Abstract
Rationale: Nocturnal asthma is a common presentation and is associated with a more severe form of the disease. However, there are few epidemiologic studies of nocturnal asthma, particularly in minority populations.
Objectives: To identify factors associated with nocturnal asthma, including the contribution of self-identified race/ethnicity and genetic ancestry.
Methods: The analysis included individuals from the Study for Asthma Phenotypes and Pharmacogenomic Interactions by Race-ethnicity (SAPPHIRE) cohort. Nocturnal asthma symptoms were assessed by questionnaire. Genome-wide genotype data were used to estimate genetic ancestry in a subset of African American participants. Logistic regression was used evaluate the association of various factors with nocturnal asthma, such as self-identified race/ethnicity and genetic ancestry.
Measurement and Main Results: The study comprised 3,380 African American and 1,818 European Americans individuals with asthma. After adjusting for other potential explanatory variables, including controller medication use, African Americans were more than twice as likely (odds ratio, 2.56; 95% confidence interval, 2.24–2.93) to report nocturnal asthma when compared with European American individuals. Among the subset of African American participants with genome-wide genotype data (n = 1,040), estimated proportion of African ancestry was also associated with an increased risk of nocturnal asthma (P = 0.007). Differences in lung function explained a small, but statistically significant (P = 0.02), proportion of the relationship between genetic ancestry and nocturnal asthma symptoms.
Conclusions: Both self-identified race/ethnicity and African ancestry appear to be independent predictors of nocturnal asthma. The mechanism by which genetic ancestry contributes to population-level differences in nocturnal asthma appears to be largely independent of lung function.
Keywords: asthma, nocturnal symptoms, race/ethnicity, lung function, genetic ancestry
At a Glance Commentary
Scientific Knowledge on the Subject
Nocturnal asthma is a common presentation and is associated with a more severe form of the disease. However, there are few epidemiologic studies of nocturnal asthma in minority populations, and none have evaluated the genetic component of ancestry in relationship to the risk of developing nocturnal asthma.
What This Study Adds to the Field
In a large, ethnically diverse cohort of individuals from southeastern Michigan with asthma, African American individuals were more likely to report nocturnal asthma when compared with European American individuals. Within the subset of African Americans, the estimated proportion of African ancestry was associated with higher likelihood of symptoms, although this relationship was only partially explained by differences in lung function.
Asthma is a common respiratory disorder affecting more than 30 million people in the United States (1) and 300 million people worldwide (2–4). Nocturnal asthma is a common disease presentation, with studies of asthma reporting up to 75% of patients with nocturnal symptoms (5). Nocturnal asthma has also been associated with disease severity and is accompanied by higher rates of morbidity (6) and mortality (7, 8). Furthermore, although asthma mortality is relatively low, there is a higher chance of dying at night relative to during the daytime (9), suggesting that nocturnal asthma is a major medical and public health concern.
Nocturnal asthma is diagnosed in patients based on reported sleep disturbance due to asthma-related symptoms. However, lung function studies have shown that affected individuals may demonstrate reduced FEV1 both at night and throughout the day (10, 11). The relative decrease appears to be greatest during the nighttime hours (12) and may be accompanied by increased eosinophil inflammation in the lung (13). Individuals with nocturnal asthma may also have higher levels of blood eosinophils and neutrophils relative to individuals with asthma without nocturnal symptoms (14). These findings suggest that nocturnal symptoms may constitute a specific subset of individuals with asthma. Nevertheless, little work has been done to characterize the epidemiology of nocturnal asthma, particularly among African American individuals, who as a group suffer disproportionately from asthma-related complications (15–17).
The current study reports results from an epidemiologic investigation of nocturnal asthma in a multiethnic cohort, the Study for Asthma Phenotypes and Pharmacogenomic Interactions by Race-Ethnicity (SAPPHIRE) cohort. Using this large cohort, we were able to identify race/ethnicity differences in both the frequency of nocturnal symptoms and the characteristics associated with nocturnal asthma.
Methods
Study Subjects
This study was approved by the Institutional Review Board of Henry Ford Hospital and was in compliance with its Health Insurance Portability and Accountability Act policy. The SAPPHIRE cohort is an ongoing study to identify the genetic predictors of asthma controller medication response among a population-based sample of individuals with asthma. Specifically, the cohort includes members of a large health system, which serves southeast Michigan and the greater Detroit metropolitan area. Participants are identified from health care claims and recorded asthma diagnoses in the electronic medical record. The eligibility criteria for SAPPHIRE participants are as follows: age 12 to 56 years, a prior clinical diagnosis of asthma, and no recorded diagnosis of chronic obstructive pulmonary disease or congestive heart failure. Individuals, who consent to participation undergo a detailed enrollment evaluation.
Measurement of Nocturnal Asthma Symptoms and Accompanying Covariates
All participants completed a detailed staff-administered survey at the time of enrollment. The Asthma Control Test (ACT) (QualityMetric Inc., Lincoln, RI) was included on the survey. The ACT included the following question about nocturnal asthma symptoms: “During the past 4 weeks, how often did your asthma symptoms (wheezing, coughing, shortness of breath, chest tightness or pain) wake you up at night or earlier than usual in the morning?” (18) The possible responses to this question were not at all (= 0), once or twice (= 1), once a week (= 2), two to three nights a week (= 3), or four or more times a week (= 4). We collapsed these responses into a single dichotomous variable for nocturnal symptoms to compare no symptoms (= 0) with any nocturnal symptoms (= 1).
The survey also asked participants to identify all race/ethnicity categories that applied to themselves. Patients were also queried about other demographic characteristics and environmental exposures, including age, smoking history, and age of asthma onset. Field staff obtained the participants’ height and weight, and these measures were used to calculate body mass index (BMI) in kilograms divided by meters squared (kg/m2). Patients had their lung function assessed, and this spirometry was performed according to 2005 American Thoracic Society/European Respiratory Society recommendations using a Fleisch-type pneumotachometer (19). Bronchodilator reversibility (BDR) was quantified as the percentage change in FEV1 after administration of 360 μg of inhaled albuterol hydroxyfluoroalkane delivered by metered dose inhaler using an AeroChamber Plus Flow-Vu spacer (Monahan Medical Corp., Plattsburgh, NY).
We assessed West African ancestry (heretofore, referred to as African ancestry) in individuals whose self-reported race-ethnicity was African American. To measure genetic ancestry, genomic DNA was first isolated from a blood specimen taken at the time of the initial study visit. Genome-wide genotyping was performed using the Axiom genome-wide AFR array (Affymetrix, Santa Clara, CA) on 1,040 SAPPHIRE subjects with asthma (20). We assessed local ancestry (i.e., African and European ancestry) at each autosomal single nucleotide polymorphism location (21, 22), and we estimated the total percentage of African ancestry (i.e., global ancestry) in African American individuals as the proportion of African alleles.
Statistical Analysis
We restricted the analysis to African American and European American individuals. The primary outcome variable was nocturnal asthma (i.e., any nocturnal symptoms vs. no symptoms). To test for heterogeneity by race/ethnicity, we compared models with and without a covariate-by-race interaction term—a 1 degree of freedom likelihood ratio test comparing a model with the interaction term to a reduced model with just the individual covariates of interest and a term for self-reported race/ethnicity (i.e., two-variable models). We used forward-stepwise selection to separately build multivariable models of nocturnal asthma for African American and European American individuals. Variables were entered into the model one at a time based on their likelihood ratio P value. A threshold P value of 0.05 was used to determine whether variables entered into the model were retained; however, sex, age, and height were included in the model regardless of statistical significance.
Causal mediation analysis (23, 24) was used to test whether the relationship between genetic African ancestry and nocturnal asthma was mediated by lung function (i.e., FEV1 or FVC). For these models, percent African ancestry was considered the treatment, lung function was considered the mediator, and dichotomous nocturnal asthma status was used as the outcome. As the treatment must be dichotomous for this model, the continuous variable of percent African ancestry was divided into quintiles, and mediation effects were estimated using the subjects in the lowest quintile of percent African ancestry (≤73.7%) as the “control” group. Each successively higher quintile was a “treatment” group. Two equations were used to model each mediator and outcome. The mediator equation was estimated using a linear model of lung function with the primary predictor being African ancestry. The outcome equation was estimated using a logistic model for the binary outcome of nocturnal asthma. These models were also adjusted for additional variables associated with nocturnal asthma in African Americans identified from the forward stepwise selection procedure. The confidence intervals (CIs) were based on the nonparametric bootstrap with 1,500 samples.
As a sensitivity analysis, we assessed whether adjusting for inhaled corticosteroid (ICS) and long-acting β-agonist (LABA) medication use at baseline affected the relationships between nocturnal asthma and both self-reported race/ethnicity and African ancestry. We also assessed the effect of adjusting for asthma controller medication adherence in the 6-month period preceding symptom assessment. Medication adherence was quantified using pharmacy claims data in the manner described by us previously (25). We also reassessed the relationship of race/ethnicity and nocturnal asthma symptoms after stratifying by BMI and adjusting for different measures of tobacco smoke exposure.
A flowchart of the analyses performed and the numbers available for each analysis are shown in Figure E1 of the online supplement. The causal mediation analysis was conducted using the “mediation” package (26) implemented in the R statistical programing language (version 3.0.2) (27). We used SAS version 9.2 (SAS institute Inc., Cary, NC) for all other analyses.
Results
The demographic and clinical characteristics of the study participants are presented in Table 1. Self-identified African American individuals composed 65% of the total study population. The African American and European American groups were similar in age and sex. Relative to European American participants, African Americans had a higher average BMI (31.8 vs. 28.9), were more likely to have smoked (31% vs. 25%), were more likely to have had asthma before the age of 12 years (64 vs. 56%), and were nearly twice as likely to report a prior hospitalization for asthma (46% vs. 24%). Regarding lung function, mean percent of predicted FEV1 was lower in African American individuals as compared with European American individuals (87.4% vs. 90.5%), whereas having a BDR value greater than 12% was approximately twice as high in the former group (29.4% vs. 16.9%).
Table 1.
Characteristics of Study Participants at the Time of Study Enrollment (n = 5,198)
| Combined (n = 5,198) | African American (n = 3,380) | European American (n = 1,818) | P Value* | |
|---|---|---|---|---|
| Age, yr | 33.4 ± 14.4 | 32.8 ± 14.0 | 34.5 ± 15.1 | <0.001 |
| Female, | 3,324 (64) | 2,183 (65) | 1,151 (63) | 0.202 |
| Height, m | 1.7 ± 0.1 | 1.67 ± 0.1 | 1.68 ± 0.1 | <0.001 |
| African American race/ethnicity | 3,380 (65) | — | — | — |
| BMI, kg/m2 | 30.8 ± 9.3 | 31.8 ± 9.5 | 28.9 ± 8.4 | <0.001 |
| Smoking, ever smoker | 1,518 (29) | 1,061 (31) | 457 (25) | <0.001 |
| Age at diagnosis, yr | 13.8 ± 13.7 | 12.9 ± 13.5 | 15.5 ± 13.8 | <0.001 |
| Age at diagnosis > 12 yr | 2,027 (39) | 1,223 (36.2) | 804 (44.2) | <0.001 |
| Ever hospitalized | 1,995 (38) | 1,556 (46) | 439 (24) | <0.001 |
| FEV1, L | 2.8 ± 0.9 | 2.6 ± 0.8 | 3.1 ± 0.8 | <0.001 |
| FVC, L | 3.6 ± 1.0 | 3.3 ± 0.9 | 4.0 ± 1.0 | <0.001 |
| FEV1/FVC ratio | 76.8 ± 9.5 | 76.2 ± 9.8 | 77.8 ± 8.8 | <0.001 |
| Percent of predicted FEV1† | 88.5 ± 18.9 | 87.4 ± 19.5 | 90.5 ± 17.6 | <0.001 |
| Percent of predicted FVC† | 96.2 ± 17.2 | 96.3 ± 17.7 | 95.9 ± 16.2 | 0.382 |
| BDR‡ | 8.3 ± 12.6 | 9.2 ± 13.6 | 6.7 ± 10.1 | <0.001 |
Definition of abbreviations: BDR = bronchodilator reversibility; BMI = body mass index.
Data are presented as mean ± SD or n (%).
P values for tests of differences between African American and European American individuals.
Based on standardized equations found in Hankinson et al. (48).
BDR was measured as the change in FEV1 after albuterol administration according to the following formula: BDR = ([FEV1(post-bronchodilator) − FEV1(pre-bronchodilator)]/FEV1(pre-bronchodilator)) × 100.
Table 2 presents the unadjusted univariable associations with nocturnal asthma. Higher measures of lung function (i.e., higher FEV1, FVC, and FEV1/FVC values) were associated with a lower likelihood of nocturnal asthma symptoms. Conversely, increasing age, female sex, higher BMI, older age at asthma diagnosis, higher BDR, a positive smoking history, and self-reported African American race-ethnicity were all associated with a higher likelihood of reporting nocturnal asthma symptoms. African American individuals were approximately three times more likely to report nocturnal asthma symptoms when compared with European American individuals (odds ratio [OR], 2.95; 95% CI, 2.61–3.34).
Table 2.
Assessment of Factors for Association with Nocturnal Asthma and Potential Differences by Race/Ethnicity
| All Subjects |
African American |
European American |
Pint† | ||||
|---|---|---|---|---|---|---|---|
| OR* | 95% CI | OR* | 95% CI | OR* | 95% CI | ||
| Age at enrollment, yr | 1.02 | 1.02–1.03 | 1.03 | 1.03–1.04 | 1.02 | 1.01–1.03 | 1.000 |
| Race/ethnicity, African American | 2.95 | 2.61–3.34 | — | — | — | — | — |
| Sex, female | 1.61 | 1.43–1.82 | 1.64 | 1.43–1.89 | 1.91 | 1.30–2.00 | 0.940 |
| Height, m | 0.22 | 0.13–0.40 | 0.32 | 0.16–0.64 | 0.23 | 0.08–0.68 | 0.620 |
| BMI, kg/m2 | 1.04 | 1.03–1.05 | 1.03 | 1.02–1.03 | 1.05 | 1.04–1.07 | <0.001 |
| Smoking, ever smoker | 2.51 | 2.22–2.83 | 2.71 | 2.32–3.16 | 2.00 | 1.60–2.51 | 0.028 |
| Age at diagnosis, yr | 1.01 | 1.00–1.01 | 1.01 | 1.01–1.02 | 1.01 | 1.00–1.02 | 0.230 |
| Age at diagnosis > 12 yr | 1.28 | 1.15–1.44 | 1.49 | 1.29–1.71 | 1.31 | 1.06–1.61 | 0.378 |
| Ever hospitalized, yes | 2.22 | 1.98–2.49 | 1.78 | 1.55–2.04 | 2.17 | 1.73–2.73 | 0.142 |
| FEV1, L | 0.47 | 0.43–0.50 | 0.52 | 0.48–0.57 | 0.54 | 0.47–0.62 | 0.668 |
| FVC, L | 0.60 | 0.57–0.64 | 0.68 | 0.63–0.73 | 0.67 | 0.60–0.75 | 0.917 |
| FEV1/FVC ratio | 0.96 | 0.95–0.96 | 0.96 | 0.95–0.97 | 0.97 | 0.96–0.98 | 0.164 |
| Bronchodilator reversibility | 1.03 | 1.03–1.04 | 1.03 | 1.02–1.03 | 1.05 | 1.04–1.06 | 0.002 |
Definition of abbreviations: BMI = body mass index; CI = confidence interval; OR = odds ratio; Pint = P value from a 1 degree of freedom likelihood ratio test of the multiplicative interaction between self-reported race/ethnicity and each variable.
The outcome variable for the association analyses was nocturnal asthma defined as having any night with disrupted sleep due to asthma in the preceding 4 weeks.
ORs represent the univariable relationship with nocturnal asthma. These estimates reflect the effect of a one-unit increase in the variable of interest.
The P value for the likelihood ratio test of interaction between race/ethnicity and each variable in a bivariable model, which includes a covariate for self-reported race/ethnicity.
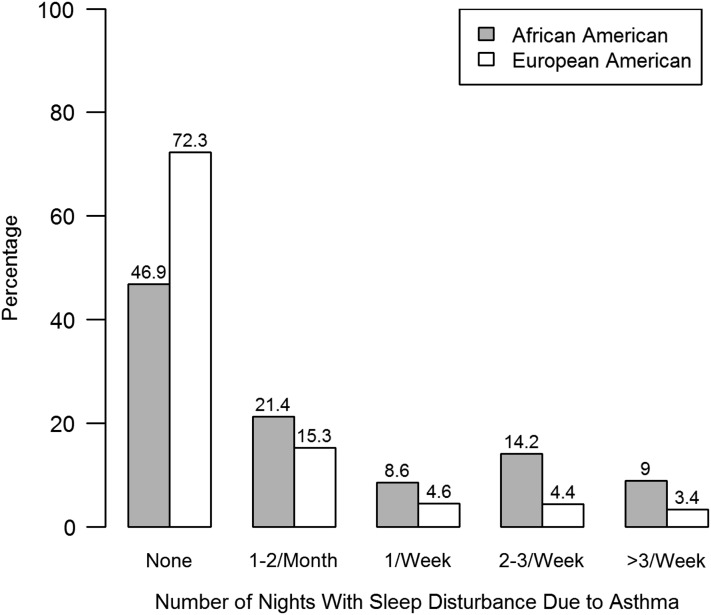
As shown in Figure 1, African American subjects also reported greater nights with disrupted sleep from asthma when compared with European American participants (P < 0.001). For example, relative to those not experiencing nocturnal symptoms, African American individuals were approximately four times as likely to report having nocturnal symptoms four or more times per week (OR, 4.05; 95% CI, 3.04–5.45) when compared with European American individuals.
Figure 1.
Percentage of study participants reporting nocturnal asthma symptoms by race/ethnicity. Strata represent the number of nights with asthma-related sleep disturbance in the preceding month. The reported relationships are unadjusted for other variables.
To determine if the effect of race-ethnicity could be explained by other factors associated with nocturnal asthma, we constructed two multivariable models. The first model included all factors, and the second was constructed using a forward-stepwise variable selection approach (see Table E2). In both models, African American race/ethnicity was associated with nocturnal symptoms (OR, 2.48; 95% CI, 2.12–2.89; and OR, 2.56; 95% CI, 2.24–2.93, respectively). Together, these results suggested that race-ethnicity had an independent association with nocturnal asthma and could not be fully explained by the other factors.
In light of the aforementioned differences by race/ethnicity, we used forward-stepwise variable selection to determine the factors associated with nocturnal asthma for African American patients and European American patients, separately (Table 3). Factors associated with nocturnal symptoms in African American individuals with asthma included current age, sex, BMI, smoking status, history of asthma hospitalization, BDR, FEV1, and FVC. With the exceptions of FVC and age, the same factors were associated with nocturnal asthma in European American individuals (i.e., sex, BMI, smoking status, history of asthma hospitalization, BDR, and FEV1), and the magnitude of the effect estimates was also similar.
Table 3.
Factors Associated with Nocturnal Asthma after Stepwise Selection among Race/Ethnic Groups
| Covariate | African American* |
European American* |
||||
|---|---|---|---|---|---|---|
| OR† | 95% CI | P Value | OR | 95% CI | P Value | |
| Age at enrollment, yr | 1.01 | 1.01–1.02 | <0.001 | 1.00 | 1.00–1.01 | 0.620 |
| Sex, female | 1.32 | 1.09–1.61 | 0.006 | 1.32 | 0.97–1.79 | 0.075 |
| Height, m | 1.30 | 0.42–4.03 | 0.644 | 1.52 | 0.27–8.46 | 0.633 |
| BMI, kg/m2 | 1.01 | 1.01–1.02 | <0.001 | 1.04 | 1.03–1.05 | <0.001 |
| Smoking, ever smoker | 2.13 | 1.80–2.52 | <0.001 | 1.55 | 1.22–1.97 | <0.001 |
| Age at diagnosis, yr | — | — | — | — | — | — |
| Age at diagnosis > 12 yr | — | — | — | — | — | — |
| Ever hospitalized, yes | 1.56 | 1.35–1.81 | <0.001 | 1.75 | 1.37–2.23 | <0.001 |
| FEV1, L | 0.55 | 0.41–0.74 | <0.001 | 0.75 | 0.62–0.93 | 0.009 |
| FVC, L | 1.29 | 1.09–1.65 | 0.043 | — | — | — |
| FEV1/FVC ratio | — | — | — | — | — | — |
| Bronchodilator reversibility | 1.01 | 1.01–1.02 | <0.001 | 1.03 | 1.02–1.05 | <0.001 |
Definition of abbreviations: BMI = body mass index; CI = confidence interval; OR = odds ratio.
The outcome variable for the association analyses was nocturnal asthma defined as having any night with disrupted sleep due to asthma in the preceding 4 weeks. P value from a 1 degree of freedom likelihood ratio test for each variable.
Forward stepwise used for variable selection for the race/ethnicity specific models. Covariates without values (i.e., —) were those not retained in the final model.
ORs reflect the estimated effect of a one-unit increase in the variable of interest, accounting for the other variables retained in the model.
Given the differences in nocturnal asthma by race/ethnicity, we also assessed whether African ancestry was associated with nocturnal symptoms among African American study participants. For this analysis, the sample was restricted to the subset of African American participants with genome-wide genotype data (n = 1,040). Association analysis revealed an increasing trend in proportion of African ancestry with increasing frequency of nocturnal asthma symptoms (P = 0.007). The average proportion of African Ancestry was 0.79 (±0.10 SD), 0.80 (±0.09 SD), and 0.81 (±0.10 SD) for patients reporting no symptoms, symptoms less than one night per week, and symptoms one or more nights per week, respectively. After adjusting for all variables retained in the stepwise model for African American individuals (Table 3), increasing African ancestry was positively associated with nocturnal symptoms (OR, 3.47; 95% CI, 0.90–13.39; P = 0.069), although the statistical significance was reduced. This reduction in statistical significance may have been due to the effect of outlier individuals with a lower proportion of African ancestry (Figure E2). Mitigating the effect of outliers by grouping individuals into quintiles based on their proportion of African ancestry (i.e., 20th percentile = 73.7%; 40th percentile = 79.2%; 60th percentile = 83.2%; 80th percentile = 87.0%) resulted in a statistically significant association between African ancestry and nocturnal asthma (P = 0.045), even after accounting for potential confounders.
We used causal mediation analysis to examine whether the observed relationship between African ancestry and nocturnal symptoms was likely to be a direct effect or mediated by changes in lung function (as measured by FEV1 or FVC). Due to the high correlation between FEV1 and FVC, these measures were examined in separate models. The results of these analyses are presented in Table 4. For both FEV1 and FVC, the findings support a small, but statistically significant, mediating effect of lung function on the relationship between African ancestry and nocturnal asthma. The strongest support for a mediating effect was achieved for FVC, with statistically significant evidence (P ≤ 0.02) across all four quintiles (i.e., the “treatment” groups) relative to the lowest quintile (i.e., the “control” group). For the group with the highest proportion of African ancestry, 13% of the total effect of ancestry on nocturnal asthma appeared to be mediated by FVC (P = 0.04) (Table 4). The mediating effect of lung function appeared to be consistent across all of the other quintile groups defined by African ancestry (Table 4). These finding support an independent effect of African ancestry on nocturnal asthma.
Table 4.
Causal Mediation Analysis Results Evaluating FEV1 and FVC as Potential Mediating Traits between African Ancestry and Nocturnal Asthma among African American Participants (n = 1,040)
| Mediator | % African Ancestry* | Average Mediation Effect |
Average Direct Effect |
% Mediated |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | P Value | Estimate | 95% CI | P Value | % | P Value | ||
| FEV1 | >20th to 40th | 0.002 | 6.1 × 10−5 to 0.01 | 0.04 | 0.021 | −4.5 × 10−4 to 0.04 | 0.06 | 9.0 | 0.08 |
| >40th to 60th | 0.004 | 5.4 × 10−5 to 0.01 | 0.05 | 0.042 | 2.1 × 10−3 to 0.04 | 0.04 | 8.9 | 0.07 | |
| >60th to 80th | 0.007 | −1.1 × 10−4 to 0.02 | 0.05 | 0.063 | 4.0 × 10−3 to 0.12 | 0.03 | 8.8 | 0.07 | |
| >80th | 0.009 | −1.9 × 10−4 to 0.20 | 0.06 | 0.081 | 5.3 × 10−3 to 0.16 | 0.04 | 8.7 | 0.08 | |
| FVC | >20th to 40th | 0.003 | 5.0 × 10−4 to 0.01 | 0.01 | 0.020 | −1.2 × 10−4 to 0.04 | 0.05 | 12.4 | 0.04 |
| >40th to 60th | 0.006 | 9.8 × 10−4 to 0.01 | 0.02 | 0.040 | −2.5 × 10−3 to 0.08 | 0.06 | 12.7 | 0.05 | |
| >60th to 80th | 0.009 | 1.5 × 10−3 to 0.02 | 0.01 | 0.060 | 3.4 × 10−4 to 0.12 | 0.05 | 12.7 | 0.03 | |
| >80th | 0.012 | 2.1 × 10−3 to 0.01 | 0.02 | 0.079 | −9.8 × 10−4 to 0.16 | 0.05 | 13.0 | 0.04 | |
Definition of abbreviation: CI = confidence interval.
Nocturnal asthma was defined as having any night with disrupted sleep due to asthma in the preceding 4 weeks. Mediation effects were estimated using the subjects in the lowest quintile of percent African ancestry (i.e., ≤73.7%) as the “control” group and each successive higher quintile as the “treatment” group. The CIs for both the mediation and direct effect estimates were based on the nonparametric bootstrap with 1,500 samples. The mediation effect was estimated using a linear model of lung function (i.e., FEV1 or FVC) and a logistic model for the binary outcome of nocturnal asthma.
The quintiles of % African ancestry were defined as follows: 20th percentile = 73.7%; 40th percentile = 79.2%; 60th percentile = 83.2%; and 80th percentile = 87.0%.
We performed a number of sensitivity analyses to test whether our findings were robust. First, we assessed whether use of ICS or LABAs may have confounded the association that we found between race-ethnicity and nocturnal asthma symptoms. For this analysis, we analyzed the subset of individuals for whom we had longitudinal clinical records of medication use (992 African American patients and 637 European American patients). Among the African American patients, 59 (6.0%) were using an ICS alone and 112 (11.3%) were using an ICS/LABA combination inhaler. Among the European American patients, 54 (8.5%) were using an ICS alone and 90 (14.1%) were using an ICS/LABA combination inhaler. Accounting for ICS and LABA use had almost no effect on the association between self-reported African American race/ethnicity and nocturnal asthma symptoms (OR, 1.96 for African American vs. European American race-ethnicity; 95% CI, 1.50–2.56; P < 0.001, before adjusting for ICS and LABA use; and OR, 1.96; 95% CI, 1.50–2.56; P < 0.001, after adjusting for ICS and LABA use). Similarly, African ancestry was still significantly associated with nocturnal symptoms among African American individuals after adjusting for ICS and LABA use (P = 0.027). Among individuals who filled an ICS prescription, accounting for the level of medication adherence did not substantively affect the relationship between race/ethnicity and nocturnal symptoms (OR, 2.24 for African American vs. European American race/ethnicity; 95% CI, 1.22–4.13; P = 0.009).
Using different measures of tobacco smoke exposure (i.e., cigarette pack-years and separate variables for past and current cigarette smoking) also did not substantively change the relationship between race-ethnicity and nocturnal symptoms (data not shown). Moreover, restricting the sample to normal-weight individuals (i.e., BMI, 18.5–24.9; n = 1,440), we found that race-ethnicity was even more strongly associated with nocturnal symptoms (OR, 2.95 for African American vs. European American race/ethnicity; 95% CI, 2.20–3.94; P < 0.001); however, among overweight individuals (BMI ≥ 25), race/ethnicity was still associated with nocturnal asthma (OR, 1.94; 95% CI, 1.63–2.30; P < 0.001).
Discussion
The current study has demonstrated large and statistically significant differences in nocturnal asthma symptoms between African American and European American individuals enrolled in the SAPPHIRE cohort. Moreover, we show that genetic ancestry may independently contribute to the risk of having nocturnal asthma.
To our knowledge, there has been only one previous study that has compared nocturnal asthma rates between African Americans and European Americans. In contrast to our present findings, Trochtenberg and colleagues studied 27 individuals and reported a higher overall prevalence of nocturnal symptoms (82%), with European American patients having a higher occurrence when compared with African American patients (100% vs. 67%, respectively) (28). However, the average age of these study participants was higher (53 and 44 yr for African American and European Americans, respectively) when compared with those enrolled in the SAPPHIRE cohort. As we have found increasing age to be positively associated with the risk of reporting nocturnal asthma symptoms, demographic factors may explain a portion of the between study differences. Furthermore, the estimates of this earlier study are inherently less precise given the smaller number of participants.
The current study is also the first to investigate the possible role of genetic ancestry as a risk factor for nocturnal asthma. To date, genetic studies of nocturnal asthma have focused primarily on candidate genes, such as the β2-adrenergic receptor (29). Our findings suggest genetic differences between nocturnal and nonnocturnal asthma that may be spread throughout the genome and that this genetic variation is associated with ancestral genetic background. Genome-wide admixture mapping and association studies of nocturnal asthma have not been published to date but appear warranted.
In addition, we show that the effect of genetic ancestry on nocturnal symptoms appears to be mediated in part, but not completely, through its association with lung function (i.e., FVC and FEV1). These results are consistent with our earlier work, which showed African ancestry to be inversely associated with measured FVC and FEV1 (30) and with results showing nighttime lung function to be particularly decreased in individuals with nocturnal asthma relative to those without nocturnal symptoms (10). Although the causal mediation analysis demonstrated a marginally more significant result for FVC as compared with FEV1, both FVC and FEV1 are highly correlated. Therefore, we are unable to conclude which of these lung function measures is the primary mediator of nocturnal symptoms. However, the overall consistency of these findings suggests that the genetic predictors of lung function may be partially shared with the phenotype of nocturnal asthma. As such, a future genome-wide study might benefit from a multitrait mapping approach that includes lung function, given the potential mediating effect of lung function identified and power increases associated with the multitrait over single-trait approaches (31, 32).
According to current U.S. guidelines, individuals in the age range of SAPPHIRE participants (i.e., ≥12 yr of age) should consider step-up therapy for uncontrolled or moderately severe persistent asthma when nighttime asthma awakenings occur more than once per week (33). The guidelines also indicate that combination ICS and LABA therapy be considered for individuals with this level of asthma severity. However, these recommendations are tempered by conflicting findings in the literature, which suggest both a beneficial (34, 35) and detrimental effect (36) of supplemental LABA use, particularly among African American individuals. As a sensitivity analysis in a subset of SAPPHIRE participants with longitudinal prescription information, we found that self-reported African American race-ethnicity was still a risk factor for nocturnal symptoms even after accounting for LABA and ICS use. Therefore, it is unlikely that differences in asthma controller medication use alone could explain the observed differences in nocturnal symptoms.
Current asthma treatment guidelines use a symptom-based approach for assessing asthma severity and directing therapeutic decisions (33), and it is not clear that these treatment algorithms would be improved by incorporating information on patient race/ethnicity or genetic ancestry. Nevertheless, our observations provide the impetus for investigating the mechanisms through which race/ethnicity and genetic ancestry contribute to nocturnal asthma. The knowledge gained by these additional studies and this one may, in turn, identify unique and actionable disease mechanisms.
This study is not without other limitations. First, the study population comprised individuals from a single, large health system serving southeast Michigan and metropolitan Detroit. However, we have previously shown that our patient population is representative of the census population of the region (37), and the proportion of African and European ancestry in our African American participants is similar to that which has been described for African American communities in other parts of the United States (38, 39). Second, it is possible that some cases of nocturnal asthma were incorrectly diagnosed. We would expect that this misclassification would decrease our power to detect associations. However, differences in symptom frequency by race-ethnicity could still be attributable to other factors contributing to nocturnal symptoms, such as obstructive sleep apnea and gastroesophageal reflux disease (40, 41). Given the relationship of these conditions with body weight (42, 43), we stratified our analyses by BMI. However, the association of race/ethnicity with nocturnal symptoms was still present among individuals with BMI less than 25. Population-level differences in allergic sensitization by race/ethnicity are well described (44, 45) and also could contribute to asthma severity (46, 47). Therefore, future analyses could investigate these and other potential mechanisms (e.g., sleep studies, allergic sensitivity testing, and nocturnal spirometry). Last, our definition of nocturnal asthma was based on responses to an ACT question assessing nighttime or early morning awakenings over the course of 4 weeks (18). It is uncertain whether this is the correct time course for labeling one as having nocturnal asthma, because undoubtedly some patients may have had previous symptoms. Nevertheless, we have no reason to believe that the time course for assessment would have influential differences by race-ethnicity, especially because our findings appeared to be independent of patient age and asthma duration.
In conclusion, the current study provides further insight into the epidemiological factors associated with nocturnal asthma among African American and European American individuals. In particular, this study found that self-identified race/ethnicity appears to be an independent risk factor for nocturnal asthma, even after accounting for likely confounders, such as patient age, sex, BMI, age of asthma onset, lung function, and controller treatment. We also found African ancestry to be associated with nocturnal symptoms among African American individuals, further supporting our findings. Therefore, further studies are needed to dissect the genetic architecture of nocturnal asthma and the mechanisms contributing to the observed disparity.
Footnotes
Supported by the National Institutes of Allergy and Infectious Diseases grant R01AI079139; the National Heart, Lung, and Blood Institute grants R01HL079055 and R01HL118267; and the National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK64695 of the National Institutes of Health; the Fund for Henry Ford Hospital; and the American Asthma Foundation (L.K.W.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Author Contributions: Each author had full access to the data and takes responsibility for the integrity and accuracy of the analysis. Conception and design: A.M.L. and L.K.W.; analysis and interpretation: A.M.L., Y.W., K.E.W., B.P., J.J.Y., E.G.B., and L.K.W.; and drafting the manuscript for important intellectual content: A.M.L., Y.W., K.E.W., B.P., J.J.Y., E.G.B., and L.K.W. All authors contributed to and approved of the final submitted manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201402-0204OC on June 17, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Centers for Disease Control and Prevention (CDC) Self-reported asthma prevalence and control among adults—United States, 2001. MMWR Morb Mortal Wkly Rep. 2003;52:381–384. [PubMed] [Google Scholar]
- 2.Global Initiative for AsthmaGlobal Strategy Asthma Management and Prevention NHLBI/WHO Workshop Report, March 1993. Bethesda, MD: National Institutes of Health, National Heart, Lung, and Blood Institute; 1993. NIH publication 95–3659
- 3.National Heart, Lung, and Blood InstituteGlobal Initiative for Asthma: Global Strategy for Asthma Management and Prevention 2002. Bethesda, MD: National Institutes of Health, National Heart, Lung, and Blood Institute; 2002. NIH publication 02–3659
- 4.Tse SM, Tantisira K, Weiss ST. The pharmacogenetics and pharmacogenomics of asthma therapy. Pharmacogenomics J. 2011;11:383–392. doi: 10.1038/tpj.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turner-Warwick M. Epidemiology of nocturnal asthma. Am J Med. 1988;85:6–8. doi: 10.1016/0002-9343(88)90231-8. [DOI] [PubMed] [Google Scholar]
- 6.Diette GB, Markson L, Skinner EA, Nguyen TT, Algatt-Bergstrom P, Wu AW. Nocturnal asthma in children affects school attendance, school performance, and parents’ work attendance. Arch Pediatr Adolesc Med. 2000;154:923–928. doi: 10.1001/archpedi.154.9.923. [DOI] [PubMed] [Google Scholar]
- 7.Calhoun WJ. Nocturnal asthma. Chest. 2003;123:399S–405S. doi: 10.1378/chest.123.3_suppl.399s. [DOI] [PubMed] [Google Scholar]
- 8.Hetzel MR, Clark TJ, Branthwaite MA. Asthma: analysis of sudden deaths and ventilatory arrests in hospital. BMJ. 1977;1:808–811. doi: 10.1136/bmj.1.6064.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Douglas NJ. Asthma at night. Clin Chest Med. 1985;6:663–674. [PubMed] [Google Scholar]
- 10.Sutherland ER. Nocturnal asthma: underlying mechanisms and treatment. Curr Allergy Asthma Rep. 2005;5:161–167. doi: 10.1007/s11882-005-0091-z. [DOI] [PubMed] [Google Scholar]
- 11.Martin RJ, Cicutto LC, Ballard RD. Factors related to the nocturnal worsening of asthma. Am Rev Respir Dis. 1990;141:33–38. doi: 10.1164/ajrccm/141.1.33. [DOI] [PubMed] [Google Scholar]
- 12.Irvin CG, Pak J, Martin RJ. Airway-parenchyma uncoupling in nocturnal asthma. Am J Respir Crit Care Med. 2000;161:50–56. doi: 10.1164/ajrccm.161.1.9804053. [DOI] [PubMed] [Google Scholar]
- 13.Kraft M, Djukanovic R, Wilson S, Holgate ST, Martin RJ. Alveolar tissue inflammation in asthma. Am J Respir Crit Care Med. 1996;154:1505–1510. doi: 10.1164/ajrccm.154.5.8912772. [DOI] [PubMed] [Google Scholar]
- 14.Nadif R, Siroux V, Oryszczyn MP, Ravault C, Pison C, Pin I, Kauffmann F Epidemiological study on the Genetics and Environment of Asthma (EGEA) Heterogeneity of asthma according to blood inflammatory patterns. Thorax. 2009;64:374–380. doi: 10.1136/thx.2008.103069. [DOI] [PubMed] [Google Scholar]
- 15.Mannino DM, Homa DM, Akinbami LJ, Moorman JE, Gwynn C, Redd SC. Surveillance for asthma—United States, 1980-1999. MMWR Surveill Summ. 2002;51:1–13. [PubMed] [Google Scholar]
- 16.Miller JE. The effects of race/ethnicity and income on early childhood asthma prevalence and health care use. Am J Public Health. 2000;90:428–430. doi: 10.2105/ajph.90.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention (CDC) Asthma mortality and hospitalization among children and young adults—United States, 1980-1993. MMWR Morb Mortal Wkly Rep. 1996;45:350–353. [PubMed] [Google Scholar]
- 18.Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, Murray JJ, Pendergraft TB. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann TJ, Kvale MN, Hesselson SE, Zhan Y, Aquino C, Cao Y, Cawley S, Chung E, Connell S, Eshragh J, et al. Next generation genome-wide association tool: design and coverage of a high-throughput European-optimized SNP array. Genomics. 2011;98:79–89. doi: 10.1016/j.ygeno.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sankararaman S, Sridhar S, Kimmel G, Halperin E. Estimating local ancestry in admixed populations. Am J Hum Genet. 2008;82:290–303. doi: 10.1016/j.ajhg.2007.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pasaniuc B, Sankararaman S, Kimmel G, Halperin E. Inference of locus-specific ancestry in closely related populations. Bioinformatics. 2009;25:i213–i221. doi: 10.1093/bioinformatics/btp197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imai K, Keele L, Tingley D. A general approach to causal mediation analysis. Psychol Methods. 2010;15:309–334. doi: 10.1037/a0020761. [DOI] [PubMed] [Google Scholar]
- 24.Imai K, Keele L, Yamamoto T. Identification, inference, and sensitivity analysis for causal mediation effects. Stat Sci. 2010;25:51–71. [Google Scholar]
- 25.Williams LK, Peterson EL, Wells K, Ahmedani BK, Kumar R, Burchard EG, Chowdhry VK, Favro D, Lanfear DE, Pladevall M.Quantifying the proportion of severe asthma exacerbations attributable to inhaled corticosteroid nonadherence. J Allergy Clin Immunol2011. 128:1185–1191.e2 [DOI] [PMC free article] [PubMed]
- 26.Tingley D, Yamamoto T, Hirose K, Keele L, Imai K.mediation: R package for causal mediation analysis. R package version 4.3.1. 2013. [accessed 2013 Dec 11]. Available from: http://CRAN.R-project.org/package=mediation [Google Scholar]
- 27.R Core TeamR: A language and environment for statistical computingVienna, Austria: R Foundation for Statistical Computing; 2013 [Google Scholar]
- 28.Trochtenberg DS, BeLue R, Piphus S, Washington N. Differing reports of asthma symptoms in African Americans and Caucasians. J Asthma. 2008;45:165–170. doi: 10.1080/02770900701847076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenberg H, Cohen RI. Nocturnal asthma. Curr Opin Pulm Med. 2012;18:57–62. doi: 10.1097/MCP.0b013e32834d098e. [DOI] [PubMed] [Google Scholar]
- 30.Kumar R, Seibold MA, Aldrich MC, Williams LK, Reiner AP, Colangelo L, Galanter J, Gignoux C, Hu D, Sen S, et al. Genetic ancestry in lung-function predictions. N Engl J Med. 2010;363:321–330. doi: 10.1056/NEJMoa0907897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klei L, Luca D, Devlin B, Roeder K. Pleiotropy and principal components of heritability combine to increase power for association analysis. Genet Epidemiol. 2008;32:9–19. doi: 10.1002/gepi.20257. [DOI] [PubMed] [Google Scholar]
- 32.O’Reilly PF, Hoggart CJ, Pomyen Y, Calboli FC, Elliott P, Jarvelin MR, Coin LJ. MultiPhen: joint model of multiple phenotypes can increase discovery in GWAS. PLoS ONE. 2012;7:e34861. doi: 10.1371/journal.pone.0034861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120:S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 34.Wells KE, Peterson EL, Ahmedani BK, Severson RK, Gleason-Comstock J, Williams LK.The relationship between combination inhaled corticosteroid and long-acting beta-agonist use and severe asthma exacerbations in a diverse population. J Allergy Clin Immunol2012. 129:1274–1279.e2 [DOI] [PMC free article] [PubMed]
- 35.Lemanske RF, Jr, Mauger DT, Sorkness CA, Jackson DJ, Boehmer SJ, Martinez FD, Strunk RC, Szefler SJ, Zeiger RS, Bacharier LB, et al. Childhood Asthma Research and Education (CARE) Network of the National Heart, Lung, and Blood Institute. Step-up therapy for children with uncontrolled asthma receiving inhaled corticosteroids. N Engl J Med. 2010;362:975–985. doi: 10.1056/NEJMoa1001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM SMART Study Group. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15–26. doi: 10.1378/chest.129.1.15. [DOI] [PubMed] [Google Scholar]
- 37.Williams LK, Joseph CL, Peterson EL, Moon C, Xi H, Krajenta R, Johnson R, Wells K, Booza JC, Tunceli K, et al. Race-ethnicity, crime, and other factors associated with adherence to inhaled corticosteroids. J Allergy Clin Immunol. 2007;119:168–175. doi: 10.1016/j.jaci.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 38.Yaeger R, Avila-Bront A, Abdul K, Nolan PC, Grann VR, Birchette MG, Choudhry S, Burchard EG, Beckman KB, Gorroochurn P, et al. Comparing genetic ancestry and self-described race in African Americans born in the United States and in Africa. Cancer Epidemiol Biomarkers Prev. 2008;17:1329–1338. doi: 10.1158/1055-9965.EPI-07-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parra EJ, Marcini A, Akey J, Martinson J, Batzer MA, Cooper R, Forrester T, Allison DB, Deka R, Ferrell RE, et al. Estimating African American admixture proportions by use of population-specific alleles. Am J Hum Genet. 1998;63:1839–1851. doi: 10.1086/302148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Julien JY, Martin JG, Ernst P, Olivenstein R, Hamid Q, Lemière C, Pepe C, Naor N, Olha A, Kimoff RJ. Prevalence of obstructive sleep apnea-hypopnea in severe versus moderate asthma. J Allergy Clin Immunol. 2009;124:371–376. doi: 10.1016/j.jaci.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 41.Gislason T, Janson C, Vermeire P, Plaschke P, Björnsson E, Gislason D, Boman G. Respiratory symptoms and nocturnal gastroesophageal reflux: a population-based study of young adults in three European countries. Chest. 2002;121:158–163. doi: 10.1378/chest.121.1.158. [DOI] [PubMed] [Google Scholar]
- 42.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 43.El-Serag HB, Graham DY, Satia JA, Rabeneck L. Obesity is an independent risk factor for GERD symptoms and erosive esophagitis. Am J Gastroenterol. 2005;100:1243–1250. doi: 10.1111/j.1572-0241.2005.41703.x. [DOI] [PubMed] [Google Scholar]
- 44.Litonjua AA, Celedón JC, Hausmann J, Nikolov M, Sredl D, Ryan L, Platts-Mills TA, Weiss ST, Gold DR. Variation in total and specific IgE: effects of ethnicity and socioeconomic status. J Allergy Clin Immunol. 2005;115:751–757. doi: 10.1016/j.jaci.2004.12.1138. [DOI] [PubMed] [Google Scholar]
- 45.Yang JJ, Burchard EG, Choudhry S, Johnson CC, Ownby DR, Favro D, Chen J, Akana M, Ha C, Kwok PY, et al. Differences in allergic sensitization by self-reported race and genetic ancestry. J Allergy Clin Immunol2008. 122:820–827.e9 [DOI] [PMC free article] [PubMed]
- 46.Gruchalla RS, Pongracic J, Plaut M, Evans R, III, Visness CM, Walter M, Crain EF, Kattan M, Morgan WJ, Steinbach S, et al. Inner City Asthma Study: relationships among sensitivity, allergen exposure, and asthma morbidity. J Allergy Clin Immunol. 2005;115:478–485. doi: 10.1016/j.jaci.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 47.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, Mitchell H, McNiff-Mortimer K, Lynn H, Ownby D, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 48.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]