Abstract
Rationale: Chronic neutrophilic inflammation is a hallmark in the pathogenesis of chronic obstructive pulmonary disease (COPD) and persists after cigarette smoking has stopped. Mechanisms involved in this ongoing inflammatory response have not been delineated.
Objectives: We investigated changes to the leukotriene A4 hydrolase (LTA4H)–proline-glycine-proline (PGP) pathway and chronic inflammation in the development of COPD.
Methods: A/J mice were exposed to air or cigarette smoke for 22 weeks followed by bronchoalveolar lavage and lung and cardiac tissue analysis. Two human cohorts were used to analyze changes to the LTA4H–PGP pathway in never smokers, control smokers, COPD smokers, and COPD former smokers. PGP/AcPGP and LTA4H aminopeptidase activity were detected by mass spectroscopy, LTA4H amounts were detected by ELISA, and acrolein was detected by Western blot.
Measurements and Main Results: Mice exposed to cigarette smoke developed emphysema with increased PGP, neutrophilic inflammation, and selective inhibition of LTA4H aminopeptidase, which ordinarily degrades PGP. We recapitulated these findings in smokers with and without COPD. PGP and AcPGP are closely associated with cigarette smoke use. Once chronic inflammation is established, changes to LTA4H aminopeptidase remain, even in the absence of ongoing cigarette use. Acrolein modifies LTA4H and inhibits aminopeptidase activity to the same extent as cigarette smoke.
Conclusions: These results demonstrate a novel pathway of aberrant regulation of PGP/AcPGP, suggesting this inflammatory pathway may be intimately involved in disease progression in the absence of ongoing cigarette smoke exposure. We highlight a mechanism by which acrolein potentiates neutrophilic inflammation through selective inhibition of LTA4H aminopeptidase activity.
Clinical trial registered with www.clinicaltrials.gov (NCT 00292552).
Keywords: COPD, inflammation, PGP, leukotriene A4 hydrolase, acrolein
At a Glance Commentary
Scientific Knowledge on the Subject
Chronic neutrophilic inflammation plays a key role in the development of chronic obstructive pulmonary disease. Cigarette smoke is central to the pathogenesis of disease. Persistent inflammation following cigarette smoke cessation is poorly understood.
What This Study Adds to the Field
Here, we translate findings of the leukotriene A4 hydrolase–proline-glycine-proline pathway of chronic inflammation from a smoking mouse model into two human cohorts. Acrolein, a reactive aldehyde found at sites of inflammation, selectively inhibits leukotriene A4 hydrolase aminopeptidase to the same extent as cigarette smoke and is a possible mechanism by which chronic inflammation persists following smoking cessation.
Chronic obstructive pulmonary disease (COPD) is prevalent worldwide and is now the third leading cause of death in the United States (1). Cigarette smoking is causative in most cases of COPD (2, 3). Cigarette smoke has been shown to induce a neutrophilic response in the airways and lungs of smokers and those with COPD (4–7). Although neutrophils are critical in the lung’s defense against microorganisms, halting excessive neutrophil recruitment and stimulating clearance may be necessary to limit ongoing tissue damage and remodeling. Classically, the glutamic acid-leucine-arginine (ELR+) CXC chemokines, such as IL-8, were thought to principally drive such neutrophilic inflammation (8). More recently, proline-glycine-proline (PGP), a tripeptide collagen breakdown product that shares sequence homology with a key motif found in the ELR+ CXC chemokines and binds to CXCR1 and CXCR2, was found to play an equally important role to ELR+ CXC chemokines in neutrophil chemotaxis and inflammation (9–11). PGP is generated in a sequential fashion through the activities of matrix metalloproteinases and the serine protease prolyl endopeptidase on collagen. Importantly, these enzymes and PGP are present in sputum of patients with COPD (10, 11). PGP exists in both an acetylated (AcPGP) and nonacetylated form, with the acetylated form being the more potent chemotactic peptide (12). Recently, we have shown that cigarette smoke can directly acetylate PGP (13).
One puzzling aspect of COPD is how neutrophilic inflammation persists in patients despite smoking cessation. We recently discovered that the enzyme responsible for degradation of PGP and resolution of acute inflammation is leukotriene A4 hydrolase (LTA4H) and suggested that derangement in its function could be responsible for chronic inflammation associated with COPD (13). LTA4H is a bifunctional enzyme with both proinflammatory and antiinflammatory functions. The epoxide hydrolase site catalyzes the conversion of leukotriene A4 to the proinflammatory mediator and neutrophil attractant leukotriene B4 (LTB4) (14). Additionally, LTA4H has aminopeptidase activity that degrades PGP, leading to resolution of acute inflammation (13). Based on previous studies with cigarette smoke extract, we hypothesized that smoking would inhibit the aminopeptidase activity of LTA4H without affecting the epoxide hydrolase function, shifting LTA4H toward a proinflammatory phenotype. If correct, this results in the accumulation of two proinflammatory molecules, PGP and LTB4, both of which have been implicated in COPD pathogenesis (9, 13–15). In this study, we first examined if an aberrant LTA4H–PGP pathway is observed in a smoking mouse model of COPD. Based on this preclinical evidence, we hypothesized that increased airway inflammation, manifested by high concentrations of myeloperoxidase (MPO), PGP, and LTB4, would be present in human smokers and those with COPD as a result of selective inhibition of the aminopeptidase activity of LTA4H. Furthermore, we hypothesize that alterations to the LTA4H–PGP pathway will correlate with clinical disease. Finally, we sought to determine whether once this pathway is initiated, there is ongoing selective LTA4H aminopeptidase inactivation through actions of acrolein, a component found in cigarette smoke (16, 17) and in current smokers (18–21) and former smokers with COPD (22), leading to a feed-forward process resulting in PGP accumulation and disease progression. Some of the results of these studies have been previously reported in the form of abstracts (23, 24).
Methods
Murine Smoking Model
This study was reviewed and approved by the Institutional Laboratory Animal Care and Use Committee of University of Alabama at Birmingham (Animal Protocol #120709133) and conformed with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (25). Female A/J mice 6 weeks of age were randomly assigned to either 48 minutes per day, 5 days per week, for 22 consecutive weeks of cigarette smoke exposure (smoke, n = 6) or air-exposed (control, n = 11) groups. The A/J mouse strain has been reported to be susceptible to cigarette smoke–induced lung disease (26, 27). We used mainstream whole-body cigarette smoke exposure with standard University of Kentucky 3R4F research cigarettes (9.4 mg tar per 0.726 mg nicotine, University of Kentucky) aiming for a moderate smoke exposure that would be expected to induce disease with chronic exposure (27). Cigarette smoke was delivered by the SCIREQ “InExpose” smoking system (SCIREQ, Montreal, QB, Canada) using the parameters that have been reported previously (28).
Human Cohorts
We enrolled 70 subjects in our original pilot cohort at the University of Alabama at Birmingham. All subjects provided informed consent and the study was approved by the local institutional review board (# F090427007). For inclusion, subjects were more than 40 years old and must be able to produce induced sputum. Subgroups were defined based on lung function and smoking status. Healthy control subjects had no evidence of obstruction by spirometry and less than 10 pack-year smoking histories with no tobacco use in 1 year. Control smokers had more than 10 pack-year smoking histories, including current cigarette use, and had no evidence of airflow obstruction by spirometry. COPD patients had airflow limitation as defined by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines with a FEV1/FVC ratio less than 0.70 (29). Spirometry was performed following American Thoracic Society/European Respiratory Society standards (30) using the KoKo spirometer (nSpire Health, Longmont, CO). To confirm preliminary findings observed in our pilot cohort, sputum samples from current and former smoking COPD patients enrolled in the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study (Clinicaltrials.gov identifier NCT00292552, GSK Study No. SCO104960) were evaluated. Details regarding the methods for the ECLIPSE study have been previously published (31). Samples were deidentified and processed by investigators masked to each subgroup. Chronic bronchitis was defined by patient-reported positive answers to questions in a modified ATS-DLD-78 indicating cough with productive sputum most days for 3 consecutive months or more during the year for at least 2 years (32). Emphysema was defined by a computed tomography low-attenuation area value of less than −950 Hounsfield units on inspiratory scans as previously reported. A % low-attenuation area threshold of greater than 10% was considered indicative of significant emphysema (33).
PGP, AcPGP, and LTB4 Detection
Large-molecular-weight proteins were removed from sputum samples via 100-kD MW cutoff filters and were analyzed by liquid chromatography–electrospray ionization–tandem mass spectrometry as described previously (9–11, 13). Specifics on LTA4H aminopeptidase activity, MPO ELISA, LTA4H ELISA, Western blot analyses, and processing are accessible from this issues’ table of content online at www.atsjournals.org.
Statistical Analysis
Baseline data from our patient cohort are expressed as means with standard deviations for normally distributed values. Bivariate analyses were conducted with two-tailed Fisher exact test for categorical data and two tailed t tests or Wilcoxon rank sum test for continuous data where appropriate. One-way analysis of variance was used to compare the means between multiple sample groups. Analysis of covariance was used to compare differences between linear slopes for Ala-pNA detected LTA4H aminopeptidase inactivation by cigarette smoke condensate and acrolein. All analyses were performed with SPSS software (version 20.0; IBM, Armonk, NY) and P values less than 0.05 were designated as statistically significant.
Results
Changes to LTA4H–PGP Balance Occur in Mice with Chronic Cigarette Smoke Exposure
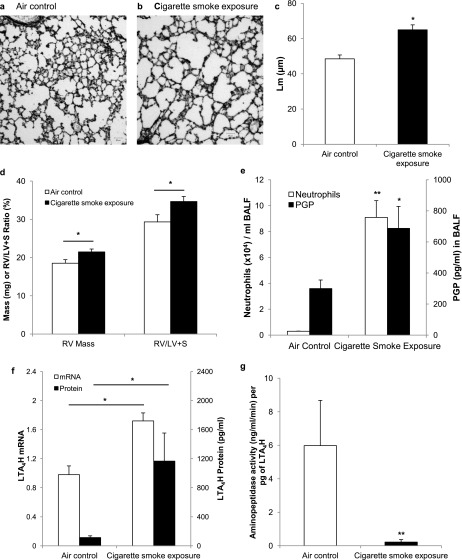
Female AJ mice were exposed to whole-body cigarette smoke (n = 6) or air control (n = 11) for 22 weeks. The mice exposed to cigarette smoke developed an emphysematous phenotype, manifest by increased linear intercept (61.9 ± 2.8 vs. 47.1 ± 2.2 μm; P < 0.05) (Figures 1a–1c) accompanied by reductions in alveolar septal volume and surface density (see Table E1 in the online supplement) and signs of cor pulmonale with development of right ventricular hypertrophy (Figure 1d) consistent with previously published results for PGP administration to the lung (9). Bronchoalveolar neutrophils increased from 0.30 ± 0.01 × 104 cells/ml in air control animals to 9.1 ± 1.29 × 104 cells/ml (P < 0.001) (Figure 1e) in cigarette smoke–exposed mice, correlating to an increased from 1.5% to 26% (P = 0.004). PGP increased from 300 ± 55 to 688 ± 141 pg/ml (P = 0.03) (Figure 1e) in bronchoalveolar lavage fluid of cigarette smoke–exposed mice, paralleling the increase in neutrophils.
Figure 1.
A/J mice exposed to once-daily whole-body cigarette smoke for 22 weeks develop an emphysematous phenotype and alterations to the leukotriene A4 hydrolase (LTA4H)–proline-glycine-proline (PGP) pathway. Representative hematoxilin and eosin stain of air control (n = 11) (a) and cigarette smoke–exposed (n = 6) (b) mouse and resultant increase in mean linear intercept (Lm) (c) in the lungs of cigarette smoke–exposed mice, and development of right ventricular (RV) hypertrophy measured by increased RV mass and increased RV/LV ratio as seen in d. Neutrophil burden and PGP amounts are increased in bronchoalveolar lavage fluid (BALF) of cigarette smoke–exposed mice as seen in e. Additionally, LTA4H mRNA amounts are increased as is the amount of enzyme detected by ELISA, depicted in f. Despite these increases in enzyme amount, the aminopeptidase function is inactivated by more than 95% in cigarette smoke–exposed mice as seen in g, preventing the degradation of PGP in cigarette smoke–exposed mice. *P < 0.05, **P < 0.01 by two-sided t test, with error bars representing SEM.
The amount of LTA4H mRNA isolated from lung tissue was increased by 1.75-fold in cigarette smoke–exposed mice compared with air control animals as seen in Figure 1f. This increase was accompanied by an increase in LTB4 from 154 ± 39 to 270 ± 35 pg/ml (P = 0.03) in the cigarette smoke–exposed group, reflecting intact intracellular epoxide hydrolase activity. Extracellular LTA4H increased from 114 ± 24 to 1165 ± 387 pg/ml (P = 0.03) and aminopeptidase activity decreased from 489 to 132 ng/ml/min (P = 0.03) in cigarette smoke–exposed mice. This equates to a standard enzyme activity of 5.91 and 0.25 ng/ml/min/pg of enzyme (P = 0.01) (Figure 1g), a finding that more accurately reflects the degree of selective aminopeptidase inhibition of extracellular LTA4H caused by cigarette smoke.
Baseline Patient Characteristics
To determine whether the effects observed in the mouse model were recapitulated in humans, subjects were recruited into a pilot cohort at a single center (n = 66) and divided into groups of never smokers (n = 18), control smokers (no airflow obstruction, n = 25), COPD current smokers (n = 13), or former smokers (n = 10). Patient characteristics are outlined in Table 1. There were no differences in race across all groups, but there were significantly more female never smokers than control smokers or COPD subjects (P = 0.02). The COPD groups were also older than both the never smokers (P < 0.01) and control smokers (P < 0.05). There was no difference in pack-year history between control smokers and those with COPD, and no difference in FEV1 between never smokers and control smokers. Levels of dyspnea and sputum production were higher in those with COPD.
Table 1.
Baseline Clinical Characteristics for the Pilot Cohort
| Never Smoker (n = 18) | Control Smoker (n = 25) | COPD Current Smoker (n = 13) | COPD Former Smoker (n = 10) | |
|---|---|---|---|---|
| Median age, yr | 47 ± 7* | 51 ± 5† | 55 ± 6 | 59 ± 8 |
| Male sex | 22% (4)† | 56% (14) | 54% (8) | 80% (8) |
| Non-Hispanic white race | 50% (9) | 28% (7) | 47% (7) | 40% (4) |
| Pack-year history | — | 44 ± 21 | 53 ± 23 | 52 ± 20 |
| FEV1, % predicted | 102 ± 11* | 96 ± 15* | 72 ± 18 | 76 ± 15 |
| FEV1/FVC | 0.82* | 0.79* | 0.59 | 0.61 |
| MMRC, median | 0* | 1 | 1 | 2 |
| BCSS, median | 0* | 4 | 5 | 3.5 |
Definition of abbreviations: BCSS = breathless, coughing, and sputum scale, range 0–12 with higher scores indicating greater burden of symptoms related to sputum production and a MCID of 1; COPD = chronic obstructive pulmonary disease; MMRC = modified Medical Research Council questionnaire, range 0–4 with higher scores indicating greater dyspnea.
Values are reported as percent (number) or mean ± standard deviation unless otherwise specified. Baseline characteristics are separated based on smoking status and the presence of COPD. Race is self-reported.
P < 0.01 compared with other groups.
P < 0.05 compared with other groups.
To validate the observations from the pilot cohort, we evaluated changes to AcPGP, LTA4H, and aminopeptidase activity in 214 participants enrolled in the ECLIPSE study. This cohort included 107 current smokers and 107 former smokers with COPD. In the ECLIPSE study cohort, current smokers were younger than former smokers. There were no differences in sex, race, pack-year smoking duration, or lung function between current and former smokers with COPD as seen in Table 2.
Table 2.
Baseline Clinical Characteristics for the ECLIPSE Study Cohort
| COPD Current Smoker (n = 107) | COPD Former Smokers (n = 107) | P Value | |
|---|---|---|---|
| Median age, yr | 62 ± 7 | 65 ± 6 | 0.008 |
| Male sex | 58% (62) | 69% (70) | 0.26 |
| Non-Hispanic white race | 100% (107) | 97% (104) | 0.08 |
| Pack-year history | 48 ± 27 | 52 ± 32 | 0.36 |
| FEV1, % predicted | 50 ± 14 | 49 ± 16 | 0.33 |
| FEV1/FVC | 0.45 ± 0.11 | 0.44 ± 0.11 | 0.45 |
| Median GOLD stage | 3 | 3 | 0.42 |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; GOLD = Global Initiative for Chronic Obstructive Lung Disease.
Values are reported as percent (number) or mean ± standard deviation unless otherwise specified. Baseline characteristics are separated based on smoking status and the presence of COPD. Race is self-reported.
Changes to the LTA4H–PGP Pathway Occur in Smokers without COPD
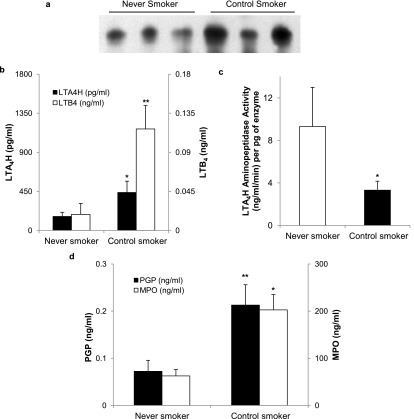
LTA4H was increased in sputum of control smokers compared with never smokers when measured by Western blot (2,046 ± 493 vs. 696 ± 280 arbitrary OD units; P = 0.03) (Figure 2a) and ELISA (438 ± 129 vs. 162 ± 48 pg/ml; P = 0.05) (Figure 2b). Despite the increase in enzyme concentration, aminopeptidase activity is reduced by approximately 65% in smokers compared with never smokers (3.32 ± 0.85 vs. 9.31 ± 3.67 ng/ml/min/pg of enzyme; P = 0.03) (Figure 2c).
Figure 2.
Changes in sputum leukotriene A4 hydrolase (LTA4H) amount and activity affect proline-glycine-proline (PGP) in smokers and never smokers. Sputum samples from never smokers (n = 18) and control smokers (n = 25) underwent immunoprecipitation and Western blot analysis demonstrating increased amounts of LTA4H in smokers compared with never smokers (a, representative blot). (b) The increase was quantified and confirmed by ELISA. The amount of leukotriene B4 (LTB4) in the sputum of smokers was increased in smokers to the same extent that was seen in LTA4H (b), suggesting that the epoxy-hydrolase activity is intact. Aminopeptidase activity is significantly inhibited in the sputum of smokers compared with never smokers as seen in c, suggesting that the aminopeptidase function is selectively inactivated. Levels of PGP are threefold elevated in the sputum of smokers, similar to changes seen in levels of the neutrophil marker myeloperoxidase (MPO) as depicted in d. *P < 0.05, **P ≤ 0.01, with error bars representing SEM.
LTB4 concentrations were higher in smokers compared with never smokers from 19 ± 12 to 117 ± 27 pg/ml (P = 0.004) (Figure 2b), equating to an increase in mole ratio of LTB4/LTA4H from 32.7 to 54.5, suggesting the epoxide hydrolase function of the enzyme remains intact in the setting of active cigarette smoking. These findings confirm observations from cigarette smoke–exposed mice. The neutrophil marker MPO is elevated in control smokers compared with never smokers (203 ± 33 vs. 63 ± 13 ng/ml; P = 0.01). PGP was threefold higher (212 ± 43 vs. 72 ± 23 pg/ml; P = 0.02) in control smokers compared with never smokers as seen in Figure 2d.
Further Derangements to the LTA4H–PGP Pathway Occur in COPD and Persist in the Absence of Ongoing Cigarette Smoking
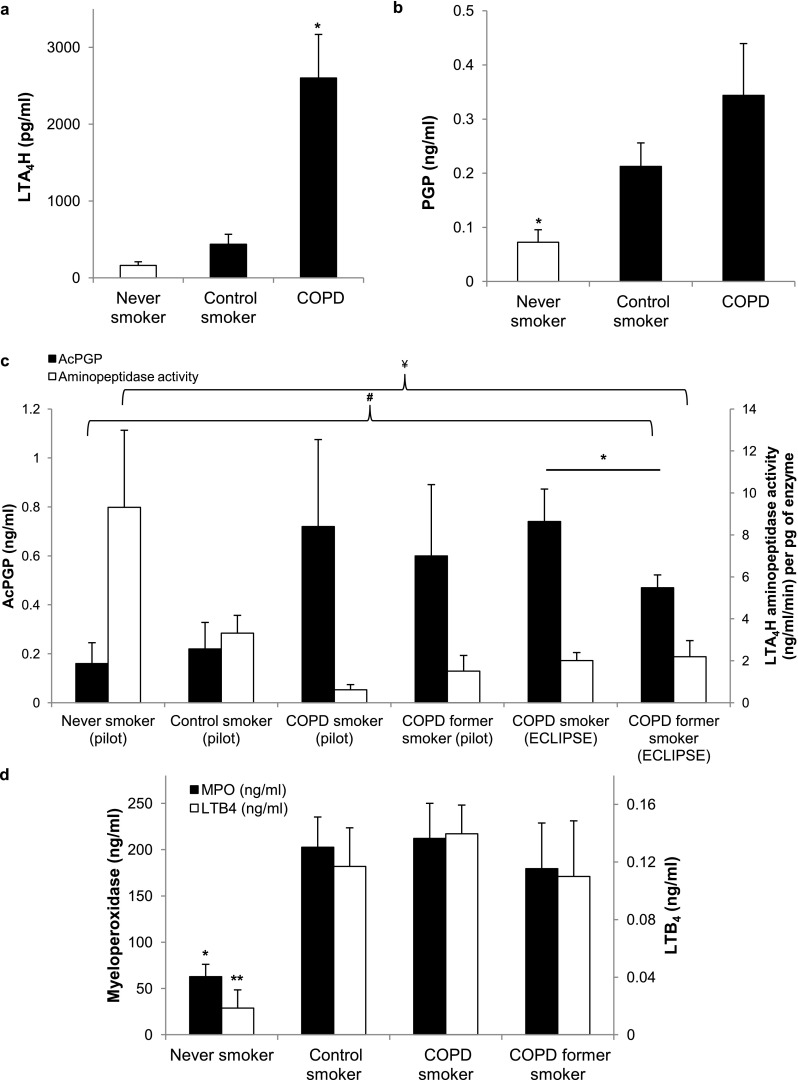
Total LTA4H is increased in the sputum of COPD subjects compared with smokers and never smokers measured by ELISA (2,601 ± 567 vs. 438 ± 129 pg/ml and 161 ± 48 pg/ml, respectively; P = 0.04 by one-way analysis of variance) (Figure 3a). Among subjects with COPD, LTA4H is elevated to similar extents (2,870 ± 745 vs. 2,252 ± 907 pg/ml; P = 0.60) in both current and former smokers. Although amounts of total enzyme are increased, aminopeptidase activity is further inhibited in the sputum of subjects with COPD compared with that of smokers and never smokers (1.02 ± 0.37 vs. 3.32 ± 0.85 ng/ml/min per pg of enzyme [P = 0.02] and 9.31 ± 3.68 ng/ml/min per pg of enzyme, P = 0.015, respectively) (Figure 3c). The degree of aminopeptidase inhibition in subjects with COPD was similar between current and former smokers (P = 0.24) (Figure 3c).
Figure 3.
Selective aminopeptidase inhibition leads to proline-glycine-proline (PGP) accumulation in sputum of smokers and in patients with chronic obstructive pulmonary disease (COPD) independent of smoking status. (a) Leukotriene A4 hydrolase (LTA4H) amounts are increased in subjects with COPD (n = 23) compared with never smokers (n = 18) and control smokers (n = 25). (b) In the pilot cohort, PGP levels are elevated in COPD subjects compared with never smokers and elevated to a similar extent as that seen in control smokers. (c) Aminopeptidase activity is decreased by more than 80% in COPD subjects, independent of ongoing cigarette use. Acetylated PGP (AcPGP) is elevated in both current and former smokers in the pilot cohort, with a trend toward lower AcPGP in former smokers. In the ECLIPSE group, AcPGP amounts are elevated in current COPD smokers (n = 107) compared with former smokers with COPD (n = 107; P = 0.05). (d) In the pilot cohort, sputum myeloperoxidase (MPO) and leukotriene B4 (LTB4) levels are increased to similar extents in healthy smokers and COPD subjects compared with never smokers. *P ≤ 0.05, **P < 0.01 by two-sided t test, #P = 0.015 for AcPGP by one-way analysis of variance, ¥P < 0.001 for aminopeptidase activity by one-way analysis of variance. Error bars represent SEM.
PGP amounts are elevated in sputum of COPD subjects compared with never smokers (343 ± 96 vs. 72 ± 23 pg/ml; P = 0.03) but levels are not significantly different from those seen in control smokers (P = 0.21) (Figure 3b). In the pilot cohort, AcPGP is higher in the sputum of all subjects with COPD compared with control smokers (0.65 ± 22 vs. 0.22 ± 0.11 ng/ml; P = 0.05), and remains elevated in the sputum of former smokers with COPD (0.60 ± 0.29 vs. 0.72 ± 0.35 ng/ml in COPD current smokers; P = 0.80) (Figure 3c). Sputum LTB4 and MPO amounts are higher in subjects with COPD compared with never smokers (P < 0.001 and P = 0.004, respectively) and are similar between control smokers and those with COPD, as seen in Figure 3d. Based on the elevated LTB4 levels in COPD, it seems that, as with control smokers, the epoxide hydrolase function of LTA4H is intact. AcPGP and MPO were correlated (r = 0.40; P = 0.004), consistent with previous findings (34). There were no correlations between PGP/AcPGP and LTB4 amounts.
To build on these observations, sputum samples from COPD current and former smokers enrolled in ECLIPSE were used to validate the changes observed in AcPGP, LTA4H, and aminopeptidase activity in the pilot cohort. In ECLIPSE, AcPGP is higher in COPD current smokers compared with former smokers (0.74 ± 0.13 vs. 0.47 ± 0.05 ng/ml; P = 0.05) (Figure 3c). LTA4H enzyme amounts are elevated to similar extents in current and former smokers (3,007 ± 742 vs. 3,692 ± 615 pg/ml; P = 0.48) and there is no difference in aminopeptidase activity (2.01 ± 0.39 vs. 2.19 ± 0.77 ng/ml/min per pg of enzyme; P = 0.83) (Figure 3c). The levels observed in ECLIPSE were similar to those observed in the pilot cohort and demonstrate similar levels of altered enzyme function.
Clinical Implications of an Altered LTA4H–AcPGP Pathway in COPD
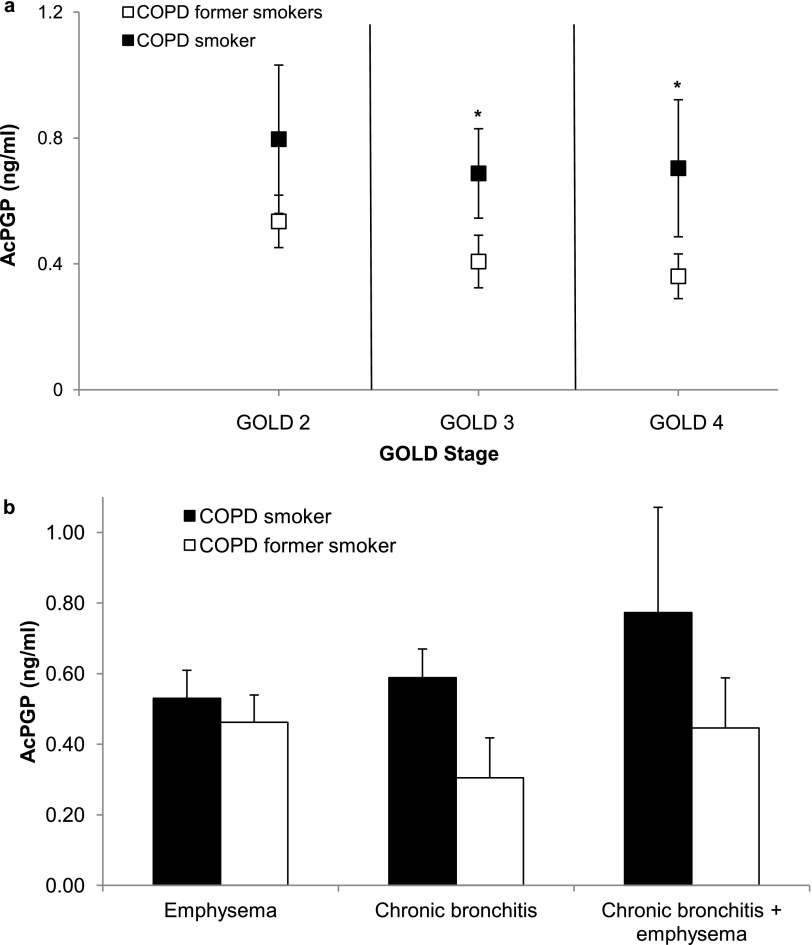
In the ECLIPSE cohort, log AcPGP levels were strongly correlated with cigarette smoking in a univariate logistic model (odds ratio, 3.04; 95% confidence interval, 1.40–6.61; P = 0.005). In fact, current smokers had similar AcPGP levels at GOLD stage 2, but were higher than AcPGP in former smokers in severe disease (GOLD 3 and 4) as seen in Figure 4a. As reported for the entire cohort above, LTA4H enzyme amounts and aminopeptidase activity were similar between current and former smokers despite GOLD stage. The increase in AcPGP in current smokers suggests that increases are the result of increased AcPGP production and not caused by alterations in PGP/AcPGP breakdown. This phenomenon of sustained inactivation of LTA4H aminopeptidase in former smokers led to exploration of potential mechanisms for its inactivation. Furthermore, the association between AcPGP levels and chronic bronchitis versus emphysema phenotype was evaluated in subjects from the ECLIPSE study. As seen in Figure 4b, AcPGP levels were similar between smokers and former smokers with emphysema alone (0.53 ± 0.08 vs. 0.46 ± 0.08 ng/ml; P = 0.54) but trended toward higher values in those with chronic bronchitis (0.59 ± 0.08 vs. 0.31 ± 0.11 ng/ml; P = 0.06). In patients with both chronic bronchitis and emphysema, there were no differences in AcPGP between smokers and former smokers (0.77 ± 0.30 vs. 0.44 ± 0.14 ng/ml; P = 0.33).
Figure 4.
Differences in acetylated proline-glycine-proline (AcPGP) between current and former smokers with chronic obstructive pulmonary disease (COPD) according to disease severity and phenotype. In the ECLIPSE cohort, AcPGP levels are elevated in both current and former smokers with COPD. (a) In severe COPD (Global Initiative for Chronic Obstructive Lung Disease [GOLD] stage 3 and 4), AcPGP is higher in current smokers (P = 0.045 for GOLD 3 and P = 0.035 for GOLD 4) compared with former smokers with similar airflow obstruction. (b) Sputum AcPGP is higher in COPD smokers with chronic bronchitis compared with former smokers (P = 0.06). Sputum AcPGP levels are similar between current and former smokers with either significant emphysema alone or in emphysema in combination with chronic bronchitis.
Acrolein Inhibits LTA4H Aminopeptidase Activity Similar to Cigarette Smoke, and LTA4H Is Acroleinated in Sputum of Smokers and Subjects with COPD
Although cigarette smoking readily explains the inactivation of LTA4H aminopeptidase activity and elevated PGP levels in COPD subjects who smoke, it is not known what drives the persistence of this phenotype in former smokers with COPD. One possibility is the reactive aldehyde acrolein, a component of cigarette smoke. Acrolein can be generated endogenously at sites of inflammation by the action of MPO and is markedly elevated in former smokers with COPD (35).
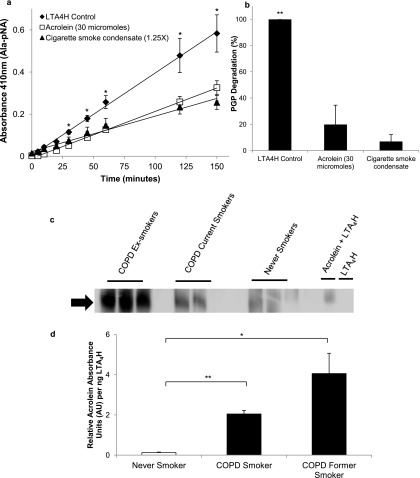
Interestingly, we have found that acrolein can mimic cigarette smoke by inactivating the aminopeptidase of LTA4H (P < 0.001 compared with control) (Figure 5a). This corresponds to the same degree of inhibition that is seen with cigarette smoke condensate at both 2.5 hours (49% activity compared with 57% in cigarette smoke condensate, P = 0.40; and P < 0.001 with control) and 24 hours of exposure (20% activity compared with 7% activity in cigarette smoke condensate, P = 0.24; and P < 0.01 with control) (Figure 5b), consistent with prior reports (13). Consequently, we tested whether LTA4H in COPD ex-smokers was acroleinated. Representative sputum samples from never smokers, COPD current smokers, and former smokers were immunoprecipated along with control LTA4H enzyme in and out of the presence of acrolein. All samples were probed for acrolein through Western blot analysis and standardized to LTA4H enzyme amount. The band present in the samples with COPD represents acroleinated LTA4H (Figure 5c). These values correlate to 0.13 ± 0.02 relative acrolein absorbance units per nanogram of LTA4H in never smokers, 2.05 ± 0.18 relative acrolein absorbance units per nanogram of LTA4H in COPD current smokers (P < 0.001 compared with never smokers), and 4.06 ± 1.01 relative acrolein absorbance units per nanogram of LTA4H in COPD former smokers (P = 0.008 compared with never smokers; P = 0.005 between all groups) (Figure 5d). The presence of acroleinated LTA4H in the absence of ongoing cigarette smoke exposure suggests that this process continues, possibly through endogenous acrolein production at sites of ongoing inflammation (18, 22, 35).
Figure 5.
Acrolein inhibits leukotriene A4 hydrolase (LTA4H) aminopeptidase activity and is detectable in the sputum of patients with chronic obstructive pulmonary disease (COPD), even in the absence of ongoing cigarette use. In an in vitro setting, cigarette smoke and acrolein significantly inhibit LTA4H aminopeptidase activity when measured over time by Ala-pNA assay (a) or at 24 hours when measured by proline-glycine-proline (PGP) degradation (b). Acroleinated LTA4H is present in the sputum of patients with COPD. (c) Representative Western blots (n = 4–5 per group) of LTA4H probed for acrolein. LTA4H was isolated from the sputum of smokers and former smokers with COPD by immunoprecipitation. The band densities shown were standardized to the amount of LTA4H enzyme concentration as detected by ELISA and then standardized to the band density of the LTA4H + acrolein control. (d) Relative acrolein absorbance units (AU) per nanogram of LTA4H was significantly higher in both COPD smokers (n = 4) and COPD former smokers (n = 4) compared with never smokers (n = 5). *P < 0.05, **P < 0.01, error bars represent SEM.
Discussion
Our results are the first to translate observed alterations to the LTA4H–PGP pathway in a murine model of chronic cigarette smoke exposure into clinical disease. Furthermore, we demonstrate that AcPGP is strongly associated with current cigarette smoking across all levels of COPD disease severity. Finally, we offer an explanation for observed continued LTA4H aminopeptidase inactivation and concomitant inflammation in this disease through the effect of acrolein. Indeed, cigarette smoke exposure selectively inhibits LTA4H aminopeptidase activity in the airways of smokers and in those with COPD, which initiates PGP accumulation and chronic neutrophilic inflammation. Once COPD is established, these effects persist, even in the absence of further cigarette smoke exposure.
The current findings build on prior observations in an influenza model of acute pulmonary neutrophilic inflammation (13). Elevations in amounts of LTA4H in the setting of cigarette smoke exposure is likely caused by increased transcription of LTA4H to halt acute inflammation caused by the cascade cigarette smoke induces, including neutrophil chemotaxis, collagen breakdown, and generation of inflammatory mediators LTB4 and PGP. However, because of the selective inactivation of the aminopeptidase function of LTA4H, there is an accumulation of PGP/AcPGP and increased generation of LTB4 from the preserved epoxide hydrolase site in the setting of higher enzyme concentrations. AcPGP levels are highest in COPD smokers compared with former smokers, a trend that becomes more apparent at higher GOLD stages of disease. These differences are caused by both increased PGP/AcPGP generation through cigarette smoke–mediated up-regulation of matrix metalloproteinase and prolyl endopeptidase activity (27, 36) and selective LTA4H aminopeptidase inactivation. Interestingly, once smokers develop COPD, these derangements observed in LTA4H function persist even in the absence of ongoing cigarette use. Former smokers with COPD no longer have the increased stimuli for PGP/AcPGP generation, but are unable to degrade the existing PGP leading to elevated levels below what is seen in COPD smokers with similar lung function. This may be in part explained by endogenous acrolein generation through the catalysis of MPO on free threonine at sites of injury (37).
The persistent inactivation of LTA4H aminopeptidase is at least in part caused by the effect of acrolein on the enzyme. We clearly demonstrate that endogenous levels of acrolein in smoking and ex-smoking cohorts can chemically modify and thereby inhibit LTA4H aminopeptidase activity. This finding shows a unique and specific biochemistry-to-function relationship between a reactive aldehyde (acrolein) and enzyme of interest (LTA4H), correlating with changes in an important bioactive product (PGP). Therefore, our work sheds light on the capability of small reactive aldehydes as critical regulators of innate immune response in disease, thereby potentially contributing to disease progression.
Our translational clinical study was limited by the small sample size in our original pilot cohort. Although we observed differences, the true effect size of the selective inhibition of LTA4H aminopeptidase would have been underrepresented. However, we extended the findings of elevated sputum PGP levels and aminopeptidase inhibition in a second large cohort from ECLIPSE, confirming the observations from the pilot translational study and expanding our understanding by demonstrating that there are indeed differences in PGP levels in COPD subjects depending on their smoking status. This reflects the true picture of PGP and LTA4H balance in patients with COPD. Additionally, data from both populations were assessed cross-sectionally, which is subject to inherent limitations for these types of studies. However, because it is the first to examine the pathways defined by prior work (13), we now have an understanding of the prevalence and outcomes associated with LTA4H–PGP pathway dysregulation. Although LTB4 remains elevated in smokers and those with COPD, it is unknown how neutrophils and macrophages release LTA4H in response to cigarette smoke and this should be studied further. Finally, bacterial colonization has been shown to influence sputum inflammation, including LTB4, neutrophil counts, and IL-8 (38), and we did not evaluate the contribution bacteria may have on this pathway.
In conclusion, the smoke-mediated loss of LTA4H aminopeptidase activity and resultant elevation in PGP/AcPGP amount seems to be a new and prominent factor in chronic neutrophilic inflammation and the development of COPD. The implications of acrolein inhibiting the crucial step in PGP catabolism even in the setting of cigarette abstinence serves as a unique hypothesis-generating scenario into the progression of disease that may ultimately support the development of novel disease-modifying therapies for COPD.
Footnotes
Supported by a Walter B. Frommeyer Jr. Fellowship in Investigational Medicine, University of Alabama (J.M.W.); NHLBI grants HL110950, HL114439, and HL07783 (J.E.B.); NHLBI grant HL092296 (P.J.O’R.); Mosaic grant from the Netherlands Organization for Scientific Research (Nederlandse Organisatie voor Wetenschappelijk Onderzoek, The Hague, The Netherlands; grant 017.008.029 (M.A.R.). ECLIPSE was funded by GlaxoSmithKline. Funds for the operation of the Targeted Metabolomics and Proteomics Laboratory come in part from the University of Alabama O'Brien Acute Kidney Injury Center (P30 DK079337), the University of Alabama Skin Disease Research Center (P30 AR50948), the University of Alabama Lung Health Center, and the University of Alabama Center for Free Radical Biology.
Research reported in this publication was supported by the NHLBI, National Institutes of Health, and the Family Smoking Prevention and Tobacco Control Act. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration.
Author Contributions: Study design, J.M.W., P.J.O’R., S.I.R., and J.E.B. Sample acquisition and analysis, J.M.W., P.J.O’R., T.S., D.I.S., G.H., C.G., C.M.M., M.A.R., B.E.M., R.T.-S., A.G., P.L.J., and J.E.B. Data interpretation, J.M.W., P.J.O’R., B.E.M., R.T.-S., S.I.R., and J.E.B. Drafting and revision of the manuscript, all authors. Accountability agreement, J.E.B. is accountable for the accuracy and integrity of all parts of the work.
Originally Published in Press as DOI: 10.1164/rccm.201401-0145OC on May 29, 2014
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Akinbami LJ, Liu X.Chronic obstructive pulmonary disease among adults aged 18 and over in the United States, 1998–2009. NCHS Data Brief2011631–8 [PubMed] [Google Scholar]
- 2.Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, Conway WA, Jr, Enright PL, Kanner RE, O'Hara P, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA. 1994;272:1497–1505. [PubMed] [Google Scholar]
- 3.Centers for Disease Control. The Surgeon General's 1989 report on reducing the health consequences of smoking: 25 years of progress. MMWR Morb Mortal Wkly Rep. 1989;38:1–32. [PubMed] [Google Scholar]
- 4.McCusker K. Mechanisms of respiratory tissue injury from cigarette smoking. Am J Med. 1992;93:18S–21S. doi: 10.1016/0002-9343(92)90622-i. [DOI] [PubMed] [Google Scholar]
- 5.Jeffery PK. Structural and inflammatory changes in COPD: a comparison with asthma. Thorax. 1998;53:129–136. doi: 10.1136/thx.53.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med. 2000;343:269–280. doi: 10.1056/NEJM200007273430407. [DOI] [PubMed] [Google Scholar]
- 7.O'Donnell R, Breen D, Wilson S, Djukanovic R. Inflammatory cells in the airways in COPD. Thorax. 2006;61:448–454. doi: 10.1136/thx.2004.024463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thatcher TH, McHugh NA, Egan RW, Chapman RW, Hey JA, Turner CK, Redonnet MR, Seweryniak KE, Sime PJ, Phipps RP. Role of CXCR2 in cigarette smoke-induced lung inflammation. Am J Physiol Lung Cell Mol Physiol. 2005;289:L322–L328. doi: 10.1152/ajplung.00039.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weathington NM, van Houwelingen AH, Noerager BD, Jackson PL, Kraneveld AD, Galin FS, Folkerts G, Nijkamp FP, Blalock JE. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med. 2006;12:317–323. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]
- 10.Gaggar A, Jackson PL, Noerager BD, O'Reilly PJ, McQuaid DB, Rowe SM, Clancy JP, Blalock JE. A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation. J Immunol. 2008;180:5662–5669. doi: 10.4049/jimmunol.180.8.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Reilly PJ, Hardison MT, Jackson PL, Xu X, Snelgrove RJ, Gaggar A, Galin FS, Blalock JE. Neutrophils contain prolyl endopeptidase and generate the chemotactic peptide, PGP, from collagen. J Neuroimmunol. 2009;217:51–54. doi: 10.1016/j.jneuroim.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haddox JL, Pfister RR, Muccio DD, Villain M, Sommers CI, Chaddha M, Anantharamaiah GM, Brouillette WJ, DeLucas LJ. Bioactivity of peptide analogs of the neutrophil chemoattractant, N-acetyl-proline-glycine-proline. Invest Ophthalmol Vis Sci. 1999;40:2427–2429. [PubMed] [Google Scholar]
- 13.Snelgrove RJ, Jackson PL, Hardison MT, Noerager BD, Kinloch A, Gaggar A, Shastry S, Rowe SM, Shim YM, Hussell T, et al. A critical role for LTA4H in limiting chronic pulmonary neutrophilic inflammation. Science. 2010;330:90–94. doi: 10.1126/science.1190594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haeggstrom JZ, Kull F, Rudberg PC, Tholander F, Thunnissen MM. Leukotriene a4 hydrolase. Prostaglandins Other Lipid Mediat. 2002;68–69:495–510. doi: 10.1016/s0090-6980(02)00051-5. [DOI] [PubMed] [Google Scholar]
- 15.Corhay JL, Henket M, Nguyen D, Duysinx B, Sele J, Louis R. Leukotriene b4 contributes to exhaled breath condensate and sputum neutrophil chemotaxis in COPD. Chest. 2009;136:1047–1054. doi: 10.1378/chest.08-2782. [DOI] [PubMed] [Google Scholar]
- 16.Moretto N, Bertolini S, Iadicicco C, Marchini G, Kaur M, Volpi G, Patacchini R, Singh D, Facchinetti F. Cigarette smoke and its component acrolein augment IL-8/CXCl8 mRNA stability via p38 MAPK/MK2 signaling in human pulmonary cells. Am J Physiol Lung Cell Mol Physiol. 2012;303:L929–L938. doi: 10.1152/ajplung.00046.2012. [DOI] [PubMed] [Google Scholar]
- 17.Uchiyama S, Inaba Y, Kunugita N. Determination of acrolein and other carbonyls in cigarette smoke using coupled silica cartridges impregnated with hydroquinone and 2,4-dinitrophenylhydrazine. J Chromatogr A. 2010;1217:4383–4388. doi: 10.1016/j.chroma.2010.04.056. [DOI] [PubMed] [Google Scholar]
- 18.Dalle-Donne I, Carini M, Vistoli G, Gamberoni L, Giustarini D, Colombo R, Maffei Facino R, Rossi R, Milzani A, Aldini G. Actin cys374 as a nucleophilic target of alpha,beta-unsaturated aldehydes. Free Radic Biol Med. 2007;42:583–598. doi: 10.1016/j.freeradbiomed.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 19.Eiserich JP, van der Vliet A, Handelman GJ, Halliwell B, Cross CE. Dietary antioxidants and cigarette smoke-induced biomolecular damage: a complex interaction. Am J Clin Nutr. 1995;62:1490S–1500S. doi: 10.1093/ajcn/62.6.1490S. [DOI] [PubMed] [Google Scholar]
- 20.Andreoli R, Manini P, Corradi M, Mutti A, Niessen WM. Determination of patterns of biologically relevant aldehydes in exhaled breath condensate of healthy subjects by liquid chromatography/atmospheric chemical ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2003;17:637–645. doi: 10.1002/rcm.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Annovazzi L, Cattaneo V, Viglio S, Perani E, Zanone C, Rota C, Pecora F, Cetta G, Silvestri M, Iadarola P. High-performance liquid chromatography and capillary electrophoresis: methodological challenges for the determination of biologically relevant low-aliphatic aldehydes in human saliva. Electrophoresis. 2004;25:1255–1263. doi: 10.1002/elps.200305843. [DOI] [PubMed] [Google Scholar]
- 22.Deshmukh HS, Shaver C, Case LM, Dietsch M, Wesselkamper SC, Hardie WD, Korfhagen TR, Corradi M, Nadel JA, Borchers MT, et al. Acrolein-activated matrix metalloproteinase 9 contributes to persistent mucin production. Am J Respir Cell Mol Biol. 2008;38:446–454. doi: 10.1165/rcmb.2006-0339OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wells JM, Jackson P, Hardison MT, Blalock JE, O'Reilly P. Blunted leukotriene a4 hydrolase aminopeptidase activity in smokers: implications for COPD. Am J Respir Crit Care Med. 2011;183:A1311. [Google Scholar]
- 24.Wells JM, Jackson P, O'Reilly P, Blalock JE.Selective inhibition of leukotriene a4 hydrolase aminopeptidase activity occurs in COPD and reflects clinical outcomes [abstract]. Am J Respir Crit Care Med2012. 185:A1422 [Google Scholar]
- 25.Clark JD, Gebhart GF, Gonder JC, Keeling ME, Kohn DF. Special report: the 1996 guide for the care and use of laboratory animals. ILAR J. 1997;38:41–48. doi: 10.1093/ilar.38.1.41. [DOI] [PubMed] [Google Scholar]
- 26.Guerassimov A, Hoshino Y, Takubo Y, Turcotte A, Yamamoto M, Ghezzo H, Triantafillopoulos A, Whittaker K, Hoidal JR, Cosio MG. The development of emphysema in cigarette smoke-exposed mice is strain dependent. Am J Respir Crit Care Med. 2004;170:974–980. doi: 10.1164/rccm.200309-1270OC. [DOI] [PubMed] [Google Scholar]
- 27.Braber S, Koelink PJ, Henricks PA, Jackson PL, Nijkamp FP, Garssen J, Kraneveld AD, Blalock JE, Folkerts G. Cigarette smoke-induced lung emphysema in mice is associated with prolyl endopeptidase, an enzyme involved in collagen breakdown. Am J Physiol Lung Cell Mol Physiol. 2011;300:L255–L265. doi: 10.1152/ajplung.00304.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Talukder MA, Johnson WM, Varadharaj S, Lian J, Kearns PN, El-Mahdy MA, Liu X, Zweier JL. Chronic cigarette smoking causes hypertension, increased oxidative stress, impaired no bioavailability, endothelial dysfunction, and cardiac remodeling in mice. Am J Physiol Heart Circ Physiol. 2011;300:H388–H396. doi: 10.1152/ajpheart.00868.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, et al. Global Initiative for Chronic Obstructive Lung D. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 30.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 31.Vestbo J, Anderson W, Coxson HO, Crim C, Dawber F, Edwards L, Hagan G, Knobil K, Lomas DA, MacNee W, et al. Investigators E. Evaluation of COPD longitudinally to identify predictive surrogate end-points (eclipse) Eur Respir J 200831869–873. [DOI] [PubMed] [Google Scholar]
- 32.Ferris BG. Epidemiology standardization project (American Thoracic Society) Am Rev Respir Dis. 1978;118:1–120. [PubMed] [Google Scholar]
- 33.Johannessen A, Skorge TD, Bottai M, Grydeland TB, Nilsen RM, Coxson H, Dirksen A, Omenaas E, Gulsvik A, Bakke P. Mortality by level of emphysema and airway wall thickness. Am J Respir Crit Care Med. 2013;187:602–608. doi: 10.1164/rccm.201209-1722OC. [DOI] [PubMed] [Google Scholar]
- 34.O'Reilly P, Jackson PL, Noerager B, Parker S, Dransfield M, Gaggar A, Blalock JE. N-alpha-PGP and PGP, potential biomarkers and therapeutic targets for COPD. Respir Res. 2009;10:38. doi: 10.1186/1465-9921-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vasilyev N, Williams T, Brennan ML, Unzek S, Zhou X, Heinecke JW, Spitz DR, Topol EJ, Hazen SL, Penn MS. Myeloperoxidase-generated oxidants modulate left ventricular remodeling but not infarct size after myocardial infarction. Circulation. 2005;112:2812–2820. doi: 10.1161/CIRCULATIONAHA.105.542340. [DOI] [PubMed] [Google Scholar]
- 36.Overbeek SA, Braber S, Koelink PJ, Henricks PA, Mortaz E, LoTam Loi AT, Jackson PL, Garssen J, Wagenaar GT, Timens W, et al. Cigarette smoke-induced collagen destruction; key to chronic neutrophilic airway inflammation? PLoS ONE. 2013;8:e55612. doi: 10.1371/journal.pone.0055612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson MM, Hazen SL, Hsu FF, Heinecke JW. Human neutrophils employ the myeloperoxidase-hydrogen peroxide-chloride system to convert hydroxy-amino acids into glycolaldehyde, 2-hydroxypropanal, and acrolein. A mechanism for the generation of highly reactive alpha-hydroxy and alpha,beta-unsaturated aldehydes by phagocytes at sites of inflammation. J Clin Invest. 1997;99:424–432. doi: 10.1172/JCI119176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hill AT, Campbell EJ, Hill SL, Bayley DL, Stockley RA. Association between airway bacterial load and markers of airway inflammation in patients with stable chronic bronchitis. Am J Med. 2000;109:288–295. doi: 10.1016/s0002-9343(00)00507-6. [DOI] [PubMed] [Google Scholar]