Abstract
Rationale: Secondhand smoke exposure (SHSe) is a significant modifiable risk for respiratory health in children. Although SHSe is declining overall, it has increased for low-income and minority populations. Implementation of effective SHSe interventions within community organizations has the potential for significant public health impact.
Objectives: To evaluate the effectiveness of motivational interviewing (MI) delivered in the context of a SHS education reduction initiative within Head Start to reduce preschool children’s SHSe.
Methods: A total of 350 children enrolled in Baltimore City Head Start whose caregivers reported a smoker living in the home were recruited. Caregivers were randomized to MI + education or education alone. Assessments were conducted at baseline, 3, 6, and 12 months.
Measurements and Main Results: The primary outcome measure was household air nicotine levels measured by passive dosimeters. Secondary outcomes included child salivary cotinine, self-report of home smoking ban (HSB), and smoking status. Participants in the MI + education group had significantly lower air nicotine levels (0.29 vs. 0.40 mg), 17% increase in prevalence of caregiver-reported HSBs, and a 13% decrease in caregiver smokers compared with education-alone group (all P values < 0.05). Although group differences in salivary cotinine were not significant, among all families who reported having an HSB, salivary cotinine and air nicotine levels declined in both groups (P < 0.05).
Conclusions: MI may be effective in community settings to reduce child SHSe. More research is needed to identify ways to tailor interventions to directly impact child SHSe and to engage more families to make behavioral change.
Clinical trial registered with www.clinicaltrials.gov (NCT 00927264).
Keywords: secondhand smoke, preschool children, community engaged research, randomized controlled trial
At a Glance Commentary
Scientific Knowledge on the Subject
Secondhand smoke exposure in children is a significant modifiable risk for respiratory health. Although efficacious behavioral interventions have been identified, they are rarely evaluated in settings that serve the most at-risk populations.
What This Study Adds to the Field
This study presents the results of a randomized trial evaluating motivational interviewing to reduce Head Start children’s’ secondhand smoke exposure.
Secondhand smoke exposure (SHSe) is a significant threat to children’s respiratory health (1). Young children are particularly at risk to the detrimental effects of SHSe because they are still growing, have higher respiration rates, and are more frequently near adults who smoke (2). SHSe during early life is causally associated with the development of a variety of respiratory tract problems (1), including asthma (3, 4), sudden infant death syndrome (5), middle ear disease (6), pneumonia, and bronchitis (7, 8).
Because low-income and minority families are at increased risk for SHSe, it is important to develop and evaluate tailored interventions for these groups (9). Counseling interventions are efficacious in reducing SHSe and encouraging parental smoking cessation (10). One strategy, motivational interviewing (MI), a patient-centered counseling technique (11), has shown promise in helping low-income and urban caregivers of children to quit smoking and/or reduce their children’s SHSe (12–17). Despite the efficacy of MI, it has not been routinely implemented in community settings targeting low-income populations.
Head Start programs serve approximately 1 million low-income, predominantly minority preschool children and families nationwide. In 2007, the Office of Head Start acknowledged the importance of reducing SHSe by entering into a Memorandum of Understanding with the Environmental Protection Agency (EPA) to develop cooperative activities and materials that promote awareness of the health effects of SHS (18). This initiative presented a unique opportunity to evaluate the effectiveness of implementing an efficacious behavioral intervention, MI, within a community setting that serves the population most at risk for high SHSe.
This project evaluated a SHSe reduction intervention with demonstrated efficacy (MI) (12, 16) within the context of Head Start SHSe education based on the EPA initiative. We hypothesized that the MI + education group would have significantly greater reduction in SHSe by the implementation of home smoking bans (HSBs) or smoking cessation compared with the education-alone group. The primary study outcome measure was changes in objectively measured household levels of air nicotine over time between groups. Secondary outcomes included caregiver-reported household smoking bans, child’s salivary cotinine levels, and caregiver-reported smoking status. Some of the results of these studies have been previously reported in the form of an abstract (19).
Methods
Participants
Participants were recruited from 16 Baltimore City Head Start programs from April 2009 to August 2012 with final data collection ending August 2013. Eligible caregivers had to be the parent or legal guardian of a child aged 6 months to 6 years, who reported one or more smokers living in the home, and who spoke English. The enrolled caregiver did not have to be the smoker. An eligibility screening questionnaire was given to all enrolled students annually by Head Start staff. When 80% of a class was screened, staff was compensated $50 for their effort; payment was provided for questionnaire completion, which included the option not to complete the form and regardless of study eligibility or enrollment. The forms asked if the Head Start student lived with a smoker, if they had an HSB, interest in being contacted about the research study, and contact information. Eligible families who gave permission were contacted by telephone to confirm eligibility and schedule the first home visit to obtain written informed consent from the child’s caregiver.
Procedures
The Johns Hopkins School of Medicine Institutional Review Board approved the study. Assessments were completed at baseline, 3, 6, and 12 months. Research assistants conducted two home visits at each time point. During the first home visit, the research assistant collected two salivary cotinine samples from the child and attached two air nicotine monitors. The second home visit was completed 7 days later when the research assistant collected two more child salivary cotinine samples, detached the air nicotine monitors, and conducted a caregiver survey. Caregivers were offered compensation ($50) for completing the full assessment at each time point. Families were randomized to either the MI + education group or the education-alone group using a block randomization scheme of groups of 10 to ensure equal group sizes. Randomization schema was developed by statistician using a random number generator. Randomization assignments were placed into sealed envelopes, which were opened after families completed baseline surveys. Research assistants that completed assessments were not masked to intervention condition.
Intervention Conditions
SHSe Head Start education program.
Each school year all Head Start students, in both conditions, received EPA Smoke Free Home educational activities and materials (available at http://www.epa.gov/smokefree/publications.html). Table E1 in the online supplement outlines which Head Start programs received any of the two components discussed next.
Staff training workshops.
Yearly workshops were offered to Head Start staff to discuss the health risks of SHSe to young children; the importance of limiting children’s SHSe; and specific behavioral strategies for educating, motivating, and assisting families to create a smoke-free home.
Expert facilitation of Head Start educational activities.
Faculty served as expert consultants for Head Start to develop and implement SHSe activities. Each program was encouraged to develop individual activities to meet their program’s needs for caregiver education days, or SHS awareness programs.
MI + Education
Participants randomized to MI + education were eligible to participate in the education activities during the school year and to receive MI. The MI intervention was adapted from previous efficacious programs (12, 16). Over 3 months we offered the caregivers four counseling sessions (∼15–30 min in length) plus one booster session (15 min) after the 3-month assessment for a total of five sessions. The Health Counselor’s (HC) overarching goal for the intervention was to motivate and assist the caregiver in reducing the child’s SHSe using the methods discussed next.
Feedback on SHSe.
The HC provided individualized feedback on child salivary cotinine levels at two time points: at baseline and at the booster visit. This feedback was provided in a nonthreatening manner consistent with MI principles and visual diagrams were used to facilitate understanding.
Discussing child SHSe.
The HC engaged the caregiver in a conversation about the child’s SHSe (20). Within this context the HC asked open-ended questions to understand the caregiver’s perceptions of the risk of SHSe. The HC sought to elicit self-motivational statements to build the connection between SHSe and their child’s health.
Awareness building and exploring ambivalence.
The HC and caregiver discussed ways to reduce the child’s SHSe. The HC asked the caregiver to rate how motivated and confident he or she was to change on a scale of 1–10. The HC also asked about the pros and cons of making changes and then provided a summary of the caregiver’s ambivalence, highlighting discrepancies between current behavior and what is most important to them (21).
Implementation of HSB.
The primary goal of the intervention was to help caregivers implement an HSB to reduce the child’s SHSe. Communication training was provided to all caregivers on how to ask other people not to smoke in the home and no-smoking signs were provided.
Smoking cessation.
If any family member indicated an interest in quitting smoking, the HC referred the family to appropriate educational materials and assisted in developing a quit plan. Referrals were provided to both local and state resources for cessation counseling and concomitant nicotine-replacement therapy. To the extent possible, the HC attempted to engage all smokers living in the home in smoking cessation, if applicable.
The HCs received 2 full days of in-person training in MI by a certified Motivational Interviewing Trainer (B.B.), which included didactics, demonstrations, role plays, reading assignments, and video. Skill acquisition was assessed via role playing, simulated study participant interviews, and pilot participants. HCs used a treatment manual to ensure standardization. Over 20% of the counseling sessions were reviewed for reliable MI adherence to the treatment protocol during supervision sessions. Additional training was conducted every 6 months to promote the maintenance of skills and to minimize drift from the intervention protocol. All HCs had previous counseling training and at least bachelors’ degrees.
The intervention was originally developed so that sessions 1 and 2 would be completed in person and sessions 3–5 would be completed by telephone. Unfortunately, one HC witnessed a homicide on the way to a home visit. To accommodate staff safety concerns, the protocol was changed to have all five sessions delivered by telephone. Eighty-three participants (50%) were offered home and telephone visits, whereas the remaining participants were offered only telephone visits.
Measures
Two objective indices of SHSe were collected: air nicotine and salivary cotinine. These are recognized as the best currently available biomarkers of SHSe (22, 23). Previous research has demonstrated that although these measures are correlated they are not identical and measure different aspects of SHSe (household compared with child level of exposure) (24).
Primary Outcome
Air nicotine was monitored using passive sampling dosimeters (25) that have been shown to be both reliable and valid (26). Monitors were placed for 7 days in the location where the child slept and another room identified as a “major activity room” by the caregiver. The air monitor relies on passive diffusion of nicotine to the filter where it is trapped. Monitors were analyzed by the Johns Hopkins Bloomberg School of Public Health for nicotine concentrations (mg/m3) using gas chromatography with nitrogen-selective detection. The detection limit of the air monitor is 0.01 μg and the coefficient of variability is 0.11 (25). For quality control a fixed random sampling procedure was used to collect a blank and duplicate sample for every 10 samples. A review of all field blank monitors recorded 0 mg and a comparison of the duplicate monitors showed greater than 0.95 correlations at all times points demonstrating high reliability. For analyses, means of the two air nicotine monitors were calculated at each time point.
Secondary Outcomes
Salivary cotinine.
Salivary cotinine analyses served as a secondary measure of child’s SHSe, which has been shown to be a well-accepted measurement of SHSe in young children (27). Saliva samples were collected with two sorbettes using a standard protocol developed for children. Samples were immediately placed on ice and then frozen within hours at −20°C. They were stored frozen, batched, and transported on ice to the Johns Hopkins University Center for Interdisciplinary Salivary Bioscience Research where they were stored frozen at −80°C until assay. Samples were assayed for cotinine using a commercially available enzyme immunoassay without modification to the manufacturer’s protocol (Salimetrics, State College, PA). The test used 20 ml of sample (10 μl saliva diluted in 90 μl of assay diluent); had a lower limit of sensitivity of 0.05 ng/ml; range of sensitivity from 0.05 to 200 ng/ml; and average intraassay and interassay coefficients of variation of less than 10% and 15%, respectively. For analyses the two cotinine samples taken 7 days apart were averaged together at each time point.
HSBs.
Caregivers were asked to select which statement best reflects the household rule about smoking in the home: (1) no smoking is allowed in my home with no exceptions, (2) smoking is sometimes allowed in my home, or (3) there are no rules about where or when people can smoke in my home. HSB questions such as these have been shown to be associated with urine cotinine levels in a Head Start population (28) and degree of reported smoking among inner-city black smokers (29). A response of (1) was coded as a present complete HSB and responses (2) and (3) were coded as an absent complete HSB.
Caregiver smoking status.
Caregivers were asked to if they had smoked any cigarettes, even a puff, in the last 7 days at each assessment time point to determine if they were a current smoker or not.
Statistical Analyses
Baseline characteristics were compared across the groups. Initial intent-to-treat (ITT) analyses compared the MI + education group with the education-alone group at each time-point for outcome measures. Generalized estimating equation (GEE) was used to estimate the group population average for each outcome over time to control for the correlation among longitudinal measures within an individual, while adjusting for baseline level of each outcome. For each outcome, time and intervention group were modeled as categorical fixed effects of outcomes. To determine an intervention effect at each time point the GEE interaction of treatment × time was evaluated. Both air nicotine and salivary cotinine were log transformed because of nonnormal distribution. For binary and continuous outcomes, Bernoulli and normal models were specified, respectively. All models were conducted controlling for baseline number of people who smoked in the home as a covariate. Post hoc analyses were conducted with families who still reported a sustained HSB at 12 months in the MI + education group to determine if there were reductions in SHSe for these families in air nicotine or salivary cotinine. Weighted GEE analyses were conducted to evaluate for the impact of missing data for all of the models. Because of the change in intervention delivery modality, differences in completion rates and outcomes were compared within the MI group between those who received home and telephone or telephone-only visits. Stata 11 (College Station, TX) was used for all data analyses.
Results
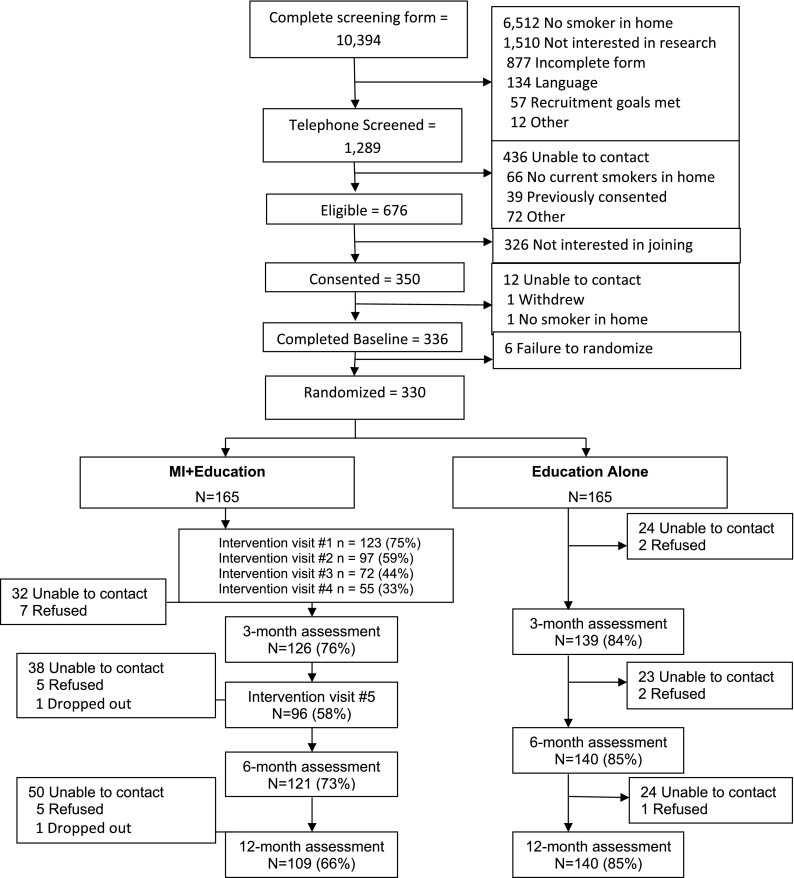
A total of 10,394 (88% of all children enrolled at Head Start) caregivers completed screening. Twenty-eight percent of children lived with a smoker; 1,289 (12%) were both eligible and provided permission to be contacted for the study (Figure 1). Overall, 350 caregivers of children who reported a smoker living in the home were consented, 336 completed the baseline assessment, and 330 were randomized to either the intervention arm (MI + education) or to the control arm (education alone). Baseline demographic characteristics are shown in Table 1. All participants had some detectable measure of SHSe at baseline (Table 2). Over 70% of enrolled caregivers were smokers, with an average of 1.4 ± 1.2 smokers per home. Only 24% had an HSB at baseline. There were group differences in the number of people who smoked in the home, air nicotine, and salivary cotinine at baseline, with the MI + education group having more people who smoked in the home and higher levels of air nicotine and salivary cotinine. Families who received the MI intervention via telephone only had significantly higher intervention visit completion rates (P < 0.01) than families who had home and telephone visits but there were no differences on the outcome variables.
Figure 1.
CONSORT diagram. MI = motivational interviewing.
Table 1.
Baseline Demographics of Randomized Sample
| Group Comparison |
||||
|---|---|---|---|---|
| Characteristics | Overall (N = 330) N (%) | MI + Education (N = 165) N (%) | Education Alone (N = 165) N (%) | P Value |
| Child characteristics | ||||
| Age, mean (SD) | 3.76 ± 0.8 | 3.81 ± 0.8 | 3.71 ± 0.8 | 0.25 |
| African American | 302 (91.5) | 153 (92.7) | 149 (90.3) | 0.43 |
| Female | 165 (50.0) | 87 (52.7) | 78 (47.3) | 0.32 |
| Diagnosis of asthma | 94 (29) | 45 (27.4) | 49 (29.7) | 0.7 |
| Caregiver characteristics | ||||
| Age, mean (SD) | 32.1 (8.9) | 32.07 (8.6) | 32.12 (9.2) | 0.96 |
| Number of smokers in the home, mean (SD) | 1.8 ± 0.06 | 1.9 ± 0.08 | 1.6 ± 0.07 | 0.01 |
| Caregiver smoker | 232 (70) | 122 (73.9) | 110 (66.7) | 0.03 |
| Relationship to child | 0.44 | |||
| Mother | 252 (76.4) | 124 (75.2) | 128 (77.6) | |
| Father | 40 (12.1) | 23 (13.9) | 17 (10.3) | |
| Grandmother | 22 (6.7) | 11 (6.7) | 11 (6.7) | |
| Other | 16 (4.8) | 7 (4.2) | 9 (5.4) | |
| Caregiver education | 0.94 | |||
| Some high school | 97 (29.3) | 49 (29.6) | 48 (29.1) | |
| High school/GED | 115 (34.9) | 54 (32.7) | 61 (36.9) | |
| Some college or trade school | 97 (29.4) | 50 (30.3) | 47 (28.5) | |
| Four-yr college | 10 (3.0) | 5 (3.0) | 5 (3.0) | |
| Missing | 11 (3.3) | 7 (4.2) | 4 (2.4) | |
| Household income | 0.15 | |||
| <$10,000/yr | 98 (29.7) | 43 (26.1) | 55 (33.3) | |
| $10,000–20,000/yr | 83 (25.2) | 45 (27.3) | 38 (23.0) | |
| $20,000–30,000/yr | 60 (18.2) | 33 (20.0) | 27 (16.4) | |
| $30,000–40,000/yr | 52 (15.8) | 30 (18.2) | 22 (13.3) | |
| >$40,000/yr | 29 (8.79) | 10 (6.0) | 19 (11.5) | |
| Missing | 8 (2.42) | 4 (2.4) | 4 (2.4) | |
Definition of abbreviation: MI = motivational interviewing.
Table 2.
Descriptive Statistics of Outcome Variables and GEE Results by Group over Time
| MI + Education | Education Alone | Parameter Estimate | 95% Confidence Interval | |
|---|---|---|---|---|
| Air nicotine, median (IQR) | ||||
| Baseline | 1.15 (0.24 to 2.92) | 0.58 (0.08 to 2.01) | 0.63 | 0.20 to 1.06 |
| 3 mo | 0.71 (022 to 1.88) | 0.51 (0.06 to 1.72) | −0.16 | −0.5 to 0.17 |
| 6 mo | 0.75 (0.22 to 2.08) | 0.52 (0.05 to 1.61) | −0.07 | −0.41 to 0.26 |
| 12 mo | 0.55 (0.07 to 1.37) | 0.32 (0.04 to 1.50) | −0.33 | −0.67 to −0.01 |
| Salivary cotinine, median (IQR) | ||||
| Baseline | 4.14 (1.18 to 7.51) | 3.00 (1.34 to 6.26) | 0.27 | 0.03 to 0.51 |
| 3 mo | 4.14 (1.63 to 6.97) | 3.25 (1.24 to 6.42) | −0.02 | −0.21 to 0.17 |
| 6 mo | 3.61 (1.55 to 7.41) | 3.05 (1.32 to 5.92) | −0.11 | −0.33 to 0.11 |
| 12 mo | 4.04 (2.14 to 7.17) | 2.84 (1.38 to 6.07) | −0.02 | −0.26 to 0.22 |
| Complete home smoking ban, n (%) | ||||
| Baseline | 34 (20.6) | 47 (28.5) | 0.65 | 0.39 to 1.08 |
| 3 mo | 46 (36.5) | 49 (35.5) | 1.72 | 1.05 to 2.80 |
| 6 mo | 43 (35.6) | 57 (40.7) | 1.38 | 0.81 to 2.37 |
| 12 mo | 44 (39.3) | 57 (40.7) | 1.48 | 0.85 to 2.60 |
| Caregiver smoker status, n (%) | ||||
| Baseline | 122 (73.9) | 110 (66.7) | 1.42 | 0.88 to 2.28 |
| 3 mo | 83 (65.9) | 85 (61.6) | 0.51 | 0.27 to 0.94 |
| 6 mo | 76 (62.8) | 88 (62.9) | 0.71 | 0.36 to 1.40 |
| 12 mo | 68 (60.7) | 84 (60.0) | 0.42 | 0.51 to 0.83 |
Definition of abbreviations: GEE = generalized estimating equation; IQR = interquartile range; MI = motivational interviewing.
A total of 165 families were each randomized to the MI + education or education-alone (Figure 1). Most of the families (75%) completed session one of the MI intervention but only 33% completed all four sessions and 58% completed the booster session five. The Head Start Education program was implemented at all Head Start programs (see Table E1). Investigators and research staff attended routine staff health advisory meetings (n = 14 programs), staffed booths at health fairs to distribute information about the harmful effects of SHSe (n = 12 programs), or implemented activities to promote smoke-free days (n = 6 programs).
Follow-up assessment retention differed significantly between groups (P < 0.01) with 73% and 66% of the MI + education group completing the 6- and 12-month assessments compared with 85% of the education-alone group completing both assessments. Baseline characteristics were examined to determine if they predicted missingness. Caregiver smokers were less likely to have data at 12 months (P < 0.01) and households having a higher number of smokers in the home were less likely to have data at 6 months (P < 0.05). Baseline levels of air nicotine, salivary cotinine, presence of an HSB, race, age, income, and employment status were not associated. Weighted GEE analyses were conducted to evaluate the impact of missing data on the models (30). There were no differences in the P values for any of the models, so the unweighted GEE models are presented next.
Air Nicotine
A total of 2,557 monitors were placed and 107 (4.2%) were returned with damage with no group or time differences. There was an overall decline in air nicotine for both groups at 12 months (b = −0.34; P < 0.01). There also was a significant time × treatment interaction for the MI + education group at 12 months compared with education-alone group in ITT analysis (b = −0.33; P < 0.05) (Table 2 for GEE results and Figure 2A) The nicotine monitor in the bedroom had significant declines in the MI group at 12 months (b = −0.38; P < 0.03). In post hoc analyses of air nicotine levels, there was a significant time × presence of HSB interaction among families in the MI + education group who reported an HSB at 6 months (b = −0.67; P < 0.05) and at 12 months (b = −1.04; P < 0.001).
Figure 2.
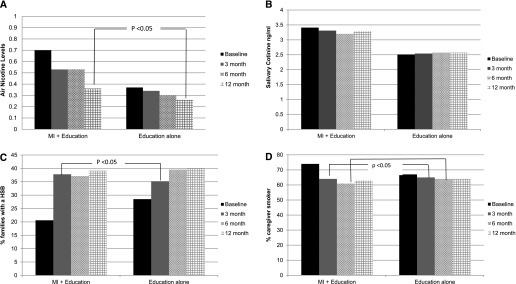
Generalized estimating equation predicted means of secondhand smoke exposure outcomes by intervention group over time. (A) Reduction in air nicotine predicted means by intervention group over time. (B) Reduction in salivary cotinine predicted means by intervention group over time. (C) Predicted prevalence of home smoking bans (HSB) by intervention group over time. (D) Predicted prevalence of caregiver smoker status by intervention group over time. MI = motivational interviewing.
Salivary Cotinine
For child salivary cotinine, a significant time × treatment interaction was not observed at any time point in ITT analysis on either the mean of the two samples or individual measures of cotinine. In post hoc analyses there was a significant reduction in child salivary cotinine at 12 months (b = −0.39; P < 0.05) (Table 2 for GEE results and Figure 2B) or families in the MI + education group who reported an HSB.
HSB
The prevalence of HSBs significantly increased for both groups at 6 and 12 months (odds ratio [OR], 1.92, P < 0.01; OR, 2.02, P < 0.01) (Table 2 for GEE results and Figure 2C). There was a significant time × treatment interaction for the MI + education group with a 17% increase (37% total) in prevalence of HSBs at 3 months compared with the education-alone group (OR, 1.7; P < 0.05). The proportion of families reporting an HSB remained the same at 6 months and increased to 39% at 12 months but was no longer significant because the education-alone group had a 7% increase in HSB at 3 months (total of 35%) and an additional increase of 4–39% at 12 months.
Caregiver Smoking Status
The number of caregivers who reported smoking decreased significantly in the MI + education group compared with the education-alone group at 3 and 6 months (OR, 0.65, P < 0.04; OR, 0.63; P < 0.03; respectively) (Table 2 for GEE results and Figure 2D). Furthermore, in post hoc analyses, caregivers who reported having an HSB at 12 months were more likely to not be a smoker at 12 months (OR, 0.44; P < 0.04) with a trend at 3 months (OR, 0.53; P < 0.08).
Discussion
The purpose of this clinical trial was to evaluate the effectiveness of implementing MI within a community setting of Head Start. The MI + education program had a modest effect in reducing household air nicotine levels, increasing the prevalence of HSBs and caregiver smoking cessation. Although there was no direct effect of child salivary cotinine levels in ITT analyses, families who implemented an HSB had significantly lower air nicotine and salivary cotinine, indicating that families who made a recommended behavior change reduced objective measures of SHSe. Although the proportion of families who made behavioral changes were small, this is an at-risk population that were not seeking intervention and may have significant health impact on not only the child but all family members.
For interventions to have the most significant public health impact, they need to be implemented within settings that serve the target populations. Therefore, partnering with Baltimore City Head Start allowed us to implement and evaluate the effectiveness of MI with a high-risk population. Implementation of universal SHSe screening for all Head Start children allowed us to not only identify and recruit families for the clinical trial but also to assess prevalence of SHSe among this high-risk population. Furthermore, the MI intervention was able to significantly reduce Head Start children’s SHSe, which is a population at high risk for respiratory illnesses, such as asthma, which are strongly associated with SHSe (31).
Adaption and implementation of efficacious SHSe interventions into established community settings offers the potential to have broad, positive public health impact for vulnerable populations, such as low-income children. The Head Start Education program was integrated into the programs and the education-alone group had improvements in HSB implementation, although less than the MI + education group.
Although our intervention was successful in reducing household air nicotine levels and increasing the prevalence of HSB, there was no difference in child SHSe as measured by salivary cotinine. This result is similar to other behavioral studies that evaluated MI to reduce child SHSe (14, 32). One possible explanation is that low-income children are exposed to SHS in multiple settings beyond their home, including relatives, caregivers, and even in outside urban settings (e.g., bus stops), such as porch stoops where families gather in the summer. In addition, young children may need to accompany the caregiver when they smoke outside for safety reasons. Another explanation is that the presence of thirdhand smoke on the caregiver, carpeting, or household surfaces could raise a child’s salivary cotinine measurement (33). Future community intervention studies need to focus on how to increase full implementation of SHSe protection for children across all settings, and how to successfully promote smoking cessation for low-income families (34).
There are limitations to this study. Because smoking cessation was not the primary outcome of the intervention, caregiver smoking status was not biochemically verified. It is notable that the level of interest and participation at the population level was low indicating a significant overall lack of interest of families in participating in an SHSe reduction intervention. However, there are no other studies to compare with because previous trials were not designed to assess and/or report the overall reach of their intervention (10). Our comprehensive Head Start SHSe screening program provided important new data on both children’s exposure and the challenge of engaging families in SHSe reduction Our study sample was all low-income, with over 50% earning less than $20,000 a year and predominately (91%) African American. Although this may limit our generalizability to other samples, it can be seen as a strength of the study because it identified an effective intervention for a highly vulnerable population.
SHSe is a substantial yet modifiable threat to children’s health that could be significantly reduced with more systematic implementation of effective behavioral interventions with families that are at risk. Community settings that serve high-risk children, such as Head Start, are ideally suited for implementing behavioral and educational interventions that help families reduce children’s SHSe. Next steps would include partnering with the federal Office of Head Start to develop implementation efforts on a larger scale to have a broader impact. Additional research is needed on the cost effectiveness of these interventions to guide implementation efforts and the impact of MI on smoking cessation in this population. However, to achieve successful implementation of SHSe interventions within fiscally challenged community programs, such as Head Start, it will require the will to change policy and a commitment to the funding necessary to ensure sustainability to improve the health of the children they serve.
Acknowledgments
Acknowledgment
The authors acknowledge Catherine Deangelis for her comments in preparing this manuscript and Amanda Diaz, Angela Green, and Alana Ridge for their assistance in conducting the study.
Footnotes
Supported by National Heart Lung Blood Institute grant HL092901.
Author Contributions: M.N.E. contributed to study design, supervised data collection and analyses, and authored most of the manuscript. A.B. oversaw data collection and conducted all the statistical analyses. C.S.R. contributed to the study design, oversaw data collection, and assisted in manuscript preparation. B.B. contributed to study design, designed and supervised the intervention, and assisted in manuscript preparation. M.H. contributed to study design and assisted in manuscript preparation. K.A.R. contributed to study design, supervised study implementation, and assisted in manuscript preparation. All authors read and approved the final version of the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.201404-0618OC on May 12, 2014
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Treyster Z, Gitterman B. Second hand smoke exposure in children: environmental factors, physiological effects, and interventions within pediatrics. Rev Environ Health. 2011;26:187–195. doi: 10.1515/reveh.2011.026. [DOI] [PubMed] [Google Scholar]
- 2.US Department of Health and Human ServicesThe health consequences of involuntary exposure to tobacco smoke: a report of the Surgeon GeneralAtlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006 [Google Scholar]
- 3.Burke H, Leonardi-Bee J, Hashim A, Pine-Abata H, Chen Y, Cook DG, Britton JR, McKeever TM. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics. 2012;129:735–744. doi: 10.1542/peds.2011-2196. [DOI] [PubMed] [Google Scholar]
- 4.Neogi T, Neher JO, Safranek S. Clinical inquiry. How does smoking in the home affect children with asthma? J Fam Pract. 2012;61:292–293. [PubMed] [Google Scholar]
- 5.Mitchell EA, Milerad J. Smoking and the sudden infant death syndrome. Rev Environ Health. 2006;21:81–103. doi: 10.1515/reveh.2006.21.2.81. [DOI] [PubMed] [Google Scholar]
- 6.Jones LL, Hassanien A, Cook DG, Britton J, Leonardi-Bee J. Parental smoking and the risk of middle ear disease in children: a systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2012;166:18–27. doi: 10.1001/archpediatrics.2011.158. [DOI] [PubMed] [Google Scholar]
- 7.Moskowitz WB, Schwartz PF, Schieken RM Medical College of Virginia. Childhood passive smoking, race, and coronary artery disease risk: the MCV Twin Study. Arch Pediatr Adolesc Med. 1999;153:446–453. doi: 10.1001/archpedi.153.5.446. [DOI] [PubMed] [Google Scholar]
- 8.Jones LL, Hashim A, McKeever T, Cook DG, Britton J, Leonardi-Bee J. Parental and household smoking and the increased risk of bronchitis, bronchiolitis and other lower respiratory infections in infancy: systematic review and meta-analysis. Respir Res. 2011;12:5. doi: 10.1186/1465-9921-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.US Environmental Protection Agency (EPA)National Survey on Environmental Management of Asthma and Children's Exposure to Environmental Tobacco Smoke (NSEMA/CEE). Washington, DC: US Environmental Protection Agency; 2004 [Google Scholar]
- 10.Rosen LJ, Noach MB, Winickoff JP, Hovell MF. Parental smoking cessation to protect young children: a systematic review and meta-analysis. Pediatrics. 2012;129:141–152. doi: 10.1542/peds.2010-3209. [DOI] [PubMed] [Google Scholar]
- 11.Miller WR, Rollnick S.Motivational interviewing: preparing people for change2nd ed.New York: Guilford Press; 2002 [Google Scholar]
- 12.Borrelli B, McQuaid EL, Novak SP, Hammond SK, Becker B. Motivating Latino caregivers of children with asthma to quit smoking: a randomized trial. J Consult Clin Psychol. 2010;78:34–43. doi: 10.1037/a0016932. [DOI] [PubMed] [Google Scholar]
- 13.Blaakman S, Tremblay PJ, Halterman JS, Fagnano M, Borrelli B.Implementation of a community-based secondhand smoke reduction intervention for caregivers of urban children with asthma: process evaluation, successes and challenges Health Educ Res 201328141–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emmons KM, Hammond SK, Fava JL, Velicer WF, Evans JL, Monroe AD. A randomized trial to reduce passive smoke exposure in low-income households with young children. Pediatrics. 2001;108:18–24. doi: 10.1542/peds.108.1.18. [DOI] [PubMed] [Google Scholar]
- 15.Halterman JS, Szilagyi PG, Fisher SG, Fagnano M, Tremblay P, Conn KM, Wang H, Borrelli B. Randomized controlled trial to improve care for urban children with asthma: results of the School-Based Asthma Therapy trial. Arch Pediatr Adolesc Med. 2011;165:262–268. doi: 10.1001/archpediatrics.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borrelli B, McQuaid EL, Becker B, Hammond K, Papandonatos G, Fritz G, Abrams D. Motivating parents of kids with asthma to quit smoking: the PAQS project. Health Educ Res. 2002;17:659–669. doi: 10.1093/her/17.5.659. [DOI] [PubMed] [Google Scholar]
- 17.Hettema JE, Hendricks PS. Motivational interviewing for smoking cessation: a meta-analytic review. J Consult Clin Psychol. 2010;78:868–884. doi: 10.1037/a0021498. [DOI] [PubMed] [Google Scholar]
- 18.US Environmental Protection AgencyUS Department of Health and Human Services. Memorandum of Understanding (MOU) to Reduce Health Risks. Washington, DC: USEPA/USDHHS; 2007
- 19.Eakin MN, Bilderback A, Borrelli B, et al. Effectiveness of motivational interviewing to reduce Head Start children's secondhand smoke exposure [abstract] Am J Respir Crit Care Med. 2013;187:A2333. doi: 10.1164/rccm.201404-0618OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rollnick S, Mason P, Butler C.Health behavor change: a guide for practitionersNew York: Churchill Livingston; 1999 [Google Scholar]
- 21.Miller WR, Rollnick S.Motivational interviewing: preparing people to change addictive behaviorNew York: Guilford Press; 1991 [Google Scholar]
- 22.Apelberg BJ, Hepp LM, Avila-Tang E, Gundel L, Hammond SK, Hovell MF, Hyland A, Klepeis NE, Madsen CC, Navas-Acien A, et al. Environmental monitoring of secondhand smoke exposure Tob Control 201322147–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Priest N, Roseby R, Waters E, Polnay A, Campbell R, Spencer N, Webster P, Ferguson-Thorne G. Family and carer smoking control programmes for reducing children’s exposure to environmental tobacco smoke. Cochrane Database Syst Rev. 2008;(4):CD001746. doi: 10.1002/14651858.CD001746.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Hovell MF, Zakarian JM, Matt GE, Liles S, Jones JA, Hofstetter CR, Larson SN, Benowitz NL. Counseling to reduce children’s secondhand smoke exposure and help parents quit smoking: a controlled trial. Nicotine Tob Res. 2009;11:1383–1394. doi: 10.1093/ntr/ntp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammond SK, Leaderer BP. A diffusion monitor to measure exposure to passive smoking. Environ Sci Technol. 1987;21:494–497. doi: 10.1021/es00159a012. [DOI] [PubMed] [Google Scholar]
- 26.Leaderer BP, Hammond SK. Evaluation of vapor-phase nicotine and respirable suspended particle mass as markers for environmental tobacco smoke. Environ Sci Technol. 1991;25:770–777. [Google Scholar]
- 27.Bernert JT, Jr, McGuffey JE, Morrison MA, Pirkle JL. Comparison of serum and salivary cotinine measurements by a sensitive high-performance liquid chromatography-tandem mass spectrometry method as an indicator of exposure to tobacco smoke among smokers and nonsmokers. J Anal Toxicol. 2000;24:333–339. doi: 10.1093/jat/24.5.333. [DOI] [PubMed] [Google Scholar]
- 28.Yousey YK. Household characteristics, smoking bans, and passive smoke exposure in young children. J Pediatr Health Care. 2006;20:98–105. doi: 10.1016/j.pedhc.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Okah FA, Okuyemi KS, McCarter KS, Harris KJ, Catley D, Kaur H, Ahluwalia JS. Predicting adoption of home smoking restriction by inner-city black smokers. Arch Pediatr Adolesc Med. 2003;157:1202–1205. doi: 10.1001/archpedi.157.12.1202. [DOI] [PubMed] [Google Scholar]
- 30.Preisser JS, Lohman KK, Rathouz PJ. Performance of weighted estimating equations for longitudinal binary data with drop-outs missing at random. Stat Med. 2002;21:3035–3054. doi: 10.1002/sim.1241. [DOI] [PubMed] [Google Scholar]
- 31.Eakin MN, Rand CS, Bilderback A, Bollinger ME, Butz A, Kandasamy V, Riekert KA.Asthma in Head Start children: effects of the Breathmobile program and family communication on asthma outcomes. J Allergy Clin Immunol2012129664–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stotts AL, Green C, Northrup TF, Dodrill CL, Evans P, Tyson J, Velasquez M, Hammond SK, Hovell MF. Feasibility and efficacy of an intervention to reduce secondhand smoke exposure among infants discharged from a neonatal intensive care unit. J Perinatol. 2013;33:811–816. doi: 10.1038/jp.2013.43. [DOI] [PubMed] [Google Scholar]
- 33.Matt GE, Quintana PJ, Destaillats H, Gundel LA, Sleiman M, Singer BC, Jacob P, Benowitz N, Winickoff JP, Rehan V, et al. Thirdhand tobacco smoke: emerging evidence and arguments for a multidisciplinary research agenda. Environ Health Perspect. 2011;119:1218–1226. doi: 10.1289/ehp.1103500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hilliard ME, Riekert KA, Hovell MF, Rand CS, Welkom JS, Eakin MN. Family beliefs and behaviors about smoking and young children's secondhand smoke exposure. Nicotine Tob Res. (In press) doi: 10.1093/ntr/ntu250. [DOI] [PMC free article] [PubMed] [Google Scholar]