Abstract
Rationale: Respiratory syncytial virus (RSV) and Streptococcus pneumoniae are major respiratory pathogens. Coinfection with RSV and S. pneumoniae is associated with severe and often fatal pneumonia but the molecular basis for this remains unclear.
Objectives: To determine if interaction between RSV and pneumococci enhances pneumococcal virulence.
Methods: We used confocal microscopy and Western blot to identify the receptors involved in direct binding of RSV and pneumococci, the effects of which were studied in both in vivo and in vitro models of infection. Human ciliated respiratory epithelial cell cultures were infected with RSV for 72 hours and then challenged with pneumococci. Pneumococci were collected after 2 hours exposure and changes in gene expression determined using quantitative real-time polymerase chain reaction.
Measurements and Main Results: Following incubation with RSV or purified G protein, pneumococci demonstrated a significant increase in the inflammatory response and bacterial adherence to human ciliated epithelial cultures and markedly increased virulence in a pneumonia model in mice. This was associated with extensive changes in the pneumococcal transcriptome and significant up-regulation in the expression of key pneumococcal virulence genes, including the gene for the pneumococcal toxin, pneumolysin. We show that mechanistically this is caused by RSV G glycoprotein binding penicillin binding protein 1a.
Conclusions: The direct interaction between a respiratory virus protein and the pneumococcus resulting in increased bacterial virulence and worsening disease outcome is a new paradigm in respiratory infection.
Keywords: respiratory syncytial virus, pneumococcus, cilia, virulence, G protein
At a Glance Commentary
Scientific Knowledge on the Subject
Respiratory syncytial virus infection is known to increase pneumococcal infection but it is unclear how this occurs.
What This Study Adds to the Field
Here we show the mechanism of direct interaction between respiratory syncytial virus and the pneumococci, via specific proteins on each organism. The result of the binding together is that the genes for pneumococcal virulence are up-regulated.
Respiratory syncytial virus (RSV) and Streptococcus pneumoniae, also known as the pneumococcus, are major respiratory pathogens causing a huge global healthcare burden, predominantly in young children and the elderly (1). Global RSV disease burden is estimated at 64 million cases and 160,000 deaths every year (2). Pneumococcal pneumonia results in more than 1 million deaths each year worldwide with approximately half a million in children younger than 5 years (3). There is increasing evidence that RSV and the pneumococcus interact to increase the severity of respiratory disease (4–6). Seasonal increases in infections with RSV in young children are strongly associated with subsequent increased hospital admissions with invasive pneumococcal disease (7–9). Studies in mice also show a significant increase in pneumococcal sepsis and decreased clearance of pneumococci from the lung of mice previously infected with RSV (10). With increasing evidence of interplay between the bacterial and viral pathogens it is important to understand the molecular mechanisms involved to develop interventions.
Here we present a new paradigm that RSV markedly increases the virulence of the pneumococcus by directly binding to a pneumococcal cell surface protein and increasing pneumococcal virulence gene expression.
Methods
Virus and Bacterial Growth Conditions
RSV subgroup A strain A2 (originally from R. Channock, National Institutes of Health, Bethesda, MD) was prepared in BSC-1 cells (epithelial cells of African green monkey kidney origin) (multiplicity of infection, 0.01 pfu/cell) for 7–10 days in antibiotic-free Glasgow modified essential medium with nonessential amino acids supplemented with 2% fetal calf serum. Infected cells were disrupted with glass beads and centrifuged at 1,000 × g for 5 minutes. Uninfected BSC-1 cell lysate was collected using the same method. The supernatants were then purified by centrifugation through a polyethersulphone membrane as previously described (11). The virus fractions (5 × 105 pfu/ml) were collected in bronchial epithelial basal medium.
S. pneumoniae strain D39 (NCTC 7466) was chosen because it is a well-characterized strain. We have previously shown it to be virulent in in vivo (12) and ex vivo studies with ciliated epithelia (13, 14). The D39 pneumolysin-deficient mutant (∆PLY) and wild-type strains were propagated in tryptic soy broth medium as described previously (15).
Human Airway Epithelial Cell Culture
Human nasal ciliated epithelium was obtained from nine healthy control subjects who had no history of respiratory disease. None of the subjects were taking medications or had a symptomatic upper respiratory tract infection in the preceding 6 weeks. All individuals gave their consent to use their cells and local ethical approval was obtained from the University of Leicester Committee for Research Ethics. Respiratory ciliated cells were cultured from these samples as previously described (16). Briefly, the brush biopsy cells were grown in basal epithelial growth media at 37°C. Basal cells were seeded onto collagen-coated Transwell inserts (Corning) and cultured at air–liquid interface using differentiation medium (50:50 BEBM and Dulbecco’s modified Eagle medium) and 10 nM trans-retinoic acid.
Infection of Airway Epithelial Cell Cultures
Cell cultures were rinsed with antibiotic-free medium. RSV (multiplicity of infection, 0.01 pfu/cell) in 400 μl was applied to the apical surface for 1 hour at 37°C and then removed. Mock-infected cells received uninfected BSC-1 cell lysate as a control. The infection was continued for 72 hours. Cells were then incubated with 107 cfu S. pneumoniae in 200 μl BEBM for 2 hours at 37°C. Changes in ciliary beat frequency and pattern were recorded using high-speed digital video microscopy (17, 18). After 2 hours the supernatants were harvested and the bacteria recovered by centrifugation. Bacteria were resuspended in RNAlater (Sigma) and stored at −80°C. The concentration of airway inflammation markers in the airway surface liquid was measured as before (19–21) using a 96-well multispot assay (Meso Scale Discovery, MD) according to the manufacturer’s instructions. Cytokines were measured using a human Th1/Th2 standard 10-spot plate and human chemokines were measured using a high-band MS6000 10-spot plate, using SECTOR Imager 6000 (MSD, MD).
Direct Interaction Assay
S. pneumoniae (108 cfu/ml in phosphate-buffered saline [PBS]) was resuspended in 100 μl of purified RSV strain A2 (diluted 1:10 in PBS), or UV-inactivated RSV A2 (diluted 1:10 in PBS). UV inactivation removed all detectable infectivity, determined by plaque assay. Alternatively, the bacteria were incubated with 2 μg/ml RSV secreted G protein from a subgroup B strain 1734 expressed by baculovirus-insect cell cultures (SinoBiological, Beijing, China) or 2 μg/ml bovine serum albumin (BSA; Sigma) in 100 μl BEBM for 30 minutes or 2 hours (for animal studies) at 37°C on a rotating rack. Bacteria were washed in PBS.
Immunofluorescence Microscopy
Bacteria were incubated with 3% BSA in PBS for 10 minutes and washed three times with PBS. All subsequent antibody incubations were performed in PBS containing 1% BSA. Reagents used for immunofluorescence in this study were rabbit antipneumococcal capsular polysaccharide (16745; 40 μg/ml; Statens Serum Institut, Copenhagen, Denmark) and goat anti-RSV conjugated to fluorescein isothiocyanate (ab20391; 40 μg/ml; Abcam, Cambridge, UK). All antibody incubations were performed for 2 hours, followed by three washes with PBS. Bound pneumococcal polysaccharide primary antibodies were detected using AlexaFluor 594 conjugated donkey antirabbit antibody (1:250; Invitrogen, Paisley, UK). Following three final washes in PBS, bacteria were cytospun at 2,000 × g for 5 minutes, air dried on a clean microscope slide, and mounted under coverslips in 80% glycerol, 3% n-propylgallate (in PBS) mounting medium. Optical sections were obtained using a Nikon C1Si confocal microscope. Images were rendered by Imaris Software (Bitplane AG, Geneva, Switzerland) using maximum intensity projection filters.
ELISA
Ninety-six–well microtiter plates (Maxisorp, Nunc, Denmark) were coated overnight at 4°C with 100 μl per well RSV (1 μg/ml) in coating buffer (50 nM NaHCO3 pH 9.6; 0.02% [wt/vol] NaN3). The next day, plates were blocked with blocking buffer (5% [wt/vol] nonfat dry milk; 0.05% [vol/vol] Tween20 in PBS) for 1 hour at 37°C and washed three times with washing buffer (50 mM TrisHCl pH 7.5; 150 mM NaCl; 0.05% [vol/vol] Tween20). A bacterial suspension (100 μl, containing 107 cfu in blocking buffer) was added to the wells and incubated for 1 hour at 37°C. The plates were washed three times as before and 100 μl of anticapsular antibody (1 μg/ml) added to the well for 2 hours at 37°C. Binding of the antibodies to bacteria was detected using alkaline phosphatase-conjugated goat antirabbit IgG secondary antibody (1/5,000; Sigma) and 1 mg/ml p-nitrophenyl phosphate (Sigma) dissolved in 1 M diethanolamine pH 9.8, 0.5 M MgCl2. Absorbance was read at 405 nm after 1 hour at 37°C. To determine the specificity of binding, wells were preincubated with anti-RSV G antibodies (Abcam).
Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis and ON BLOT Binding Assay
A total of 200 ml of D39 was grown directly from a frozen stock until OD600 of 1.6 was reached. The bacteria were centrifuged andwashed twice in PBS. The pellet was then resuspended in digestion buffer (330 units/ml of mutanolysin, 20% of sucrose, 2.5 mM MgCl2, 5 mM TRIS-HCl pH 7.5, 10 ml/ml of protease inhibitors) and incubated statically at 37°C for 1 hour. Lysate was centrifuged at 12,000 rpm for 30 minutes and the supernatant was frozen at −80°C. The pellet containing the protoplast was resuspended in EB buffer (Envelope buffer: 10 mM TRIS-HCl pH 7.5), frozen at −80°C for 3 hours, and sonicated (four cycles of 15-s sonication and 45-s cooling at 6–8 μm). The membrane and cytoplasmic proteins were separated by ultracentrifugation at 50,000 rpm for 10 minutes using a Beckman TL-100. Protein concentration was determined using a spectrophotometer (Nanodrop) at 280 nm.
Equal concentration of protein extracts were boiled and separated by electrophoresis on a 12% polyacrylamide gel at 50 mA constant. Half of the gel was stained with colloidal Coomassie for protein sequencing and the other half was blotted onto a polyvinylidene fluoride membrane for 1 hour at 250 mA. The membrane was washed for 5 minutes in Tris-buffered saline (TBS) and incubated in blocking buffer (5% BSA in TBS-Tween20) at room temperature for 2 hours. The membrane was then washed three times in TBS-Tween20 for 10 minutes and incubated for 4 hours with 0.1 mg/ml of RSV G protein in blocking buffer at room temperature (or RSV virus). The blot was further incubated in 1:5,000 dilution of anti-RSV primary antibodies (Abcam, ab20745). Three further washes were performed and the samples incubated with 1:20,000 dilution of antigoat IgG peroxidase conjugate developed in rabbit (SIGMA A0545) secondary antibodies. The final three washes were performed in TBS-Tween20 and the potential binding target protein was detected by ECL and sent for identification to the University of Leicester sequencing service (the Protein Nucleic Acid Chemistry Laboratory).
DNA Microarray Analysis
D39 were exposed to four different kinds of treatments: BSA, RSV A2 virions, or purified preparations of RSV (A2) fusion (F) glycoprotein (expressed by baculovirus-insect cell cultures) and RSV G protein. Three biologic replicates of each treatment were performed in medium (BEBM). Cells were harvested for RNA isolation after the 2 hours of treatment. All other procedures regarding cell disruption, RNA isolation, RNA quality testing, cDNA synthesis, labeling with dyes (Cy3 and Cy5), hybridization, and scanning were performed as described (22, 23).
Transcriptome data were analyzed as described (23, 24). For identification of differentially expressed genes a Bayesian P value less than 0.001 and fold change cut-off of 2 was applied. Microarray data have been submitted to Gene Expression Omnibus (GEO) under accession number GSE58427.
RNA Extraction, Reverse Transcription, and Quantitative Real-Time Reverse Transcriptase Polymerase Chain Reaction
Bacterial RNA was extracted using a High Pure Total RNA Isolation System Kit (Roche) according to the manufacturer’s instructions. Samples were immediately processed for reverse transcription using the Transcriptor First Strand cDNA Synthesis Kit (Roche) according to the manufacturer’s instructions. Quantitative real-time polymerase chain reaction (qRT-PCR) was performed as previously described (25) using the Light Cycler DNA-Master SYBR Green I Kit (Roche). Primers for gyrB (SP0806), comX (SP0014), nanA (SP1693), nanB (SP1687), nox (SP1469), sodA (SP0766), and ply (SP1923) were as previously described (25, 26). The relative gene expression was analyzed using the 2-∆∆CT method (27) using the reference gene of gyrB and the reference condition of pneumococci exposed to tissue culture medium (BEBM) alone. For each analysis, five distinct biologic replicas were done and each qPCR reaction was completed in triplicate. A P value of less than 0.05 was considered statistically significant.
Mouse Pneumonia Model
Groups of 10 outbred pathogen-free female mice (9-wk old) strain MF1 (HarlanOlac, Bicester, UK) were anesthetized using isoflurane and inoculated intranasally with 1 × 106 to 5 × 106 cfu/50 μl pneumococci. This dose was previously shown not to cause disease in naive mice (100% survival at 7 d postinfection). As a control, one group of mice was inoculated intranasally with 5 × 104 pfu/50 μl RSV A2 alone in PBS. Following infection, the health status of animals was monitored for up to 5 days and their symptoms scored according to the scheme of Morton and Griffiths (28). Six hours postinfection three mice from each group were killed by cervical dislocation and the lungs were harvested into 10 ml of sterile PBS, weighed, and homogenized. Viable counts in lung homogenates and blood were determined by serial dilution in sterile PBS and plating onto blood agar plates (Oxoid, Basingstoke, UK) supplemented with 5% (vol/vol) horse blood. All animal work was conducted in accordance with the UK national regulations.
Results
Exposure to RSV-infected Differentiated Epithelial Cells Alters the Pneumococcal and Host Response to Infection
We investigated whether pneumococcal virulence is altered during its interaction with RSV-infected human ciliated nasal epithelial cells. This system closely resembles the physiologic conditions of the nasopharynx, which is a natural environment for pneumococcal-RSV interactions (29). Ciliated respiratory cell cultures were infected with RSV or mock-infected, and then challenged 3 days later with S. pneumoniae. This follows the time course of secondary bacterial infection following RSV infection (10, 30). Results showed that S. pneumoniae adhered to the ciliated epithelium in greater numbers (Figure 1A) and increased (P < 0.05) the degree of ciliary dyskinesia (Figure 1B) in ciliated cultures pretreated with RSV compared with mock-infected (control) cultures after 2-hour exposure. Pneumococci were also shown to form significant clumps of bacteria associated with the ciliary tips of RSV-infected ciliated cells (Figure 1D), which was not observed in control cells (Figure 1C). High-speed videography showed that cilia with clumps of pneumococci adhered to them had a reduction in ciliary beat frequency. Ciliary dyskinesia and reduced ciliary beat frequency are known to impair mucociliary clearance in the airways of infected patients, which may contribute to enhanced symptoms of disease (31).
Figure 1.
The effect of respiratory syncytial virus (RSV) and Streptococcus pneumoniae alone and together on ciliated epithelial cells. (A) The adherence of pneumococci after 1 hour to ciliated epithelial cells previously mock-infected or infected with RSV for 72 hours. (B) The mean (SEM) level of ciliated cells with dyskinetic cilia expressed as a percentage of the total motile ciliated cells after 1 hour. Data were collected from seven independent experiments. Cells were infected with RSV, mock infected followed by pneumococci, or infected with RSV followed by pneumococci. Control cells were treated with bovine serum albumin alone. (C and D) Relative quantification of pneumococcal adherence (C) or toxicity-associated gene expression (D) when exposed to naive (green bars) or RSV-infected (blue bars) human ciliated epithelial cells. The fold changes in gene expression were measured by quantitative real-time polymerase chain reaction and calculated using the 2–∆∆CT method (27). The internal control gene was gyrB and the reference condition was pneumococci exposed to naive respiratory epithelial cells obtained from the same donor. Data were analyzed using a parametric paired t test. A P value of less than 0.05 was statistically significant. n = 5 individual experiments. *P < 0.05; **P < 0.001. (See Videos 1A and 1B.)
Video 1A.
Slow-motion video highlighting regions of pneumococcal clumping around motile cilia of mock (Video 1A) or RSV-infected (Video 1B) air–liquid interface cell cultures. See Figure 1 for further details.
Video 1B.
Coinfection also enhanced the inflammatory response of the ciliated epithelium. The addition of pneumococci to RSV-infected cells led to significant (P < 0.05) increases in the apical secretion of IL-1β, IL-12p70, tumor necrosis factor-α, IL-5, IL-13, CCL11, CCL17, and CCL22 (Table 1) (compared with cells infected with RSV alone). Compared with cells exposed to pneumococci alone, coinfection led to significant increases (P < 0.05) in levels of tumor necrosis factor-α, IL-5, and CXCL8 (Table 1). The increase in CXCL8 is of particular interest because this chemokine is responsible for the recruitment of neutrophils into the airways of patients infected with RSV. In our system, CXCL8 was ninefold higher following coinfection (median, 271 pg/ml; P < 0.05) compared with uninfected control epithelial cells (30 pg/ml). This compares with a threefold increase following pneumococcal infection alone (98 pg/ml; P > 0.05), and a fivefold increase following RSV infection alone (159 pg/ml; P > 0.05). This suggests that dual infection of respiratory epithelium may lead to a further increase in the number of inflammatory cells and increased lung cellular inflammation.
Table 1.
Secreted Cytokine and Chemokine Concentrations in Cell Culture Supernatant after 2-Hour Exposure of Streptococcus pneumoniae to Mock or RSV-infected Human Ciliated Epithelial Cells
| Chemokine cytokine (pg/ml) | Mock* |
RSV* |
||
|---|---|---|---|---|
| Control† | Pneumococcus† | Control† | Pneumococcus† | |
| IFN-γ | 12 (10–15) | 20 (15–20) | 17 (14–25) | 20 (19–25) |
| IL-1β | 2 (2–4) | 4 (3–4) | 6 (5–12) | 5 (4–6) |
| IL-12p70 | 2 (2–2) | 3 (3–4) | 2 (2–3) | 4 (3–4) |
| TNF-α | 7 (6–8) | 10 (10–15) | 12 (9–19) | 19 (14–19)‡ |
| IL-5 | 3 (2–3) | 4 (4–4) | 5 (4–16) | 6 (4–6)‡ |
| IL-13 | 6 (5–7) | 10 (8–11) | 6 (5–22) | 10 (9–11) |
| CCL11 | 72 (68–73) | 92 (90–93) | 68 (63–153) | 93 (93–99) |
| CCL4 | 1 (1–1) | 2 (2–3) | 2 (2–12) | 3 (3–4) |
| CCL17 | 35 (26–36) | 48 (48–49) | 30 (23–89) | 52 (51–58) |
| CCL22 | 91 (91–101) | 131 (123–138) | 141 (122–335) | 167 (128–191) |
| CXCL8 | 30 (26–62) | 98 (83–152) | 159 (104–1,319) | 271 (93–457)‡ |
Definition of abbreviations: RSV = respiratory syncytial virus; TNF = tumor necrosis factor.
Data are shown as median and interquartile range (in parentheses).
Statistical differences from the “Mock-control” sample were calculated using a paired t test. Significant changes (P < 0.05) from the mock control are highlighted in bold.
Primary infection (for 72 h).
Secondary infection (for 2 h).
Significant difference (P < 0.05) between the “Mock-pneumococcus” and “RSV-pneumococcus” samples (n = 5).
Exposure to RSV-infected Differentiated Epithelial Cells Alters Pneumococcal Gene Expression
To determine whether the increased inflammatory response was linked to increased pneumococcal virulence we recovered the bacterial RNA from these cultures for analysis of specific virulence gene expression by qRT-PCR. The low quantity of bacterial RNA recovered restricted the number of genes we could study. Analysis showed five out of the six genes studied were expressed at a higher level compared with pneumococci exposed to mock-infected epithelium (Figures 1E and 1F). There was a sixfold (6.0 ± 2.3 mean ± SEM) increase in expression of the gene for the pneumococcal toxin pneumolysin (ply) and a fourfold (4.0 ± 1.6) increase of expression of the gene for neuraminidase A (nanA) in the presence of RSV-infected ciliated cells. Expression of neuraminidase B (nanB), nicotinamide adenine dinucleotide phosphate reduced oxidase (nox), and competence-stimulating peptide (comX) genes was also higher than detected in pneumococci exposed to mock-infected ciliated epithelium.
Pneumococcal Penicillin-Binding Protein 1a Binds RSV G Protein
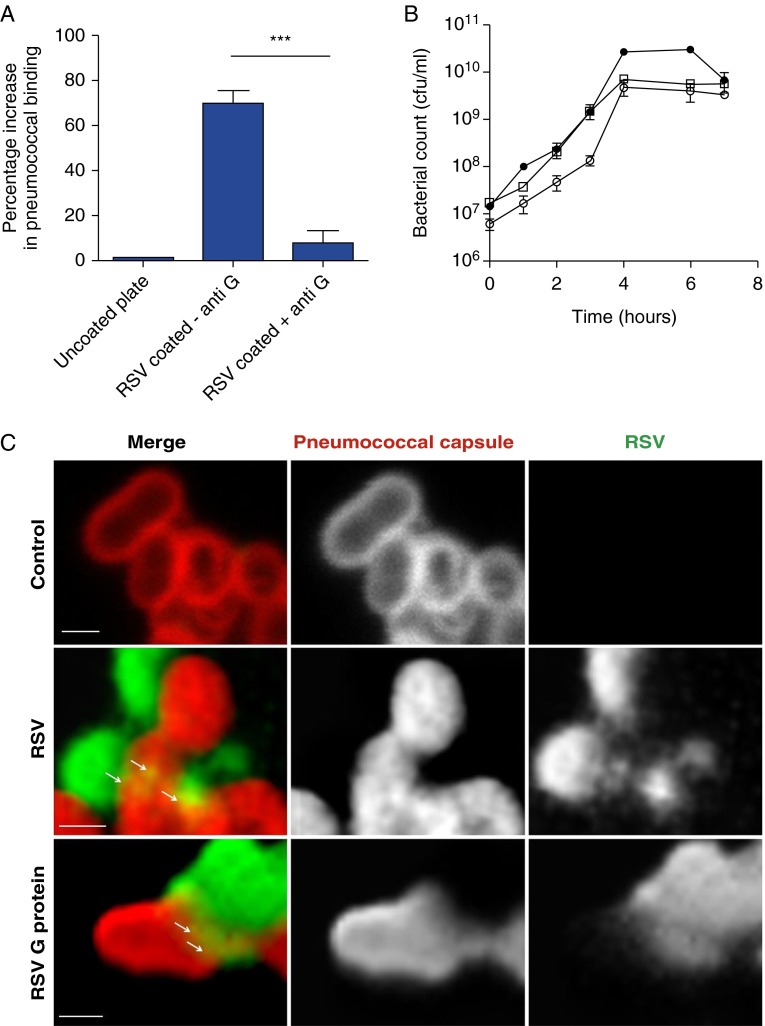
The RSV virion carries only three surface proteins: the attachment (G) protein, the fusion (F) protein, and a small hydrophobic protein. The RSV attachment G protein has been proposed to act as a receptor for pneumococci on infected cells and facilitate bacterial invasion of the cell (32–34). Figure 2A shows that the ability of pneumococcus, detected by ELISA, to bind to immobilized RSV was inhibited by preincubation with anti-RSV G antibodies confirming the ability of the G protein to bind pneumococcus (for a table showing in detail the exact genes that are up-regulated and down-regulated in the transcriptome of S. pneumoniae, see Figure 3). We confirmed this interaction using antigen colocalization and confocal imaging, which showed that RSV G protein directly binds to the surface of the pneumococcus (indicated by the arrowed yellow regions in Figure 2C). Western blotting analysis of a S. pneumoniae membrane lysate showed that RSV G protein bound to a single pneumococcal protein with a molecular weight of approximately 80 kD (Figure 2D). The reactive region of the polyacrylamide gel containing the pneumococcal protein was excised from two separate gels and mass spectrometry detected only pneumococcal penicillin-binding protein 1a (PBP1a) (Figure 2E).
Figure 2.
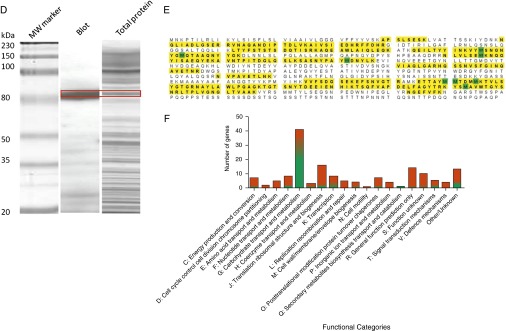
Respiratory syncytial virus (RSV) directly binds to Streptococcus pneumoniae. (A) ELISA showing inhibition of pneumococcus binding to an RSV-coated plate by prior treatment of the plate with an anti-RSV G antibody. Bound pneumococci were detected using an anticapsular polysaccharide antibody. (B) Growth curve of pneumococci exposed to bovine serum albumin (BSA; empty squares), RSV (filled circles), or purified RSV G protein (empty circles). (C) Confocal images of pneumococci exposed to BSA, RSV, or RSV G protein. Bacterial preparations were stained with an antibody specific for RSV (green) and an antipneumococcal capsule antibody (red). Areas of antigen colocalization are shown in yellow (and indicated by arrows). Scale bar = 1 µm. (D) Western blot of pneumococcal cell membrane extract incubated with purified RSV G protein. The bound G protein was detected using a monoclonal antibody. The band excised for mass spectrometry identification is indicated by the red box. (E) Peptide fragment alignment of MALDI-time of flight mass spectrometry of the excised band from the Western blot of S. pneumoniae RSV G protein-binding protein obtained after digestion with trypsin. The data show the identification of S. pneumoniae RSV G protein binding protein. The protein sequencing was performed as described in Methods in the online supplement from two individual sequencings, which resulted in 29 peptide matches. Database searching showed that the peptides covered 43% of a 79.7-kD protein that showed a greater than 99% match probability to the S. pneumoniae PBP1A protein. Normal font, no highlight = not sequenced; bold font and yellow highlight = 100% sequence match to PBP1a; bold font and green highlight = random amino acid mismatch. (F) Summary of changes in the transcriptome of S. pneumoniae following incubation with RSV. The data indicate the number of genes in each category and the proportion showing increased (red) or decreased (green). ***Statistically significant change (P < 0.0001).
Figure 3.
Table showing in detail the exact genes that are up-regulated (red) and down-regulated (green) in the transcriptome of S. pneumoniae. See Figure 2 for further details.
Binding RSV Alters the Pneumococcal Transcriptome
To determine how binding RSV affects pneumococcal gene expression we isolated bacterial RNA from pneumococci bound to RSV G protein or BSA for microarray analysis. A viable count following the direct interaction with RSV and RSV G protein showed that pneumococcal CFU were unchanged from those exposed to BSA (∼109 cfu/ml) indicating that RSV does not affect pneumococcal viability. Incubation of pneumococci with RSV or purified RSV G protein did not affect bacterial viability or growth characteristics when compared with a BSA-treated control (Figure 2B). However, pneumococci exposed to RSV G protein displayed significant differences in the transcriptome compared with pneumococci exposed to BSA. Figure 2F shows the number of genes that were affected in the transcriptome profiling experiment in response to RSV G protein treatment. The genes are grouped into Cluster of Orthologous Groups categories based on the putative functions of corresponding proteins (shown in Figure 2F). In total, expression of 157 genes were altered of which 99 were up-regulated and 58 were down-regulated in response to RSV G protein treatment (see full list in Table E1 in the online supplement). Of particular note was the significant up-regulation of the pneumolysin (ply) gene because the toxin it encodes has been shown to be critical for the development of invasive pneumococcal disease (35–39). Interestingly, expression of the gene encoding PBP1a (the pneumococcal receptor for RSV) was also up-regulated in pneumococci following exposure to RSV G protein (see Table E1), which is consistent with our mass spectrometry results.
Exposure to RSV Increases Pneumococcal Virulence In Vivo
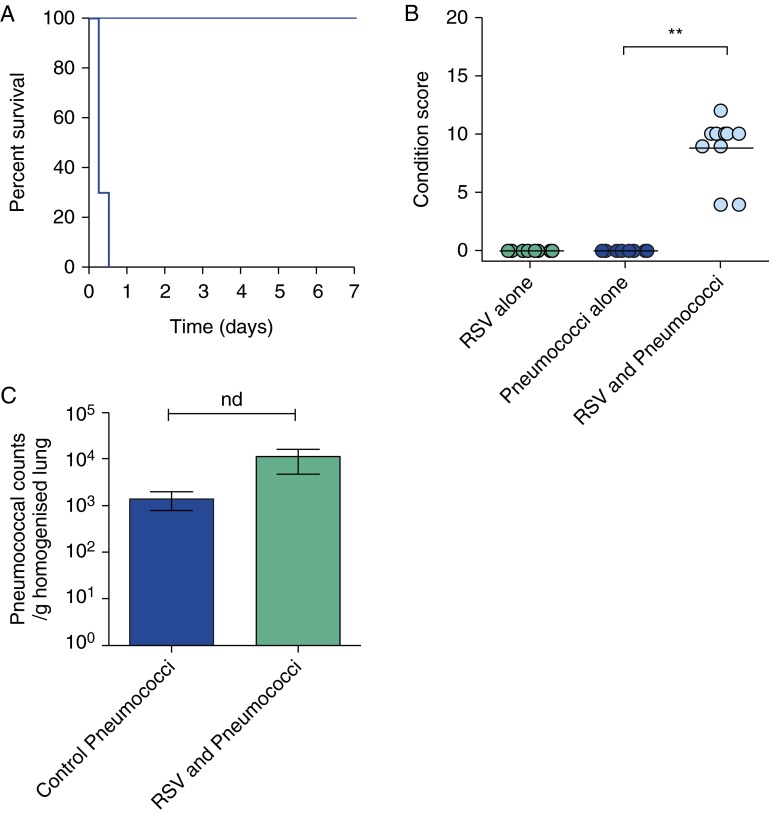
To test our hypothesis that binding RSV directly enhances the virulence of the pneumococcus, we incubated pneumococci with a purified preparation of RSV and used these pneumococci to challenge mice. Intranasal infection with a sublethal dose of RSV-exposed pneumococci resulted in the development of pneumococcal disease by 6 hours postinfection. All mice reached the predefined endpoint and were culled by 12 hours (Figure 4A). In contrast, all mice receiving the same dose of pneumococci alone (exposed to BSA) survived the 7-day challenge without any signs of disease (Figure 4B). Comparison of the condition scores of mice challenged with RSV-exposed pneumococci showed that these mice fared significantly worse (P < 0.05) than the group infected with pneumococci alone (Figure 4B). Viable counts of bacteria in the lungs of mice challenged with RSV-exposed and control pneumococci after 6 hours showed the bacteria had replicated to similar levels (P > 0.05) (Figure 4C) suggesting that enhanced virulence was not a property of increased bacterial replication in the lungs. This surprisingly rapid development of disease implies an immune-mediated response. A previous study showed that mice receiving sequential infection by RSV then pneumococci had significantly greater inflammation and high levels of accumulated neutrophils compared with control mice and mice that were inoculated with each pathogen separately (10). Mice infected with RSV (strain A2) alone did not develop any signs of illness over the 7-day study period (Figure 4B). This eliminated the possibility of a toxic effect from the RSV preparation.
Figure 4.
Prior exposure to respiratory syncytial virus (RSV) increases pneumococcal virulence in vivo. (A) The survival curve of mice challenged intranasally with 1 × 105 cfu Streptococcus pneumoniae D39 previously exposed to RSV (dark blue), exposed to RSV alone (light blue), or exposed to bovine serum albumin (BSA) alone. The RSV and BSA alone data overlap completely (pale blue). (B) The condition score of MF1 mice 6 hours after challenge with either wild-type S. pneumoniae previously exposed to RSV, exposed to RSV alone, or exposed to pneumococci alone for 2 hours. **Statistically significant change (P < 0.001). (C) Viable counts of S. pneumoniae D39 recovered from the lungs of mice 6 hours after challenge with 1 × 105 cfu S. pneumoniae D39 previously exposed to either BSA (Control Pneumococci) or RSV (RSV+Pneumococci) for 2 hours. nd = no significant difference (P > 0.05); n = 3.
Discussion
Here we demonstrate for the first time that the direct interaction of RSV and S. pneumoniae increases bacterial virulence. Using a human respiratory epithelial cell and mouse model of infection we made the major findings that binding of RSV to pneumococci (1) is mediated by the extracellular domain of the RSV G protein and pneumococcal PBP1a protein, (2) increases pneumococcal virulence gene expression, and (3) leads to increased fatalities in mice and increased bacterial adherence and infection of human differentiated ciliated respiratory epithelium.
Unlike previous studies that used monolayer human cell culture models (10, 30, 32, 34), this study used fully differentiated ciliated human respiratory epithelial cells as the system to study the effect of coinfection. This system closely resembles the physiologic conditions of the nose and airways of the lungs, which is the natural environment for pneumococcal-RSV interactions (19, 40). The data show that coinfection led to increased ciliary dyskinesia, which is known to impair mucociliary clearance in the airways of infected patients and may contribute to enhanced symptoms of disease (31). There was also an increase in apical secretion of CXCL8, which is of particular interest because this chemokine is responsible for the recruitment of neutrophils into the airways of patients infected with RSV. The data suggest that the increase in cytokine release and epithelial cell ciliary dysfunction caused by RSV treatment is additive rather than synergistic with the response to pneumococcus alone. This would imply that the RSV induces changes in the pneumococcus in a dose-dependent fashion and that the level of RSV present may influence the disease outcome. This suggests that coinfection of respiratory epithelium by RSV and the pneumococcus may lead to a further increase in the number of inflammatory cells and increased lung cellular inflammation.
S. pneumoniae is thought to initiate infection by adhering to epithelial cells that line the nasopharynx and pneumococci adhered to human ciliated epithelial cells in greater numbers and formed bacterial aggregates associated with the ciliary tips of RSV-infected ciliated cultures. The aggregates and bacteria appeared to move in time with the beating of the cilia suggesting they were attached to the cilia. The bacterial aggregates were not observed in control cultures. Increased pneumococcal adherence could result from increased expression of the enzyme neuraminidase in these bacteria. Neuraminidase is a major pneumococcal virulence factor involved in biofilm formation and adherence to host respiratory tract epithelial cells, possibly by clearing host cell surface structures (41). Bacterial RNA recovered following exposure to RSV-infected cells showed higher levels of neuraminidase A (nanA) and neuraminidase B (nanB) gene expression compared with pneumococci exposed to control cultures. Alternatively, the increased pneumococcal adherence could be caused by the presence of RSV G protein on infected host cells. Previous studies suggest that RSV acts as a direct coupling particle between bacteria and epithelial cells (10, 30, 32, 34) and our previous work showed that RSV G protein is expressed on the ciliary tips (19), consistent with RSV G protein acting as a bacterial receptor to increase bacterial adherence at this site.
A second form of RSV G protein is secreted from infected cells and therefore has the potential to directly alter the virulence of pneumococci present in the nasopharynx. The RSV G protein directly binds pneumococcal PBP1a and binding RSV G protein led to significant changes to the pneumococcal transcriptome (in the absence of infected cells) including the up-regulation of genes encoding CiaR and CiaH, which together regulate virulence and competence in S. pneumoniae (42). In addition the gene for pneumolysin (ply) was also up-regulated following exposure to RSV G protein, consistent with our previous qRT-PCR results showing that bacteria recovered from RSV-infected ciliated cell cultures displayed significant up-regulation of ply. This is a major new finding because the toxin it encodes has been shown to be critical for the development of invasive pneumococcal disease (35–39) and blockade of pneumolysin represents a new target for antipneumococcal therapy (43). Mutants deficient in pneumolysin are not lethal to mice (35, 38) and pretreatment of such a mutant with RSV did not result in increased virulence (see Figure E1). This indicates that the RSV-induced increase in pneumococcal virulence requires pneumolysin expression.
It has been shown that prior infection with RSV increases the susceptibility of mice to subsequent bacterial pneumonias with a decrease in pneumococcal clearance (10). In this study simultaneous treatment with RSV and the pneumococcus led to a significant disease and rapid death. Histopathologic examination of mice lungs following 6-hour pneumococcal challenge (Figure 4C) showed that lung pathology was absent in all groups of mice except the mice challenged with pneumococci exposed to RSV. Only one mouse (from a group of five) had extensive immune cell recruitment, indicating an immune mediated process in the development of mortality.
In considering the significance of the interaction of PBP1a and RSV G protein on pneumococcal virulence and the extensive sequence variation between G proteins from different RSV isolates two questions immediately arise: Is the enhancement of pneumococcal virulence seen with clinical RSV isolates? Is there is any variation in the response of the bacteria to G proteins from different clinical strains of RSV? The whole virus experiments reported here used a subgroup A virus, whereas a purified subgroup B G protein was used for the interaction assays. Both generated the enhancement of disease suggesting that the G proteins of both subgroups have the capacity to enhance pneumococcal disease. However, investigation of potential inherent variation in this interaction will permit us to uncover the key molecular motifs and identify high-risk serotypes and pathotypes and this is currently being investigated.
In conclusion, these data show for the first time that the direct interaction of RSV and S. pneumoniae induces expression of pathogenicity determinants that leads to fatal infection in mice and increased pathogenesis in a human respiratory system. The data also show that the resulting disease process requires a functional pneumolysin toxin. This study provides novel insights into the molecular mechanisms underlying secondary pneumococcal disease and the wider implications of the interplay between respiratory pathogens.
Footnotes
Supported by grants from Action Medical Research (SP4499 and SP4118).
Author Contributions: Study design, C.M.S., R.A.H., A.J.E., P.W.A., and C.O’C. Conception, C.M.S., A.J.E., P.W.A., and C.O’C. Data acquisition, C.M.S., S. Sandrini, S.D., P.F., S. Shafeeq, P.R., R.A.H., and S.M.G. Technical support, G.W. Data analysis, C.M.S., S. Sandrini, S.D., P.F., P.R., R.A.H., S.M.G., O.P.K., A.J.E., P.W.A., and C.O’C. Manuscript preparation, C.M.S., R.A.H., A.J.E., P.W.A., and C.O’C.
Originally Published in Press as DOI: 10.1164/rccm.201311-2110OC on June 18, 2014
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
This article has embedded videos, which play in place from both the online PDF or HTML versions. If you cannot view Flash videos on your device, please try playing the videos in the online PDF, or access the original uncompressed videos at http://www.atsjournals.org/doi/suppl/10.1164/rccm.201311-2110OC. To play, mouse over the image and click on the arrow that will appear in the center or on the lower left.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA. 1999;282:1440–1446. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization Initiative for Vaccine Research (IVR). Respiratory syncytial virus and parainfluenza viruses. Disease Burden 2009. Available from: http://apps.who.int/vaccine_research/diseases/ari/en/index2.html
- 3.World Health Organization Estimated Hib and pneumococcal deaths for children under 5 years of age 2008. Available from: http://www.who.int/immunization_monitoring/burden/Pneumo_hib_estimates/en/index.html
- 4.Stensballe LG, Hjuler T, Andersen A, Kaltoft M, Ravn H, Aaby P, Simoes EA. Hospitalization for respiratory syncytial virus infection and invasive pneumococcal disease in Danish children aged <2 years: a population-based cohort study. Clin Infect Dis. 2008;46:1165–1171. doi: 10.1086/529438. [DOI] [PubMed] [Google Scholar]
- 5.Madhi SA, Klugman KP Vaccine Trialist Group. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat Med. 2004;10:811–813. doi: 10.1038/nm1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ampofo K, Bender J, Sheng X, Korgenski K, Daly J, Pavia AT, Byington CL. Seasonal invasive pneumococcal disease in children: role of preceding respiratory viral infection. Pediatrics. 2008;122:229–237. doi: 10.1542/peds.2007-3192. [DOI] [PubMed] [Google Scholar]
- 7.Kim PE, Musher DM, Glezen WP, Rodriguez-Barradas MC, Nahm WK, Wright CE. Association of invasive pneumococcal disease with season, atmospheric conditions, air pollution, and the isolation of respiratory viruses. Clin Infect Dis. 1996;22:100–106. doi: 10.1093/clinids/22.1.100. [DOI] [PubMed] [Google Scholar]
- 8.Talbot TR, Poehling KA, Hartert TV, Arbogast PG, Halasa NB, Edwards KM, Schaffner W, Craig AS, Griffin MR. Seasonality of invasive pneumococcal disease: temporal relation to documented influenza and respiratory syncytial viral circulation. Am J Med. 2005;118:285–291. doi: 10.1016/j.amjmed.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Watson M, Gilmour R, Menzies R, Ferson M, McIntyre P New South Wales Pneumococcal Network. The association of respiratory viruses, temperature, and other climatic parameters with the incidence of invasive pneumococcal disease in Sydney, Australia. Clin Infect Dis. 2006;42:211–215. doi: 10.1086/498897. [DOI] [PubMed] [Google Scholar]
- 10.Stark JM, Stark MA, Colasurdo GN, LeVine AM. Decreased bacterial clearance from the lungs of mice following primary respiratory syncytial virus infection. J Med Virol. 2006;78:829–838. doi: 10.1002/jmv.20631. [DOI] [PubMed] [Google Scholar]
- 11.Bataki EL, Evans GS, Everard ML. Respiratory syncytial virus and neutrophil activation. Clin Exp Immunol. 2005;140:470–477. doi: 10.1111/j.1365-2249.2005.02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith CM, Lo Passo C, Scuderi A, Kolberg J, Baxendale H, Goldblatt D, Oggioni MR, Felici F, Andrew PW. Peptide mimics of two pneumococcal capsular polysaccharide serotypes (6B and 9V) protect mice from a lethal challenge with Streptococcus pneumoniae. Eur J Immunol. 2009;39:1527–1535. doi: 10.1002/eji.200839091. [DOI] [PubMed] [Google Scholar]
- 13.Mohammed BJ, Mitchell TJ, Andrew PW, Hirst RA, O’Callaghan C. The effect of the pneumococcal toxin, pneumolysin on brain ependymal cilia. Microb Pathog. 1999;27:303–309. doi: 10.1006/mpat.1999.0306. [DOI] [PubMed] [Google Scholar]
- 14.Smith CM, Fadaee-Shohada MJ, Sawhney R, Baker N, Williams G, Hirst RA, Andrew PW, O’Callaghan C. Ciliated cultures from patients with primary ciliary dyskinesia do not produce nitric oxide or inducible nitric oxide synthase during early infection. Chest. 2013;144:1671–1676. doi: 10.1378/chest.13-0159. [DOI] [PubMed] [Google Scholar]
- 15.Kadioglu A, Gingles NA, Grattan K, Kerr A, Mitchell TJ, Andrew PW. Host cellular immune response to pneumococcal lung infection in mice. Infect Immun. 2000;68:492–501. doi: 10.1128/iai.68.2.492-501.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirst RA, Rutman A, Williams G, O’Callaghan C. Ciliated air-liquid cultures as an aid to diagnostic testing of primary ciliary dyskinesia. Chest. 2010;138:1441–1447. doi: 10.1378/chest.10-0175. [DOI] [PubMed] [Google Scholar]
- 17.Chilvers MA, O’Callaghan C. Analysis of ciliary beat pattern and beat frequency using digital high speed imaging: comparison with the photomultiplier and photodiode methods. Thorax. 2000;55:314–317. doi: 10.1136/thorax.55.4.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chilvers MA, McKean M, Rutman A, Myint BS, Silverman M, O’Callaghan C. The effects of coronavirus on human nasal ciliated respiratory epithelium. Eur Respir J. 2001;18:965–970. doi: 10.1183/09031936.01.00093001. [DOI] [PubMed] [Google Scholar]
- 19.Smith CM, Kulkarni H, Radhakrishnan P, Rutman A, Bankart MJ, Williams G, Hirst RA, Easton AJ, Andrew PW, O’Callaghan C. Ciliary dyskinesia is an early feature of respiratory syncytial virus infection. Eur Respir J. 2014;43:485–496. doi: 10.1183/09031936.00205312. [DOI] [PubMed] [Google Scholar]
- 20.Smith CM, Fadaee-Shohada MJ, Sawhney R, Baker N, Willliams G, Hirst RA, Andrew PW, O'Callaghan C. Ciliated cultures from patients with primary ciliary dyskinesia do not produce nitric oxide or inducible nitric oxide synthase during early infection. Chest. 2013;144:1671–1676. doi: 10.1378/chest.13-0159. [DOI] [PubMed] [Google Scholar]
- 21.Tsang KW, Leung R, Fung PC, Chan SL, Tipoe GL, Ooi GC, Lam WK. Exhaled and sputum nitric oxide in bronchiectasis: correlation with clinical parameters. Chest. 2002;121:88–94. doi: 10.1378/chest.121.1.88. [DOI] [PubMed] [Google Scholar]
- 22.Shafeeq S, Yesilkaya H, Kloosterman TG, Narayanan G, Wandel M, Andrew PW, Kuipers OP, Morrissey JA. The cop operon is required for copper homeostasis and contributes to virulence in Streptococcus pneumoniae. Mol Microbiol. 2011;81:1255–1270. doi: 10.1111/j.1365-2958.2011.07758.x. [DOI] [PubMed] [Google Scholar]
- 23.Shafeeq S, Kloosterman TG, Kuipers OP. Transcriptional response of Streptococcus pneumoniae to Zn2+) limitation and the repressor/activator function of AdcR. Metallomics. 2011;3:609–618. doi: 10.1039/c1mt00030f. [DOI] [PubMed] [Google Scholar]
- 24.van Hijum SA, García de la Nava J, Trelles O, Kok J, Kuipers OP. MicroPreP: a cDNA microarray data pre-processing framework. Appl Bioinformatics. 2003;2:241–244. [PubMed] [Google Scholar]
- 25.Oggioni MR, Iannelli F, Ricci S, Chiavolini D, Parigi R, Trappetti C, Claverys JP, Pozzi G. Antibacterial activity of a competence-stimulating peptide in experimental sepsis caused by Streptococcus pneumoniae. Antimicrob Agents Chemother. 2004;48:4725–4732. doi: 10.1128/AAC.48.12.4725-4732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oggioni MR, Trappetti C, Kadioglu A, Cassone M, Iannelli F, Ricci S, Andrew PW, Pozzi G. Switch from planktonic to sessile life: a major event in pneumococcal pathogenesis. Mol Microbiol. 2006;61:1196–1210. doi: 10.1111/j.1365-2958.2006.05310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Morton DB, Griffiths PHM. Guidelines on the recognition of pain, distress and discomfort in experimental animals and an hypothesis for assessment. Vet Rec. 1985;116:431–436. doi: 10.1136/vr.116.16.431. [DOI] [PubMed] [Google Scholar]
- 29.Wright AKA, Ferreira DM, Gritzfeld JF, Wright AD, Armitage K, Jambo KC, Bate E, El Batrawy S, Collins A, Gordon SB. Human nasal challenge with Streptococcus pneumoniae is immunising in the absence of carriage. PLoS Pathog. 2012;8:e1002622. doi: 10.1371/journal.ppat.1002622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Avadhanula V, Rodriguez CA, Devincenzo JP, Wang Y, Webby RJ, Ulett GC, Adderson EE. Respiratory viruses augment the adhesion of bacterial pathogens to respiratory epithelium in a viral species- and cell type-dependent manner. J Virol. 2006;80:1629–1636. doi: 10.1128/JVI.80.4.1629-1636.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stannard WA, Chilvers MA, Rutman AR, Williams CD, O’Callaghan C. Diagnostic testing of patients suspected of primary ciliary dyskinesia. Am J Respir Crit Care Med. 2010;181:307–314. doi: 10.1164/rccm.200903-0459OC. [DOI] [PubMed] [Google Scholar]
- 32.Hament JM, Aerts PC, Fleer A, Van Dijk H, Harmsen T, Kimpen JL, Wolfs TF. Enhanced adherence of Streptococcus pneumoniae to human epithelial cells infected with respiratory syncytial virus. Pediatr Res. 2004;55:972–978. doi: 10.1203/01.PDR.0000127431.11750.D9. [DOI] [PubMed] [Google Scholar]
- 33.Levine S, Klaiber-Franco R, Paradiso PR. Demonstration that glycoprotein G is the attachment protein of respiratory syncytial virus. J Gen Virol. 1987;68:2521–2524. doi: 10.1099/0022-1317-68-9-2521. [DOI] [PubMed] [Google Scholar]
- 34.Avadhanula V, Wang Y, Portner A, Adderson E. Nontypeable Haemophilus influenzae and Streptococcus pneumoniae bind respiratory syncytial virus glycoprotein. J Med Microbiol. 2007;56:1133–1137. doi: 10.1099/jmm.0.47086-0. [DOI] [PubMed] [Google Scholar]
- 35.Canvin JR, Marvin AP, Sivakumaran M, Paton JC, Boulnois GJ, Andrew PW, Mitchell TJ. The role of pneumolysin and autolysin in the pathology of pneumonia and septicemia in mice infected with a type 2 pneumococcus. J Infect Dis. 1995;172:119–123. doi: 10.1093/infdis/172.1.119. [DOI] [PubMed] [Google Scholar]
- 36.Alexander JE, Lock RA, Peeters CC, Poolman JT, Andrew PW, Mitchell TJ, Hansman D, Paton JC. Immunization of mice with pneumolysin toxoid confers a significant degree of protection against at least nine serotypes of Streptococcus pneumoniae. Infect Immun. 1994;62:5683–5688. doi: 10.1128/iai.62.12.5683-5688.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts P, Jeffery PK, Mitchell TJ, Andrew PW, Boulnois GJ, Feldman C, Cole PJ, Wilson R. Effect of immunization with Freund’s adjuvant and pneumolysin on histologic features of pneumococcal infection in the rat lung in vivo. Infect Immun. 1992;60:4969–4972. doi: 10.1128/iai.60.11.4969-4972.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirst RA, Gosai B, Rutman A, Guerin CJ, Nicotera P, Andrew PW, O’Callaghan C. Streptococcus pneumoniae deficient in pneumolysin or autolysin has reduced virulence in meningitis. J Infect Dis. 2008;197:744–751. doi: 10.1086/527322. [DOI] [PubMed] [Google Scholar]
- 39.Hirst RA, Sikand KS, Rutman A, Mitchell TJ, Andrew PW, O’Callaghan C. Relative roles of pneumolysin and hydrogen peroxide from Streptococcus pneumoniae in inhibition of ependymal ciliary beat frequency. Infect Immun. 2000;68:1557–1562. doi: 10.1128/iai.68.3.1557-1562.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang L, Peeples ME, Boucher RC, Collins PL, Pickles RJ. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J Virol. 2002;76:5654–5666. doi: 10.1128/JVI.76.11.5654-5666.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parker D, Soong G, Planet P, Brower J, Ratner AJ, Prince A. The NanA neuraminidase of Streptococcus pneumoniae is involved in biofilm formation. Infect Immun. 2009;77:3722–3730. doi: 10.1128/IAI.00228-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ibrahim YM, Kerr AR, McCluskey J, Mitchell TJ. Control of virulence by the two-component system CiaR/H is mediated via HtrA, a major virulence factor of Streptococcus pneumoniae. J Bacteriol. 2004;186:5258–5266. doi: 10.1128/JB.186.16.5258-5266.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andrew PW.New methods of treatment of antibiotic-resistant pneumococcal disease. Available from: http://cordis.europa.eu/search/index.cfm?fuseaction=lib.document&DOC_LANG_ID=EN&DOC_ID=129641381&q=; 2011