Abstract
Purpose
The natural history of primary bladder carcinoma in situ (CIS) has not been well described. We describe patterns of disease recurrence and progression and identify clinical-outcome predictors for primary CIS after BCG therapy.
Materials and Methods
A retrospective analysis of 155 patients diagnosed with isolated primary high-grade CIS in a tertiary center from 1990 to 2008 who underwent transurethral resection followed by intravesical BCG therapy. The endpoints included time to disease recurrence, time to progression to invasive disease (≥cT1) or to muscle-invasive disease (≥cT2), or early radical cystectomy (RC). Predictors included gender, age, race, smoking history, presenting symptoms, CIS pattern (focal, multiple or diffuse), and response to BCG.
Results
In total, 155 patients received BCG therapy within 6 mo. Five-yr cumulative incidence of progression to ≥cT1 was 45% (95% CI, 37%–55%), to ≥cT2 was 17% (95% CI, 12%–25%), adjusting for the competing risk of RC. Of 130 patients evaluated for response to BCG, 81 (62%) were considered responders. Response to BCG was significantly associated with progression to ≥cT1/RC (HR: 0.59; 95% CI, 0.36–0.95; p=0.029) and to ≥cT2/RC (HR: 0.53; 95% CI, 0.32–0.88; p=0.015). This association was largely driven by the higher rate of early RC among non-responders.
Conclusions
Despite BCG therapy and early RC, patients with primary CIS had a high rate of disease progression. Response to BCG was significantly associated with a lower rate of disease progression or early RC.
Keywords: Bladder Cancer, Carcinoma in Situ, BCG
1. Introduction
Carcinoma in situ (CIS) of the bladder has highly variable clinical behaviour.1, 2 Recent studies have suggested differences in biological mechanism of disease between primary CIS (isolated CIS with no prior or concomitant papillary tumors), secondary CIS (diagnosed after a prior papillary tumor), and concomitant CIS (simultaneous diagnosis of CIS and papillary tumor).1, 3, 4
Our understanding of the natural history of primary CIS has been limited by its rarity and the lack of adoption of a uniform definition, leading to studies of limited size and heterogenous case mix (primary CIS mixed with secondary or concomitant CIS and other non–muscle-invasive tumors).1, 5–7 Consequently, it has not yet been clearly shown that these definitions are associated to oncologic clinical outcomes, and no evidence has been shown in the literature to confirm its applicability in clinic. To fill part of this gap, we sought to describe the natural history of primary CIS and to identify predictors of outcomes to allow improved clinical decision-making. Towards this end, we evaluated response to bacillus Calmette-Guerin (BCG) therapy, time to recurrence, and time to progression in a large cohort of patients treated for primary CIS receiving therapy at a tertiary cancer center.
2. Materials and Methods
2. 1 Patients
We performed a retrospective review of the Memorial Sloan-Kettering Cancer Center (MSKCC) database to identify patients with primary CIS of the bladder, as approved by the institutional review board. The original diagnosis of CIS was based on urine cytology, cystoscopy with biopsy or transurethral resection (TUR), bimanual examination, as well as pathologic evaluation by a dedicated genito-urinary pathologist at MSKCC. We excluded patients whose pathology slides had been unavailable for review. Patients were followed every 3 months with urine cytology and cystoscopy. Random biopsies and repeat TURs were performed in all suspicious cases. BCG therapy consisted of an induction course of 6 weekly intravesical instillations and 1 reinduction as necessary.
This yielded a cohort of 281 patients diagnosed between 1990 and 2008 with primary bladder CIS, defined as an isolated high-grade cTis on the first TUR without prior or concomitant papillary tumor. Our primary aim was to describe patterns of recurrence in CIS patients after receiving BCG therapy. Therefore, patients who were not followed (n=2), did not receive BCG therapy (n=57), were missing the date they received BCG therapy (n=9), received therapy more than 6 months after their initial TUR (n=28) or progressed before receiving BCG therapy (n=30) were excluded, leaving 155 patients available for analysis.
We used the TNM system of the International Union Against Cancer and the World Health Organization/International Society of Urological Pathology 1998 grading system.8 The medical records were reviewed for clinical information related to patient characteristics and predictors of outcome. Surgery and pathology reports were used to define the CIS pattern. We classified as focal when only one biopsy core was positive, multiple when two or three cores from distinct areas of the bladder were positive, and diffuse when more than three from distinct areas were positive.
2.2 Objectives and Statistical Methods
Our primary aim was to describe the patterns of progression of patients with primary CIS who received BCG therapy and to identify predictors of progression to both ≥cT1 and ≥cT2. We were interested in analyzing two separate endpoints: progression to invasive bladder cancer, defined as cT1 or higher (≥cT1) and progression to muscle-invasive disease, defined as cT2 or higher (≥cT2). As early radical cystectomy (RC) is an unfavorable outcome, we considered the earlier of either event (RC or progression) as a single endpoint in this analysis. We created separate univariate Cox regression models for the two endpoints (≥cT1/RC and ≥cT2/RC). We used as predictors: age, gender, race (white non-Hispanic vs. all others), smoking history (none, former, or current), presenting symptoms (asymptomatic/micro-hematuria, gross hematuria, voiding symptoms [irritative or obstructive], or unknown), CIS pattern (focal, multiple, or diffuse), or BCG response. We defined as either responders or non-responders to BCG patients who had no evidence of disease within 6 mo of BCG therapy or presented disease recurrence in this initial period, respectively. In order to analyze response to BCG therapy as a predictor of progression, we conducted a landmark time analysis. The clock was started at 6 mo after BCG therapy and patients were excluded if they had progressed before the 6-month point or had not been followed for at least 6 mo following BCG therapy.
As some patients had undergone RC before progression to either cT1 or cT2, we illustrated risk of progression using the cumulative incidence function, which is used to calculate the risk of an event (progression to pT1 or progression to pT2) in the presence of a competing risk (radical cystectomy).
For our second aim, we used univariate logistic regression to determine whether any of the predictors used in the above analyses predicted whether a patient would respond to BCG therapy. Our third aim was to describe the patterns of recurrence in BCG responders and to identify predictors of recurrence in these patients. We used the same predictors as those used in the analyses of progression, with the exception of BCG response. All analyses were conducted using Stata 10.1 (Stata Corp., College Station, TX) and R (R Foundation for Statistical Computing, http://www.R-project.org) with the cmprsk package.
3. Results
In total, 155 patients received BCG therapy within 6 mo after an initial TUR. The majority of patients were male (85%; n=132) and white (95%; n=147) and were classified as either former (59%; n=91) or current (7%; n=11) smokers. The most common presenting symptoms were gross hematuria (34%; n=52) and irritative or obstructive voiding symptoms (30%; n=46). At initial TUR, 79 patients (51%) had multiple or diffuse CIS pattern. Patient characteristics, stratified by BCG response status are shown in Table 1. We found no evidence that age, gender, race, smoking history, or CIS pattern were significantly associated with response to BCG therapy.
Table 1.
Characteristics of 155 patients who received BCG within 6 mo of TUR. All values are median (IQR) or frequency (proportion). P value is for the difference between responders and non-responders.
| All patients N=155 |
Responders* | Non-Responders* | ||
|---|---|---|---|---|
|
| ||||
| N=81 | N=49 | P valueэ | ||
| Age at first TUR (yr) | 69 (62, 74) | 65 (61, 72) | 72 (65, 77) | 0.096 |
| Male | 132 (85%) | 70 (86%) | 41 (84%) | 0.7 |
| White (non-Hispanic) | 147 (95%) | 76 (94%) | 46 (94%) | 1 |
| Smoking history | 0.6 | |||
| None | 44 (28%) | 23 (28%) | 14 (29%) | |
| Current | 11 (7%) | 7 (9%) | 2 (4%) | |
| Former | 91 (59%) | 47 (58%) | 29 (59%) | |
| Missing | 9 (6%) | 4 (5%) | 4 (8%) | |
| Presenting symptoms | 0.5 | |||
| Asymptomatic, micro hematuria | 36 (23%) | 22 (27%) | 10 (20%) | |
| Gross hematuria | 52 (34%) | 25 (31%) | 19 (39%) | |
| Voiding symptoms (irritative or obstructive) | 46 (30%) | 24 (30%) | 11 (22%) | |
| Unknown | 21 (14%) | 10 (12%) | 9 (18%) | |
| CIS pattern | 0.5 | |||
| Focal | 41 (26%) | 22 (27%) | 14 (29%) | |
| Multiple | 65 (42%) | 39 (48%) | 15 (31%) | |
| Diffuse | 14 (9%) | 6 (7%) | 3 (6%) | |
| Missing | 35 (23%) | 14 (17%) | 17 (35%) | |
25 patients who progressed within 6 mo of BCG or were not followed for 6 mo were excluded.
P value obtained from univariate logistic models where BCG response was the outcome
Sixty-nine patients experienced bladder cancer progression: 47 progressed to cT1 and 27 to cT2 (5 patients who progressed to cT1 subsequently progressed to cT2). Of the remaining 86 patients, 19 underwent RC without progression of disease. The median follow up was 3.3 years and 4.0 years for those who did not progress to ≥cT1/RC or ≥cT2/RC, respectively. Overall, 57 patients recurred as cTa, of which 21 patients had two or more repeat diagnosis during follow-up as either low or high grade. Twelve patients had only low grade cTa recurrence, 37 had high grade cTa, and 8 recurred as both. Twenty patients developed distant metastasis and 18 had upper tract urothelial carcinoma.
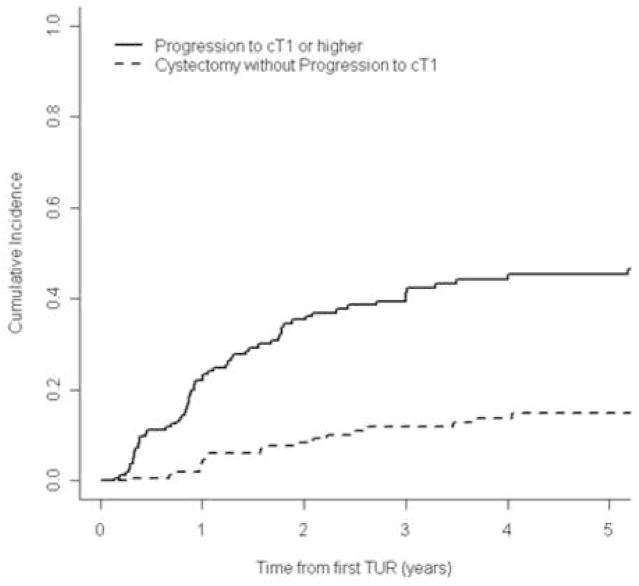
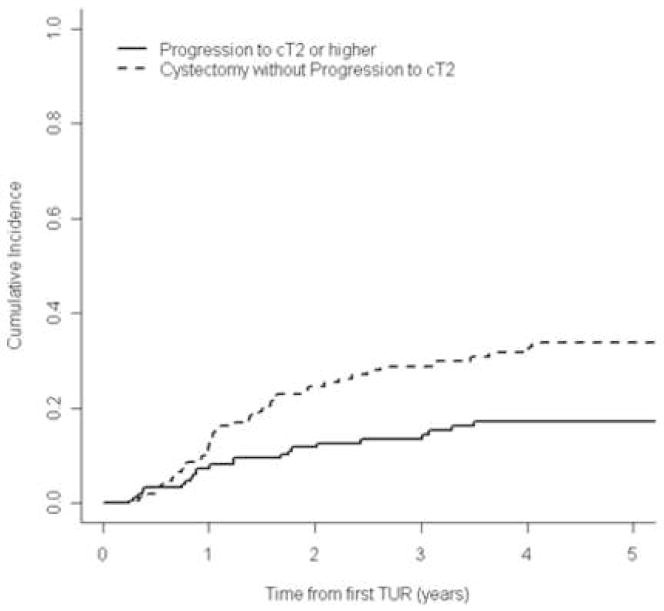
Figures 1 and 2 show the cumulative incidence of progression to invasive disease or muscle-invasive disease, respectively, accounting for the event of RC before progression. The 5-yr cumulative incidence of progression to invasive disease (≥cT1) was 45% (95% CI, 37%–55%); and to muscle-invasive disease (≥cT2) was 17% (95% CI, 12%–25%). Figure 2 shows that a large proportion of patients (approximately 35% by 5 yr) underwent RC before progression to muscle-invasive disease could occur. Therefore, without the early RC, the 5-yr cumulative incidence of progression to cT2 could have been as high as 50%.
Figure 1.
Cumulative incidence of progression to invasive disease (≥cT1) or early radical cystectomy for all patients after BCG therapy (n=155).
Figure 2.
Cumulative incidence of progression to muscle-invasive disease (≥cT2) or early radical cystectomy for all patients after BCG therapy (n=155).
We found no evidence that age, gender, race, smoking history, or CIS pattern were associated with progression to ≥cT1/RC or to ≥cT2/RC in all patients (Table 2). Initial presenting symptoms showed a trend towards being associated with progression to ≥cT1/RC (global p=0.067), but not to ≥cT2/RC (p=0.19). When analyzing the timing of BCG administration after TUR, a variable indicating whether BCG was received before or after 4 weeks was not significantly associated with either outcome (p=0.6 for both endpoints).
Table 2.
Univariate models for progression to invasive disease/radical cystectomy or to muscle-invasive disease/radical cystectomy in 155 patients (unless otherwise indicated)
| Progression to invasive disease (≥cT1) or radical cystectomy | Progression to muscle- invasive disease (≥cT2) or radical cystectomy | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Male | 0.654 | 0.379, 1.13 | 0.13 | 0.698 | 0.389, 1.25 | 0.2 |
| Age (per 10-yr increase) | 1.02 | 0.827, 1.27 | 0.8 | 1.04 | 0.828, 1.31 | 0.7 |
| Race (white non-Hispanic vs. all others) | 0.810 | 0.328, 2.00 | 0.6 | 0.739 | 0.298, 1.83 | 0.5 |
| Smoking history (n=146) | 0.9 | 0.5 | ||||
| None | Ref | Ref | Ref | Ref | ||
| Current | 0.860 | 0.351, 2.11 | 0.536 | 0.183, 1.57 | ||
| Former | 1.02 | 0.629, 1.66 | 1.02 | 0.608, 1.71 | ||
| Presenting symptoms | 0.067 | 0.19 | ||||
| Asymptomatic, micro-hematuria | Ref | Ref | Ref | Ref | ||
| Gross hematuria | 1.83 | 0.988, 3.38 | 1.89 | 0.986, 3.63 | ||
| Voiding symptoms (irritative or obstructive) | 2.29 | 1.21, 4.32 | 1.94 | 0.975, 3.87 | ||
| Unknown | 1.40 | 0.668, 2.91 | 1.36 | 0.621, 2.97 | ||
| CIS pattern (n=120) | 0.4 | 0.9 | ||||
| Focal | Ref | Ref | Ref | Ref | ||
| Multiple | 1.05 | 0.626, 1.78 | 1.03 | 0.598, 1.79 | ||
| Diffuse | 1.69 | 0.779, 3.66 | 1.25 | 0.527, 2.94 | ||
| BCG responder (n=130)* | 0.587 | 0.363, 0.947 | 0.029 | 0.532 | 0.320, 0.884 | 0.015 |
25 patients who progressed within 6 mo of BCG therapy or were not followed for 6 mo were excluded.
We also looked at response to BCG therapy as a predictor of subsequent progression. For these analyses, patients were excluded if they were not followed for at least 6 months following BCG therapy (n=7) or had progressed before 6 mo (n=18) and the follow up was started at 6 months after BCG therapy. Of the 130 patients evaluable for response to BCG therapy, 81 (62%) were responders (no recurrence or progression within 6 mo of BCG) and 49 (38%) were non-responders. BCG responders were significantly less likely to progress to to ≥cT1/RC (HR: 0.59; 95% CI, 0.36–0.95; p=0.029) or to ≥cT2/RC (HR: 0.53; 95% CI, 0.32–0.89; p=0.015).
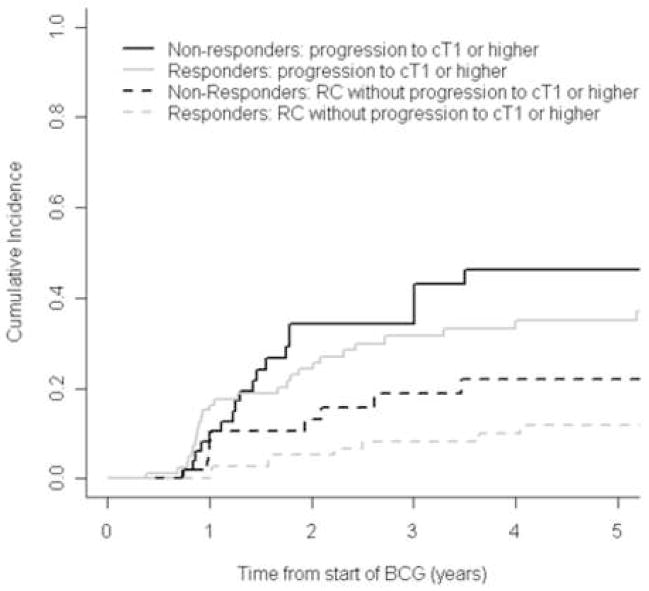
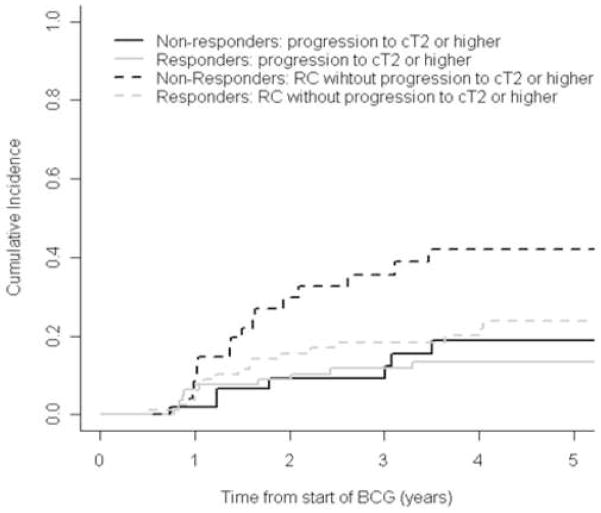
Figures 3 and 4 show the cumulative incidence of progression to invasive disease or muscle-invasive disease, separately for patients classified as BCG responders or non-responders. Progression to cT1 or higher was plotted in figure 3, demonstrating that BCG responders had a slightly lower cumulative incidence of progression to invasive disease than non-responders: 3-yr cumulative incidence was 31.5% (95% CI: 22.5%, 44.1%) and 34.2% (95% CI: 22.4%, 52.1%), respectively. Figure 4 shows the evident higher incidence of RC in BCG non-responders, when comparing to progression to muscle-invasive disease in either BCG responders and non-responders. Also, we found a similar cumulative incidence of progression to muscle-invasive disease between responders (3-yr cumulative incidence was 9.2% [95% CI, 3.5%–23.9%]) and non-responders (11.8% [95% CI, 6.3%–21.9%]). Nonetheless, these findings appear to be driven by the higher rate of RC in BCG non-responders after progression to invasive disease.
Figure 3.
Cumulative incidence of progression to invasive disease (≥cT1) or early radical cystectomy (RC) in BCG responders (grey lines) or non-responder (black lines).
Figure 4.
Cumulative incidence of progression to muscle-invasive disease (≥cT2) or early radical cystectomy (RC) in BCG responders (grey lines) or non-responder (black lines).
Among the 81 patients who responded to BCG, 59 experienced a recurrence or progression during follow-up. In this group, the 5-yr cumulative incidence of recurrence was 88% (95% CI, 80%–97%). No patients progressed to cT2 before recurring. We found no evidence that age, gender, race, smoking history, initial presenting symptoms, or CIS pattern were associated with recurrence of disease in BCG responders (Table 3).
Table 3.
Univariate models for recurrence/radical cystectomy in BCG responders only (n=81 unless otherwise indicated)
| HR | 95% CI | P value | |
|---|---|---|---|
| Male | 0.928 | 0.437, 1.97 | 0.8 |
| Age (per 10-yr increase) | 0.961 | 0.736, 1.25 | 0.8 |
| Race (white non-Hispanic vs. all others) | 0.629 | 0.227, 1.75 | 0.4 |
| Smoking history (n=77) | 0.9 | ||
| None | Ref | Ref | |
| Current | 1.22 | 0.463, 3.21 | |
| Former | 1.16 | 0.621, 2.18 | |
| Presenting symptoms | 0.3 | ||
| Asymptomatic, micro- hematuria | Ref | Ref | |
| Gross hematuria | 1.74 | 0.868, 3.50 | |
| Voiding symptoms (irritative or obstructive) | 1.25 | 0.593, 2.65 | |
| Unknown | 0.953 | 0.367, 2.48 | |
| CIS pattern (n=67) | 0.16 | ||
| Focal | Ref | Ref | |
| Multiple | 0.971 | 0.504, 1.87 | |
| Diffuse | 2.51 | 0.885, 7.12 |
Discussion
The natural history of primary CIS is unclear and its management remains a challenge. The small size and the case mix of previous studies have limited our understanding.3, 8 In the present study, we describe the clinical course of primary CIS at a high-volume cancer center.
Results from several previous studies have identified the presence of CIS (primary, secondary, or concomitant) as a poor prognostic factor, as it increases both the risk of progression to muscle-invasive disease and of death from bladder cancer.1, 9–11 However, very few authors have aimed at identifying differences between the clinical impact of primary, secondary, and concomitant CIS, separately.6 Studies failed to analyze primary CIS patients as a distinct entity either by mixing clinical stages (CIS, cTa, and cT1 in the same group), by combining primary and secondary CIS, or by analyzing small cohorts of patients.1
Our findings showed a high rate of CIS progression directly to muscle-invasive bladder cancer without the intermediate development of cT1 disease. Although the high rate of early RC for cT1 may have reduced the number who would have subsequently progressed from cT1 to cT2, it also enhances the assumption that the total progression to cT2 could have been significantly higher in the absence of early RC. These findings suggest that a more frequent surveillance protocol or combined diagnostic tools may be required for primary CIS.
This aggressive behavior may be due to the mechanism of primary CIS invasion. Historically, Farrow et al. studied 69 patients with primary CIS in a pre-BCG era, with results suggesting two pathogenic forms of bladder cancer, although follow-up was reported only in 12 patients.12 Subsequently, Utz and Farrow classified the pattern of CIS invasion into papillary or flat sessile.13, 14 The flat CIS may present a higher risk of developing a microinvasion pattern, since a timely diagnosis will occur less often, and this allows progression to muscle invasion when a conservative treatment is chosen.12, 15 These routes may even coexist, but the current lack of understanding of the mechanism of CIS invasion, due partly to the lack of attention given to CIS as an independent entity, may explain the unexpectedly high percentage of direct progression to muscle-invasive disease found in our study.
We demonstrated that BCG response within 6 mo was a predictor of progression or radical cystectomy in patients with primary CIS. Previously, Herr and Dalbagni showed that response to BCG therapy after 6 mo significantly predicted recurrence during a 24-mo follow-up in a cohort of patients with non–muscle-invasive bladder cancer.16 In our study, the association was driven largely by the higher rate of RC in patients who had failed BCG therapy; however, in the absence of RC, it is possible that rates of progression would be higher in these patients.
We found that a high proportion of primary CIS patients eventually failed BCG therapy despite initially responding to treatment. Even among BCG responders, there was a high rate of recurrence and progression. By five years, almost half of all the patients had progressed to invasive disease and nearly one-fifth to muscle-invasive bladder cancer. Moreover, the high rate of RC in patients without overt progression in this series likely lowered the estimated progression rate. Early RC has been shown to play a significant role in the management of high-risk non–muscle-invasive disease, giving support to the frequent use of early surgical treatment seen in our series.17, 18 Nevertheless, no study has specifically evaluated this management strategy in a homogenous primary CIS cohort after BCG therapy.
Studies that evaluated the impact of age on clinical outcomes in non–muscle-invasive bladder cancer have suggested a higher response to BCG therapy in the younger population.19 In our study, responders to BCG therapy were younger than non-responders; however, this difference was not statistically significant.
There are several important limitations to our study. The study was retrospective and included patients treated over a 19-year period. During this time, a shift towards earlier RC may have influenced our results. Also, patients treated at our tertiary hospital may differ from bladder cancer patients treated at community centers.
Conclusions
Primary CIS of the bladder is a potentially lethal disease that requires aggressive management. A large number of patients with primary CIS will experience disease progression within a short period of time. The aggressiveness of this disease entity is further supported by its frequent direct progression to muscle-invasive disease. Patients who fail BCG therapy are more likely to undergo early RC, as this treatment is thought to abrogate the natural progression of the disease. Further studies are necessary to devise the optimal evidence-based management of primary CIS and to describe the different biological and clinical behavior of other distinct entities of CIS (secondary and concomitant CIS).
Acknowledgments
Supported by: The Sidney Kimmel Center for Prostate and Urologic Cancers. Dr. Chade is a research fellow in urologic oncology supported by CAPES (pós-doutorado). Dr. Shariat is a research fellow in urologic oncology supported by NIH T32-CA82088.
Key of Abbreviations
- CIS
Carcinoma in Situ
- BCG
bacillus Calmette-Guerin
- TUR
transurethral resection
- RC
radical cystectomy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sylvester RJ, van der Meijden A, Witjes JA, et al. High-grade Ta urothelial carcinoma and carcinoma in situ of the bladder. Urology. 2005;66:90. doi: 10.1016/j.urology.2005.06.135. [DOI] [PubMed] [Google Scholar]
- 2.van Rhijn BW, Burger M, Lotan Y, et al. Recurrence and Progression of Disease in Non-Muscle-Invasive Bladder Cancer: From Epidemiology to Treatment Strategy. Eur Urol. 2009 doi: 10.1016/j.eururo.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 3.Lamm D, Herr H, Jakse G, et al. Updated concepts and treatment of carcinoma in situ. Urol Oncol. 1998;4:130. doi: 10.1016/s1078-1439(99)00020-4. [DOI] [PubMed] [Google Scholar]
- 4.Nese N, Gupta R, Bui MH, et al. Carcinoma in situ of the urinary bladder: review of clinicopathologic characteristics with an emphasis on aspects related to molecular diagnostic techniques and prognosis. J Natl Compr Canc Netw. 2009;7:48. doi: 10.6004/jnccn.2009.0004. [DOI] [PubMed] [Google Scholar]
- 5.Andius P, Damm O, Holmang S. Prognostic factors in patients with carcinoma in situ treated with intravesical bacille Calmette-Guerin. Scand J Urol Nephrol. 2004;38:285. doi: 10.1080/00365590410028692. [DOI] [PubMed] [Google Scholar]
- 6.Gofrit ON, Pode D, Pizov G, et al. The natural history of bladder carcinoma in situ after initial response to bacillus Calmette-Guerin immunotherapy. Urol Oncol. 2009;27:258. doi: 10.1016/j.urolonc.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Sylvester RJ. Natural history, recurrence, and progression in superficial bladder cancer. Scientific World Journal. 2006;6:2617. doi: 10.1100/tsw.2006.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall MC, Chang SS, Dalbagni G, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol. 2007;178:2314. doi: 10.1016/j.juro.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Althausen AF, Prout GR, Jr, Daly JJ. Non-invasive papillary carcinoma of the bladder associated with carcinoma in situ. J Urol. 1976;116:575. doi: 10.1016/s0022-5347(17)58916-8. [DOI] [PubMed] [Google Scholar]
- 10.Lamm DL. Carcinoma in situ. Urol Clin North Am. 1992;19:499. [PubMed] [Google Scholar]
- 11.Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49:466. doi: 10.1016/j.eururo.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 12.Farrow GM, Utz DC, Rife CC, et al. Clinical observations on sixty-nine cases of in situ carcinoma of the urinary bladder. Cancer Res. 1977;37:2794. [PubMed] [Google Scholar]
- 13.Utz DC, Farrow GM. Carcinoma in situ of the urinary tract. Urol Clin North Am. 1984;11:735. [PubMed] [Google Scholar]
- 14.Utz DC, Farrow GM, Rife CC, et al. Carcinoma in situ of the bladder. Cancer. 1980;45:1842. [PubMed] [Google Scholar]
- 15.Farrow GM, Utz DC, Rife CC. Morphological and clinical observations of patients with early bladder cancer treated with total cystectomy. Cancer Res. 1976;36:2495. [PubMed] [Google Scholar]
- 16.Herr HW, Dalbagni G. Defining bacillus Calmette-Guerin refractory superficial bladder tumors. J Urol. 2003;169:1706. doi: 10.1097/01.ju.0000062605.92268.c6. [DOI] [PubMed] [Google Scholar]
- 17.Denzinger S, Fritsche HM, Otto W, et al. Early versus deferred cystectomy for initial high-risk pT1G3 urothelial carcinoma of the bladder: do risk factors define feasibility of bladder-sparing approach? Eur Urol. 2008;53:146. doi: 10.1016/j.eururo.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 18.Herr HW, Sogani PC. Does early cystectomy improve the survival of patients with high risk superficial bladder tumors? J Urol. 2001;166:1296. [PubMed] [Google Scholar]
- 19.Herr HW. Age and outcome of superficial bladder cancer treated with bacille Calmette-Guerin therapy. Urology. 2007;70:65. doi: 10.1016/j.urology.2007.03.024. [DOI] [PubMed] [Google Scholar]