To the Editor:
Quadriceps weakness and atrophy is present in approximately 30% of patients with chronic obstructive pulmonary disease (COPD) in secondary care (1, 2). The quadriceps also displays a shift in fiber type so that there are fewer type I (oxidative) fibers and more type II (glycolytic) fibers (3). Pulmonary rehabilitation only partially addresses this fiber shift (4). Muscle mass (5) and strength (6) are both associated with increased mortality, but the prognostic significance of fiber shift is unknown.
In a retrospective multicenter analysis of 392 patients from four sites (see Tables E1–E4 in the online supplement), mortality data were collated, as part of audit procedures, on outpatients with stable COPD who had undergone a vastus lateralis biopsy between 1995 and 2013. Data from these subjects have been previously published (e.g., References 2, 4, 5). Fiber proportion, reported as the percentage of type II fibers (type II fiber %), was established by immunohistochemistry. Fiber shift, evaluated as a dichotomous variable, was considered to have occurred when the proportion of type II fibers was greater than 68% (men) or greater than 65% (women) based on normal ranges established from an age-matched healthy population published by Natanek and colleagues (3). Body mass index (BMI), fat-free mass index (FFMI), dominant leg isometric quadriceps maximum voluntary contraction (QMVC and QMVC/BMI), mid-thigh cross-sectional area determined by computed tomography scan (MTCSA), residual volume normalized to total lung capacity (RV/TLC), and percent predicted value for the carbon monoxide transfer factor corrected for hemoglobin (TLCOc), when available, were included in subanalyses. Data were analyzed for the whole dataset and also after splitting the group into those with an FEV1 less than 50% predicted and those with an FEV1 greater than or equal to 50% predicted. Further details on the methodology and statistical analyses are presented in the online supplement. Some of the results of this study have been previously reported in abstract form (7).
Patients were followed up for a median of 1,699 days (127–6,601 d); 102 of 392 (26.7%) patients died during follow-up (Table E6). Cohort characteristics are presented in Tables 1 and 2 and Tables E1–E5. One hundred fifty-one patients had Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage I/II disease and 241 had GOLD stage III/IV disease. Those who died were older and had a lower FEV1 % predicted, and there was a greater male preponderance (Table E6A). One hundred seventy-seven (45.1%) of the patients had fiber shift. The patients who died had a higher percentage of type II fibers (69.5% [62.2, 76.3%] vs. 66.0% [54.0, 74.2%]; P = 0.002) and a higher proportion of them exhibited fiber shift (58% vs. 41%, P = 0.004). BMI, FFMI, QMVC, MTCSA, and TLCOc were all lower, and RV/TLC higher, in those who died (Table E6B).
Table 1.
Core Characteristics of the Cohort (n = 392) in Addition to Univariate and Multivariate Analyses Including Type II Fiber Proportion Dichotomized into the Occurrence of Fiber Shift
| Parameter | %/Mean (SD)/Median (IQR) | Univariate HR (95% CI) | P Value | Multivariate HR (95% CI) | P Value |
|---|---|---|---|---|---|
| Age, yr | 65.9 (8) | 1.045 (1.018, 1.072) | 0.001 | 1.070 (1.040, 1.101) | <0.0001 |
| Male sex, % | 73 | 1.346 (0.805, 2.252) | 0.257 | — | — |
| FEV1 % predicted | 41.6 (28.0, 61.0) | 0.970 (0.957, 0.982) | <0.0001 | 0.965 (0.951, 0.979) | <0.0001 |
| Fiber shift present | 45 | 2.073 (1.390, 3.091) | 0.001 | 1.598 (1.056, 2.419) | 0.027 |
Definition of abbreviations: CI = confidence interval; HR = hazard ratio; IQR = interquartile range.
Table 2.
Core Characteristics of the Cohort (n = 392) in Addition to Univariate and Multivariate Analyses Including Type II Fiber Proportion as a Continuous Measure
| Parameter | %/Mean (SD)/Median (IQR) | Univariate HR (95% CI) | P Value | Multivariate HR (95% CI) | P Value |
|---|---|---|---|---|---|
| Age, yr | 65.9 (8) | 1.045 (1.018, 1.072) | 0.001 | 1.072 (1.041, 1.103) | <0.0001 |
| Male sex, % | 73 | 1.346 (0.805, 2.252) | 0.257 | — | — |
| FEV1 % predicted | 41.6 (28.0, 61.0) | 0.970 (0.957, 0.982) | <0.0001 | 0.965 (0.951, 0.979) | <0.0001 |
| % Type II fibers | 66.6 (56.0, 75.0) | 1.024 (1.009, 1.039) | 0.002 | 1.014 (0.998, 1.030) | 0.088 |
Definition of abbreviations: CI = confidence interval; HR = hazard ratio; IQR = interquartile range.
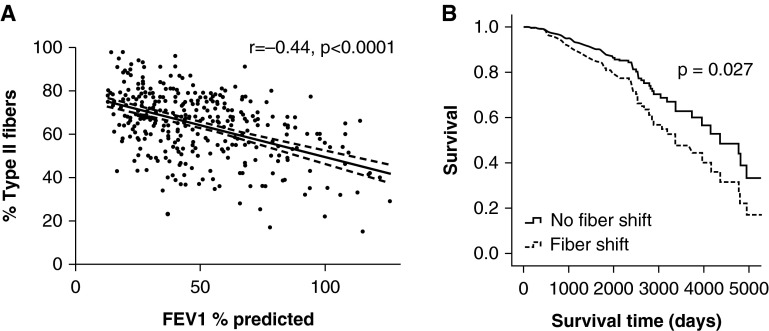
In the cohort considered as a whole, both type II fiber % and the presence of fiber shift were univariate predictors of mortality, as were age and FEV1 % predicted (Tables 1 and 2). In a multivariate analysis including fiber shift as a dichotomous variable, fiber shift was retained, as were age and FEV1 % predicted, Table 1. When age, FEV1 % predicted, and type II fiber % were entered into a multivariate analysis, age and FEV1 % predicted were retained as independent predictors, but the association between fiber type and mortality just missed statistical significance (Table 2). The relationship between FEV1 and fiber proportion is shown in Figure 1A, and survival as a function of fiber shift, adjusted for age and FEV1, is shown in Figure 1B. Additional data regarding other lung function and muscle parameters are presented in Tables E7–E10. FEV1 expressed in liters and TLCOc were also univariate predictors of mortality; however, RV/TLC was not. When including TLCOc in the analysis (n = 209), fiber shift, age, FEV1 % predicted, and TLCOc were all independent predictors of mortality. In other subanalyses, BMI, FFMI, QMVC, QMVC/BMI, and MTCSA were not univariate predictors of mortality.
Figure 1.
(A) The relationship between type II fiber percentage and FEV1 % predicted (dashed lines demonstrate the 95% confidence interval), and (B) survival curves for those with fiber shift (n = 177) and those without fiber shift (n = 215) after adjusting for age and FEV1 % predicted as covariates.
When limiting the analysis to those with an FEV1 greater than or equal to 50% predicted, age was the only predictor of mortality (hazard ratio [HR], 1.16; 95% CI, 1.07, 1.25; P < 0.0001; Table E11). In a multivariate analysis confined to those with an FEV1 less than 50%, fiber shift was retained as an independent predictor (HR, 1.71; 95% CI, 1.08, 2.71; P = 0.02), as were age (HR, 1.06; 95% CI, 1.03, 1.09; P < 0.0001), and FEV1 % predicted (HR, 0.96; 95% CI, 0.94, 0.99; P = 0.002; Table E12A). In a separate analysis confined to those with an FEV1 less than 50%, type II fiber % was not retained as an independent predictor (HR, 1.014; 95% CI, 0.996, 1.032; P = 0.13), whereas age and FEV1 % predicted were (Table E12B).
Fiber shift in the vastus lateralis of patients with COPD was associated with increased mortality, although this association was weaker when lung function and age were included in the analysis. This finding was pronounced in patients with GOLD stage III/IV disease but undetectable in those with GOLD stage I/II disease. The relationship between skeletal muscle atrophy (5) and weakness (6) with mortality has been previously noted in COPD. However, we believe the present analysis is timely because we (3) and others (8) have recently shown that the nature of skeletal muscle involvement in COPD is heterogeneous rather than uniform. No prior study has related quadriceps biopsy appearances to long-term outcome in COPD.
Given the known relationship between exercise capacity and survival (9), these data are consistent with our prior studies, which demonstrated a relationship between fiber shift (although not fiber atrophy) and impaired exercise capacity (3) and functional performance (10). Nevertheless, it remains unclear whether fiber shift causes poor exercise tolerance or is a manifestation of exercise intolerance and reduced physical activity, which are both associated with increased mortality in COPD (9, 11). Both concepts can be supported by in vivo models that demonstrate that muscle disuse results in type I to type II fiber shift (12) and that fiber shift toward a type I fiber predominance increases exercise performance (13). Due to the retrospective nature of the current analysis, exercise performance and physical activity data were not available for inclusion in this report, so a causative role for fiber shift in mortality cannot be demonstrated from this study. A prospective study would have been preferable and could also have considered other factors of relevance, including pulmonary rehabilitation over the intervening period. Despite the limitations of the current study, it is doubtful that a prospective study of comparable size and duration will ever be done.
Interest in pharmacological management of skeletal muscle dysfunction is growing (14), and addressing fiber shift may eventually become a therapeutic possibility. Further studies to address whether the reversal of fiber shift is of benefit are of value.
Acknowledgments
Acknowledgment
The authors thank Gemma Marsh and other members of the lung function and muscle laboratory departments at the Royal Brompton & Harefield Hospitals, Annie Dubé for her technical assistance in the Québec cohort, and the Pulmonary Rehabilitation Units at Evangelismos and Sotiria Hospitals in Athens.
Footnotes
Supported by the National Institute for Health Research Respiratory Biomedical Research Unit at the Royal Brompton & Harefield National Health Service Foundation Trust and Imperial College London, who partly fund M.I.P.’s salary. S.A.N. was supported by the Wellcome Trust.
Author Contributions: M.S.P. drafted the manuscript. M.S.P., S.A.N., G.S., S.P., J.M.-L., G.T., J.G., I.V., and F.M. recruited the patients and collected the data. All authors contributed to the analysis of data and preparation of the final manuscript. M.I.P. conceived the idea and developed it with I.V., J.G., and F.M. M.I.P. is the primary investigator who takes responsibility for the integrity of the work as a whole, from inception to published article.
This letter has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Seymour JM, Spruit MA, Hopkinson NS, Natanek SA, Man WD, Jackson A, Gosker HR, Schols AM, Moxham J, Polkey MI, et al. The prevalence of quadriceps weakness in COPD and the relationship with disease severity. Eur Respir J. 2010;36:81–88. doi: 10.1183/09031936.00104909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shrikrishna D, Patel M, Tanner RJ, Seymour JM, Connolly BA, Puthucheary ZA, Walsh SL, Bloch SA, Sidhu PS, Hart N, et al. Quadriceps wasting and physical inactivity in patients with COPD. Eur Respir J. 2012;40:1115–1122. doi: 10.1183/09031936.00170111. [DOI] [PubMed] [Google Scholar]
- 3.Natanek SA, Gosker HR, Slot IG, Marsh GS, Hopkinson NS, Man WD, Tal-Singer R, Moxham J, Kemp PR, Schols AM, et al. Heterogeneity of quadriceps muscle phenotype in chronic obstructive pulmonary disease (COPD); implications for stratified medicine? Muscle Nerve. 2013;48:488–497. doi: 10.1002/mus.23784. [DOI] [PubMed] [Google Scholar]
- 4.Vogiatzis I, Simoes DC, Stratakos G, Kourepini E, Terzis G, Manta P, Athanasopoulos D, Roussos C, Wagner PD, Zakynthinos S. Effect of pulmonary rehabilitation on muscle remodelling in cachectic patients with COPD. Eur Respir J. 2010;36:301–310. doi: 10.1183/09031936.00112909. [DOI] [PubMed] [Google Scholar]
- 5.Marquis K, Debigaré R, Lacasse Y, LeBlanc P, Jobin J, Carrier G, Maltais F. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:809–813. doi: 10.1164/rccm.2107031. [DOI] [PubMed] [Google Scholar]
- 6.Swallow EB, Reyes D, Hopkinson NS, Man WD, Porcher R, Cetti EJ, Moore AJ, Moxham J, Polkey MI. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax. 2007;62:115–120. doi: 10.1136/thx.2006.062026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel MS, Natanek SA, Shrikrisha D, Gea J, Terzis J, Hopkinson NS, Vogiatzis I, Maltais F, Polkey MI. Vastus lateralis fiber shift is predictive of mortality in COPD [abstract] Am J Respir Crit Care Med. 2014;189:A3646. doi: 10.1164/rccm.201404-0713LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vanfleteren LE, Spruit MA, Groenen M, Gaffron S, van Empel VP, Bruijnzeel PL, Rutten EP, Op ’t Roodt J, Wouters EF, Franssen FM. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:728–735. doi: 10.1164/rccm.201209-1665OC. [DOI] [PubMed] [Google Scholar]
- 9.Oga T, Nishimura K, Tsukino M, Sato S, Hajiro T. Analysis of the factors related to mortality in chronic obstructive pulmonary disease: role of exercise capacity and health status. Am J Respir Crit Care Med. 2003;167:544–549. doi: 10.1164/rccm.200206-583OC. [DOI] [PubMed] [Google Scholar]
- 10.Patel MS, Mohan D, Andersson YM, Baz M, Kon SS, Canavan JL, Jackson SG, Clark AL, Hopkinson NS, Natanek SA, et al. Phenotypic characteristics associated with reduced short physical performance battery score in chronic obstructive pulmonary disease. Chest. 2014;145:1016–1024. doi: 10.1378/chest.13-1398. [DOI] [PubMed] [Google Scholar]
- 11.Waschki B, Kirsten A, Holz O, Müller KC, Meyer T, Watz H, Magnussen H. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest. 2011;140:331–342. doi: 10.1378/chest.10-2521. [DOI] [PubMed] [Google Scholar]
- 12.Guillot C, Steinberg JG, Delliaux S, Kipson N, Jammes Y, Badier M. Physiological, histological and biochemical properties of rat skeletal muscles in response to hindlimb suspension. J Electromyogr Kinesiol. 2008;18:276–283. doi: 10.1016/j.jelekin.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Wang YX, Zhang CL, Yu RT, Cho HK, Nelson MC, Bayuga-Ocampo CR, Ham J, Kang H, Evans RM. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol. 2004;2:e294. doi: 10.1371/journal.pbio.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steiner MC, Roubenoff R, Tal-Singer R, Polkey MI. Prospects for the development of effective pharmacotherapy targeted at the skeletal muscles in chronic obstructive pulmonary disease: a translational review. Thorax. 2012;67:1102–1109. doi: 10.1136/thoraxjnl-2012-201765. [DOI] [PubMed] [Google Scholar]