Abstract
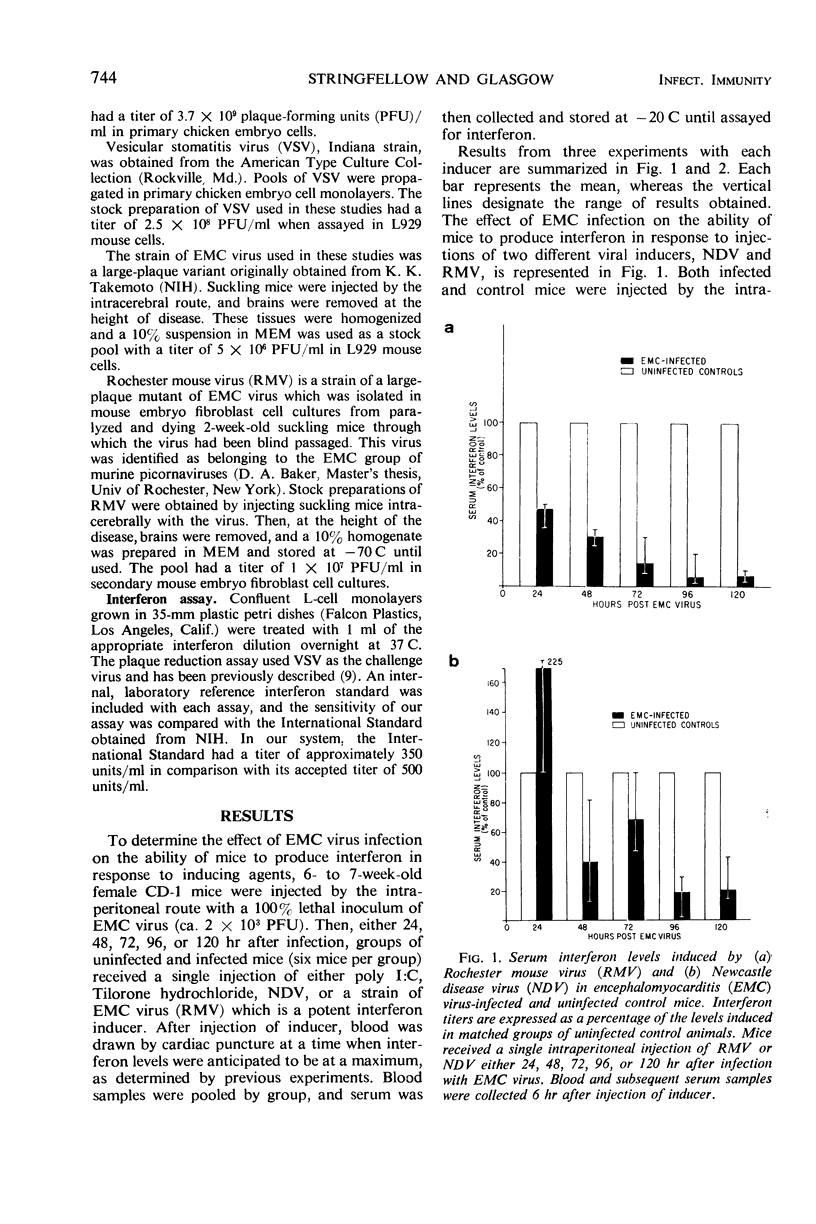
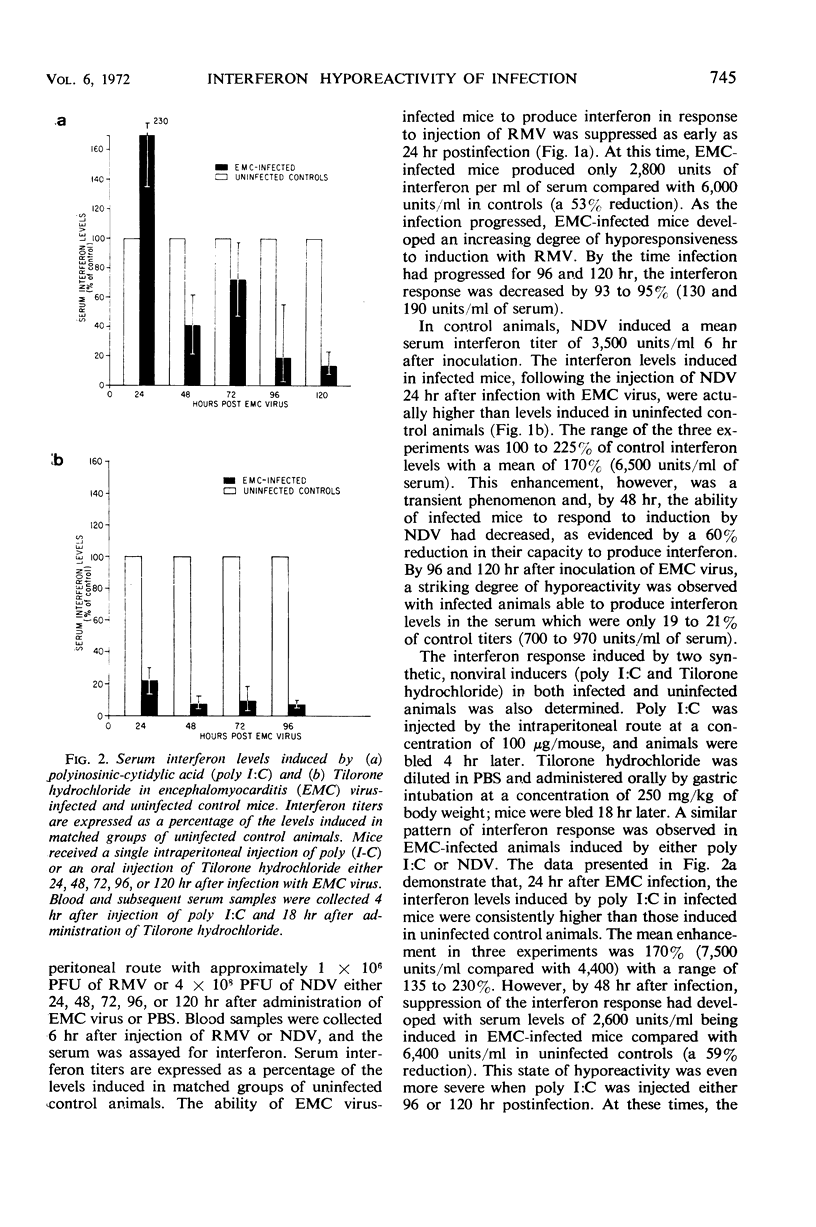
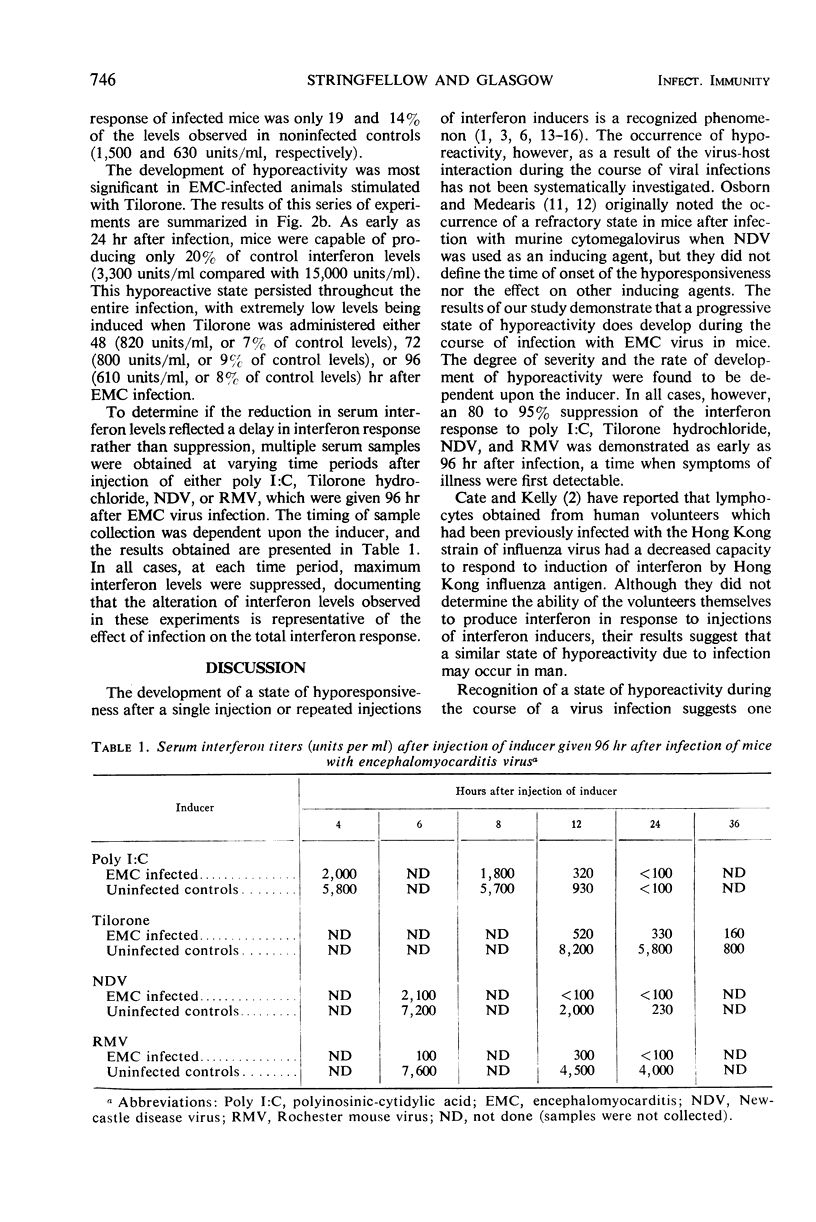
Interferon inducers are generally most effective as antiviral agents when used prophylactically. One possible explanation for this is that animals develop a state of hyporeactivity during the course of a virus infection. Such a progressive loss of capacity to produce interferon was observed with a representative group of interferon-inducing agents (polyinosinic-cytidylic acid, Tilorone hydrochloride, New-castle disease virus, or a strain of encephalomyocarditis virus) during the course of a model picornavirus infection in mice.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buckler C. E., DuBuy H. G., Johnson M. L., Baron S. Kinetics of serum interferon response in mice after single and multiple injections of polyI-poly C. Proc Soc Exp Biol Med. 1971 Feb;136(2):394–398. doi: 10.3181/00379727-136-35272. [DOI] [PubMed] [Google Scholar]
- Cate T. R., Kelly J. R. Hong Kong influenza antigen sensitivity and decreased interferon response of peripheral lymphocytes. Antimicrob Agents Chemother (Bethesda) 1970;10:156–160. [PubMed] [Google Scholar]
- Colby C., Morgan M. J. Interferon induction and action. Annu Rev Microbiol. 1971;25:333–360. doi: 10.1146/annurev.mi.25.100171.002001. [DOI] [PubMed] [Google Scholar]
- De Clercq E., Merigan T. C. Current concepts of interferon and interferon induction. Annu Rev Med. 1970;21:17–46. doi: 10.1146/annurev.me.21.020170.000313. [DOI] [PubMed] [Google Scholar]
- Hilleman M. R. Prospects for the use of double-stranded ribonucleic acid (poly I:C) inducers in man. J Infect Dis. 1970 Feb;121(2):196–211. doi: 10.1093/infdis/121.2.196. [DOI] [PubMed] [Google Scholar]
- ISAACS A., LINDENMANN J. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci. 1957 Sep 12;147(927):258–267. doi: 10.1098/rspb.1957.0048. [DOI] [PubMed] [Google Scholar]
- Lindh H. F., Lindsay H. L., Mayberry B. R., Forbes M. Polyinosinic-cytidylic acid complex (poly I:C) and viral infections in mice. Proc Soc Exp Biol Med. 1969 Oct;132(1):83–87. doi: 10.3181/00379727-132-34153. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Glasgow L. A. Factors modifying host resistance to viral infection. 3. Effect of whole body x-irradiation on experimental encephalomyocarditis virus infection in mice. J Exp Med. 1968 May 1;127(5):1035–1052. doi: 10.1084/jem.127.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemes M. M., Tytell A. A., Lampson G. P., Field A. K., Hilleman M. R. Inducers of interferon and host resistance. VI. Antiviral efficacy of poly I:C in animal models. Proc Soc Exp Biol Med. 1969 Nov;132(2):776–783. doi: 10.3181/00379727-132-34308. [DOI] [PubMed] [Google Scholar]
- Osborn J. E., Medearis D. N., Jr Studies of relationship between mouse cytomegalovirus and interferon. Proc Soc Exp Biol Med. 1966 Mar;121(3):819–824. doi: 10.3181/00379727-121-30897. [DOI] [PubMed] [Google Scholar]
- Osborn J. E., Medearis D. N., Jr Suppression of interferon and antibody and multiplication of Newcastle disease virus in cytomegalovirus infected mice. Proc Soc Exp Biol Med. 1967 Feb;124(2):347–353. doi: 10.3181/00379727-124-31740. [DOI] [PubMed] [Google Scholar]
- Park J. H., Baron S. Herpetic keratoconjunctivitis: therapy with synthetic double-stranded RNA. Science. 1968 Nov 15;162(3855):811–813. doi: 10.1126/science.162.3855.811. [DOI] [PubMed] [Google Scholar]
- VILCEK J., RADA B. Studies on an interferon from tickborne encephalitis virus-infected cells (IF). III. Antiviral action of IF. Acta Virol. 1962 Jan;6:9–16. [PubMed] [Google Scholar]
- Youngner J. S., Stinebring W. R. Interferon appearance stimulated by endotoxin, bacteria, or viruses in mice pre-treated with Escherichia coli endotoxin or infected with Mycobacterium tuberculosis. Nature. 1965 Oct 30;208(5009):456–458. doi: 10.1038/208456a0. [DOI] [PubMed] [Google Scholar]



