06 | 0.07 |
| RV/TLC | 12.2 (−12.4 to 36.9) | −22.0 (−47.5 to 3.4) | 0.06 | 0.06 |
| DlCO | −0.2 (−8.6 to 8.2) | −2.9 (−12.2 to 6.4) | 0.65 | 0.64 |
Definition of abbreviations: CI = confidence interval; DlCO = diffusing capacity of carbon monoxide; ERV = expiratory reserve volume; IC = inspiratory capacity; RV = residual volume; SVC = slow vital capacity.
Values are % predicted mean difference and 95% CI.
P values shown are for group × visit interaction results from mixed model repeated measures analyses of variance.
Adjusted P value with body mass index as a covariate in the mixed model repeated measures analysis of variance.
Figure 3.
Change in airway reactivity to methacholine (MCh) versus change in FRC with weight loss in subjects with asthma. PC20 = provocative concentration of methacholine causing a 20% drop in FEV1.
Effects of Weight Loss on Respiratory System Impedance
Weight loss had a significantly different effect on respiratory system impedance in the group with asthma compared with the control group.
Control subjects had a parallel shift and significant decrease in resistance at all frequencies after weight loss (Figure 2A; P < 0.05, comparing control subjects before and after). By contrast, patients with asthma exhibited a decreased dependence of resistance on frequency such that resistance was elevated at 5 Hz compared with control subjects but remained essentially the same by 35 Hz. When the impedance spectra were normalized to FRC, there was a significant group–visit interaction (P = 0.03 after transformation to achieve normality of distribution, which did not change when body mass index [BMI] was included in the model) for resistance, indicating that there was a significantly different response to weight loss in patients with asthma compared with control subjects. Change in resistance was significantly related to change in BMI in control subjects only (see Figure E1 in the online supplement).
Reactance became less negative in control subjects at all frequencies with weight loss (Figure 2B; P < 0.05 comparing control subjects before and after weight loss). Reactance became less negative in patients with asthma at 5 Hz with weight loss (Figure 2A; P < 0.01), with similar tendencies at the other frequencies. Overall, patients with asthma and control subjects responded similarly to weight loss: there was no significant group–visit interaction (P = 0.68) for change in reactance with weight loss (log transformation not needed).
Modeling Changes in Respiratory System Impedance
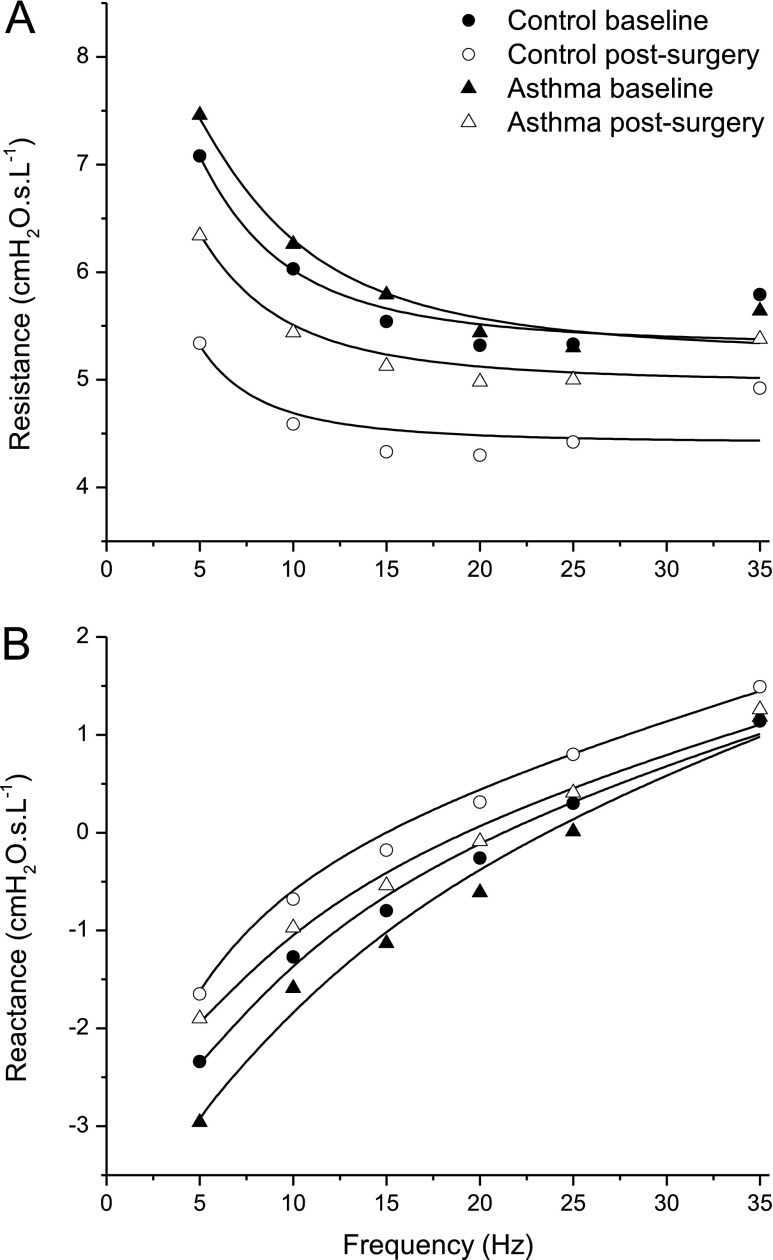
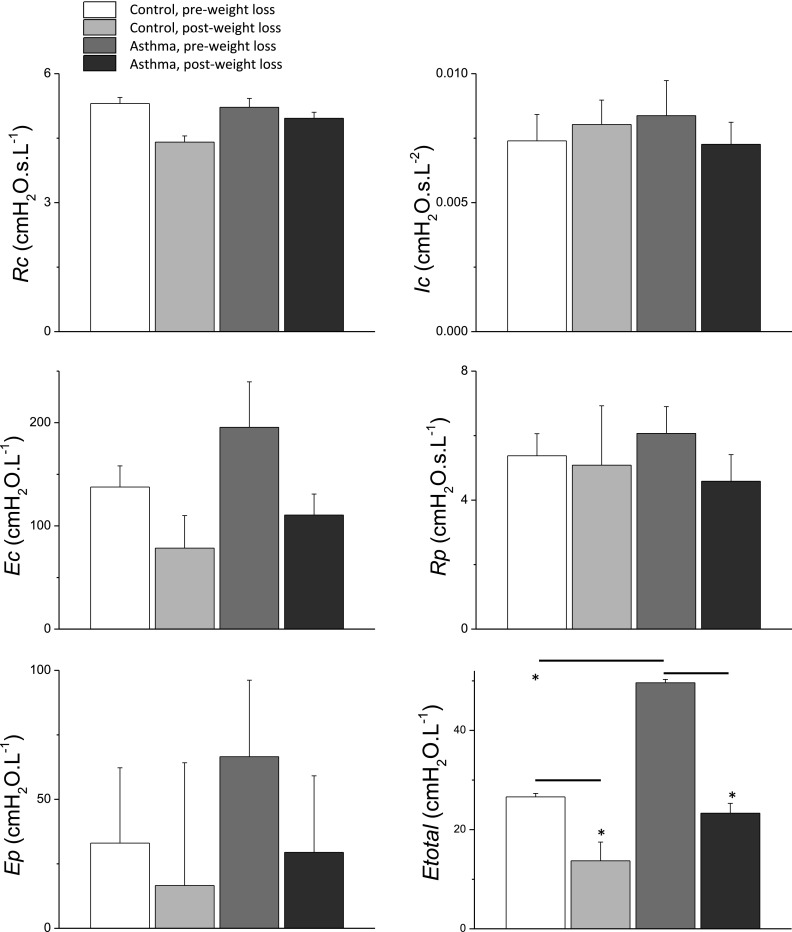
The impedance spectra obtained by fitting the model in Figure 1 to the mean data in Figure 2 are shown in Figure 4. The best-fit model parameter values are shown in Figure 5 along with their estimated standard deviations. The best-fit parameters vary considerably between the groups largely as a result of the vertical shifts in reactance between the groups, which translate into substantial differences in the two elastance parameters in the model. For example, weight loss caused peripheral elastance (Ep in Figure 5) to decrease in subjects with and without asthma. We interpret these changes as reflecting relief from lung compression. That is, a more compressed lung has less parenchymal tissue in communication with the airway opening and thus appears to be stiffer compared with a less compressed lung. A similar picture pertains to central elastance (Ec in Figure 5), and to a lesser extent to peripheral airway resistance (Rp in Figure 5). The only significant finding in any of these fitted model parameters, however, was the 17% decrease in central airway resistance (Rc in Figure 5) that occurred after weight loss in the control subjects, compared with virtually no change in the patients with asthma. However, if one assumes flow in the central airway to be laminar (22), a 17% decrease in resistance corresponds to a decrease in central airway radius of only 4.5%.
Figure 4.
Respiratory system resistance (A) and reactance (B) obtained by fitting the model illustrated in Figure 3 (solid lines) to the means of the experimental measurements (symbols). The best-fit model parameter values are shown in Figure 5.
Figure 5.
Parameter values determined by fitting the model shown in Figure 1 to the impedance data shown in Figure 2. The model parameter values were obtained by fitting the model to the mean impedance data, whereas the standard deviations shown by the error bars were obtained using a Monte Carlo approach described in the online supplement. The only significant difference among the individual model parameters is Rc in the control group after weight loss, which is different than the other three groups. Etotal, however, was significantly reduced by weight loss in both the control subjects and the patients with asthma, and was significantly greater in the patients with asthma compared with the control subjects before weight loss (*P < 0.05). Ec = central elastance; Ep = peripheral elastance; Etotal = total elastance; Ic = gas inertance; Rc = central resistance; Rp = peripheral resistance.
The lack of significant differences in model parameters between the various groups means that these parameters are quite sensitive to variations in the impedance data. This applies particularly to Ep, which controls the fraction of the imposed flow impulses that are shunted into the central airways. Changes in this fraction can be accommodated by relatively modest variations in Ec. This means that variations in the value of Ep are readily compensated for by changes in Ec, resulting in relatively little effect on how well the model fits the impedance data. We therefore calculated the total elastance of the model, Etotal, as a reflection of the combined effects of Ep and Ec (see online supplement for details of this calculation). In contrast to the individual model parameters, Etotal was significantly reduced by weight loss in both control subjects and patients with asthma, and was significantly greater in patients with asthma compared with control subjects before weight loss (Figure 5).
Discussion
The obese patients with asthma and obese control subjects in our study exhibited similar abnormalities in baseline lung function (Table 1), but differing responses to weight loss (Tables 2–4). These differences manifested most clearly as contrasting changes in respiratory impedance measured during normal breathing (Figure 2). In particular, obese control subjects experienced a parallel shift in both resistance and reactance with weight loss (Figure 2B). In contrast, obese patients with asthma had a significantly different change in resistance (Figure 2A) with weight loss compared with obese control subjects. Our modeling results (Figures 4 and 5) suggest that weight loss led to greater reductions in lung elastance in the patients with asthma, and that the patients with asthma started with greater elastance before weight loss. Etotal, which can be taken as a measure of overall lung derecruitment, was significantly elevated in the asthmatic postsurgery group, and was significantly reduced by weight loss in both groups (Figure 5). Taken together, these modeling results suggest that late-onset obese asthma is characterized by a lung periphery that is abnormally prone to collapse.
We anticipated finding that improvements in airway reactivity in patients with asthma would be related to increasing lung volume, and we initially hypothesized that these effects would be different compared with nonpatients with asthma. Interestingly, although we did find different effects of weight loss on lung volume, it was only in the nonpatients with asthma that FRC and TLC increased significantly with weight loss (Table 2). We also found no relationship between change in methacholine sensitivity and change in lung volume in our subjects (Figure 3), which is curious given that methacholine sensitivity and responsiveness have previously been shown to have a strong inverse dependence on FRC (12, 23). These findings seem to contradict our hypothesis that lung compression is behind the nonallergic form of obese asthma.
However, conventional lung function requires that subjects take a deep breath, which may mask phenomena of interest that arise when subtle changes in lung volume are at play. Accordingly, we also measured respiratory system impedance in our subjects using a method that avoided taking deep breaths. The impedance measurements showed significant differences in the responses to weight loss between patients with asthma and control subjects, but are somewhat difficult to interpret on their own. One possible explanation is differences in bronchodilator tone in patients with asthma with weight loss, but because change in FEV1 with bronchodilator did not change with weight loss, this is unlikely. Therefore, to help us understand the physiologic significance of these differences in impedance, we invoked a mathematical model of the respiratory system (Figure 1). This model mimics the main frequency-dependent features of both resistance and reactance, particularly in terms of the negative frequency dependence of resistance (Figure 4A) and the vertical shifts in reactance between the various groups (Figure 4B), allowing us to hypothesize that variations in peripheral lung collapsibility are what distinguishes the patients with asthma from the control subjects. Taken together, the effects of surgery on the model parameters shown in Figure 5 lead us to hypothesize that the peripheral airways and parenchyma of the asthmatic lungs were more compliant than those of the control lungs, making them more easily collapsed by lung compression. Such differences in collapsibility could have been caused by differences in the intrinsic stiffness of the airway wall. It is possible, therefore, that there exists within the normal population a range of airway wall stiffness for which lung function is normal when lung volumes are normal, but that the compliant end of this distribution becomes asthmatic when lung volumes are reduced through the effects of obesity.
The previous explanation ascribes the pathogenesis of nonallergic obese asthma purely to the effects of reduced lung volume, but other possibilities include (1) increased collapsibility of the airways caused by mechanical decoupling from the parenchyma, as can occur either as a result of peribronchial or alveolar fat accumulation (24) or during sleep (25); (2) abnormalities of surfactant function (26) leading to increased alveolar instability and collapse; and (3) the production by adipose tissue of mediators that may have both direct and indirect effects on the airways (27). These various explanations for nonallergic asthma cannot be distinguished using the data of the present study, but represent an important question that needs to be resolved.
Of course, none of the computational findings shown in Figures 4 and 5 prove that the mechanisms embodied in the model in Figure 1 are actually responsible for the experimental data, because we can never be sure that there is no other model with the same descriptive capability. Probably most at issue in this regard is the role of regional ventilation heterogeneity, such as would be modeled by two or more peripheral compartments acting in parallel, and which has been shown to accompany imposed reductions in lung volume (23). Parallel heterogeneity also increases the negative frequency dependence of resistance, but these effects occurred in our impedance data over the frequency range 5–35 Hz (Figure 2), which means that at least one of the compartments involved must have had a time-constant in the range 1/5–1/35 = 0.029–0.200 seconds. Such short time-constants are unlikely to arise from peripheral compartments experiencing compressive increases in airway resistance, which would be likely to increase time-constants above the normal value of about 0.2 seconds. Indeed, we have previously found time-constants of several seconds for the asthmatic lung periphery (28). A more likely source for a short time-constant is a high-elastance low-resistance compartment representing central airway stiffness, as in the model in Figure 1. Thus, although we certainly do not claim that parallel ventilation heterogeneity did not exist in our subjects, we believe that the observed frequency dependence of resistance above 5 Hz in these subjects was most likely caused by the effects of the imposed flow impulses being shunted into the central airways. We must also acknowledge that the model in Figure 1 does not account for every feature of the experimental data. For example, it does not reproduce the increases in resistance above 20 Hz (Figure 2), which probably reflect the effects of tissue inertance that give rise to a resonant peak in resistance around 80 Hz (29), which the model is not able to mimic.
The onset of airway hyperresponsiveness at low lung volumes has been investigated extensively in nonobese subjects (12, 23, 30, 31), and is often explained as being caused by an impaired ability to stretch the airway wall (32). However, although obese individuals do breathe at reduced FRC, they typically have increased tidal volumes and minute ventilations compared with lean individuals (33), and so their airway smooth muscle may actually undergo more deformation during tidal breathing than control subjects. These considerations further support the notion that there is something mechanically different about the lungs of those subjects destined to become asthmatic with obesity, compared with those who remain nonasthmatic. Our hypothesis about increased collapsibility of the asthmatic lungs also potentially explains the curious finding that FRC and TLC did not change after surgery in the patients with asthma (Table 3), whereas they both increased in control subjects (Table 2). That is, more than usually collapsible airways in the patients with asthma might have resulted in persistent derecruitment of peripheral airways even when the compressive influence of excessive adipose tissue was relieved.
Finally, we must be aware of certain limitations of our study that could have impacted the results, quite apart from any assumptions made in the modeling of impedance discussed previously. We did not measure the anatomic site of weight loss, which might have differed between patients with asthma and control subjects. We measured lung volumes by gas dilution (because not all subjects could fit into the body plethysmograph), so we would not have measured any gas trapped behind closed airways that would otherwise have added to our estimates of total lung volume. Also, the group with asthma remained significantly heavier than the control subjects after surgery (Table 1). It is thus possible that the patients with asthma did not lose enough weight to yield a significant improvement in lung volumes. It is also possible that some of the differences in Etotal shown in Figure 5 could reflect intergroup differences in BMI. Nevertheless, both groups lost a large amount of weight and the patients with asthma were lighter after surgery than the control subjects were at baseline. Also, the patients with asthma improved significantly in terms of airways responsiveness after surgery, so we are confident that the differences in their behaviors relative to the control subjects do indeed signify the presence of some specific pathophysiologic mechanism related to obesity.
In summary, in the small sample of obese subjects we studied we found that late-onset nonallergic asthma is not purely a consequence of breathing at low lung volumes. However, arguments based on changes in respiratory system impedance with weight loss, and what may happen to lung volumes in the presence of recalcitrant lung derecruitment, lead us to the novel hypothesis that obese patients with asthma are distinguished from obese control subjects by having excessive collapsibility of the lung periphery, perhaps as a consequence of reduced distal airway wall stiffness. Therapies aimed at recruiting the lung, and keeping it recruited, may thus have a place in the management of obese late-onset nonallergic patients with asthma.
Footnotes
Supported by National Institutes of Health grants P30 GM103532, M01 RR00109, and RR019965.
Author Contributions: A.A.-A. assisted with patient recruitment and procedures, and analysis, drafting, and revision of the manuscript. J.H.T.B. assisted with design of the study, analysis of data, modeling of data, and writing and revision of the manuscript. D.G.C. assisted with interpretation of data, and drafting and revision of the manuscript. D.A.K. assisted with design of the study, supervision of patient procedures, interpretation of data, and drafting and revision of the manuscript. M.J.D. assisted with data analysis, and drafting and revision of the manuscript. C.G.I. assisted with study design, interpretation of data, and drafting and revision of the manuscript. A.E.D. conceived the study, recruited patients, supervised patient procedures, assisted with analysis and interpretation of data, and drafting and revision of the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.201401-0178OC on May 12, 2014
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Chen Y, Dales R, Jiang Y. The association between obesity and asthma is stronger in nonallergic than allergic adults. Chest. 2006;130:890–895. doi: 10.1378/chest.130.3.890. [DOI] [PubMed] [Google Scholar]
- 2.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175:661–666. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dixon AE, Holguin F, Sood A, Salome CM, Pratley RE, Beuther DA, Celedón JC, Shore SA American Thoracic Society Ad Hoc Subcommittee on Obesity and Lung Disease. An official American Thoracic Society Workshop report: obesity and asthma. Proc Am Thorac Soc. 2010;7:325–335. doi: 10.1513/pats.200903-013ST. [DOI] [PubMed] [Google Scholar]
- 4.Taylor B, Mannino D, Brown C, Crocker D, Twum-Baah N, Holguin F. Body mass index and asthma severity in the National Asthma Survey. Thorax. 2008;63:14–20. doi: 10.1136/thx.2007.082784. [DOI] [PubMed] [Google Scholar]
- 5.Mosen DM, Schatz M, Magid DJ, Camargo CA., JrThe relationship between obesity and asthma severity and control in adults. J Allergy Clin Immunol2008. 122:507–511, e6 [DOI] [PubMed]
- 6.Peters-Golden M, Swern A, Bird SS, Hustad CM, Grant E, Edelman JM. Influence of body mass index on the response to asthma controller agents. Eur Respir J. 2006;27:495–503. doi: 10.1183/09031936.06.00077205. [DOI] [PubMed] [Google Scholar]
- 7.Camargo CA, Jr, Boulet LP, Sutherland ER, Busse WW, Yancey SW, Emmett AH, Ortega HG, Ferro TJ. Body mass index and response to asthma therapy: fluticasone propionate/salmeterol versus montelukast. J Asthma. 2010;47:76–82. doi: 10.3109/02770900903338494. [DOI] [PubMed] [Google Scholar]
- 8.Dixon A. The treatment of asthma in obesity. Expert Rev Respir Med. 2012;6:331–340. doi: 10.1586/ers.12.22. [DOI] [PubMed] [Google Scholar]
- 9.Dixon AE, Pratley RE, Forgione PM, Kaminsky DA, Whittaker-Leclair LA, Griffes LA, Garudathri J, Raymond D, Poynter ME, Bunn JY, et al. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. J Allergy Clin Immunol2011. 128:508–515, e1 [DOI] [PMC free article] [PubMed]
- 10.Holguin F, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Erzurum SC, Fitzpatrick AM, Gaston B, Israel E, Jarjour NN, et al. Obesity and asthma: an association modified by age of asthma onset. J Allergy Clin Immunol2011. 127:1486–1493, e2 [DOI] [PMC free article] [PubMed]
- 11.Sideleva O, Suratt BT, Black KE, Tharp WG, Pratley RE, Forgione P, Dienz O, Irvin CG, Dixon AE. Obesity and asthma: an inflammatory disease of adipose tissue not the airway. Am J Respir Crit Care Med. 2012;186:598–605. doi: 10.1164/rccm.201203-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding DJ, Martin JG, Macklem PT. Effects of lung volume on maximal methacholine-induced bronchoconstriction in normal humans. J Appl Physiol (1985) 1987;62:1324–1330. doi: 10.1152/jappl.1987.62.3.1324. [DOI] [PubMed] [Google Scholar]
- 13.Chapman DG, Berend N, King GG, Salome CM. Increased airway closure is a determinant of airway hyperresponsiveness. Eur Respir J. 2008;32:1563–1569. doi: 10.1183/09031936.00114007. [DOI] [PubMed] [Google Scholar]
- 14.Mahadev S, Farah CS, King GG, Salome CM. Obesity, expiratory flow limitation and asthma symptoms. Pulm Pharmacol Ther. 2013;26:438–443. doi: 10.1016/j.pupt.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Salome CM, Munoz PA, Berend N, Thorpe CW, Schachter LM, King GG. Effect of obesity on breathlessness and airway responsiveness to methacholine in non-asthmatic subjects. Int J Obes (Lond) 2008;32:502–509. doi: 10.1038/sj.ijo.0803752. [DOI] [PubMed] [Google Scholar]
- 16.Mahadev S, Salome CM, Berend N, King GG. The effect of low lung volume on airway function in obesity. Respir Physiol Neurobiol. 2013;188:192–199. doi: 10.1016/j.resp.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 17.Al-Alwan A, Kaminsky DA, Bates JHT, Irvin CG, Dixon AE. Differential effects of bariatric surgery on lung function in asthmatics and controls. Am J Respir Crit Care Med. 2012;185:A3953. [Google Scholar]
- 18.Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, MacIntyre NR, McKay RT, Wanger JS, Anderson SD, et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161:309–329. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 19.Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, Casaburi R, Crapo R, Enright P, van der Grinten CP, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26:511–522. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 20.American Thoracic Society. 1994 Update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 21.Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–735. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 22.Bates JHT.Lung mechanics. An inverse modeling approach. Cambridge: Cambridge University Press; 2009 [Google Scholar]
- 23.Chapman DG, Berend N, Horlyck KR, King GG, Salome CM. Does increased baseline ventilation heterogeneity following chest wall strapping predispose to airway hyperresponsiveness? J Appl Physiol (1985) 2012;113:25–30. doi: 10.1152/japplphysiol.01582.2011. [DOI] [PubMed] [Google Scholar]
- 24.Foster DJ, Ravikumar P, Bellotto DJ, Unger RH, Hsia CC. Fatty diabetic lung: altered alveolar structure and surfactant protein expression. Am J Physiol Lung Cell Mol Physiol. 2010;298:L392–L403. doi: 10.1152/ajplung.00041.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irvin CG, Pak J, Martin RJ. Airway-parenchyma uncoupling in nocturnal asthma. Am J Respir Crit Care Med. 2000;161:50–56. doi: 10.1164/ajrccm.161.1.9804053. [DOI] [PubMed] [Google Scholar]
- 26.Brown LA, Longmore WJ. Altered phospholipid secretion in type II pneumocytes isolated from streptozotocin-diabetic rats. Biochim Biophys Acta. 1986;878:258–265. doi: 10.1016/0005-2760(86)90154-2. [DOI] [PubMed] [Google Scholar]
- 27.Sideleva O, Dixon A. The many faces of asthma in obesity. J Cell Biochem. 2014;115:421–426. doi: 10.1002/jcb.24678. [DOI] [PubMed] [Google Scholar]
- 28.Kaminsky DA, Bates JH, Irvin CG. Effects of cool, dry air stimulation on peripheral lung mechanics in asthma. Am J Respir Crit Care Med. 2000;162:179–186. doi: 10.1164/ajrccm.162.1.9806079. [DOI] [PubMed] [Google Scholar]
- 29.Jackson AC, Giurdanella CA, Dorkin HL. Density dependence of respiratory system impedances between 5 and 320 Hz in humans. J Appl Physiol (1985) 1989;67:2323–2330. doi: 10.1152/jappl.1989.67.6.2323. [DOI] [PubMed] [Google Scholar]
- 30.Chapman DG, Berend N, King GG, Salome CM. Effect of deep inspiration avoidance on ventilation heterogeneity and airway responsiveness in healthy adults. J Appl Physiol (1985) 2011;110:1400–1405. doi: 10.1152/japplphysiol.00855.2010. [DOI] [PubMed] [Google Scholar]
- 31.Skloot G, Permutt S, Togias A. Airway hyperresponsiveness in asthma: a problem of limited smooth muscle relaxation with inspiration. J Clin Invest. 1995;96:2393–2403. doi: 10.1172/JCI118296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fredberg JJ. Frozen objects: small airways, big breaths, and asthma. J Allergy Clin Immunol. 2000;106:615–624. doi: 10.1067/mai.2000.109429. [DOI] [PubMed] [Google Scholar]
- 33.Matos CM, Moraes KS, França DC, Tomich GM, Farah MW, Dias RC, Parreira VF. Changes in breathing pattern and thoracoabdominal motion after bariatric surgery: a longitudinal study. Respir Physiol Neurobiol. 2012;181:143–147. doi: 10.1016/j.resp.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 34.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 35.Goldman HI, Becklake MR. Respiratory function tests; normal values at median altitudes and the prediction of normal results. Am Rev Tuberc. 1959;79:457–467. doi: 10.1164/artpd.1959.79.4.457. [DOI] [PubMed] [Google Scholar]
- 36.Gaensler EA, Wright GW. Evaluation of respiratory impairment. Arch Environ Health. 1966;12:146–189. doi: 10.1080/00039896.1966.10664355. [DOI] [PubMed] [Google Scholar]