Asthma is a highly prevalent chronic lung condition affecting upward of 300 million people worldwide (1). An increase in asthma incidence has been observed that is not fully understood but is perhaps attributable to environmental factors such as increased air pollution or allergens, or is potentially related to the obesity epidemic and the accompanying increase in sleep apnea. Nocturnal asthma is characterized by coughing, wheezing, or dyspnea that interrupts and disturbs sleep and serves as an indicator of a more severe and indolent asthma phenotype. Importantly, data have accrued underscoring the role of nocturnal asthma in contributing to increased mortality (2). Moreover, diurnal patterns are intrinsic to asthma pathophysiology, such that most deaths related to asthma symptoms occur during the night (3). Diurnal mechanisms even independent from sleep are likely culprits in nocturnal asthma, given that airway resistance and decrements in peak expiratory flow have been shown to increase throughout the night (4). Sleep-specific physiologic increases in airway parasympathetic tone, reduction in lung volume, and airway smooth muscle unloading are also likely key contributing factors in nocturnal asthma risk in those individuals with underlying predisposition. Despite the scant, albeit illuminating, work performed thus far, there remains a relative dearth of information from both epidemiologic and mechanistic perspectives relative to nocturnal asthma, particularly in terms of race-based susceptibilities. Disentangling the underlying risks and modifying factors of this potentially lethal disorder therefore represents a critical area of investigation.
In this issue of the Journal, Levin and colleagues (pp. 266–273) undertake an examination of the Study for Asthma Phenotypes and Pharmacogenomic Interactions by Race-ethnicity (SAPPHIRE) cohort, a large cohort enriched with African Americans (total n = 5,198; 65% African American), to address existing knowledge gaps by evaluating the risks of nocturnal asthma with a focus on the contribution of race-ethnicity, based on self-identification and also genetic ancestry (5). Compared with European Americans, African Americans were younger and more obese and had younger age at asthma diagnosis, more compromised pulmonary function, increased bronchodilator reversibility, and increased hospitalizations. Even after taking into consideration a host of confounding factors including smoking and obesity, African Americans were approximately 2-fold more likely to report nocturnal asthma symptoms compared with European Americans, based on response to the Asthma Control test question. African Americans also reported more nights with disrupted sleep from asthma symptoms compared with European-American individuals. Furthermore, increasing African ancestry, when examined in the restricted subset of African Americans with genome-wide genotype data, was associated with an increase in nocturnal asthma symptoms. Another interesting finding was that lung function appeared to serve as at least a partial mediator of the relationship of genetic ancestry and nocturnal asthma, with the findings being strongest for FVC mediation of 13%.
These findings contribute to the growing body of knowledge of asthma disparities. It is recognized that African Americans with asthma disproportionately suffer greater morbidity and mortality compared with European Americans in terms of emergency department visits, hospitalizations, and mortality (6). The current work adds to our understanding that African Americans are potentially at greater risk for nocturnal asthma, which is of key importance, given its association with increased morbidity and mortality. These findings, in particular, deserve to be highlighted, given that African Americans have been relatively under-represented in the existing data examining the pathophysiologic bases specific to nocturnal asthma. Although the authors did take into account confounders such as smoking and obesity, the confounding influences of environmental exposures, including allergens and socioeconomic status, were not considered.
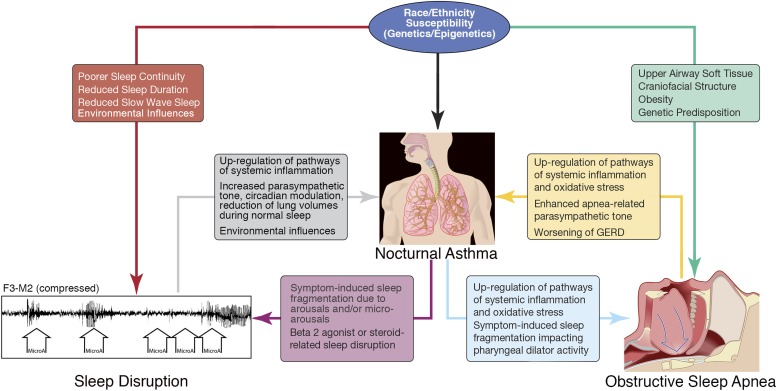
A key facet that cannot be ignored is the potential interrelationship not only with global sleep disruption vulnerabilities but also with obstructive sleep apnea occurring in nocturnal asthma and potentially contributing to predisposition in African Americans (7, 8). The authors attempted to take this into consideration by statistical adjustment, stratification, and sensitivity analyses of body mass index to assess for the direct influence of obesity, as well as indirectly serving as a limited surrogate of obstructive sleep apnea risk. Stratifying on the basis of a body mass index of less than versus more than 25 kg/m2, the association of African American race with nocturnal asthma persisted in the less obese group, as well as when analyses were restricted to normal-weight individuals. The risk burden for obstructive sleep apnea, however, operates through both obesity-dependent and obesity-independent pathways. For example, soft tissue characteristics in terms of volume and structure of the tongue and soft palate are factors that appear to predispose African Americans to obstructive sleep apnea, which would not necessarily be captured by considering only obesity as the representative factor for sleep apnea (9, 10). Genetic predisposition may also play a role in race-based susceptibilities in sleep apnea; for example, among African Americans, the lysophosphatidic acid receptor, a factor with an inherent proinflammatory role, has been identified as a susceptibility locus that was significantly associated with the apnea hypopnea index. Interestingly, these findings were more pronounced in leaner individuals, suggesting independence of obesity (11). Potential synergies may also be present, with observed increased nocturnal cardiac death noted in obstructive sleep apnea and the increased vulnerability of those with nocturnal asthma to increased nighttime death. Figure 1 provides a conceptual diagram outlining multidirectional biologically plausible relationships among race/ethnicity, sleep disruption, obstructive sleep apnea, and nocturnal asthma.
Figure 1.
A conceptual paradigm of the interplay of factors contributing to nocturnal asthma, including race/ethnicity susceptibilities as they relate to sleep disruption and obstructive sleep apnea.
Overall, the findings of this study are compelling in terms of identifying self-reported African American race as a factor associated with nocturnal asthma. Particularly from the standpoint of informing nocturnal asthma risk stratification and public health guidelines, further investigation should focus on the reproducibility of these findings, effectively dissecting the interplay of obesity and obstructive sleep apnea, as well as environmental exposures pertaining to this relationship and gaining a better understanding of autonomic, physiologic, and circadian underpinnings.
Footnotes
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Braman SS. The global burden of asthma. Chest. 2006;130:4S–12S. doi: 10.1378/chest.130.1_suppl.4S. [DOI] [PubMed] [Google Scholar]
- 2.Braido F, Baiardini I, Ghiglione V, Fassio O, Bordo A, Cauglia S, Canonica GW. Sleep disturbances and asthma control: a real life study. Asian Pac J Allergy Immunol. 2009;27:27–33. [PubMed] [Google Scholar]
- 3.Tough SC, Hessel PA, Ruff M, Green FH, Mitchell I, Butt JC. Features that distinguish those who die from asthma from community controls with asthma. J Asthma. 1998;35:657–665. doi: 10.3109/02770909809048968. [DOI] [PubMed] [Google Scholar]
- 4.Spengler CM, Shea SA. Endogenous circadian rhythm of pulmonary function in healthy humans. Am J Respir Crit Care Med. 2000;162:1038–1046. doi: 10.1164/ajrccm.162.3.9911107. [DOI] [PubMed] [Google Scholar]
- 5.Levin AM, Wang Y, Wells KE, Padhukasahasram B, Yang JJ, Burchard EG, Williams LK.Nocturnal asthma and the importance of race/ethnicity and genetic ancestry Am J Respir Crit Care Med 2014190266–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akinbami LJ, Moorman JE, Liu X.Asthma prevalence, health care use, and mortality: United States, 2005–2009. National health statistics reports; no 32. Hyattsville, MD: National Center for Health Statistics; 2011 [accessed 2014 Jun 12]. Available from: http://www.cdc.gov/nchs/data/nhsr/nhsr032.pdf [PubMed]
- 7.Nunes J, Jean-Louis G, Zizi F, Casimir GJ, von Gizycki H, Brown CD, McFarlane SI. Sleep duration among black and white Americans: results of the National Health Interview Survey. J Natl Med Assoc. 2008;100:317–322. doi: 10.1016/s0027-9684(15)31244-x. [DOI] [PubMed] [Google Scholar]
- 8.Ruiter ME, Decoster J, Jacobs L, Lichstein KL. Normal sleep in African-Americans and Caucasian-Americans: a meta-analysis. Sleep Med. 2011;12:209–214. doi: 10.1016/j.sleep.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Cakirer B, Hans MG, Graham G, Aylor J, Tishler PV, Redline S. The relationship between craniofacial morphology and obstructive sleep apnea in whites and in African-Americans. Am J Respir Crit Care Med. 2001;163:947–950. doi: 10.1164/ajrccm.163.4.2005136. [DOI] [PubMed] [Google Scholar]
- 10.Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spry K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med. 1997;155:186–192. doi: 10.1164/ajrccm.155.1.9001310. [DOI] [PubMed] [Google Scholar]
- 11.Patel SR, Goodloe R, De G, Kowgier M, Weng J, Buxbaum SG, Cade B, Fulop T, Gharib SA, Gottlieb DJ, et al. Association of genetic loci with sleep apnea in European Americans and African-Americans: the Candidate Gene Association Resource (CARe) PLoS ONE. 2012;7:e48836. doi: 10.1371/journal.pone.0048836. [DOI] [PMC free article] [PubMed] [Google Scholar]