Abstract
Rationale: Whether allergic airway inflammation mediates the association between overweight or obesity and childhood asthma is unknown.
Objectives: To examine adiposity, asthma, and fractional exhaled nitric oxide (FeNO) in U.S. children.
Methods: Cross-sectional study of indicators of adiposity or obesity, FeNO (a biomarker of eosinophilic airway inflammation), and asthma in 2,681 children aged 6–17 years in the 2007–2010 National Health and Nutrition Examination Survey. Adiposity measures included body mass index (BMI), percent body fat (PBF), and waist circumference (WC).
Measurements and Main Results: BMI, PBF, and WC were associated with asthma among children with low FeNO (odds ratio, 1.54–1.68; P < 0.01), but not among children with increased FeNO. Among children without asthma, BMI, PBF, and WC were associated with higher FEV1 and FVC, and lower FEV1/FVC. Among children with asthma and a high FeNO, all adiposity indicators were associated with decreased FEV1/FVC (β = −1.5% to −1.7% per z score) but not with FEV1 or FVC. Higher BMI or PBF was associated with worse asthma severity or control in children with asthma and increased FeNO, but not in children with asthma and low FeNO. Similar results were obtained in a secondary multivariate analysis of overweight or obesity (defined as BMI ≥85th percentile) and asthma or indicators of asthma severity or control, stratified by FeNO level.
Conclusions: Adiposity indicators are associated with asthma in children with low FeNO. Among children with asthma, adiposity indicators are associated with worse asthma severity or control in those with high FeNO.
Keywords: asthma, airway inflammation, adiposity, obesity, NHANES
At a Glance Commentary
Scientific Knowledge on the Subject
Childhood asthma and obesity are major public health problems in the United States. To be overweight or obese has been consistently associated with childhood asthma. Whether allergic airway inflammation mediates this association is unclear.
What This Study Adds to the Field
We report that having increased adiposity or being overweight/obese is associated with asthma only in children with low fractional exhaled nitric oxide (a marker of eosinophilic airway inflammation). Among children with asthma, however, having increased adiposity or being overweight/obese was associated with markers of increased asthma severity or poor asthma control only in those with increased fractional exhaled nitric oxide.
Over the last few decades, the parallel epidemics of asthma and obesity have become major public health issues in industrialized countries, such as the United States. In 2010, approximately 16% of U.S. children were obese and approximately 9% had asthma (1, 2). Epidemiologic studies have shown that obesity is associated with asthma, asthma morbidity or control, and decreased response to inhaled corticosteroids during childhood (3–6), and that increased weight precedes the development of asthma symptoms (7).
Although an association between obesity and asthma has been well established, the mechanisms behind this link are less than clear. Various pathways have been proposed, including mechanical changes from excessive weight (8); existence of comorbidities, such as obstructive sleep apnea; a proinflammatory milieu that exists in obesity; and shared genetic polymorphisms (9, 10). It is becoming progressively clear that the “obese asthmatic” phenotype is likely complex and multifactorial.
A recent study reported that obesity-induced airway hyperresponsiveness in a murine model of obesity is mediated by nonallergic mechanisms, including inflammasome activation and production of interleukin-17 by innate lymphoid cells in the lung (11, 12). Consistent with these findings, obesity has been shown to be predominantly associated with nonatopic asthma in adult subjects (13, 14). However, the role of atopy in “obese asthma” in children is less clear: several (15, 16) but not all (4, 17) studies have shown a stronger association between obesity and asthma in nonatopic children. Whether obesity influences childhood asthma through noneosinophilic airway inflammation is largely unknown.
Fractional exhaled nitric oxide (FeNO) is a sensitive marker of ongoing eosinophilic airway inflammation, with FeNO levels less than 20 ppb denoting low likelihood of eosinophilic airway inflammation or corticosteroid responsiveness (18). We hypothesized that increased adiposity or overweight/obesity (as measured by body mass index [BMI], percent body fat [PBF], and waist circumference [WC]) would cause asthma through noneosinophilic airway inflammation, as indicated by a low FeNO. On the basis of our recent findings (4), we further hypothesized that, once asthma is established, increased adiposity would worsen asthma control in children with eosinophilic airway inflammation (as indicated by an elevated FeNO). We examined these hypotheses in a nationwide study of 2,681 children aged 6–17 years in the United States.
Methods
Subject Recruitment and Study Procedures
The National Health and Nutrition Examination Survey (NHANES) is a cross-sectional nationwide survey designed to assess the health and nutritional status of noninstitutionalized children and adults in the United States (19). NHANES combines interviews and physical examinations of study participants by highly trained personnel. Study participants are selected using a stratified multistage probability design and are thus a representative sample of the U.S. population. As part of the study design, ethnic minorities (African Americans and Mexican Americans) are oversampled to increase statistical power for data analysis in these groups. We included children ages 6–17 years who participated in the 2007–2008 and 2009–2010 NHANESs in this analysis. The NHANES was approved by the institutional review board of the National Center for Health Statistics of the CDC. Informed consent was obtained from all participants.
Measures of adiposity and obesity were collected by trained health technicians and followed guidelines from the Anthropometric Standardization Reference Manual (20). BMI was calculated as weight in kilograms divided by height (in meters) squared. PBF was calculated from tricipital and subscapular skinfolds. For data analysis, all measures were transformed to z scores to obtain standardized and comparable coefficients and odds ratios (ORs): BMI z scores were calculated using equations based on the 2000 CDC growth charts (21), PBF z scores were calculated by using a recent study on reference equations for U.S. children (22), and WC was standardized by using the distribution of this measure in our study population.
Spirometry was performed following American Thoracic Society recommendations (23). The best FEV1 and FVC values were selected for data analysis. Participants were not eligible for spirometry if they were on supplemental oxygen or had painful ear infections, current chest pain or a physical problem with forceful expiration, recent surgery (of the eye, chest, or the abdomen), heart disease, or tuberculosis. FeNO was measured using the Aerocrine NIOX MINO, a portable, hand-held NO analyzer (Aerocrine AB, Solna, Sweden). The NHANES protocol required two valid FeNO measurements that were reproducible. Further details on NHANES measurements and procedures may be found in the online supplement or in the NHANES website (http://www.cdc.gov/nchs/nhanes.htm).
Statistical Analysis
Current asthma was defined as both having had asthma diagnosed by a doctor or other health professional and having had at least one asthma attack in the past year. Participants who had neither diagnosed asthma nor an asthma attack in the past year were selected as control subjects. Children who reported a lifetime diagnosis of asthma but no asthma attacks in the past year were excluded from this analysis (n = 175). Indicators of asthma severity or control during the previous year included (1) at least one emergency care (EC; meaning emergency room or urgent care center) visit for asthma; (2) number of school days lost because of asthma symptoms (≤1 wk vs. >1 wk); and (3) whether asthma limited usual activities (not at all vs. any activity limitation).
Primary sampling units and strata for the complex NHANES survey design were taken into account for data analysis. Sampling weights, stratification, and clusters provided in the NHANES dataset were incorporated into the analysis to obtain proper estimates and their standard errors. The SAS SURVEY procedure was used for descriptive, bivariate, and multivariate analysis (SAS Institute Inc., Cary, NC). Wald chi-square tests and t tests were used for bivariate analyses of binary and continuous variables, respectively.
After stratification by FeNO level (<10 ppb, 10–20 ppb, or ≥20 ppb), multivariate logistic or linear regression was used to examine the relationship between each indicator of adiposity or obesity (as a continuous variable and as quartiles) and current asthma or (in subjects with asthma only) indicators of asthma severity or control. All multivariate models were adjusted for age, sex, race and ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, or other), family history of asthma, annual household income, use of oral or inhaled corticosteroids in the 2 days before FeNO testing, and environmental tobacco smoke; models for FEV1 and FVC were additionally adjusted for height and height squared. In a secondary multivariate analysis stratified by FeNO level, we examined the relationship between overweight or obesity (BMI ≥ 85th and ≥ 95th percentiles for age and sex, respectively) and asthma or indicators of asthma severity or control, using the same approach as for our primary analysis (see above). All statistical analyses were conducted using SAS 9.3 software.
Results
The main characteristics of the 2,681 participating children with (n = 171) and without (n = 2,510) asthma are shown in Table 1 (see Figure E1 in the online supplement for derivation of the final cohort and details on study subpopulations). Compared with children without asthma, those with asthma were more likely to be male and to belong to a racial or ethnic group other than non-Hispanic white, and to have a household income greater than $20,000 per year, a family history of asthma, health insurance coverage, and lower FEV1 and FEV1/FVC but higher FeNO, BMI, and PBF. There were no significant differences in age, sex, or environmental tobacco smoke between children with and without asthma.
Table 1.
Characteristics of Study Participants
| Characteristics | No Asthma (n = 2,510)* | Asthma (n = 171)* | P Value for Comparison | |
|---|---|---|---|---|
| Age, yr | 11.91 ± 0.09 | 11.78 ± 0.40 | 0.728 | |
| Male sex | 1,282 (50.29) | 108 (61.59) | 0.027 | |
| Race and ethnicity | 0.002 | |||
| Non-Hispanic white | 849 (61.35) | 44 (49.56) | ||
| Non-Hispanic black | 493 (11.54) | 64 (25.81) | ||
| Hispanic | 1,029 (20.10) | 48 (15.45) | ||
| Other | 139 (7.02) | 15 (6.88) | ||
| Annual household income < $20,000 | 482 (13.58) | 42 (19.17) | 0.084 | |
| Covered by health insurance | 2,184 (90.11) | 159 (95.60) | 0.029 | |
| Family history of asthma | 618 (23.68) | 103 (57.71) | <0.001 | |
| Environmental tobacco exposure | 353 (14.14) | 27 (15.43) | 0.701 | |
| FEV1, L† | 2.71 ± 0.03 | 2.58 ± 0.12 | 0.004 | |
| FVC, L† | 3.14 ± 0.04 | 3.13 ± 0.15 | 0.841 | |
| FEV1/FVC, % | 86.69 ± 0.16 | 82.46 ± 0.82 | <0.001 | |
| Fractional exhaled nitric oxide, ppb | 15.53 ± 0.45 | 30.19 ± 2.66 | <0.001 | |
| Body mass index, z score | 0.54 ± 0.03 | 0.85 ± 0.11 | 0.008 | |
| Percent body fat, z score | 0.16 ± 0.03 | 0.37 ± 0.08 | 0.024 | |
| Waist circumference, z score | −0.12 ± 0.03 | 0.08 ± 0.08 | 0.032 |
Results are shown as mean (SE) for continuous and N (%) for binary variables.
Numbers may vary because of missingness.
Adjusted for age, sex, height, and height squared.
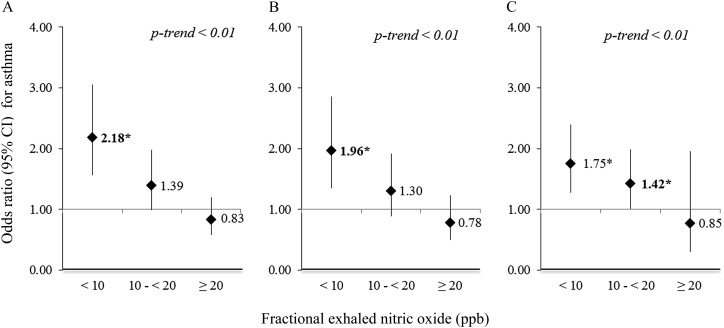
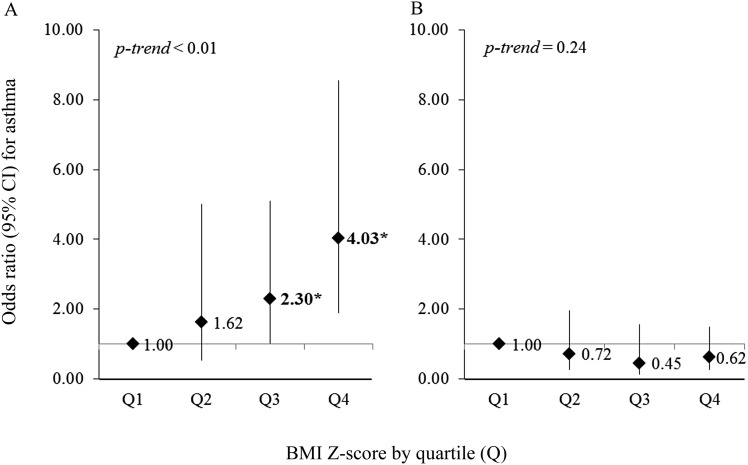
Figure 1 shows the results of the multivariate analysis of adiposity or obesity indicators and asthma. In the multivariate analysis of children with FeNO less than 10 ppb, all indicators of adiposity or obesity (BMI, PBF, and WC) were associated with (75–118%) excess odds of asthma per every 1.0 z score increment in each adiposity indicator. For children with FeNO 10–20 ppb, the increment in the odds of asthma was less prominent (30–42%). In contrast, there was no significant association between any indicator of adiposity or obesity and asthma among children with FeNO greater than or equal to 20 ppb. The linear trend was significant for all three adiposity indicators (P < 0.01) (see Table E1 for more detailed results). Similar findings were obtained in our secondary analysis of overweight or obesity and asthma (see Figure E2). Overweight or obesity was associated with asthma only in children without eosinophilic airway inflammation: the OR for BMI was 3.79, for PBF OR was 3.14, and for WC OR was 3.58 among children with FeNO less than 10 ppb (P < 0.01 for each and P < 0.01 for the linear trend). Furthermore, dividing BMI z score into quartiles showed a clear dose–response effect for children with low FeNO, but no effect among children with high FeNO (Figure 2), with similar results for PBF and WC (see Figure E3).
Figure 1.
(A–C) Multivariate analysis of adiposity indicators and asthma, stratified by fractional exhaled nitric oxide level. (A) Body mass index z score. (B) Percent body fat z score. (C) Waist circumference z score. All models adjusted for age, sex, race and ethnicity, family history of asthma, annual household income, use of oral or inhaled steroids, and environmental tobacco smoke exposure. *P < 0.05. CI = confidence interval.
Figure 2.
(A and B) Multivariate analysis of body mass index (BMI) z score quartiles and asthma, stratified by fractional exhaled nitric oxide (FeNO) level. (A) FeNO < 20 ppb; (B) FeNO ≥ 20 ppb. All models adjusted for age, sex, race and ethnicity, family history of asthma, annual household income, use of oral or inhaled steroids, and environmental tobacco smoke exposure. *P < 0.01. CI = confidence interval.
We then conducted a multivariate analysis of the relationship between indicators of adiposity or obesity and binary markers of disease severity or control in children with asthma (Table 2). Among children with asthma and eosinophilic airway inflammation, BMI and PBF were associated with at least one EC visit and school absences in the previous year. In contrast, there was no association between any adiposity indicator and markers of asthma severity or control in children with asthma but no eosinophilic airway inflammation. Similar results were obtained in a multivariate analysis of overweight or obesity and indicators of asthma severity or control (see Table E2). For example, children with asthma who were overweight or obese and had higher FeNO had fivefold greater odds of having at least one EC visit for asthma than those who were of normal weight but had an increased FeNO (95% confidence interval for OR, 1.8–18.3; P < 0.05).
Table 2.
Multivariate Analysis of Adiposity Indicators and Markers of Asthma Severity or Control, Stratified by FeNO Level
| BMI z Score | PBF z Score | WC z Score | |
|---|---|---|---|
| FeNO < 20 ppb (n = 86) | |||
| ≥1 EC visit in past year | 0.64 (0.39–1.07) | 0.64 (0.36–1.13) | 0.63 (0.34–1.16) |
| >1 wk of school days lost in past year | 1.03 (0.69–1.52) | 0.84 (0.51–1.39) | 0.81 (0.48–1.35) |
| Limited activity in past year | 0.90 (0.61–1.31) | 0.72 (0.48–1.08) | 1.05 (0.70–1.56) |
| FeNO ≥ 20 ppb (n = 85) | |||
| ≥1 EC visit in past year | 2.44 (1.18–5.06)* | 2.83 (1.26–6.35)* | 2.15 (0.96–4.81) |
| >1 wk of school days lost in past year | 2.75 (1.19–6.38)* | 4.70 (1.89–11.66)† | 1.62 (0.69–3.79) |
| Limited activity in past year | 0.80 (0.39–1.65) | 0.83 (0.44–1.56) | 0.68 (0.32–1.47) |
Definition of abbreviations: BMI = body mass index; EC = emergency care; FeNO = fractional exhaled nitric oxide; PBF = percent body fat; WC = waist circumference.
Results are shown as odds ratio (95% confidence interval) per 1.0 z score increment in each adiposity measure. All models adjusted for age, sex, race and ethnicity, family history of asthma, annual household income, use of oral or inhaled steroids, and environmental tobacco smoke exposure.
P < 0.05.
P < 0.01.
Table 3 shows the results of the multivariate analysis of indicators of adiposity or obesity and lung function, stratified by asthma and eosinophilic airway inflammation (normal-high vs. low FeNO level). Among children without asthma or eosinophilic airway inflammation, all three adiposity indicators were positively associated with FEV1 and FVC but inversely associated with FEV1/FVC (P < 0.01 in all instances). Among children without asthma but with eosinophilic airway inflammation, the three adiposity indicators had a similar inverse association with FEV1/FVC but were more strongly and positively associated with FEV1 and FVC. Among children with asthma, the only consistently significant finding was for FEV1/FVC in those with eosinophilic airway inflammation. In these children, all adiposity indicators were significantly and inversely associated with FEV1/FVC despite small sample size (P < 0.05 in all instances). Similar results were obtained in the multivariate analysis of overweight/obesity and lung function after stratification by FeNO level and asthma (see Table E3). However, all results among children with asthma were not significant because of small sample size.
Table 3.
Adiposity Indicators and Lung Function, Stratified by Current Asthma and FeNO Level
| BMI z Score | PBF z Score | WC z Score | |
|---|---|---|---|
| FeNO < 20 ppb | |||
| No asthma (n = 1,973) | |||
| FEV1, ml* | 64.61 (42.77 to 86.46)† | 27.76 (3.50 to 52.02)‡ | 49.22 (21.20 to 77.25)† |
| FVC, ml* | 112.48 (90.42 to 134.54)† | 65.84 (42.27 to 89.41)† | 111.84 (81.23 to 142.45)† |
| FEV1/FVC, % | −0.98 (−1.27 to −0.67)† | −0.92 (−1.28 to −0.56)† | −1.27 (−1.71 to −0.83)† |
| Asthma (n = 86) | |||
| FEV1, ml* | 59.36 (12.34 to 106.38)‡ | 33.67 (−31.34 to 98.68) | 34.22 (−44.68 to 113.11) |
| FVC, ml* | 89.26 (34.81 to 143.72)† | 35.91 (−46.91 to 120.74) | 63.71 (−24.68 to 152.10) |
| FEV1/FVC, % | −0.42 (−1.51 to 0.67) | −0.09 (−1.23 to 1.05) | −0.66 (−1.74 to 0.43) |
| FeNO ≥ 20 ppb | |||
| No asthma (n = 537) | |||
| FEV1, ml* | 110.89 (70.27 to 151.51)† | 92.38 (51.02 to 133.73)† | 121.36 (69.23 to 173.50)† |
| FVC, ml* | 186.02 (136.93 to 235.11)† | 150.67 (98.69 to 202.65)† | 206.33 (136.93 to 275.74)† |
| FEV1/FVC, % | −1.31 (−1.84 to −0.79)† | −1.14 (−1.82 to −0.46)† | −1.28 (−1.95 to −0.61)† |
| Asthma (n = 85) | |||
| FEV1, ml* | −57.88 (−154.75 to 38.99) | −56.43 (−161.65 to 48.39) | −56.80 (−173.38 to 59.78) |
| FVC, ml* | −4.08 (−115.80 to 107.64) | −11.12 (−136.27 to 114.03) | −2.19 (−155.65 to 151.26) |
| FEV1/FVC, % | −1.67 (−2.93 to −0.42)† | −1.54 (−2.94 to −0.14)‡ | −1.54 (−2.93 to −0.15)‡ |
Definition of abbreviations: BMI = body mass index; FeNO = fractional exhaled nitric oxide; PBF = percent body fat; WC = waist circumference.
Results shown as beta coefficient (95% confidence interval) per 1.0 z score increment in each adiposity measure. All models adjusted for age, sex, race and ethnicity, family history of asthma, annual household income, use of oral or inhaled steroids, and environmental tobacco smoke exposure.
Analyzed as absolute values; adjusted additionally for height and height squared.
P < 0.01.
P < 0.05.
Discussion
To our knowledge, this is the first study to examine indicators of adiposity or obesity and asthma in children according to their level of exhaled FeNO. We show that each of three adiposity indicators (BMI, PBF, and WC) is significantly associated with current asthma, but only in children with low FeNO. Similarly, overweight or obesity was only associated with current asthma in children with low FeNO. Interestingly, both adiposity and FeNO exhibited dose–response effects.
FeNO is a marker of eosinophilic airway inflammation, a hallmark of atopic asthma (24). Our results suggest that increased adiposity leads to nonatopic asthma in children, and are generally consistent with those of prior studies in children (15) and adults (13, 14, 25) in whom adiposity or obesity was only associated with asthma in subjects without atopy (defined by objective markers, such as skin test reactivity or a positive IgE to allergens). Obesity has also been associated with current asthma among children without other allergic diseases (16), and overweight has been associated with a 77% increased risk of new-onset asthma in nonallergic children (26). Our findings are also consistent with those of a recently published murine model, in which obesity led to increased airway responsiveness through nonatopic (e.g., TH17) mechanisms (11). Of note, TH17-mediated responses could also account for our prior observation of reduced response to inhaled corticosteroids among overweight or obese North American children with asthma (5, 27).
In mouse models of allergic airway inflammation, body weight has been correlated with airway eosinophilia, suggesting that obesity may lower the threshold for allergic sensitization and augment airway eosinophilia (28, 29). In contrast, other studies have found no association between body weight and eosinophilia in murine models of asthma (30). Our findings are consistent with those of a recent study suggesting that atopy partly mediates the detrimental effects of adiposity or obesity on asthma severity or control in children with established disease (4). This is also consistent with recent studies in adults with severe asthma that described higher FeNO levels in the cluster of obese people with asthma with uncontrolled asthma (31), and that severe obese people with asthma have increased eosinophils in bronchial submucosa (but not in sputum) (32). Thus, we hypothesize obesity may play a role in the inception of asthma via nonatopic pathways, but that in children with established atopy and asthma, obesity and atopy act synergistically and result in worsened asthma severity and control.
In adipose tissue from normal mice, eosinophils act via TH2 cytokines (IL-4, IL-5, IL-13) to sustain alternatively activated macrophages, which are necessary to maintain glucose homeostasis (33); in obese mice, decreased eosinophils in adipose tissue leads to an increase in classically activated macrophages, which foster metabolic dysregulation (33). In a murine model of obesity, type 3 innate lymphoid cells (ILC3) led to airway hyperreactivity via a macrophage-derived IL-1β pathway (11); whereas this airway hyperreactivity was mediated predominantly by neutrophils, there was concurrent airway eosinophilia (11). Conversely, aerosolized anti–IL-1β has been shown to decrease eosinophils and neutrophils in bronchoalveolar lavage, and to decrease eosinophils in peripheral blood in guinea pigs (34). Furthermore, IL-33 (a member of the IL-1 family) has been shown to activate eosinophil degranulation (35); and polymorphisms of the ILRL1 (IL-receptor like 1) gene have been associated with decreased eosinophils and lower odds of asthma (36). Hence, nonatopic pathways associated with obese asthma inception in children could also produce changes in eosinophil distribution, migration, or activity among the adipose tissue, the blood, and the airways. Moreover, the effects these nonatopic pathways exert on atopy-related eosinophilia could explain why obese children with asthma may have a more severe disease in the presence of atopy.
Obesity is known to adversely affect lung function. In adults with asthma, increased BMI is associated with a restrictive ventilatory deficit, with reduced FEV1 and FVC but normal FEV1/FVC, and low FRC (37, 38). In contrast to this finding in adults, higher BMI, WC, and total body fat are significantly associated with increased FEV1 and FVC but lower FEV1/FVC in children with and without asthma (39–41). In our study, children without asthma or allergic airway inflammation showed increasing FEV1 and FVC, but a decreasing FEV1/FVC ratio, with increasing obesity/adiposity. These findings could denote airway dysanapsis, whereby lung size is larger in obese (but otherwise healthy) children, but airway size has not yet grown proportionately. However, children with both asthma and eosinophilic airway inflammation did not show increasing FEV1 or FVC, and had a more prominent decrease in FEV1/FVC than children with asthma but no eosinophilic airway inflammation. This is consistent with true airflow obstruction caused by adiposity in children with asthma and a high FeNO.
Several other mechanisms could underlie the association between obesity and asthma. Lower FRC in obese individuals can lead to increased airway closure, and this may alter the structure and function of airway smooth muscle, making airways more prone to bronchoconstriction (8, 38). Obesity has been shown to be a proinflammatory state, and adipokines, such as leptin and adiponectin, have been linked to asthma. Compared with obese women without asthma, obese women with asthma have increased macrophage infiltration, higher expression of leptin, and lower expression of adiponectin in adipose tissue (42). Other proposed mechanisms include presence of comorbidities (43, 44), hormonal differences (45), dietary pattern changes (46, 47), and alterations in insulin or arginine metabolism (48–50).
Our study has considerable strengths, including availability of a representative sample of U.S. children, performance of standardized procedures by uniformly trained personnel, and FeNO measures in children with and without asthma. We also acknowledge several limitations. First, this is a cross-sectional study, and therefore a temporal relationship between adiposity or obesity and eosinophilic airway inflammation or asthma cannot be determined. Second, we had limited statistical power to detect an association between indicators of adiposity or obesity and binary markers of asthma severity or control because of the small number of participating children with current asthma. Third, we lacked data on puberty. Body composition changes during growth, and hormonal expression after puberty may influence the relationship between adiposity and asthma, particularly among girls. Fourth, we did not control for all factors related to adiposity or obesity and poor asthma control, such as diet. We used household income as our main proxy for socioeconomic status. Although we did not include both household income and health insurance coverage in the multivariate models because of collinearity, our results remained unchanged after replacing household income with health insurance coverage in the multivariate analysis (data not shown). Finally, some subjects with mild asthma may have had quiescent disease or failed to report symptoms, and thus could have been misclassified as unaffected.
In summary, we show that increased adiposity or overweight/obesity is only associated with asthma in children without eosinophilic airway inflammation (as indicated by low FeNO). Among children with asthma, we found that increased adiposity or overweight/obesity is associated with worse asthma severity or control only in those with eosinophilic airway inflammation (as indicated by a high FeNO). Although further mechanistic research is needed, our results emphasize the importance of maintaining a lean body weight or promoting weight loss in overweight or obese children (with and without asthma).
Footnotes
Supported by grants HL079966 and HL117191 from the National Institutes of Health (J.C.C.), by an endowment from the Heinz Foundation (J.C.C.), and by grant HD052892 from the National Institutes of Health (E.F.).
Originally Published in Press as DOI: 10.1164/rccm.201403-0565OC on June 12, 2014
Author Contributions: Y.-Y.H., E.F., and J.C.C. participated in study design and implementation and data analysis. Y.-Y.H. wrote the initial draft of the manuscript. E.F. and J.C.C. participated in the review of the manuscript and approved its final version.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Moorman JE, Akinbami LJ, Bailey CM, Zahran HS, King ME, Johnson CA, Liu L.National surveillance of asthma: United States, 2001–2010 Vital Health Stat 3 2012(35)1–67. [PubMed]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM.Prevalence of obesity in the United States, 2009–2010. NCHS data brief, no 82. Hyattsville, MD: National Center for Health Statistics; 2012 [PubMed] [Google Scholar]
- 3.Dixon AE, Holguin F, Sood A, Salome CM, Pratley RE, Beuther DA, Celedón JC, Shore SA American Thoracic Society Ad Hoc Subcommittee on Obesity and Lung Disease. An official American Thoracic Society Workshop report: obesity and asthma. Proc Am Thorac Soc. 2010;7:325–335. doi: 10.1513/pats.200903-013ST. [DOI] [PubMed] [Google Scholar]
- 4.Forno E, Acosta-Perez E, Brehm J, Han YY, Alvarez M, Colon-Semidey A, Canino G, Celedon JC.Obesity and adiposity indicators, asthma, and atopy in Puerto Rican children. J Allergy Clin Immunol 20141331308–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forno E, Lescher R, Strunk R, Weiss S, Fuhlbrigge A, Celedón JC Childhood Asthma Management Program Research Group. Decreased response to inhaled steroids in overweight and obese asthmatic children. J Allergy Clin Immunol. 2011;127:741–749. doi: 10.1016/j.jaci.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borrell LN, Nguyen EA, Roth LA, Oh SS, Tcheurekdjian H, Sen S, Davis A, Farber HJ, Avila PC, Brigino-Buenaventura E, et al. Childhood obesity and asthma control in the GALA II and SAGE II studies. Am J Respir Crit Care Med. 2013;187:697–702. doi: 10.1164/rccm.201211-2116OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flaherman V, Rutherford GW. A meta-analysis of the effect of high weight on asthma. Arch Dis Child. 2006;91:334–339. doi: 10.1136/adc.2005.080390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beuther DA, Weiss ST, Sutherland ER. Obesity and asthma. Am J Respir Crit Care Med. 2006;174:112–119. doi: 10.1164/rccm.200602-231PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melén E, Granell R, Kogevinas M, Strachan D, Gonzalez JR, Wjst M, Jarvis D, Ege M, Braun-Fahrländer C, Genuneit J, et al. Genome-wide association study of body mass index in 23,000 individuals with and without asthma. Clin Exp Allergy. 2013;43:463–474. doi: 10.1111/cea.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melen E, Himes BE, Brehm JM, Boutaoui N, Klanderman BJ, Sylvia JS, Lasky-Su J.Analyses of shared genetic factors between asthma and obesity in children J Allergy Clin Immunol 2010126631–637.e1–e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HY, Lee HJ, Chang YJ, Pichavant M, Shore SA, Fitzgerald KA, Iwakura Y, Israel E, Bolger K, Faul J, et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat Med. 2014;20:54–61. doi: 10.1038/nm.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Celedón JC, Kolls JK. An innate link between obesity and asthma. Nat Med. 2014;20:19–20. doi: 10.1038/nm.3433. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Dales R, Jiang Y. The association between obesity and asthma is stronger in nonallergic than allergic adults. Chest. 2006;130:890–895. doi: 10.1378/chest.130.3.890. [DOI] [PubMed] [Google Scholar]
- 14.Appleton SL, Adams RJ, Wilson DH, Taylor AW, Ruffin RE North West Adelaide Health Study Team. Central obesity is associated with nonatopic but not atopic asthma in a representative population sample. J Allergy Clin Immunol. 2006;118:1284–1291. doi: 10.1016/j.jaci.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Visness CM, London SJ, Daniels JL, Kaufman JS, Yeatts KB, Siega-Riz AM, Calatroni A, Zeldin DC. Association of childhood obesity with atopic and nonatopic asthma: results from the National Health and Nutrition Examination Survey 1999-2006. J Asthma. 2010;47:822–829. doi: 10.3109/02770903.2010.489388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Marcos L, Arnedo Pena A, Busquets-Monge R, Morales Suárez-Varela M, García de Andoin N, Batlles-Garrido J, Blanco-Quirós A, López-Silvarrey Varela A, García-Hernández G, Aguinaga-Ontoso I, et al. How the presence of rhinoconjunctivitis and the severity of asthma modify the relationship between obesity and asthma in children 6-7 years old. Clin Exp Allergy. 2008;38:1174–1178. doi: 10.1111/j.1365-2222.2008.02993.x. [DOI] [PubMed] [Google Scholar]
- 17.Schachter LM, Peat JK, Salome CM. Asthma and atopy in overweight children. Thorax. 2003;58:1031–1035. doi: 10.1136/thorax.58.12.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, Olin AC, Plummer AL, Taylor DR American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184:602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention2007–2008 National Health and Nutrition Examination Survey (NHANES) survey operations manuals, brochures, consent documents. 2012[accessed 2014 Feb 18]. Available from: http://www.cdc.gov/nchs/nhanes/nhanes2007–2008/current_nhanes_07_08.htm
- 20.Lohman TG, Roche AF.Anthropometric standardization reference manual. Champaign, IL: Human Kinetics Books; 1988 [Google Scholar]
- 21.Centers for Disease Control and PreventionA SAS program for the CDC growth charts. 2011[accessed 2014 Feb 18]. Available from: http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm
- 22.Slaughter MH, Lohman TG, Boileau RA, Horswill CA, Stillman RJ, Van Loan MD, Bemben DA. Skinfold equations for estimation of body fatness in children and youth. Hum Biol. 1988;60:709–723. [PubMed] [Google Scholar]
- 23.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 24.Scott M, Raza A, Karmaus W, Mitchell F, Grundy J, Kurukulaaratchy RJ, Arshad SH, Roberts G. Influence of atopy and asthma on exhaled nitric oxide in an unselected birth cohort study. Thorax. 2010;65:258–262. doi: 10.1136/thx.2009.125443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fenger RV, Gonzalez-Quintela A, Vidal C, Gude F, Husemoen LL, Aadahl M, Berg ND, Linneberg A. Exploring the obesity-asthma link: do all types of adiposity increase the risk of asthma? Clin Exp Allergy. 2012;42:1237–1245. doi: 10.1111/j.1365-2222.2012.03972.x. [DOI] [PubMed] [Google Scholar]
- 26.Gilliland FD, Berhane K, Islam T, McConnell R, Gauderman WJ, Gilliland SS, Avol E, Peters JM. Obesity and the risk of newly diagnosed asthma in school-age children. Am J Epidemiol. 2003;158:406–415. doi: 10.1093/aje/kwg175. [DOI] [PubMed] [Google Scholar]
- 27.McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, Henry A, Irvin CG, Piganelli JD, Ray A, et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008;181:4089–4097. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dietze J, Böcking C, Heverhagen JT, Voelker MN, Renz H. Obesity lowers the threshold of allergic sensitization and augments airway eosinophilia in a mouse model of asthma. Allergy. 2012;67:1519–1529. doi: 10.1111/all.12031. [DOI] [PubMed] [Google Scholar]
- 29.Calixto MC, Lintomen L, Schenka A, Saad MJ, Zanesco A, Antunes E. Obesity enhances eosinophilic inflammation in a murine model of allergic asthma. Br J Pharmacol. 2010;159:617–625. doi: 10.1111/j.1476-5381.2009.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Vries A, Hazlewood L, Fitch PM, Seckl JR, Foster P, Howie SE. High-fat feeding redirects cytokine responses and decreases allergic airway eosinophilia. Clin Exp Allergy. 2009;39:731–739. doi: 10.1111/j.1365-2222.2008.03179.x. [DOI] [PubMed] [Google Scholar]
- 31.Sutherland ER, Goleva E, King TS, Lehman E, Stevens AD, Jackson LP, Stream AR, Fahy JV, Leung DY Asthma Clinical Research Network. Cluster analysis of obesity and asthma phenotypes. PLoS ONE. 2012;7:e36631. doi: 10.1371/journal.pone.0036631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desai D, Newby C, Symon FA, Haldar P, Shah S, Gupta S, Bafadhel M, Singapuri A, Siddiqui S, Woods J, et al. Elevated sputum interleukin-5 and submucosal eosinophilia in obese individuals with severe asthma. Am J Respir Crit Care Med. 2013;188:657–663. doi: 10.1164/rccm.201208-1470OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei-xu H, Qin X, Zhu W, Yuan-yi C, Li-feng Z, Zhi-yong L, Dan H, Xiao-mu W, Guo-zhu H. Therapeutic potential of anti-IL-1β IgY in guinea pigs with allergic asthma induced by ovalbumin. Mol Immunol. 2014;58:139–149. doi: 10.1016/j.molimm.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Cherry WB, Yoon J, Bartemes KR, Iijima K, Kita H. A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J Allergy Clin Immunol. 2008;121:1484–1490. doi: 10.1016/j.jaci.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Savenije OE, Kerkhof M, Reijmerink NE, Brunekreef B, de Jongste JC, Smit HA, Wijga AH, Postma DS, Koppelman GH.Interleukin-1 receptor-like 1 polymorphisms are associated with serum IL1RL1-a, eosinophils, and asthma in childhood J Allergy Clin Immunol 2011127750–756.e1–e5 [DOI] [PubMed] [Google Scholar]
- 37.Sin DD, Sutherland ER. Obesity and the lung: 4. Obesity and asthma. Thorax. 2008;63:1018–1023. doi: 10.1136/thx.2007.086819. [DOI] [PubMed] [Google Scholar]
- 38.Salome CM, King GG, Berend N. Physiology of obesity and effects on lung function. J Appl Physiol (1985) 2010;108:206–211. doi: 10.1152/japplphysiol.00694.2009. [DOI] [PubMed] [Google Scholar]
- 39.Bekkers MB, Wijga AH, de Jongste JC, Kerkhof M, Postma D, Gehring U, Smit HA, Brunekreef B. Waist circumference, BMI, and lung function in 8-year-old children: the PIAMA birth cohort study. Pediatr Pulmonol. 2013;48:674–682. doi: 10.1002/ppul.22722. [DOI] [PubMed] [Google Scholar]
- 40.Tantisira KG, Litonjua AA, Weiss ST, Fuhlbrigge AL Childhood Asthma Management Program Research Group. Association of body mass with pulmonary function in the Childhood Asthma Management Program (CAMP) Thorax. 2003;58:1036–1041. doi: 10.1136/thorax.58.12.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y, Rennie D, Cormier Y, Dosman JA. Waist circumference associated with pulmonary function in children. Pediatr Pulmonol. 2009;44:216–221. doi: 10.1002/ppul.20854. [DOI] [PubMed] [Google Scholar]
- 42.Sideleva O, Suratt BT, Black KE, Tharp WG, Pratley RE, Forgione P, Dienz O, Irvin CG, Dixon AE. Obesity and asthma: an inflammatory disease of adipose tissue not the airway. Am J Respir Crit Care Med. 2012;186:598–605. doi: 10.1164/rccm.201203-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hancox RJ, Poulton R, Taylor DR, Greene JM, McLachlan CR, Cowan JO, Flannery EM, Herbison GP, Sears MR, Talley NJ. Associations between respiratory symptoms, lung function and gastro-oesophageal reflux symptoms in a population-based birth cohort. Respir Res. 2006;7:142. doi: 10.1186/1465-9921-7-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rothenberg S, Cowles R. The effects of laparoscopic Nissen fundoplication on patients with severe gastroesophageal reflux disease and steroid-dependent asthma. J Pediatr Surg. 2012;47:1101–1104. doi: 10.1016/j.jpedsurg.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 45.Tam A, Morrish D, Wadsworth S, Dorscheid D, Man SF, Sin DD. The role of female hormones on lung function in chronic lung diseases. BMC Womens Health. 2011;11:24. doi: 10.1186/1472-6874-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gonzalez Barcala FJ, Pertega S, Bamonde L, Garnelo L, Perez Castro T, Sampedro M, Sanchez Lastres J, San Jose Gonzalez MA, Lopez Silvarrey A. Mediterranean diet and asthma in Spanish schoolchildren. Pediatr Allergy Immunol. 2010;21:1021–1027. doi: 10.1111/j.1399-3038.2010.01080.x. [DOI] [PubMed] [Google Scholar]
- 47.Han YY, Blatter J, Brehm JM, Forno E, Litonjua AA, Celedon JC. Diet and asthma: vitamins and methyl donors. Lancet Respir Med. 2013;1:813–822. doi: 10.1016/S2213-2600(13)70126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dekkers BG, Schaafsma D, Tran T, Zaagsma J, Meurs H. Insulin-induced laminin expression promotes a hypercontractile airway smooth muscle phenotype. Am J Respir Cell Mol Biol. 2009;41:494–504. doi: 10.1165/rcmb.2008-0251OC. [DOI] [PubMed] [Google Scholar]
- 49.Wells SM, Buford MC, Migliaccio CT, Holian A. Elevated asymmetric dimethylarginine alters lung function and induces collagen deposition in mice. Am J Respir Cell Mol Biol. 2009;40:179–188. doi: 10.1165/rcmb.2008-0148OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holguin F, Comhair SA, Hazen SL, Powers RW, Khatri SS, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Fitzpatrick AM, et al. An association between L-arginine/asymmetric dimethyl arginine balance, obesity, and the age of asthma onset phenotype. Am J Respir Crit Care Med. 2013;187:153–159. doi: 10.1164/rccm.201207-1270OC. [DOI] [PMC free article] [PubMed] [Google Scholar]