Abstract
Rationale: HIV-1–induced interstitial pneumonitis (IP) is a serious complication of HIV-1 infection, characterized by inflammation and cellular infiltration in lungs, often leading to respiratory failure and death. The barrier function of the pulmonary endothelium is caused in part by tight junction (TJ) proteins, such as claudin-5. Peroxisome proliferator–activated receptor (PPAR)-γ is expressed in lung tissues and regulates inflammation. We hypothesize that HIV-1 induces vascular lung injury, and HIV-1–mediated damage of the pulmonary endothelium and IP is associated with dysregulation of PPAR-γ.
Objectives: Investigate the effects of HIV-1 infection on the pulmonary microvasculature and the modulatory effects of the PPAR-γ ligands.
Methods: Using human lung tissues, we demonstrated down-regulation of claudin-5 (marker of pulmonary barrier integrity), down-regulation of PPAR-γ transcription, and expression in lung tissues of HIV-1–infected humans with IP.
Measurements and Main Results: Human lung microvascular endothelial cells expressed the TJ proteins claudin-5, ZO-1, and ZO-2; HIV-1 decreased TJ proteins expression and induced nuclear factor-κB promoter activity, which was reversed by PPAR-γ agonist. Using two murine HIV/AIDS models, we demonstrated decreased claudin-5 expression and increased macrophage infiltration in the lungs of HIV-1–infected animals. Activation of PPAR-γ prevented HIV-1–induced claudin-5 down-regulation and significantly reduced viremia and pulmonary macrophage infiltration.
Conclusions: HIV-induced IP is associated with injury to the lung vascular endothelium, with decreased TJ and PPAR-γ expression, and increased pulmonary macrophage infiltration. PPAR-γ ligands abrogated these effects. Thus, regulation of PPAR-γ can be a therapeutic approach against HIV-1–induced vascular damage and IP in infected humans. Removal of Expression of Concern: Issues leading to the previous expression of concern for this article have been resolved after further revisions and editorial review. No further concerns exist.
Keywords: pulmonary endothelium, tight junction proteins, HIV/AIDS, macrophages, PBMC
At a Glance Commentary
Scientific Knowledge on the Subject
Peroxisome proliferator–activated receptor (PPAR)-γ has been shown to regulate inflammation, and PPAR-γ agonists possess antiinflammatory properties. HIV-induced interstitial pneumonitis (IP) is a serious complication of HIV infection, characterized by increased interstitial leukocyte infiltration in the alveolar septae, and fibrosis eventually leading to respiratory failure. However, the pathogenesis of IP associated with HIV remains unknown. We hypothesize that HIV-induced vascular lung injury and HIV-induced injury of the pulmonary endothelium and IP are associated with PPAR-γ dysregulation.
What This Study Adds to the Field
We show here for the first time that HIV-induced IP in humans is associated with decreased transcription and expression of PPAR-γ and claudin-5, a marker of endothelial and microvascular barrier integrity, in the lungs. Using in vitro and in vivo studies, we also demonstrate that PPAR-γ modulates HIV-1–induced vascular injury, nuclear factor-κB promoter activity, viral infection, and macrophage infiltration into the lungs. Thus, modulation of PPAR-γ could provide a therapeutic approach to prevent vascular injury, leukocyte infiltration into the lungs, and pneumonitis in HIV-infected individuals.
The lung is a major target for HIV-1 infection, and ongoing pulmonary complications often indicate enhanced progression to an AIDS-defining illness (1). Despite modern antiretroviral therapy, pulmonary involvement is present in 80–94% of patients with AIDS, being a major cause of morbidity and mortality, and respiratory failure is a major cause of death in patients with HIV/AIDS (2, 3). HIV-1 is present in the respiratory tract of seropositive subjects at all stages of infection, and 75–85% of AIDS autopsies showed evidence of lung pathology (1).
Interstitial pneumonitis (IP) is a serious complication of HIV-1 infection characterized by interstitial inflammation and progressive fibrosis (4, 5). A study of 250 patients with HIV/AIDS who died of acute respiratory failure showed that 40% had acute interstitial pneumonia, and 36% showed diffuse alveolar damage (3). In regions with high rates of HIV infection, the prevalence of severe pneumonia among infected children ranges from 35% to 70% (3, 6, 7), and the case fatality rate was three to eight times higher than in noninfected children (7).
Peroxisome proliferator–activated receptors (PPARs) are ligand-activated transcription factors belonging to the nuclear hormone receptor superfamily and include three subtypes (PPAR-α, PPAR-γ, and PPAR-β/δ) that exhibit differential tissue distribution and ligand specificities (8). PPAR-γ is ubiquitously expressed in many tissues and cell types, including endothelial and smooth muscle cells of the pulmonary vasculature (8, 9). PPAR-γ plays a key role in the regulation of genes mediating inflammatory responses (10, 11). Both natural and synthetic PPAR-γ agonists possess antiinflammatory properties and inhibit the expression of cytokines, chemokines, and adhesion molecules (10, 12). PPAR agonists have also been shown to inhibit HIV infection in macrophages, peripheral blood mononuclear cells (PBMC), dendritic cells, and chronically infected cell lines (13); and activation of PPARs attenuates HIV-1–induced inflammation in brain endothelial cells (14).
Claudins, transmembrane proteins, are part of tight junction (TJ) complexes present in endothelial and epithelial cells that maintain intercellular barriers (15, 16). TJ localized at the apical cell-cell contacts of endothelial and epithelial cells create a physiologic intercellular barrier, maintain cell polarity, regulate the paracellular permeability of solutes, and prevent transmigration of leukocytes (16, 17). There are 24 claudins; claudin-5, a 23-kD protein, has been shown to be specifically expressed in endothelial TJ (15) with high expression in the brain and lung vascular endothelium (15, 18). Claudin-5 is exclusively concentrated at cell-cell borders of endothelial cells of blood vessels but not at those of epithelial cells (15) indicating that claudin-5 is an endothelial cell–specific component of TJ. Analysis of human lung tissues also showed that during all developmental stages claudin-5 was strongly expressed in endothelial cells of alveolar septae (19). Lung injury was associated with decreased mRNA and protein levels of claudin-5 and other TJ proteins (20), resulting in increased permeability (21).
The pulmonary endothelium forms a semiselective barrier that regulates fluid balance and leukocyte trafficking between the vascular and extravascular spaces. PPAR-γ is ubiquitously present in lung tissue of humans and animals, is highly expressed in pulmonary endothelial cells, and plays a major role in the pathobiology of lung diseases (8, 9, 22). We hypothesize that HIV-1 induces lung injury, and HIV-1–induced injury of the pulmonary endothelium and IP in patients with HIV/AIDS is associated with dysregulation of PPAR-γ. To this end, we investigated the effect of HIV-1 infection on the pulmonary vasculature, and the modulatory effects of the PPAR-γ ligands, using human lungs tissues, human primary cells, and HIV/AIDS animal models.
Methods
Cell Isolation and Viral Infection
Monocytes, PBMC, and peripheral blood lymphocytes (PBL) were obtained by countercurrent centrifugal elutriation of leukopheresis packs from HIV-1, HIV-2, and hepatitis B–seronegative donors, and monocyte-derived macrophages (MDM) obtained as previously described (23). Cell culture, HIV-1 infection, and experiments assessing the effect of PPAR-γ on viral infection were performed as detailed in the online supplement.
RNA Isolation and Real-Time Polymerase Chain Reaction
Lung tissues from HIV-1–seropositive patients with IP or bronchopneumonia and aged-matched seronegative control subjects (Table 1) were obtained from the National NeuroAIDS Tissue Consortium and the National Disease Research Interchange (Philadelphia, PA). RNA extraction and real-time polymerase chain reaction (RT-PCR) analyses were performed as detailed in the online supplement.
Table 1.
Clinical History of Human Lung Tissue Donors
| HIV Status | Patient ID | Sex/Age (yr) | Blood CD4+ Cell Count | Blood Viral Load | Pulmonary Autopsy Diagnosis |
|---|---|---|---|---|---|
| Negative | N1 | M/50 | Normal/none | ||
| Negative | N2 | M/46 | Normal/none | ||
| Negative | N3 | M/67 | Normal/none | ||
| Negative | N4 | M/58 | Normal/none | ||
| Negative | N5 | M/46 | Normal/none | ||
| Negative | N6 | M/43 | Normal/none | ||
| Positive | P1 | M/43 | 12 | 90,258 | Interstitial pneumonitis |
| Positive | P2 | M/32 | 125 | <400 | Interstitial pneumonitis |
| Positive | P3 | M/54 | 336 | <400 | Interstitial pneumonitis, pulmonary edema |
| Positive | P4 | M/64 | 96 | 2,064 | Interstitial pneumonitis |
| Positive | P5 | F/44 | 66 | >750,000 | Interstitial pneumonitis, bronchopneumonia |
| Positive | P6 | F/38 | 10 | 223,100 | Interstitial pneumonitis |
| Positive | P7 | M/39 | 11 | 474,239 | Lymphocytic interstitial pneumonitis |
| Positive | P8 | M/70 | N/A | N/A | Diffuse alveolar damage, pulmonary edema |
| Positive | P9 | M/46 | 1,217 | 2,649 | Bronchopneumonia |
| Positive | P10 | M/47 | 150 | >750,000 | Bronchopneumonia |
| Positive | P11 | M/51 | 36 | 3,137 | Bronchopneumonia |
Protein Extraction, Western Blot Analysis, and Immunofluorescence Microscopy
Primary human lung microvascular endothelial cells (HLMEC) were purchased from Lonza (Walkersville, MD) and cultured in EGM-2 MV BulletKit media (Lonza). Protein extraction from cells and lung tissues, Western blot analyses, and microscopy were performed as previously described (23) and as detailed in the online supplement.
Human-PBL-NOD/SCID Mice Model
This model was described in our previous publications (24, 25), and experimental procedures are detailed in the online supplement.
Humanized HIV Mice Model
Humanized NOD/scid-IL-2Rγcnull (NSG) mice were developed by transplantation of newborn mice with CD34+ hematopoietic stem cells from human fetal liver as previously described (26–28), infected at 19–22 weeks of age, and viral load (VL) quantified as detailed in the online supplement. Age-matched noninfected, nonreconstituted, and reconstituted noninfected animals served as control subjects.
Flow Cytometry, Histopathologic Evaluation, and Cytotoxicity
To quantify human and murine cells in animal lung tissues, single-cell suspension was prepared from lung tissues and analyzed by fluorescence-activated cell sorter (FACS). FACS, histopathologic and cytotoxicity procedures are detailed in the online supplement.
Results
Down-regulation of Claudin-5 Transcription and Expression in the Lungs of HIV-infected Humans with IP
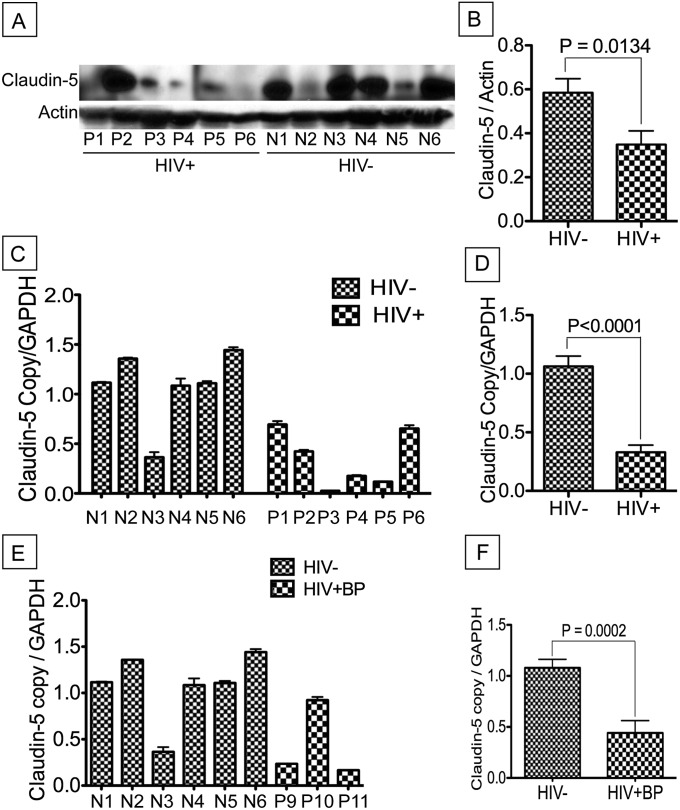
To determine whether HIV-1–induced IP was associated with alterations of pulmonary vascular endothelium in HIV-1–infected humans, we assessed the expression of claudin-5 in lung tissues of six HIV-1– and HIV-2–seronegative control subjects and 11 HIV+ patients with IP/pneumonia (HIV+IP). Table 1 shows demographic information and summary of post-mortem findings for all 17 human lung donors. The age range was 43–67 years for seronegative control subjects and 32–70 years for HIV+IP patients. Pathologists from National NeuroAIDS Tissue Consortium performed pathologic evaluation rendering HIV-IP diagnoses. Blood CD4+ T-cell count and VL were available for 10 HIV+ lung donors. CD4+ T-cell count was 12, 125, 336, 96, 66, 10, 11, 1,217, 150, and 36 /mm3, respectively, for patients P1–P7 and P9–P11. Low CD4+ T-cell count was generally associated with high VL. Patients P5 and P10 had a VL greater than 750,000 copies per milliliter; for patients P1, P6, and P7, VL was, respectively, 90,258, 223,100, and 474,239 copies per milliliter. For patients P4, P9, and P11, VL was, respectively, 2,064, 2,649, and 3,137 copies per milliliter; and for patients P2 and P3, VL was less than 400 copies per milliliter.
Western blot analyses of available protein samples showed significant decrease of claudin-5 expression in lung tissues of HIV+IP patients compared with seronegative control subjects (P = 0.0003) (Figures 1A and 1B). Quantitative RT-PCR further showed significant down-regulation of claudin-5 mRNA in lung tissues of HIV+IP patients (P < 0.0001) (Figures 1C and 1D) and HIV+ patients with bronchopneumonia (P = 0.0002) (Figures 1E and 1F) compared with HIV-seronegative control subjects.
Figure 1.
Decreased expression of claudin-5 in lung tissues of HIV-1–infected humans with interstitial pneumonitis (IP). Western blot (A) and real-time polymerase chain reaction (C and D) analyses of lung tissues from HIV-1–infected humans with IP showed overall decreased levels of claudin-5 protein (A), and decreased claudin-5 mRNA (C and D), compared with lung tissues from seronegative human control subject. Computer-assisted densitometry analyses of all protein samples and real-time polymerase chain reaction data of all samples further showed a significant decrease in claudin-5 expression (B) (P = 0.0003) and transcription (D) (P < 0.0001) in lung tissues of HIV-infected humans with IP. Additional analyses also showed decreased claudin-5 mRNA in two of the three HIV+ patients with bronchopneumonia (HIV+BP, E and F). Data shown in A–C are from 12 donors; data in E and F are from nine donors. Donors N1–N6 are control HIV-1–seronegative donors, donors P1–P6 are HIV-1–seropositive donors with IP, and P9–P11 are HIV-1–seropositive donors with bronchopneumonia. For A, M is the molecular weight marker. Demographics and clinical history of each donor are detailed in Table 1. Error bars represent the SEM. GAPDH = glyceraldehyde phosphate dehydrogenase.
HLMEC Expressed TJ Proteins and HIV-1 Decreased TJ Expression
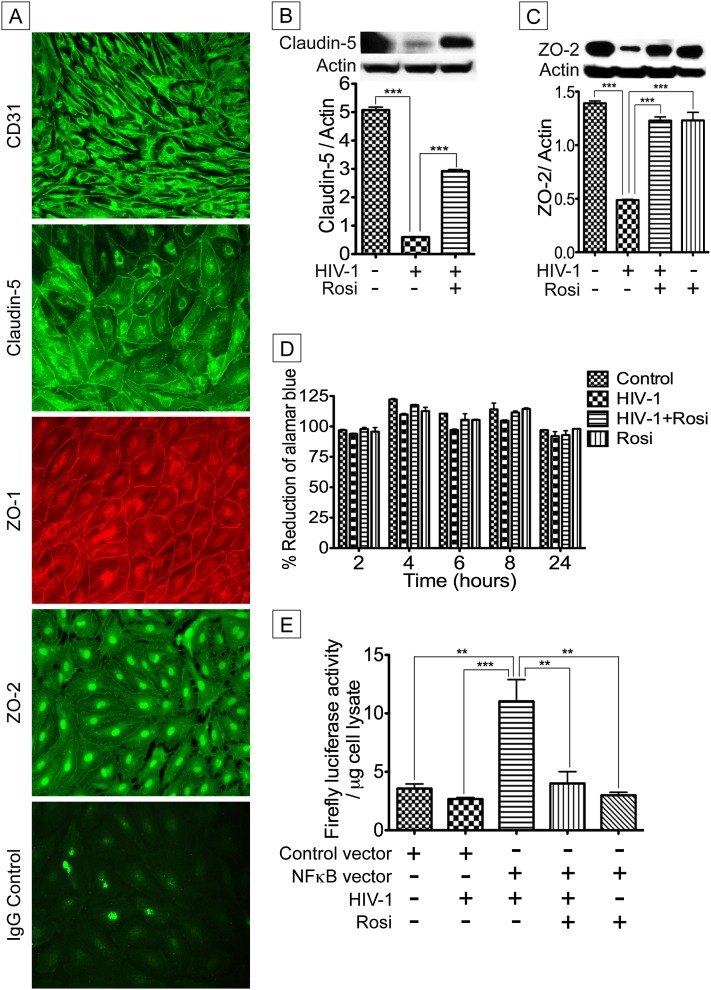
It has been demonstrated that claudin-5 is localized at cell borders of endothelial cells in blood vessels and is an endothelial-specific component of TJ strands (15). Thus, it is likely that claudin-5 down-regulation in the lungs of HIV+IP patients occurs through viral-induced endothelial damage, especially damage of TJ proteins in pulmonary endothelial cells. To test this hypothesis, we first determined whether HLMEC express TJ proteins. Immunofluorescence analyses showed that primary HLMEC expressed the TJ proteins claudin-5, ZO-1, and ZO-2 (Figure 2A). The platelet endothelial cell adhesion molecule-1 CD31, abundant at intercellular junctions of endothelial cells, is a known endothelial cell marker (29). Strong immunoreactivity of HLMEC for CD31 confirmed the endothelial nature of these cells (Figure 2A). Next, we determined the effects of HIV-1 on HLMEC, and the effects of PPAR-γ agonist on viral-induced effects. Exposure of HLMEC to HIV-1 virions (HIV-1UG001; MOI 0.01; 24 h) induced down-regulation of claudin-5 and ZO-2; the PPAR-γ agonist rosiglitazone (25 μM) partially reversed HIV-1–induced down-regulation of both TJ proteins (Figures 2B and 2C). To assess toxicity, HLMEC were exposed to HIV-1UG001 and/or rosiglitazone for 24 hours, and viability monitored using AlamarBlue assay over a 24-hour period. Results showed no HIV effect on HMLEC viability over 48 hours (Figure 2D). Cytotoxicity assays on infected and noninfected MDM, with or without exposure to rosiglitazone, also showed no significant effect of HIV-1 infection or rosiglitazone treatment on MDM over 12 days in culture compared with uninfected or untreated MDM (see Figures E1 and E2 in the online supplement).
Figure 2.
Peroxisome proliferator–activated receptor-γ modulation of HIV-1–induced down-regulation of tight junction protein expression and nuclear factor (NF)-κB promoter activity. (A) Primary human lung microvascular endothelial cells (HLMEC) express claudin-5, ZO-1, and ZO-2. Strong staining with the endothelial cell marker CD31 confirmed the endothelial nature of these cells. Additional controls consisted of HLMEC stained with isotype-matched control antibody (A). Direct exposure of HLMEC to infectious viral particles (HIV-1UG001; 24 h) significantly decreased claudin-5 (B) and ZO-2 (C) expression. Rosiglitazone (Rosi, 25 μM) significantly diminished HIV-1–induced down-regulation of claudin-5 (B). (D) Assessment of cytotoxicity over 24 hours by alamarBlue assay showed that HIV-1 and rosiglitazone did not decrease HLMEC viability compared with untreated control cells. (E) HLMEC were transfected with a control vector or a similar vector containing copies of an NF-κB response element (NF-κB–RE) that drives transcription of the luciferase reporter gene (NF-κB vector). Exposure of transfected HLMEC to HIV-1UG001 for 1 hour significantly increased luciferase activity and rosiglitazone (25 μM) significantly diminished HIV-1–induced luciferase activity. No luciferase activity was observed in transfected HLMEC exposed only to rosiglitazone or cells transfected with control vector (*P < 0.05, **P < 0.01, ***P < 0.001). For A, original magnifications are ×400. For B and C, the summary graphs represent data from three human donors; D and E show combined data from five (D) and three (E) independent experiments using five and three human donors, respectively; and for each experimental condition in D and E, each donor sample was tested in triplicate. Error bars represent SEM.
HIV-1–induced Nuclear Factor-κB Promoter Activity in HLMEC Was Abrogated by PPAR-γ Agonists
Because PPAR-γ signals through nuclear factor (NF)-κB and activation of PPAR-γ can inhibit inflammatory responses by preventing NF-κB activation (30), we determined the effects of HIV-1 and rosiglitazone on NF-κB promoter activity in primary HLMEC transfected with NF-κB or control vector. Exposure of HLMEC to HIV-1UG001 increased NF-κB promoter activity by fourfold compared with cells transfected with control vector (P < 0.001) (Figure 2E). Rosiglitazone prevented viral-induced up-regulation of NF-κB promoter activity (Figure 2E).
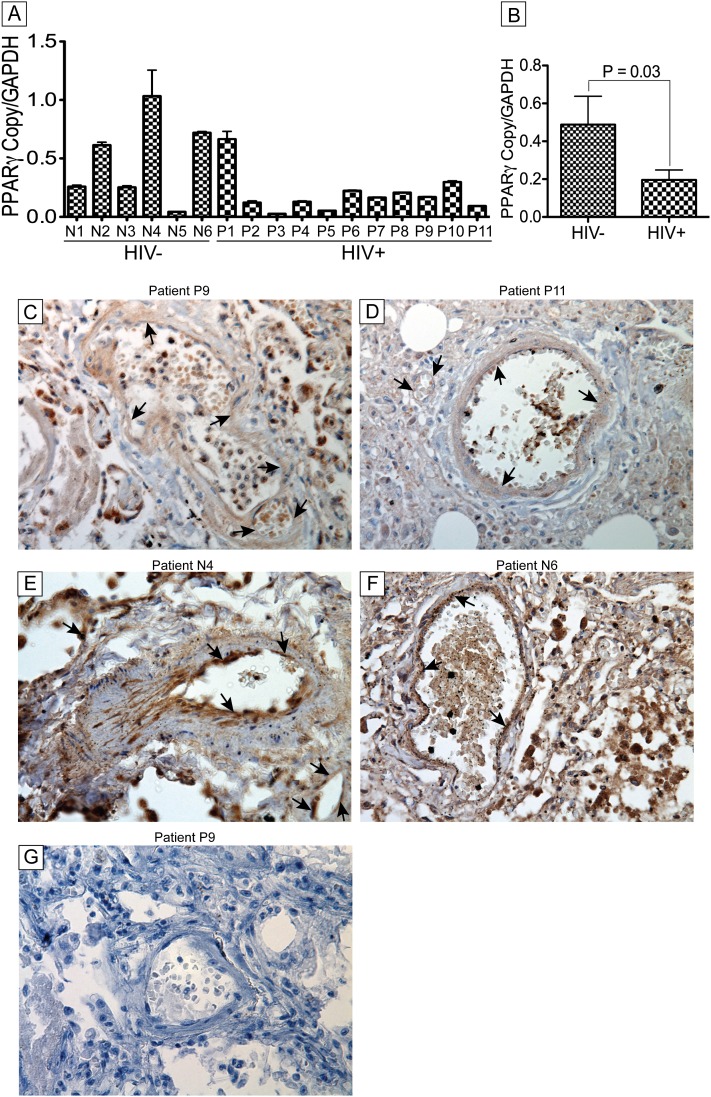
Down-regulation of PPAR-γ in the Lungs of HIV-infected Individuals with IP
HIV-induced IP is an inflammatory disease. Because PPAR-γ has been shown to be a key regulator of lung inflammation and repair (31, 32), we hypothesized that HIV-induced IP is associated with dysregulation of PPAR-γ. Quantitative RT-PCR analyses showed significant transcriptional down-regulation of PPAR-γ in lung tissues of HIV+IP patients compared with seronegative control subjects (P < 0.03) (Figures 3A and 3B). Immunohistochemistry analyses also showed decreased PPAR-γ expression in lung tissues and blood vessels (arrows) of HIV+IP patients (Figures 3C and 3D) compared with lung tissues of seronegative control subjects (Figures 3E and 3F). Data further showed increased expression of PPAR-γ in pulmonary vessels of control individuals compared with pulmonary vessels of HIV+IP patients (arrows, Figures 3C–3F). Additional control consisted of lung tissues stained with isotype-matched control antibody (antirabbit IgG) (Figure 3G).
Figure 3.
Decreased expression of peroxisome proliferator–activated receptor (PPAR)-γ in lung tissues and blood vessels of HIV-1–infected humans with interstitial pneumonitis (IP). (A) Real-time polymerase chain reaction analyses showed decreased PPAR-γ mRNA in lung tissues from HIV-infected humans with IP compared with seronegative human control subjects. (B) Combined analyses of all data by group confirmed the decreased PPAR-γ transcription in these patients (P = 0.03). Immunohistochemistry analyses showed decreased PPAR-γ expression in lung tissues and blood vessels (arrows) of HIV+ patients with IP (C and D) compared with lung tissues from seronegative human control subjects (E and F). Additional controls consisted of lung tissues stained with isotype-matched antibody (G). Data shown are from 17 human donors. Original magnifications at ×400. Error bars represent SEM. GAPDH = glyceraldehyde phosphate dehydrogenase.
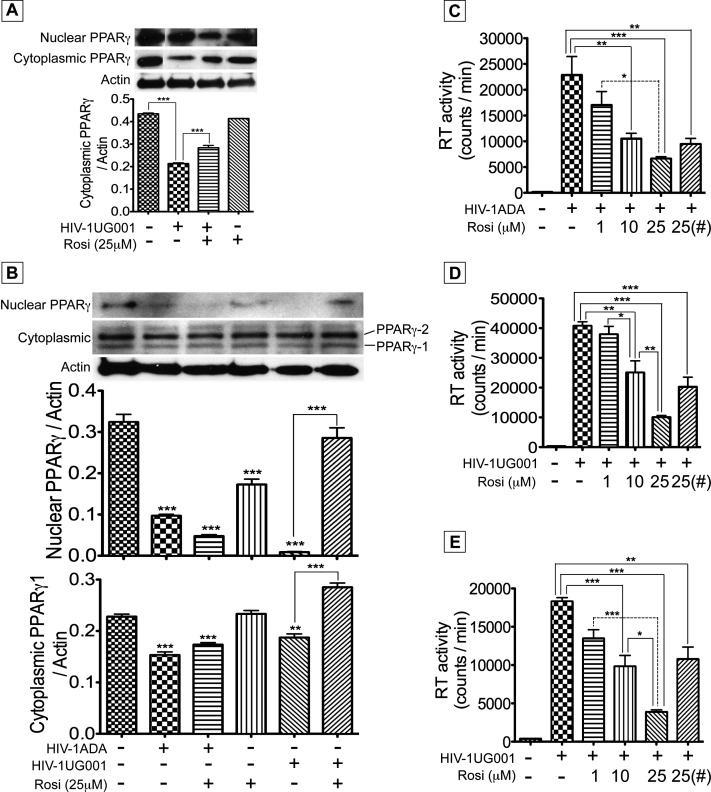
PPAR-γ Modulates HIV-1 Infection of Human Peripheral Blood Cells
Productive HIV-infected cells in the lungs of HIV+ individuals consist predominantly of infiltrating macrophages and T cells from the peripheral blood. To test the effect of PPAR-γ modulation on HIV-1 infection of these cells, we determined PPAR-γ expression in infected PBMC and MDM. HIV-1 infection decreased PPAR-γ levels in PBMC; protein fractionation showed decreased nuclear and cytoplasmic PBMC PPAR-γ levels (Figure 4A). Rosiglitazone treatment partially restored cytoplasmic and nuclear PPAR-γ levels (Figure 4A). HIV-1 infection of MDM decreased nuclear and cytoplasmic PPAR-γ levels (Figure 4B). Although rosiglitazone tended to increase cytoplasmic and nuclear PPARg1 levels in the HIV-1UG001–infected macrophages, this trend did not reach statistical significance (Figure 4B). Using two different HIV-1 strains, we further tested the effect of PPAR-γ on MDM and PBMC infection. Both the M-tropic (HIV-1ADA) (Figure 4C) and dual-tropic (HIV-1UG001) (Figure 4D) HIV-1 strains productively infected human MDM, and rosiglitazone dose-dependently decreased viral infection. At 1, 10, and 25 μM, rosiglitazone decreased HIV-1ADA infection of MDM by 1.07-, 1.46-, and 3.11-fold, respectively (Figure 4C); decreased HIV-1UG001 infection of MDM by 1.1-, 1.56-, and 3.43-fold, respectively (Figure 4D); and decreased HIV-1UG001 infection of PBMC by 2.2-, 2.15-, and 5.26-fold, respectively (Figure 4E). Even when MDM and PBMC were treated with rosiglitazone only during the 4-hour viral exposure and culture continued without rosiglitazone in the media, reverse transcriptase (RT) assay at Days 5 and 12 postinfection showed that rosiglitazone reduced virus replication in HIV-1ADA–infected MDM by twofold (P < 0.01) (Figure 4C), decreased HIV-1UG001 infection of MDM by 1.97-fold (Figure 4D), and diminished HIV-1UG001 replication in PBMC by 2.375-fold (P < 0.01) (Figure 4E).
Figure 4.
HIV-1 infection diminished peroxisome proliferator–activated receptor (PPAR)-γ expression and activation of PPAR-γ decreased infection of macrophages and peripheral blood mononuclear cells (PBMC). Human PBMC and monocyte-derived macrophages (MDM) were infected with HIV-1UG001 or HIV-1ADA, with or without rosiglitazone in the media. (A) Western blot analyses of PBMC nuclear and cytoplasmic extracts at Day 12 postinfection showed decreased expression of PPAR-γ in PBMC infected with HIV-1UG001 and rosiglitazone partially restored cytoplasmic PPAR-γ levels. (B) Western blot analyses of macrophages nuclear and cytoplasmic extracts also showed decreased expression of PPAR-γ in macrophages infected with HIV-1UG001 or HIV-1ADA. Both PPAR-γ1 and PPAR-γ2 isoforms were present in some macrophage cytoplasmic extracts (B). Although rosiglitazone tended to increase cytoplasmic and nuclear PPARγ1 levels in the HIV-1UG001–infected macrophages, this trend did not reach statistical significance (B). Reverse transcriptase assay showed that rosiglitazone significantly and dose-dependently decreased MDM (C and D) and PBMC (E) infection. (#) MDM (C and D) and PBMC (E) exposed to rosiglitazone only during the 4-hour viral exposure and cultured without rosiglitazone in the media showed significant decrease in viral replication (*P < 0.05, ** P < 0.01, *** P < 0.001, and P values in A and B are compared with controls). For A, the summary graph represents data from five independent experiments using five human donors; for B, the summary graph represent data from three (nuclear PPAR-γ) and four (cytoplasmic PPAR-γ) independent experiments using three and four human donors, respectively. C and D show combined data from three independent experiments using three human donors, E shows combined data from four independent experiments using four human donors, and for each experimental condition in C–E, each donor sample was tested in triplicate. Error bars represent SEM. GAPDH = glyceraldehyde phosphate dehydrogenase.
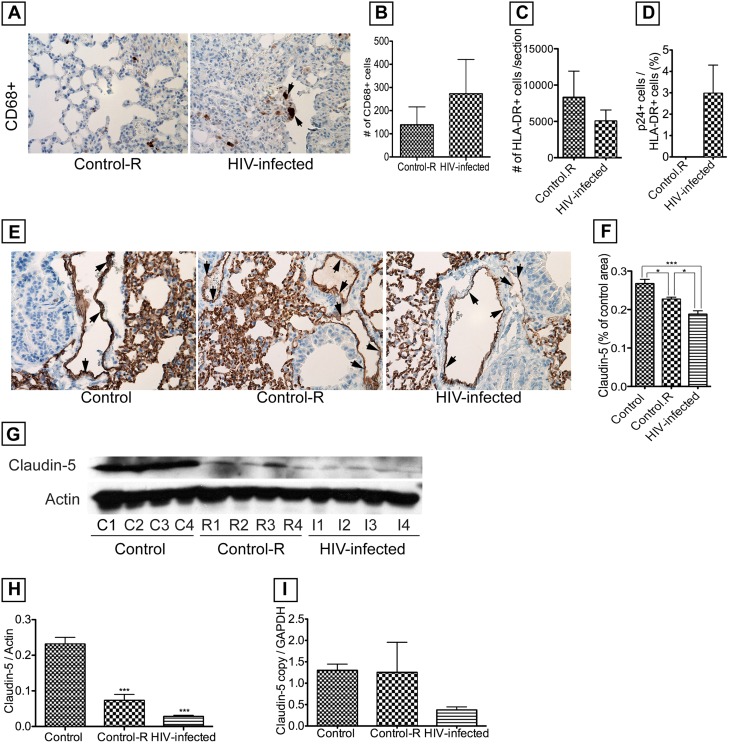
Pulmonary Cellular Infiltrates and HIV-induced Damage on Pulmonary Microvasculature in Humanized NSG Mice
These mice were previously well-characterized and shown to have a very good engraftment of human cells (26–28). HIV-1 infection was associated with increased levels of macrophages in the lungs (Figures 5A and 5B) and formation of multinucleated giant cells (Figure 5A, arrows). Our current data also showed good engraftment with the presence of human cells in blood and lung tissues. Human cells (detected by HLA-DR immunoreactivity) were present in lung tissues, with more cells in noninfected mice compared with infected mice (Figure 5C). For noninfected mice, mean HLA-DR+ cells was 8,309 ± 10,206 (SD; n = 8), whereas for infected mice, mean HLA-DR+ cells was 5,038 ± 3,412 (n = 5). However, the difference was not statistically significant in part because of large SD. HIV-1 was present in the blood of all infected mice, and VLs varied from 44,450 to 248,150 copies per milliliter: mean VL was 121,363 ± 89,687 copies per milliliter. Immunohistochemistry showed the presence of HIV-infected cells in mice lungs, whereas no infected cells were detected in noninfected mice (Figure 5D). HIV-1 infection was also associated with diminished claudin-5 expression in lung tissues and blood vessels (Figure 5E, arrows) compared with control mice engrafted with human cells and control animals without human cell engraftment. Densitometry of lung tissues from all mice (Figure 5F), Western blot analyses (Figures 5G and 5H), and RT-PCR (Figure 5I) confirmed HIV-1–induced down-regulation of claudin-5 protein and mRNA. Engrafted noninfected mice also showed decreased claudin-5 expression, however, to a lesser extent than engrafted-infected mice (Figures 5E–5H).
Figure 5.
Analyses of lung tissues from HIV-1–infected humanized NGS mice. HIV-1 infection was associated with increased macrophage infiltration into lung tissues (A and B), formation of multinucleated giant cells (A, arrows), and all mice showed good engraftment of human (HLA-DR+) cells in the lungs (C). HIV-1–infected cells were detected in lung tissues of engrafted, infected mice, whereas engrafted, noninfected animals (control-R) showed no infected cells (D). HIV-1 infection was associated with decreased expression of claudin-5 in tissues (E) and blood vessels (E, arrows), which was confirmed by semiquantitative analyses of claudin-5 staining in all lung tissues (F), and Western blot analyses (G and H). Real-time polymerase chain reaction also showed that HIV-1 infection was associated with decreased claudin-5 mRNA in mouse lung tissues (I). For F, the number of mice n = 5 in each group; for G and H, n = 4 in each group; for real-time polymerase chain reaction (I), n = 4, 4, and 8, respectively, for control, control-R, and HIV-infected group. Each sample was analyzed in triplicate. Control: nonreconstituted, noninfected mice. Control-R: mice reconstituted with human CD34+ hematopoietic stem cells, noninfected. HIV-infected: mice reconstituted with human CD34+ hematopoietic stem cells and infected. (*P < 0.05, ***P < 0.001). Original magnifications at ×400. Error bars represent SEM.
Pulmonary Cellular Infiltrates in HIV-infected NOD-SCID Mice
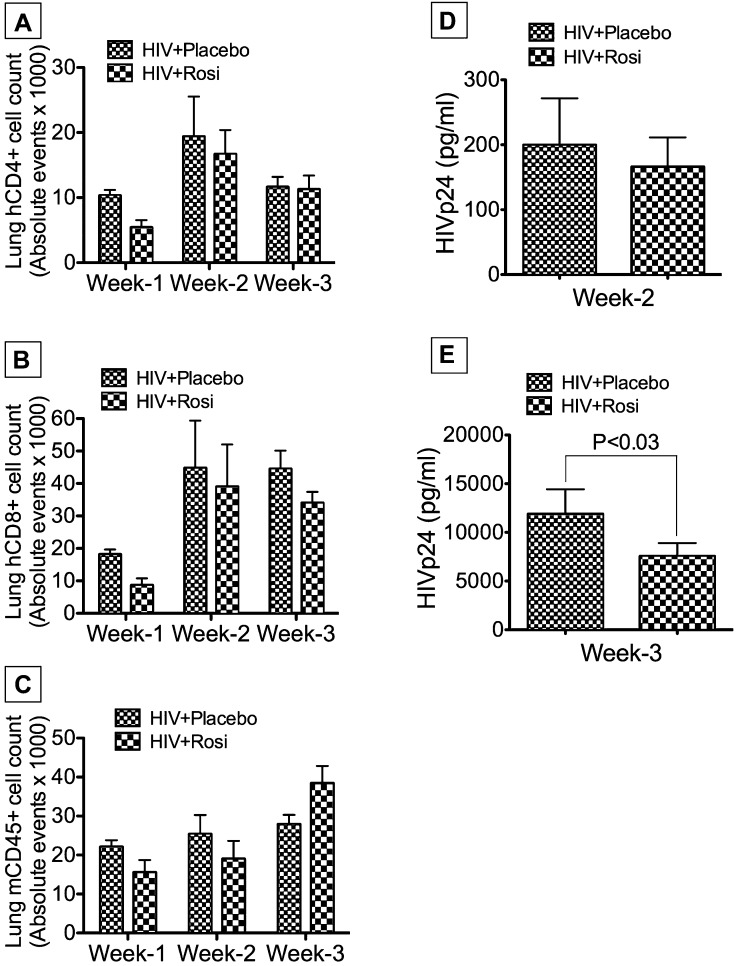
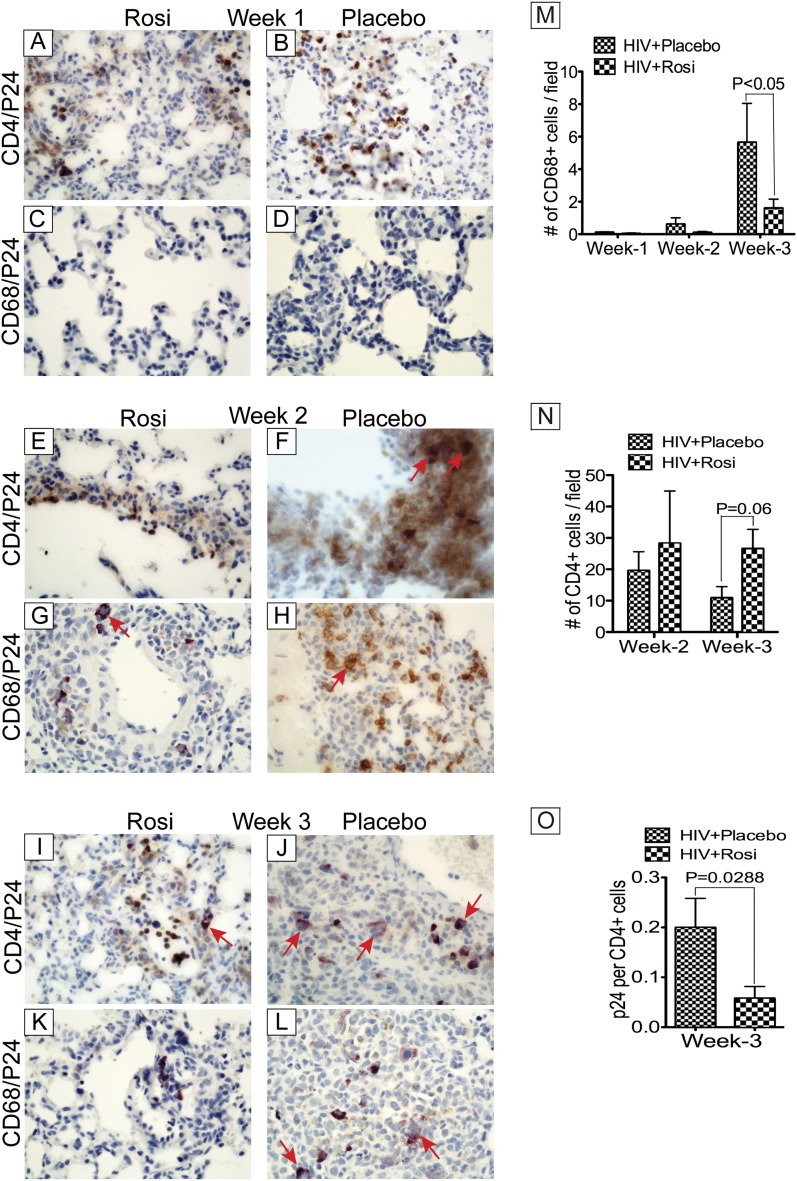
We assessed the composition of leukocyte infiltrates in the lungs of infected NOD-SCID mice, and the effects of PPAR-γ agonists. FACS analyses of pulmonary infiltrates at Weeks 1, 2, and 3 postinfection showed infiltration of CD4+ (Figure 6A) and CD8+ (Figure 6B) cells in HIV-1–infected mice. Infected rosiglitazone-treated mice showed slightly lower levels of pulmonary cellular infiltration compared with infected placebo-treated animals, but the differences were not statistically significant (Figure 6). However, additional studies using NSG mice engrafted with human PBL (Hu-PBL-NSG) showed significantly lower number of CD4+ cells in lung tissues of HIV-1–infected mice, compared with infected mice treated with rosiglitazone (see Figure E3B), suggesting that rosiglitazone prevent HIV-1–induced depletion of CD4+ cells in these Hu-PBL-NSG mice. FACS analyses using mouse CD45 monoclonal antibodies showed small increases in the numbers of murine leukocytes in lungs over time, with slightly higher levels in lungs of infected placebo-treated mice at Weeks 1 and 2, compared with infected rosiglitazone-treated mice; the differences were not statistically significant (Figure 6C). Immunostaining of lung tissues using monoclonal antibodies to human CD4 and CD68 confirmed lymphocytes and macrophages pulmonary infiltration. Double immunostaining with antibodies to HIV-1 p24 showed the presence of infected T lymphocytes and macrophages in lung tissues with higher levels of infected lymphocytes (Figures 7B, 7F, and 7J) and macrophages (Figures 7H and 7L) in lungs of infected placebo-treated mice compared with infected rosiglitazone-treated mice (Figures 7A, 7E, 7I, 7G, and 7K). Quantitative analyses of total human macrophages in lung tissues also showed higher pulmonary macrophage infiltration in infected placebo-fed mice at Weeks 2 and 3 compared with infected rosiglitazone-fed mice (Figure 7M). Although macrophage infiltration in lung tissues increased gradually from Weeks 1 to 3 postinfection in both groups, rosiglitazone-treated mice (n = 13) showed a 5.8- and 3.5-fold decrease in the number of pulmonary CD68+ cells compared with placebo-treated mice (n = 16) at Weeks 2 and 3, respectively. Quantitative analyses of total CD4+ T lymphocytes in lung tissues showed higher numbers of lymphocytes (Figure 7N) and significantly lower numbers of infected lymphocytes (Figure 7O) in the lungs of infected rosiglitazone-treated mice at Weeks 2 and 3 compared with infected placebo-treated mice. Depletion of CD4+ cells in infected placebo-treated mice correlated with increased viremia and formation of giant cells (Figures 7A–7L, arrows). The concentration of pioglitazone used (1 mg/kg) had little effect on viremia and pulmonary cellular infiltration (data not shown).
Figure 6.
Pulmonary cellular infiltrates and viremia in HIV-infected, placebo-treated, and rosiglitazone-treated Hu-PBL.NOD-SCID mice. Fluorescence-activated cell sorter analyses of lung tissues showed human CD4+ (A) and CD8+ (B) cells in the lungs at Weeks 1–3. Week 1 data showed decreased cellular infiltrates in rosiglitazone-treated mice, but Weeks 2 and 3 data showed no significant difference between the two groups. (C) Significant differences in the number of murine leukocytes in animal lung tissues were not detected between groups. Quantification of blood HIV-1 p24 antigen levels showed no differences in viremia between placebo-treated and rosiglitazone-treated mice at Week 2 (D); but viremia increased significantly at Week 3, and rosiglitazone significantly reduced HIV-1 p24 levels (E). For A–C, Week 1 includes data from four mice in each group; Week 2 includes data from six mice for the HIV+placebo group, and seven mice for the HIV+Rosi group; Week 3 includes data from six mice for the HIV+placebo group, and eight mice for the HIV+Rosi group. For D, the HIV+placebo group includes data from eight mice and the HIV+Rosi group includes data from nine mice. For E, the HIV+placebo group includes data from six mice, and the HIV+Rosi group includes data from eight mice. Error bars represent SEM.
Figure 7.
Activation of peroxisome proliferator–activated receptor-γ decreased viremia and pulmonary macrophage infiltration in Hu-PBL.NOD-SCID mice. Immunohistochemistry analyses of lung tissues from HIV-1–infected placebo- and rosiglitazone-treated Hu-PBL.NOD-SCID mice showed infected CD4+ T cells (A, B, E, F, I, and J) and macrophages (C, D, G, H, K, and L) in animal lung tissues, and multinucleated giant cells at Weeks 2 and 3 postinfection (arrows). Macrophage infiltration in the lungs increased gradually over time (M), whereas the levels of CD4+ cells decreased over time (N). Rosiglitazone-treated mice showed significantly less numbers of macrophages (M) and infected cells (O) in the lungs, and higher total CD4+ cell counts (N), correlating with reduced infection in rosiglitazone-treated mice. Original magnifications at ×400. For panels M–O, Week 1 includes data from four mice in each group; Week 2 includes data from six mice for the HIV+placebo group, and seven mice for the HIV+Rosi group; Week 3 includes data from six mice for the HIV+placebo group, and eight mice for the HIV+Rosi group. Error bars represent SEM.
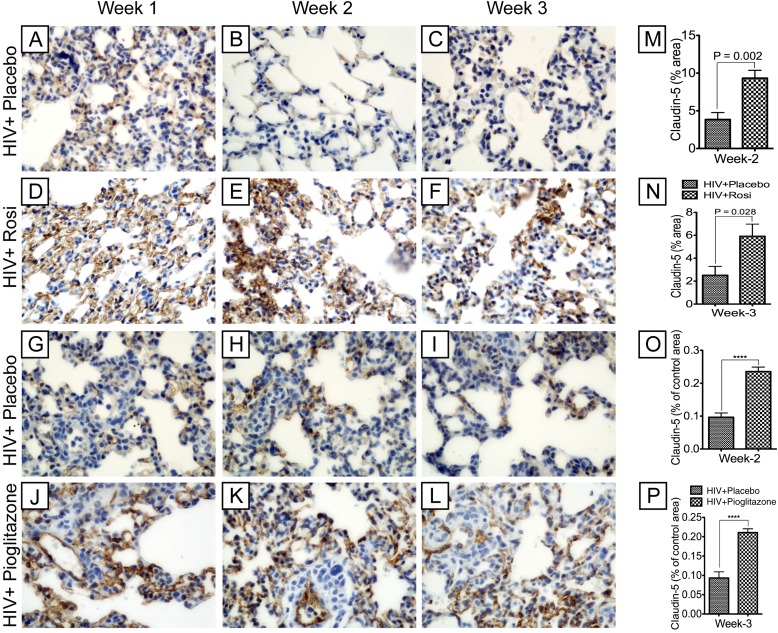
HIV-1 Infection Induced Damage of the Pulmonary Microvasculature in NOD-SCID Mice and PPAR-γ Agonists Prevent HIV-1–induced Vascular Damage
To assess the effects of HIV-1 infection and cell infiltration on the pulmonary endothelium of NOD-SCID mice, we performed immunostaining for claudin-5, a marker of endothelial microvascular barrier integrity. HIV-1 infection resulted in injury of the pulmonary microvasculature, as evidenced by loss of claudin-5 expression (Figures 8A–8C, 8G–8I). PPAR-γ agonists prevented injury of the lung microvasculature as demonstrated by preserved claudin-5 staining in rosiglitazone- (Figures 8D–8F) and pioglitazone-treated (Figure 8J-L) mice compared with placebo-treated animals (Figures 8A–8C, 8G–8I). Computer-assisted image analyses confirmed HIV-1–induced damage of the lung microvasculature and the ability of PPAR-γ agonists to reduce vascular injury. Compared with placebo-treated mice, rosiglitazone increased claudin-5 expression at Weeks 2 and 3 by 2.42-fold (Figure 8M) (P = 0.002) and 2.35-fold (Figure 8N) (P = 0.028), respectively, whereas pioglitazone increased claudin-5 expression at Weeks 2 and 3 by 2.44-fold and 2.26-fold, respectively (Figure 8O and 8P) (P < 0.0001).
Figure 8.
Peroxisome proliferator–activated receptor-γ agonists prevented HIV-1–induced damage of the lung microvasculature in Hu-PBL.NOD-SCID mice. HIV-1 infection resulted in microvascular injury, as evidenced by decreased expression of the tight junction protein claudin-5 (brown, A–C and G–I). Peroxisome proliferator–activated receptor-γ agonists prevented viral-induced microvascular injury, as demonstrated by more intense staining for claudin-5 in infected rosiglitazone-treated mice (D–F) compared with infected placebo-treated mice (A–C) and more intense staining for claudin-5 in infected pioglitazone-treated mice (J–L) compared with infected placebo-treated mice (G–I). Original magnifications are at ×400. (M and N): Semiquantitative analyses of claudin-5 staining in lung tissues from infected placebo-treated (n = 16) and rosiglitazone-treated mice (n = 13) at Weeks 2 and 3 postinfection. Rosiglitazone increased the intensity of claudin-5 staining at Week 2 by 2.42-fold (M) and by 2.35-fold at Week 3 (N). (O and P): Semiquantitative analyses of claudin-5 staining in lung tissues from infected placebo-treated (n = 15) and pioglitazone-treated mice (n = 16) at Weeks 2 and 3 postinfection; for each group, n represent the number of mice used. Pioglitazone increased the intensity of claudin-5 staining at Week 2 by 2.44-fold (O) and by 2.26-fold at Week 3 (P) (****P < 0.0001). Error bars represent SEM.
PPAR-γ Stimulation Decreased Viremia in NOD-SCID Mice
Quantification of HIV-1 p24 antigen levels in animal blood served as a measure of viremia. In the first experiment, treatment with pioglitazone (1 mg/ml) had no effect on viremia. In the second experiment, treatment with rosiglitazone (10 mg/kg) produced no detectable viremia at Week 1; by Week 2, the levels of HIV-1 p24 were similar in both groups (16.6–566 pg/ml in placebo-treated mice, 17–321 pg/ml in rosiglitazone-treated mice) (Figure 6D) (P = 0.137). A significant increase in viremia was detected at Week 3 compared with Week 2, and rosiglitazone reduced viremia by approximately 50%. HIV-1 p24 levels in placebo-treated mice were 11,890 ± 2,530 pg/ml, whereas levels in rosiglitazone-treated mice were 7,553 ± 1,358 pg/ml (Figure 6E) (P < 0.03).
HIV-1 Infection Induced Damage of the Pulmonary Microvasculature in Hu-PBL-NSG Mice and PPAR-γ Agonists Prevent HIV-1–induced Vascular Damage, Decreased Viremia, and Depletion of CD4+ Cells
To assess the effects of HIV-1 infection on the pulmonary endothelium of Hu-PBL-NSG mice, we performed immunostaining for claudin-5, a marker of endothelial microvascular barrier integrity. HIV-1 infection resulted in injury of the pulmonary microvasculature, as evidenced by loss of claudin-5 expression (see Figure E3A). PPAR-γ agonists diminished HIV-induced injury of the lung microvasculature as demonstrated by increased claudin-5 expression in infected and rosiglitazone-treated mice, compared with infected mice treated with placebo (see Figure E3A). Control noninfected mice engrafted with human PBL showed high levels of claudin-5 expression (see Figure E3A), which demonstrate that engraftment did not affect the pulmonary vasculature in these animals.
Quantitative analyses of CD4+ T lymphocytes in lung tissues showed significantly lower number of lymphocytes in lung tissues of HIV-infected Hu-PBL-NSG mice treated with placebo, compared with lung tissues of rosiglitazone-treated infected mice or control noninfected Hu-PBL-NSG mice (see Figure E3B). There was no significant difference in the levels of lung CD8+ cells among the three groups (see Figure E3C). Immunostaining for HIV-1 p24 antigen (see Figure E3D) and ELISA assays (see Figure E3E) showed higher viremia in lung tissues of HIV-infected Hu-PBL-NSG mice treated with placebo, compared with lung tissues of rosiglitazone-treated infected mice, confirming that depletion of CD4+ cells in infected placebo-treated mice correlated with increased viremia.
Discussion
This study is the first, to our knowledge, demonstrating claudin-5 down-regulation and pulmonary vascular injury in HIV-induced IP. Lymphocytic IP is common in the pediatric HIV population and is responsible for 30–40% of pulmonary disease (33). Interstitial infiltration of activated lymphocytes and macrophages into alveolar septae is seen in affected lung parenchyma and often leads to respiratory failure (4, 5). The barrier integrity of the pulmonary endothelium is caused by TJ proteins, such as claudin-5 (15, 16). Claudin-5 is an endothelial cell–specific component of TJ strands, and lung injury is associated with diminished expression of TJ proteins and increased permeability (20, 21). In the present study, we demonstrate significant down-regulation of claudin-5 transcription and expression in lung tissues of HIV-1–infected humans with IP compared with uninfected control subjects. This suggests that viral-induced injury of the pulmonary endothelium and claudin-5 down-regulation contribute to the pathogenesis of IP in HIV-infected individuals. Claudin-5 expression and translation showed heterogeneity within infected and noninfected subjects; and some patients did not show correlation between claudin-5 protein and mRNA levels, suggesting differential regulation of claudin-5 transcription and expression in those subjects. Because no previous study, to our knowledge, has analyzed claudin-5 in lung tissues of HIV+ humans, we do not know whether this phenotype is specific to HIV+ or HIV+IP individuals. Our previous studies showed significant decrease in claudin-5 expression in the brain endothelium of HIV+ individuals with encephalitis (23). Thus, it is likely that HIV infection associated with inflammatory conditions, such IP or encephalitis, induces a decrease in claudin-5 expression in the endothelium. We further showed that primary HLMEC express TJ proteins, including claudin-5; and exposure of these cells to HIV-1 down-regulated claudin-5 and ZO-2 expression. Viral-induced decrease in TJ protein expression may directly contribute to injury of the lung endothelium. Previous works from our laboratory also showed that, as in HLMEC (34, 35), HIV-1 and viral gp120 also down-regulated TJ proteins in endothelial cells from other vascular beds, including human brain endothelial cells (36). These evidences show that HIV-1 and viral proteins directly injure the endothelium.
Our findings in lung tissues of HIV-1–infected humans were further validated using three different, well-established HIV/AIDS murine models. These models use animals reconstituted with human cells; they reproduce HIV/AIDS pathobiology, allowing testing of diverse therapeutic approaches (24–28). We demonstrated significant down-regulation of claudin-5 expression, a marker of pulmonary vascular injury, in HIV-infected animals. Pulmonary cells, such as alveolar macrophages and lymphoid cells, are productively infected by HIV-1, and the lung is the organ most frequently affected in HIV-associated diseases (1–3). Our data suggest that HIV-induced down-regulation of endothelial TJ proteins and injury to the pulmonary vascular endothelium likely contributes to the development of pulmonary complications, such as IP. This virus-associated injury of the lung endothelium may also enhance inflammatory cell infiltration into lungs. In fact, IP is characterized by increased infiltration of cells, such as macrophages (4, 5), and our data, using two different HIV/AIDS animal models, showed increased macrophage infiltration in lung tissues of HIV-infected mice. Some animals also showed signs of lung pathology with infiltrating mononuclear cells in the alveolar spaces. However, recent studies showed that NOD-SCID mice that receive human PBL can develop xenogenic graft-versus-host disease because of human antimouse major histocompatibility complex class II reactivity (37). Thus, both HIV-1 infection and/or graft-versus-host disease responses inherent to the model could contribute to this pathology and represents a limitation of our animal model. However, we controlled for this in our humanized NGS mice and Hu-PBL-NSG mice by using additional control subjects consisting of reconstituted noninfected animals.
IP is an inflammatory disease, and PPAR-γ has been implicated in the regulation of inflammation and lung injury (8, 10–12, 22, 38). HIV-1 proteins have also been shown to alter PPAR-γ regulation and expression (39). Thus, we hypothesize that dysregulation of PPAR-γ is involved in HIV-1–induced IP and vascular injury. Our data showed significant down-regulation of PPAR-γ transcription and expression in lung tissues of HIV-1–infected humans with IP. This suggests that suppression of PPAR-γ expression and transcriptional activities may be involved in the pathogenesis of HIV-induced IP. Using two different HIV-1 strains and PPAR-γ agonists, including rosiglitazone, a Food and Drug Administration–approved drug for the treatment of such metabolic conditions as diabetes, we also demonstrated that HIV-1 down-regulates PPAR-γ expression; activation of PPAR-γ dose-dependently decreased HIV-1 infection of MDM and PBMC. This is in agreement with previous findings, because both natural and synthetic PPAR-γ agonists reduce HIV-1 infection in macrophages, PBMC, dendritic cells, and chronically infected cell lines (13). Activation of PPAR-γ also diminished viral infection in the NOD-SCID model of HIV encephalitis and migration of HIV-infected monocytes in an in vitro blood-brain barrier model (25). Studies using a murine preadipocytes cell line showed that HIV-1 Vpr suppressed PPAR-γ–mediated gene transcription, which may contribute to viral-induced dysregulation of metabolic function (39). Overexpression of PPAR-α and PPAR-γ attenuated HIV-induced down-regulation of TJ proteins ZO-1, occludin, and junctional adhesion molecules in brain endothelial cell lines (40), and protected against HIV- and Tat-induced expression of inflammatory cytokines and chemokines in macrophages, PBMC, and brain endothelial cells (13, 14).
In addition to HIV-induced down-regulation of lung PPAR-γ and claudin-5, PPAR-γ agonists decreased viremia and macrophage infiltration into lungs, and prevented HIV-1–induced down-regulation of claudin-5 in vitro and in vivo. This suggests that PPAR-γ activation prevents HIV-induced injury and dysfunction of the lung endothelium and cellular infiltration into the lungs. PPAR-γ is expressed in normal human and animal lungs and has been shown to play a major role in the pathogenesis of lung diseases (8, 9, 22). PPAR-γ deficiency results in reduced lung elastic recoil and abnormalities in airspace distribution (22). Analyses of lung tissues from patients with pulmonary hypertension or from rats with induced severe pulmonary hypertension showed losses or significant decreases in PPAR-γ mRNA and protein levels in pulmonary vascular lesions (9, 38). Studies in rat models of pulmonary hypertension and hypoxia showed that rosiglitazone attenuates hypoxia-induced pulmonary arterial remodeling (41) and prevented hyperoxia-induced neonatal lung injury (42). Pulmonary fibrosis and impaired lung function are the most serious complications of bleomycin treatment. In vivo studies showed that both natural and synthetic PPAR-γ agonists suppressed bleomycin-induced lung injury and fibrosis (43), and also inhibited inflammation, as well as skin and lung fibrosis in a murine model of scleroderma (44).
We were not able to correlate findings in humans with disease severity because of lack of lung specimens from HIV+ subjects without pulmonary involvement and uninfected patients with IP. However, our data are in agreement with previous studies showing that lung injury is associated with altered expression of PPAR-γ. Endotoxin-induced acute lung injury in rats is associated with decreased PPAR-γ expression in the lungs and is prevented by rosiglitazone (45, 46). Studies in murine models of acute lung injury also showed that PPAR-γ agonists significantly reduce hyperoxia-induced inflammation, whereas partial inhibition of PPAR-γ by siRNA treatment significantly exacerbates the inflammation (47).
It is likely that HIV-1–induced macrophage infiltration in the lungs, as observed in this study, contributes to disease pathobiology. In fact, lung macrophages play an important role in the regulation of pulmonary immune responses and have been shown to contribute to pulmonary infection and fibrosis (4). PPAR-γ regulates inflammatory responses in these cells because PPAR-γ ligands significantly decrease lipopolysaccharide-induced tumor necrosis factor-α production in alveolar macrophages (48), and deletion of PPAR-γ in alveolar macrophages is associated with increased pulmonary inflammatory responses (49). The mechanisms through which PPAR-γ activation diminished HIV-1 infection and viral-induced down-regulation of claudin-5 remain to be elucidated. Possible pathways include NF-κB or the signal transducer and activator of transcription (STAT) pathways, because HIV-induced endothelial injury is associated with transcriptional up-regulation of STAT-1 and NF-κB-associated genes (50), and STAT1 activation (23). Moreover, PPAR-γ ligands inhibit NF-κB and STAT-1 activation (30), HIV-1–induced NF-κB activation and binding (25). This is in agreement with our present data showing that HIV-1–induced NF-κB promoter activity in HLMEC and PPAR-γ ligand abrogated these effects. There is also evidence that PPAR-γ ligands inhibit inflammatory responses by preventing NF-κB activation (30). Studies are ongoing in our laboratory to further explore these pathways and determine the mechanisms through which PPAR-γ activation exerts its protective effects against HIV-induced lung injury.
In summary, our present study shows that HIV-1 infection and HIV-induced IP are associated with injury to the vascular endothelium, with decreased expression of claudin-5 and PPAR-γ, and increased cellular infiltration into the lungs. Furthermore, activation of PPAR-γ decreased viremia, prevented HIV-1–induced injury to the lung microvasculature, and reduced macrophage infiltration into the lungs. Thus, regulation of PPAR-γ represents an attractive strategy to treat HIV-induced pulmonary inflammation and IP, and preserve lung vasculature.
Acknowledgments
Acknowledgment
The authors thank the National NeuroAIDS Tissue Consortium and National Disease Research Interchange for providing human lung tissues specimens, the NIH AIDS Research and Reference Reagent Program for providing viral isolates. From the University of Nebraska Medical Center, they thank Drs. Larisa Poluektova and Santhi Gorantla for providing lung tissues from NSG mice; Bryan Knipe, Corey Lawson, Sidra Akhter, Brenda Morsey, and Dr. Aaron Mercer for technical assistance; Robin Taylor for excellent editorial support; and Dr. Lee Mosley for critical reading of the manuscript.
Footnotes
Supported by American Lung Association grant CI-9817-N (G.D.K.) and National Institutes of Health grants R01MH081780 (G.D.K.), R01MH65151, and R37 AA015913 (Y.P.).
Author Contributions: H.L. and S.S. performed research. R.P. and Y.P. contributed to Hu-PBL-NOD/SCID mice model experiments and design and edited the manuscript. G.D.K. designed and supervised the study, collected and analyzed the data, and wrote the paper.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201106-1151OC on February 16, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Afessa B, Green W, Chiao J, Frederick W. Pulmonary complications of HIV infection: autopsy findings. Chest. 1998;113:1225–1229. doi: 10.1378/chest.113.5.1225. [DOI] [PubMed] [Google Scholar]
- 2.Lanjewar DN, Duggal R. Pulmonary pathology in patients with AIDS: an autopsy study from Mumbai. HIV Med. 2001;2:266–271. doi: 10.1046/j.1468-1293.2001.00079.x. [DOI] [PubMed] [Google Scholar]
- 3.Soeiro AdeM, Hovnanian AL, Parra ER, Canzian M, Capelozzi VL. Post-mortem histological pulmonary analysis in patients with HIV/AIDS. Clinics (Sao Paulo) 2008;63:497–502. doi: 10.1590/S1807-59322008000400014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agostini C, Sancetta R, Cerutti A, Semenzato G. Alveolar macrophages as a cell source of cytokine hyperproduction in HIV-related interstitial lung disease. J Leukoc Biol. 1995;58:495–500. doi: 10.1002/jlb.58.5.495. [DOI] [PubMed] [Google Scholar]
- 5.Griffiths MH, Miller RF, Semple SJ. Interstitial pneumonitis in patients infected with the human immunodeficiency virus. Thorax. 1995;50:1141–1146. doi: 10.1136/thx.50.11.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakraborty R, Pulver A, Pulver LS, Musoke R, Palakudy T, D’Agostino A, Rana F. The post-mortem pathology of HIV-1-infected African children. Ann Trop Paediatr. 2002;22:125–131. doi: 10.1179/027249302125000841. [DOI] [PubMed] [Google Scholar]
- 7.Graham SM. HIV-related pulmonary disorders: practice issues. Ann Trop Paediatr. 2007;27:243–252. doi: 10.1179/146532807X245625. [DOI] [PubMed] [Google Scholar]
- 8.Huang TH, Razmovski-Naumovski V, Kota BP, Lin DS, Roufogalis BD. The pathophysiological function of peroxisome proliferator-activated receptor-gamma in lung-related diseases. Respir Res. 2005;6:102. doi: 10.1186/1465-9921-6-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ameshima S, Golpon H, Cool CD, Chan D, Vandivier RW, Gardai SJ, Wick M, Nemenoff RA, Geraci MW, Voelkel NF. Peroxisome proliferator-activated receptor gamma (PPARgamma) expression is decreased in pulmonary hypertension and affects endothelial cell growth. Circ Res. 2003;92:1162–1169. doi: 10.1161/01.RES.0000073585.50092.14. [DOI] [PubMed] [Google Scholar]
- 10.Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 11.Ricote M, Valledor AF, Glass CK. Decoding transcriptional programs regulated by PPARs and LXRs in the macrophage: effects on lipid homeostasis, inflammation, and atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:230–239. doi: 10.1161/01.ATV.0000103951.67680.B1. [DOI] [PubMed] [Google Scholar]
- 12.Cuzzocrea S, Pisano B, Dugo L, Ianaro A, Maffia P, Patel NS, Di Paola R, Ialenti A, Genovese T, Chatterjee PK, et al. Rosiglitazone, a ligand of the peroxisome proliferator-activated receptor-gamma, reduces acute inflammation. Eur J Pharmacol. 2004;483:79–93. doi: 10.1016/j.ejphar.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 13.Skolnik PR, Rabbi MF, Mathys JM, Greenberg AS. Stimulation of peroxisome proliferator-activated receptors alpha and gamma blocks HIV-1 replication and TNFalpha production in acutely infected primary blood cells, chronically infected U1 cells, and alveolar macrophages from HIV-infected subjects. J Acquir Immune Defic Syndr. 2002;31:1–10. doi: 10.1097/00126334-200209010-00001. [DOI] [PubMed] [Google Scholar]
- 14.Huang W, Rha GB, Han MJ, Eum SY, András IE, Zhong Y, Hennig B, Toborek M. PPARalpha and PPARgamma effectively protect against HIV-induced inflammatory responses in brain endothelial cells. J Neurochem. 2008;107:497–509. doi: 10.1111/j.1471-4159.2008.05626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morita K, Sasaki H, Furuse M, Tsukita S. Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol. 1999;147:185–194. doi: 10.1083/jcb.147.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amasheh S, Milatz S, Krug SM, Markov AG, Günzel D, Amasheh M, Fromm M. Tight junction proteins as channel formers and barrier builders. Ann N Y Acad Sci. 2009;1165:211–219. doi: 10.1111/j.1749-6632.2009.04439.x. [DOI] [PubMed] [Google Scholar]
- 17.Piehl C, Piontek J, Cording J, Wolburg H, Blasig IE. Participation of the second extracellular loop of claudin-5 in paracellular tightening against ions, small and large molecules. Cell Mol Life Sci. 2010;67:2131–2140. doi: 10.1007/s00018-010-0332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohtsuki S, Sato S, Yamaguchi H, Kamoi M, Asashima T, Terasaki T. Exogenous expression of claudin-5 induces barrier properties in cultured rat brain capillary endothelial cells. J Cell Physiol. 2007;210:81–86. doi: 10.1002/jcp.20823. [DOI] [PubMed] [Google Scholar]
- 19.Kaarteenaho R, Merikallio H, Lehtonen S, Harju T, Soini Y. Divergent expression of claudin -1, -3, -4, -5 and -7 in developing human lung. Respir Res. 2010;11:59. doi: 10.1186/1465-9921-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia XM, Wang FY, Wang ZK, Wan HJ, Xu WA, Lu H. Emodin enhances alveolar epithelial barrier function in rats with experimental acute pancreatitis. World J Gastroenterol. 2010;16:2994–3001. doi: 10.3748/wjg.v16.i24.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Q, Zhang Q, Wang C, Liu X, Qu L, Gu L, Li N, Li J. Altered distribution of tight junction proteins after intestinal ischaemia/reperfusion injury in rats. J Cell Mol Med. 2009;13:4061–4076. doi: 10.1111/j.1582-4934.2009.00975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simon DM, Tsai LW, Ingenito EP, Starcher BC, Mariani TJ. PPARgamma deficiency results in reduced lung elastic recoil and abnormalities in airspace distribution. Respir Res. 2010;11:69. doi: 10.1186/1465-9921-11-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaudhuri A, Yang B, Gendelman HE, Persidsky Y, Kanmogne GD. STAT1 signaling modulates HIV-1-induced inflammatory responses and leukocyte transmigration across the blood-brain barrier. Blood. 2008;111:2062–2072. doi: 10.1182/blood-2007-05-091207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Persidsky Y, Limoges J, McComb R, Bock P, Baldwin T, Tyor W, Patil A, Nottet HS, Epstein L, Gelbard H, et al. Human immunodeficiency virus encephalitis in SCID mice Am J Pathol 19961491027–1053.(see comments. [PMC free article] [PubMed] [Google Scholar]
- 25.Potula R, Ramirez SH, Knipe B, Leibhart J, Schall K, Heilman D, Morsey B, Mercer A, Papugani A, Dou H, et al. Peroxisome proliferator-activated receptor-gamma activation suppresses HIV-1 replication in an animal model of encephalitis. AIDS. 2008;22:1539–1549. doi: 10.1097/QAD.0b013e3283081e08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watanabe S, Ohta S, Yajima M, Terashima K, Ito M, Mugishima H, Fujiwara S, Shimizu K, Honda M, Shimizu N, et al. Humanized NOD/SCID/IL2Rgamma(null) mice transplanted with hematopoietic stem cells under nonmyeloablative conditions show prolonged life spans and allow detailed analysis of human immunodeficiency virus type 1 pathogenesis. J Virol. 2007;81:13259–13264. doi: 10.1128/JVI.01353-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe S, Terashima K, Ohta S, Horibata S, Yajima M, Shiozawa Y, Dewan MZ, Yu Z, Ito M, Morio T, et al. Hematopoietic stem cell-engrafted NOD/SCID/IL2Rgamma null mice develop human lymphoid systems and induce long-lasting HIV-1 infection with specific humoral immune responses. Blood. 2007;109:212–218. doi: 10.1182/blood-2006-04-017681. [DOI] [PubMed] [Google Scholar]
- 28.Gorantla S, Sneller H, Walters L, Sharp JG, Pirruccello SJ, West JT, Wood C, Dewhurst S, Gendelman HE, Poluektova L. Human immunodeficiency virus type 1 pathobiology studied in humanized BALB/c-Rag2-/-gammac-/- mice. J Virol. 2007;81:2700–2712. doi: 10.1128/JVI.02010-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newman PJ. The role of PECAM-1 in vascular cell biology. Ann N Y Acad Sci. 1994;714:165–174. doi: 10.1111/j.1749-6632.1994.tb12041.x. [DOI] [PubMed] [Google Scholar]
- 30.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 31.Liu D, Zeng BX, Shang Y. Decreased expression of peroxisome proliferator-activated receptor gamma in endotoxin-induced acute lung injury. Physiol Res. 2006;55:291–299. doi: 10.33549/physiolres.930822. [DOI] [PubMed] [Google Scholar]
- 32.Standiford TJ, Keshamouni VG, Reddy RC. Peroxisome proliferator-activated receptor-gamma as a regulator of lung inflammation and repair. Proc Am Thorac Soc. 2005;2:226–231. doi: 10.1513/pats.200501-010AC. [DOI] [PubMed] [Google Scholar]
- 33.Theron S, Andronikou S, George R, du Plessis J, Goussard P, Hayes M, Mapukata A, Gie R. Non-infective pulmonary disease in HIV-positive children. Pediatr Radiol. 2009;39:555–564. doi: 10.1007/s00247-009-1156-2. [DOI] [PubMed] [Google Scholar]
- 34.Kanmogne GD, Kennedy RC, Grammas P. Analysis of human lung endothelial cells for susceptibility to HIV type 1 infection, coreceptor expression, and cytotoxicity of gp120 protein. AIDS Res Hum Retroviruses. 2001;17:45–53. doi: 10.1089/088922201750056771. [DOI] [PubMed] [Google Scholar]
- 35.Kanmogne GD, Primeaux C, Grammas P. Induction of apoptosis and endothelin-1 secretion in primary human lung endothelial cells by HIV-1 gp120 proteins. Biochem Biophys Res Commun. 2005;333:1107–1115. doi: 10.1016/j.bbrc.2005.05.198. [DOI] [PubMed] [Google Scholar]
- 36.Kanmogne GD, Primeaux C, Grammas P. HIV-1 gp120 proteins alter tight junction protein expression and brain endothelial cell permeability: implications for the pathogenesis of HIV-associated dementia. J Neuropathol Exp Neurol. 2005;64:498–505. doi: 10.1093/jnen/64.6.498. [DOI] [PubMed] [Google Scholar]
- 37.Pino S, Brehm MA, Covassin-Barberis L, King M, Gott B, Chase TH, Wagner J, Burzenski L, Foreman O, Greiner DL, et al. Development of novel major histocompatibility complex class I and class II-deficient NOD-SCID IL2R gamma chain knockout mice for modeling human xenogeneic graft-versus-host disease. Methods Mol Biol. 2010;602:105–117. doi: 10.1007/978-1-60761-058-8_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Becker J, Delayre-Orthez C, Frossard N, Pons F. Regulation of inflammation by PPARs: a future approach to treat lung inflammatory diseases? Fundam Clin Pharmacol. 2006;20:429–447. doi: 10.1111/j.1472-8206.2006.00425.x. [DOI] [PubMed] [Google Scholar]
- 39.Shrivastav S, Kino T, Cunningham T, Ichijo T, Schubert U, Heinklein P, Chrousos GP, Kopp JB. Human immunodeficiency virus (HIV)-1 viral protein R suppresses transcriptional activity of peroxisome proliferator-activated receptor gamma and inhibits adipocyte differentiation: implications for HIV-associated lipodystrophy. Mol Endocrinol. 2008;22:234–247. doi: 10.1210/me.2007-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang W, Eum SY, András IE, Hennig B, Toborek M. PPARalpha and PPARgamma attenuate HIV-induced dysregulation of tight junction proteins by modulations of matrix metalloproteinase and proteasome activities. FASEB J. 2009;23:1596–1606. doi: 10.1096/fj.08-121624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crossno JT, Jr, Garat CV, Reusch JE, Morris KG, Dempsey EC, McMurtry IF, Stenmark KR, Klemm DJ. Rosiglitazone attenuates hypoxia-induced pulmonary arterial remodeling. Am J Physiol Lung Cell Mol Physiol. 2007;292:L885–L897. doi: 10.1152/ajplung.00258.2006. [DOI] [PubMed] [Google Scholar]
- 42.Rehan VK, Sakurai R, Corral J, Krebs M, Ibe B, Ihida-Stansbury K, Torday JS. Antenatally administered PPAR-gamma agonist rosiglitazone prevents hyperoxia-induced neonatal rat lung injury. Am J Physiol Lung Cell Mol Physiol. 2010;299:L672–L680. doi: 10.1152/ajplung.00240.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aoki Y, Maeno T, Aoyagi K, Ueno M, Aoki F, Aoki N, Nakagawa J, Sando Y, Shimizu Y, Suga T, et al. Pioglitazone, a peroxisome proliferator-activated receptor gamma ligand, suppresses bleomycin-induced acute lung injury and fibrosis. Respiration. 2009;77:311–319. doi: 10.1159/000168676. [DOI] [PubMed] [Google Scholar]
- 44.de Andrade JA, Thannickal VJ. Innovative approaches to the therapy of fibrosis. Curr Opin Rheumatol. 2009;21:649–655. doi: 10.1097/BOR.0b013e328330da9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu D, Zeng BX, Shang Y. Decreased expression of peroxisome proliferator-activated receptor gamma in endotoxin-induced acute lung injury. Physiol Res. 2006;55:291–299. doi: 10.33549/physiolres.930822. [DOI] [PubMed] [Google Scholar]
- 46.Liu D, Zeng BX, Zhang SH, Wang YL, Zeng L, Geng ZL, Zhang SF. Rosiglitazone, a peroxisome proliferator-activated receptor-gamma agonist, reduces acute lung injury in endotoxemic rats. Crit Care Med. 2005;33:2309–2316. doi: 10.1097/01.ccm.0000183161.81503.7d. [DOI] [PubMed] [Google Scholar]
- 47.Cho HY, Gladwell W, Wang X, Chorley B, Bell D, Reddy SP, Kleeberger SR. Nrf2-regulated PPARgamma expression is critical to protection against acute lung injury in mice. Am J Respir Crit Care Med. 2010;182:170–182. doi: 10.1164/rccm.200907-1047OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Asada K, Sasaki S, Suda T, Chida K, Nakamura H. Antiinflammatory roles of peroxisome proliferator-activated receptor gamma in human alveolar macrophages. Am J Respir Crit Care Med. 2004;169:195–200. doi: 10.1164/rccm.200207-740OC. [DOI] [PubMed] [Google Scholar]
- 49.Malur A, Mccoy AJ, Arce S, Barna BP, Kavuru MS, Malur AG, Thomassen MJ. Deletion of PPAR gamma in alveolar macrophages is associated with a Th-1 pulmonary inflammatory response. J Immunol. 2009;182:5816–5822. doi: 10.4049/jimmunol.0803504. [DOI] [PubMed] [Google Scholar]
- 50.Chaudhuri A, Duan F, Morsey B, Persidsky Y, Kanmogne GD. HIV-1 activates proinflammatory and interferon-inducible genes in human brain microvascular endothelial cells: putative mechanisms of blood-brain barrier dysfunction. J Cereb Blood Flow Metab. 2008;28:697–711. doi: 10.1038/sj.jcbfm.9600567. [DOI] [PubMed] [Google Scholar]