Abstract
Rationale: Ex vivo, bronchial epithelial cells from people with asthma are more susceptible to rhinovirus infection caused by deficient induction of the antiviral protein, IFN-β. Exogenous IFN-β restores antiviral activity.
Objectives: To compare the efficacy and safety of inhaled IFN-β with placebo administered to people with asthma after onset of cold symptoms to prevent or attenuate asthma symptoms caused by respiratory viruses.
Methods: A total of 147 people with asthma on inhaled corticosteroids (British Thoracic Society Steps 2–5), with a history of virus-associated exacerbations, were randomized to 14-day treatment with inhaled IFN-β (n = 72) or placebo (n = 75) within 24 hours of developing cold symptoms and were assessed clinically, with relevant samples collected to assess virus infection and antiviral responses.
Measurements and Main Results: A total of 91% of randomized patients developed a defined cold. In this modified intention-to-treat population, asthma symptoms did not get clinically significantly worse (mean change in six-item Asthma Control Questionnaire <0.5) and IFN-β treatment had no significant effect on this primary endpoint, although it enhanced morning peak expiratory flow recovery (P = 0.033), reduced the need for additional treatment, and boosted innate immunity as assessed by blood and sputum biomarkers. In an exploratory analysis of the subset of more difficult-to-treat, Step 4-5 people with asthma (n = 27 IFN-β; n = 31 placebo), Asthma Control Questionnaire-6 increased significantly on placebo; this was prevented by IFN-β (P = 0.004).
Conclusions: Although the trial did not meet its primary endpoint, it suggests that inhaled IFN-β is a potential treatment for virus-induced deteriorations of asthma in difficult-to-treat people with asthma and supports the need for further, adequately powered, trials in this population.
Clinical trial registered with www.clinicaltrials.gov (NCT 01126177).
Keywords: innate immunity, treatment, respiratory virus
At a Glance Commentary
Scientific Knowledge on the Subject
People with asthma are more likely to develop lower respiratory tract symptoms because of a cold (i.e., an upper respiratory tract infection), and there is no specific antiviral treatment that can prevent upper respiratory tract infection–induced asthma exacerbations.
What This Study Adds to the Field
Although the trial did not meet its primary endpoint, exploratory subanalysis of the difficult-to-treat-subgroup suggests that inhaled IFN-β enhances innate immunity and may impact favorably on cold-induced asthma exacerbations in this patient population.
Exacerbations of asthma, the majority caused by respiratory viruses (1, 2), are a large unmet medical need, especially in more severe disease (3–5). People with asthma are more likely to develop lower respiratory tract symptoms after an upper respiratory tract infection (URTI) even though the frequency and severity of colds in people with asthma is not higher than normal (2). Using culture of bronchial epithelial cells obtained by bronchoscopic brushings from corticosteroid-treated people with asthma, we have previously demonstrated that, when infected with rhinoviruses (RV), the asthmatic bronchial epithelium failed to mount an effective innate immune response involving IFN-β (6) and IFN-λ (7). Suboptimal activation of antiviral pathways resulted in greater viral replication and shedding, cytolysis of epithelial cells, and mediator release (6, 7). A similar defect exists in asthmatic airway macrophages (8) and airway cells of children with asthma (9, 10). Importantly, epithelial antiviral activity could be corrected ex vivo by low concentrations of exogenous IFN-β (6, 11). Some other studies have been unable to replicate such differences between people with asthma and healthy individuals, but these were conducted in mild, corticosteroid-naive people with asthma (12) or in those with well-controlled asthma (13).
To evaluate the clinical relevance of the IFN-β deficiency in asthma and explore the potential for IFN-β as a treatment for virus-induced asthma exacerbations, a series of clinical studies were conducted with inhaled IFN-β (SNG001; Synairgen Research Ltd, Southampton, UK) (see online supplement). A dose-escalating study in volunteers with asthma showed that nebulized IFN-β (6 mIU) given once daily for 14 days is well tolerated and enhances innate immune responses in the airways, as assessed by several biomarkers of IFN-β–related antiviral activity (neopterin, IFN-γ–induced protein 10 [IP-10, CXCL10], myxoma resistance protein 1, and 2’-5′ oligoadenylate synthetase) measured in induced sputum (see Figure E1 in the online supplement). This also provided evidence that CXCL10 may be a useful biomarker for clinical development of IFN-β.
A randomized placebo-controlled trial of IFN-β administered to people with asthma with a history of cold-induced exacerbations was, therefore, conducted to test the hypothesis that, when delivered by oral inhalation at the report of an URTI, IFN-β can prevent or substantially reduce the increase in asthma symptoms, thereby providing initial proof of concept for IFN-β as a potential treatment for virus-induced exacerbations.
Some of the results of these studies have been previously reported in the form of an abstract (14).
Methods
Study Design
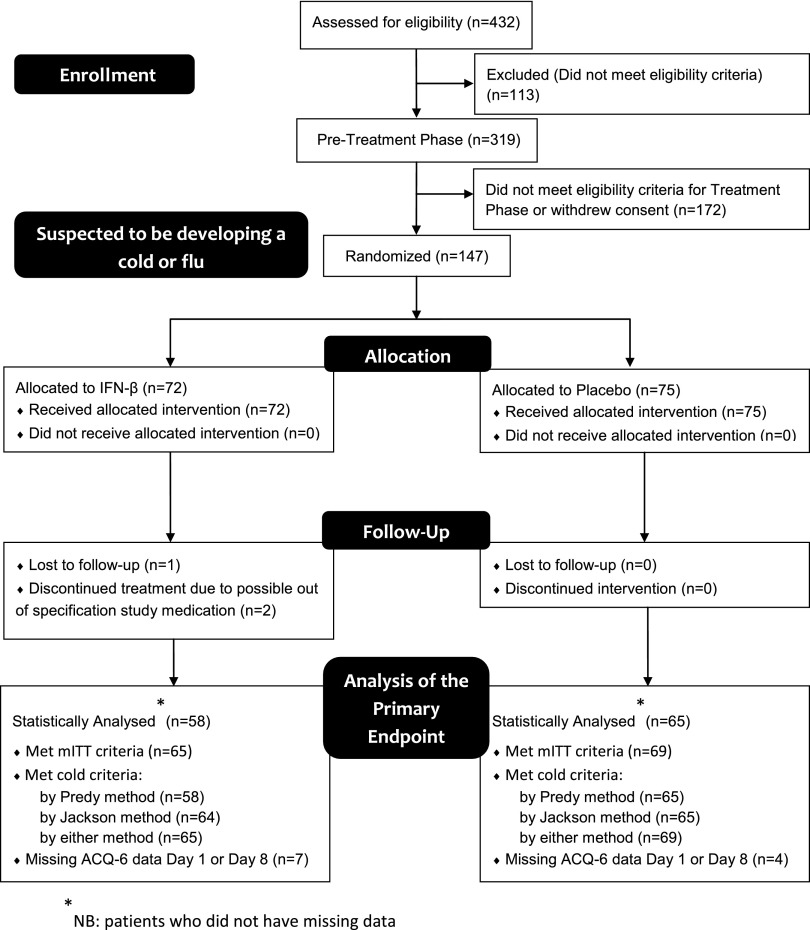
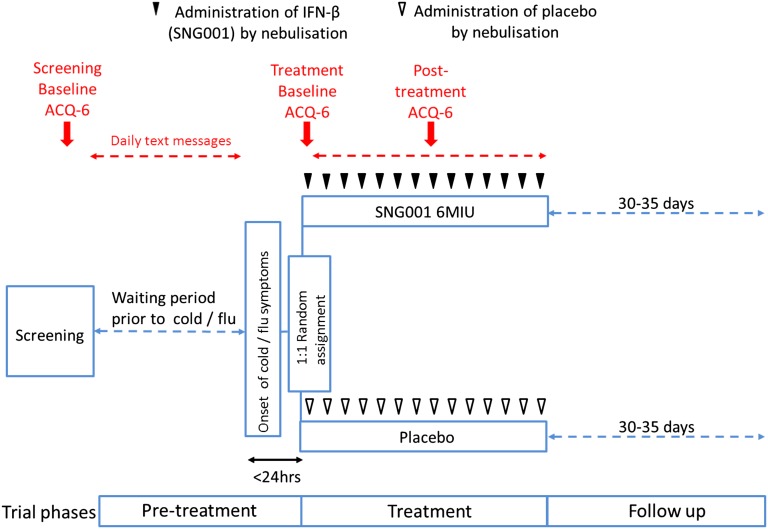
This was a randomized, double-blind, parallel, placebo-controlled trial of IFN-β (SNG001) (Figures 1 and 2) involving patients with a history of URTI-induced exacerbations, to test whether inhaled IFN-β can prevent or attenuate worsening asthma symptoms (defined as a rise in the six-item Juniper Asthma Control Questionnaire [ACQ-6] [15]) caused by respiratory viruses if administered within 24 hours after reporting cold or influenza symptoms. The primary endpoint was defined as the comparison of the mean change from baseline to Day 8 in ACQ-6 in the modified intention-to-treat (mITT) population (i.e., those patients who fulfilled the Jackson [16] or Predy [17] criteria for a cold). Patients responded daily to text message questions about URTI symptoms. If they met the preset criteria for an URTI, they attended the research units within 24 hours (Day 1) to begin treatment with inhaled IFN-β or placebo (randomized in a 1:1 ratio) given as single daily doses for 14 days. Patients were reviewed on Days 4, 7, 10, 13, and 17 and recorded daily upper and lower respiratory symptoms and peak expiratory flow (PEF) measurements at home (see Table E1 for the full study schedule).
Figure 1.
CONSORT flow diagram. ACQ = Asthma Control Questionnaire; mITT = modified intention-to-treat.
Figure 2.
Study design. During the pretreatment phase, patients responded to daily text messages inquiring about symptoms of cold. If the prespecified threshold for symptoms was reached, they visited the research unit within 24 hours to receive their first dose. Thereafter, patients received their daily treatment of IFN-β or placebo for a total of 14 days. The primary outcome, a validated shortened version of the Asthma Control Questionnaire (ACQ-6) (15), was completed during screening, before treatment began on Day 1, and 7 days later. For details of biologic samples collected, see Methods section and Table E1.
Study Treatments
SNG001 consists of recombinant IFN-β1a formulated as an aqueous solution that, unlike some other commercial preparations, does not contain mannitol or human serum albumin and is pH neutral. Patients received 6 mIU of IFN-β or placebo (formulation buffer without IFN-β) from a portable mesh nebulizer delivered over 3–4 minutes (I-neb; Philips Respironics, Chichester, UK).
Patients
The inclusion criteria for the pretreatment phase included (1) age 18–65 years, (2) asthma symptoms for greater than or equal to 2 years confirmed by history and one of either FEV1 reversibility to albuterol (≥12% and 200 ml), bronchial hyperresponsiveness (at screening or historical), emergency admission, or attendance at primary care or out-of-hours clinics for worsening asthma; (3) history of cold-induced exacerbations and greater than or equal to one exacerbation suspected to be caused by a respiratory virus in the past 24 months requiring oral corticosteroids and/or antibiotics; and (4) treatment with regular inhaled corticosteroids (ICS) (i.e., British Thoracic Society [BTS] Guidelines Step 2 and above) (18).
Patients entered the treatment phase if they fulfilled the following criteria: (1) URTI symptoms within the last 24 hours presenting as cold symptoms (blocked or runny nose and a sore or scratchy throat) or influenza-like illness (temperature > 37.8°C, plus two of either headache, cough, sore throat, myalgia), (2) patient’s belief that they have a cold or flu, (3) continued regular ICS since screening, and (4) post-bronchodilator FEV1 greater than or equal to 35% of predicted.
Clinical and Laboratory Assessments
Patients were screened by history, physical examination, lung function testing, and bronchial hyperresponsiveness (if asthma diagnosis required confirmation). To establish asthma control at screening baseline, patients completed the ACQ-6, and over 7 days, recorded twice daily PEF and responded to asthma symptom questions from the Asthma Index Questionnaire (19) (twice daily) and the Jackson Cold Score questionnaire (JCSQ) (16) (in the evening) by text message.
After establishing the screening baseline, patients responded to daily text messages asking them if they had symptoms of an URTI (see online supplement). If the response suggested that patients were experiencing cold or influenza symptoms, patients were telephoned to assess eligibility for the treatment phase using the JCSQ to confirm the onset of cold symptoms within the past 24 hours. For the next 28 days, beginning on treatment Day 1, the text messages switched to questions from the JCSQ and four questions from the Asthma Index Questionnaire (symptoms of chest tightness, wheeze, cough, and shortness of breath, scored 0–3). The ACQ-6 was completed by telephone interview on Day 1 (as treatment baseline) and on treatment Day 8.
Nasal lavage was collected during the first week and screened for a panel of respiratory viruses by quantitative real-time polymerase chain reaction (qPCR). Sputum induction was attempted at selected sites at screening and on treatment Days 4 and 7 to quantify RV virus load and antiviral biomarkers by qPCR and proinflammatory biomarkers by Luminex (Life Technologies, Paisley, UK). Blood samples were collected for measurement of serum CXCL10 by ELISA and genome-wide gene expression analysis.
Statistical Analyses
Primary analysis was conducted on data only from those patients who fulfilled the Jackson (16) or Predy (17) criteria for a cold: these patients constituted the mITT population. All statistical tests were two-sided with a 5% level of significance, with no adjustments for multiplicity. The primary hypothesis (that IFN-β is superior to placebo in respect of the change from treatment baseline ACQ-6 [Day 1] to Day 8 in the mITT population) was tested by analysis of covariance, including terms for pooled site and baseline value. Sample size calculation, based on data from a prospective multicenter study of asthma control associated with a cold (20), determined that 56 patients per treatment-arm provided 80% power to detect a mean treatment difference of 0.5 in the change from baseline in ACQ-6 8 days after onset of a cold, a change accepted as clinically relevant (21), with a between-patient standard deviation of 0.93. A planned masked sample size recalculation (22) was performed mid-study, resulting in no required modification.
Additional secondary trial endpoints are provided in the online supplement; the statistical analysis plan also stated that exploratory analyses in patient subgroups defined by asthma severity may be investigated, although the definition of severity was not prespecified.
Results
Patients
From a total of 319 recruited patients, 147 considered by the investigators to be developing a cold were randomized into the treatment phase (ITT population). Subsequent analysis showed that most (134 patients) went on to develop a cold, as judged by fulfilling either the Jackson or Predy cold criteria (16); these were, therefore, treated as the mITT population (Table 1).
Table 1.
Patient Characteristics in the mITT Population
| Whole mITT |
BTS Step 2 |
BTS Step 3 |
BTS Step 4-5 |
|||||
|---|---|---|---|---|---|---|---|---|
| Placebo (n = 69) | IFN-β (n = 65) | Placebo (n = 15) | IFN-β (n = 17) | Placebo (n = 23) | IFN-β (n = 21) | Placebo (n = 31) | IFN-β (n = 27) | |
| Age, yr, mean (range) | 39.6 (19–64) | 37.0 (19–64) | 42.3 (25–58) | 33.6 (21–61) | 40.3 (19–64) | 32.9 (19–57) | 37.8 (19–61) | 42.2 (23–64) |
| Sex, male/female, % | 30/70 | 37/63 | 33/67 | 24/76 | 30/70 | 43/57 | 29/71 | 41/59 |
| Atopy status (skin prick test), atopic/nonatopic/not tested, %* | 74/25/1 | 80/17/3 | 67/33/0 | 65/24/12 | 74/22/4 | 86/14/0 | 77/23/0 | 85/15/0 |
| Smoking status, nonsmoker/smoker, % | 87/13 | 88/12 | 87/13 | 88/12 | 83/17 | 90/10 | 90/10 | 85/15 |
| Smoking history, pack-years, current smoker/ex-smoker | 13.3/8.2 | 7.8/5.8 | 22.0/16.3 | 0.3/2.3 | 8.1/3.1 | 14.0/2.0 | 14.3/3.5 | 8.5/9.9 |
| Dose of inhaled corticosteroids (before treatment baseline), μg/d (fluticasone equivalent), median (range) | 400 (100–2,000) | 400 (50–2,000) | 200 (100–400) | 200 (50–400) | 200 (100–400) | 200 (100–400) | 750 (200–2,000)† | 800 (200–2,000)† |
| Prebronchodilator FEV1 at screening, % of predicted, mean (SD) | 90.9 (20.4) | 88.9 (19.0) | 92.7 (22.7) | 94.2 (19.5) | 91 (18.8) | 91.1 (18.7) | 90.0 (21.0) | 83.8 (18.5) |
| Prebronchodilator FEV1 at treatment baseline, % of predicted, mean (SD) | 89.1 (18.5) | 88.8 (18.4) | 88.7 (19.5) | 90.3 (19.9) | 89.0 (18.8) | 91.1 (17.6) | 89.3 (18.5) | 86.2 (18.5) |
| Post-bronchodilator FEV1 at screening, % of predicted, mean (SD) | 98.3 (18.2) | 95.6 (17.5) | 99.4 (20.3) | 101.5 (16.0) | 97.0 (17.2) | 97.8 (17.3) | 98.7 (18.3) | 90.1 (17.6) |
| Post-bronchodilator FEV1 at treatment baseline, % of predicted, mean (SD) | 96.2 (16.7) | 94.9 (16.4) | 98.1 (16.9) | 98.5 (14.1) | 95.6 (16.6) | 96.2 (15.9) | 95.8 (17.1) | 91.8 (17.9) |
| Jackson Cold Score at treatment baseline (max. 24), score, mean (SD) | 8.2 (3.7) | 9.1 (4.3) | 7.2 (3.5) | 8.9 (3.6) | 8.8 (4.5) | 10.1 (3.5) | 8.5 (3.7) | 8.1 (4.6) |
| ACQ-6 at screening (0–6), score, mean (SD) | 1.26 (0.88) | 1.20 (0.91) | 1.08 (0.69) | 0.89 (0.62) | 1.19 (0.83) | 0.90 (0.82) | 1.41 (0.99) | 1.63 (1.03) |
| ACQ-6 at treatment baseline (0–6), score, mean (SD) | 1.57 (0.98) | 1.44 (0.95) | 1.24 (0.78) | 1.23 (0.82) | 1.45 (0.97) | 1.07 (0.84) | 1.82 (1.02) | 1.87 (0.97) |
Definition of abbreviations: ACQ = Asthma Control Questionnaire; BTS = British Thoracic Society; mITT = modified intention-to-treat.
Patients who fulfilled either the Jackson or Predy criteria (16, 17) for a cold were included in the mITT group. There were no clinically important differences between patients randomized to receive IFN-β or placebo in respect of any clinical criterion, either when assessing the mITT population as a whole or when subgrouped by asthma severity, using BTS steps (18, 21).
Atopy percentages may not always add up to 100% because of rounding.
Two patients taking 200 μg/day inhaled corticosteroids (fluticasone equivalent) were taking four classes of asthma drug as part of their normal asthma medication and were, therefore, assigned to the BTS Step 4 subgroup. One subject taking 200 μg/day inhaled corticosteroids (fluticasone equivalent) was taking oral corticosteroids as part of their normal asthma medication and was assigned to the BTS Step 5 subgroup.
Relationship between Virus Infections and Asthma Symptoms
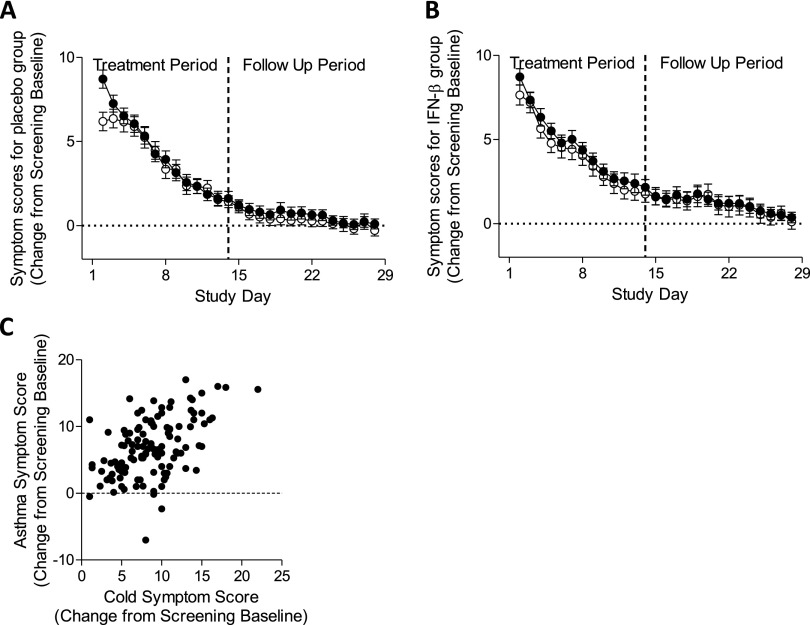
Respiratory viruses were detected by qPCR in nasal lavage in 63% of patients; more than one virus was detected in some samples. Most viruses detected were RV (68%), the rest being adenovirus (3%), bocavirus (23%), coronavirus (9%), enterovirus (4%), human metapneumovirus (1%), parainfluenza virus (4%), and respiratory syncytial virus (3%). Analysis of the time-course, conducted in the placebo group to avoid active treatment effects, showed that both asthma and cold symptoms increased markedly from screening baseline, peaking on Day 2 of the treatment phase and gradually returning to baseline (Figure 3A). There was a similar trend in the IFN-β group (Figure 3B). For most patients, complete sets of asthma symptom scores were available only from Day 2 (i.e., 1 day after starting treatment). At presentation, the URTI (cold) and asthma symptoms were positively correlated (rs = 0.48; P < 0.0001) (Figure 3C).
Figure 3.
Daily cold and asthma symptom scores in the modified intention-to-treat population. Symptoms (mean ± SEM) were analyzed in patients randomized to the placebo arm of the trial (n = 54–69) to avoid any effects of active treatment. (A) This showed that both cold (closed symbol) and asthma (open symbol) symptoms peaked at presentation, before treatment was initiated, and declined to baseline in a similar manner over a period of about 17 days. (B) There was a similar trend in the IFN-β group (n = 51–65). (C) At presentation there was a highly significant monotonic relationship (Spearman rank correlation = 0.48; P < 0.0001) between upper respiratory tract infection (cold) and asthma symptoms (n = 61).
Analysis of Data from the mITT Population
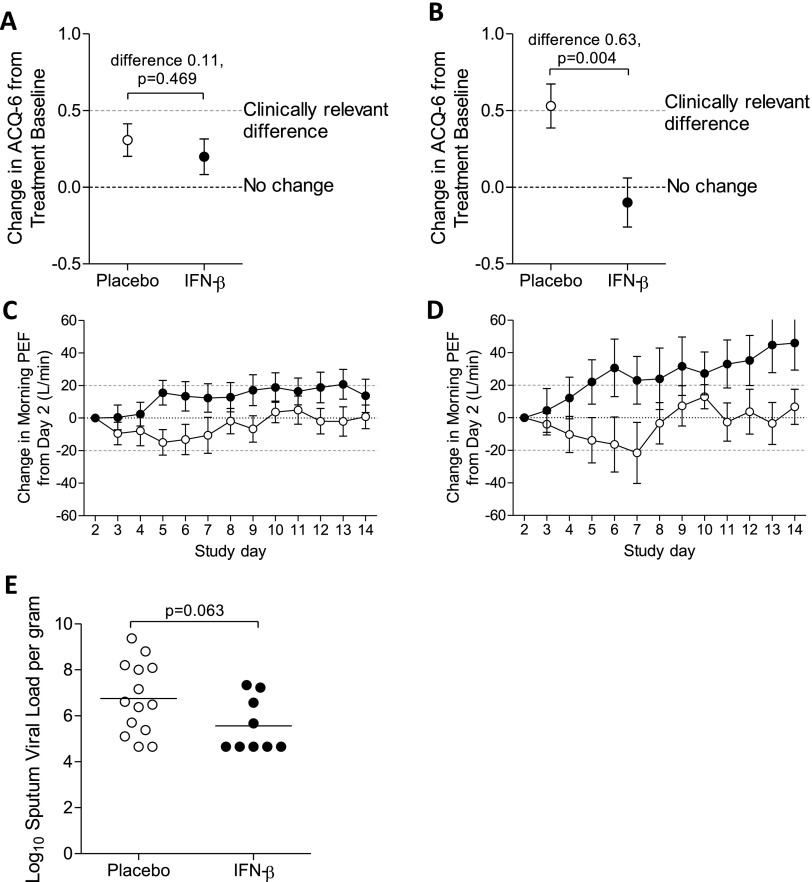
Because of missing data, 11 subjects were excluded from analysis of the primary outcome. Analysis of the effect of treatment on ACQ-6 from treatment baseline (Day 1) to Day 8, the primary outcome, showed that IFN-β was not superior to placebo in the mITT population (Figure 4A). However, IFN-β was superior to placebo when assessing morning PEF (mean between-group difference, 19.47 L/min; 95% confidence interval [CI], 1.62–37.31; P = 0.033 for area under the curve), with steady improvement during IFN-β treatment but an initial decline during the first week of placebo treatment (Figure 4C). There was also a trend toward reduced sputum RV load in the active group on Day 4 (mean between-group difference, −1.20 log10 copies/g; 95% CI, −2.46 to 0.07; P = 0.063) (Figure 4E).
Figure 4.
Effect of nebulized IFN-β on clinical outcomes. Analysis of the modified intention-to-treat population (A) showed that IFN-β treatment (n = 58) did not significantly affect the change in Asthma Control Questionnaire (ACQ-6) scores (LS mean ± SEM) at Day 8 (from treatment baseline) compared with placebo (n = 65). Further analysis of the subgroup with difficult-to-treat asthma (i.e., British Thoracic Society Step 4-5) (B) showed an increase (LS mean ± SEM) in ACQ-6 of 0.53 in the placebo group (n = 30) and a decrease of 0.10 in the IFN-β group (n = 24), a between-group difference of −0.63 (95% confidence interval [CI], −1.05 to −0.21; P = 0.004). In the whole modified intention-to-treat population, treatment with IFN-β (closed symbols) resulted in faster recovery of morning peak expiratory flow (PEF) (C), compared with placebo (open symbols) measured daily at home (P = 0.033 for area under the curve analysis; n = 56 for placebo; n = 58 for IFN-β; dashed line represents clinically relevant difference). This improvement was also seen in the British Thoracic Society Step 4-5 (D) subgroup of patients (P = 0.029 for area under the curve; n = 25 for placebo; n = 22 for IFN-β). Analysis of sputum (E) obtained on Day 4 from patients, in whom rhinovirus was detected in nasal lavage, showed a trend toward reduced rhinovirus load (P = 0.063) in IFN-β–treated patients (n = 9) compared with placebo (n = 14).
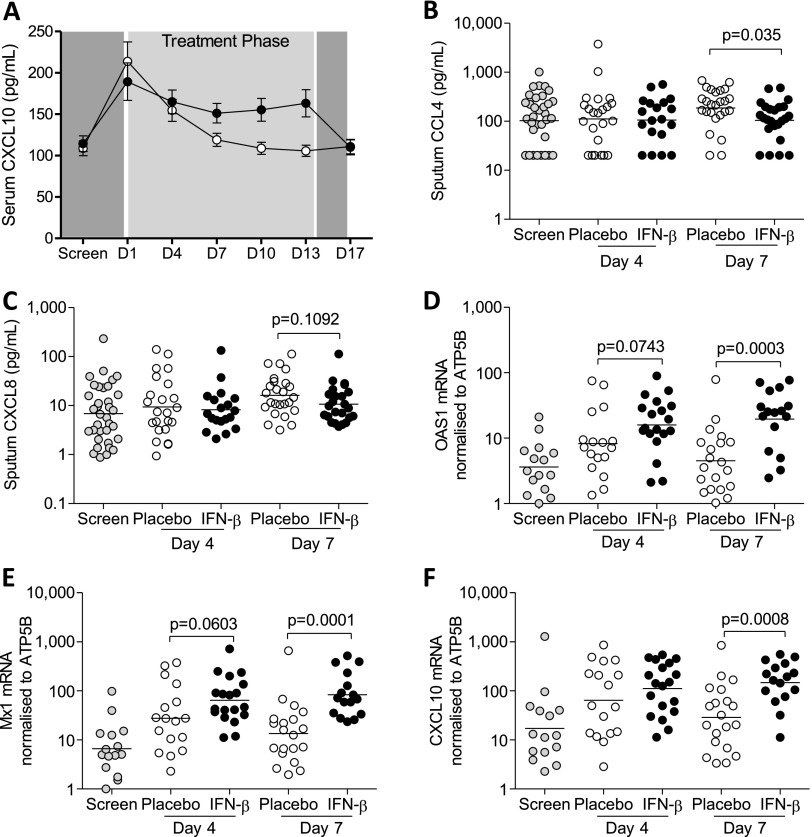
Assessment of blood and sputum biomarkers, using a combination of immunoassay and qPCR, showed changes consistent with enhanced antiviral activity and associated attenuation of proinflammatory responses in patients on IFN-β treatment. Before treatment, serum levels of the antiviral cytokine, CXCL10, increased in both the active and placebo-treated groups relative to screening but remained elevated over several days only during IFN-β treatment (Figure 5A), whereas it fell rapidly on placebo (differences were significant on Days 4, 7, 10, and 13; P = 0.037, 0.001 < 0.001, < 0.001, respectively, analyzed on the log scale via analysis of covariance, including Day 1 as a covariate). There was a significant reduction in the proinflammatory biomarker CCL4 concentration (P = 0.035) in the fluid phase of sputum from patients receiving IFN-β on treatment Day 7 (Figure 5B) and a trend toward reduced CXCL8 concentration (P = 0.109) (Figure 5C), without any between-group differences in these biomarkers on Day 4. Analysis of sputum cell pellets by qPCR showed significantly increased gene expression for antiviral biomarkers OAS1 (Figure 5D), Mx1 (Figure 5E), and CXCL10 (Figure 5F) on Day 7 (P = 0.0003, 0.0001, and 0.0008, respectively) as a consequence of treatment, with trends toward increases on Day 4 for OAS1 and Mx1 (P = 0.07, 0.06, respectively) but not CXCL10.
Figure 5.
Induction of innate immunity by IFN-β treatment. A more sustained rise (mean ± SEM) in serum concentrations of CXCL10 (A) was measured in patients treated with IFN-β (n = 58–65; closed symbols) when compared with those on placebo (n = 62–66; open symbols). Data were analyzed on the log scale via analysis of covariance, including Day 1 as a covariate (P = 0.037, 0.001, < 0.001, and < 0.001 for Days 4, 7, 10, and 13). By comparison with placebo (n = 25) treatment, the concentrations of CCL4 (B) in the sputum fluid phase measured on Day 7 was significantly (P = 0.035, data were log10 transformed and analyzed by unpaired t test) lower in patients on IFN-β (n = 24) and there was a trend toward lower CXCL8 (P = 0.109) (C). Gene expression of antiviral biomarkers OAS1 (D), Mx1 (E), and CXCL10 (F) in sputum cells from patients treated with IFN-β (n = 16) was significantly higher when compared with placebo (n = 20) on Day 7 (P = 0.0003, 0.0001, and 0.0008 respectively). Data are from patients in the modified intention-to-treat population.
Fewer patients who received IFN-β required additional treatment (i.e., oral corticosteroids or antibiotics, for worsening asthma symptoms during the treatment period). Including one subject on placebo who just failed to meet the mITT JCSQ criteria because of data missing on Day 1, five patients receiving placebo required additional treatment (four required oral corticosteroids and one required antibiotics), and one of these was hospitalized, whereas only one patient on IFN-β required oral corticosteroids. Of importance, all the placebo-treated patients who required additional treatment were from the difficult-to-treat patient population categorized as Step 4 according to the BTS severity criteria (18), in keeping with these patients having more severe exacerbations with viral infections.
Analysis of Patient Subsets Based on Difficulty to Treat
Analysis of the placebo-treated patients classified using the BTS criteria showed that only the difficult-to-treat people with asthma at Step 4-5 (i.e., not the Step 2 and 3) had developed a clinically significant increase in asthma symptoms (rise in ACQ-6 ≥ 0.5) after a cold (Figure 4B), supporting the observation that this group was more likely to require additional corticosteroid or antibiotic treatment. Further analysis of treatment effects on the primary outcome in this group of 54 patients showed a mean ACQ-6 increase of 0.53 in the placebo group (n = 30) and a decrease of 0.10 in the IFN-β group (n = 24), a between-group difference of −0.63 (P = 0.004) (Figure 4B, Table 2). Correspondingly, the percentage of patients in whom individual changes in ACQ-6 were greater than or equal to 0.5 was significantly lower for IFN-β (17%) as compared with placebo (50%) (P = 0.012). Similarly, analysis of PEF changes during the treatment period showed significant IFN-β–related effects in Step 4-5 patients (mean between-group difference, 31.42 L/min; 95% CI, 3.21–59.63; P = 0.029 for area under the curve) (Figure 4D, Table 3).
Table 2.
Analysis of ACQ-6 during Treatment Period by Asthma Severity (BTS Treatment Step) in the mITT Population
| Change in ACQ-6 from Treatment Baseline to Day
8 |
||||||||
|---|---|---|---|---|---|---|---|---|
| Whole mITT |
Step 2 |
Step 3 |
Steps 4–5 |
|||||
| Placebo | IFN-β | Placebo | IFN-β | Placebo | IFN-β | Placebo | IFN-β | |
| N | 65 | 58 | 14 | 17 | 21 | 17 | 30 | 24 |
| Least-squares mean | 0.31 | 0.20 | 0.12 | 0.52 | 0.13 | 0.32 | 0.53 | −0.1 |
| Mean difference (95% CI) | −0.11 (−0.40 to 0.19) |
0.41 (−0.15 to 0.97) |
0.19 (−0.31 to 0.70) |
−0.63 (−1.05 to −0.21) |
||||
| P value | 0.469 | 0.15 | 0.45 | 0.004 | ||||
Definition of abbreviations: ACQ = Asthma Control Questionnaire; BTS = British Thoracic Society; CI = confidence interval; mITT = modified intention-to-treat.
The ACQ-6 scores were recorded on Days 1 (treatment baseline) and 8 (see Methods).
Table 3.
Analysis of PEF during the Treatment Period by Asthma Severity (BTS Treatment Step) in the mITT Population
| Change in Morning PEF (AUC) from Day 2 to 14 |
||||||||
|---|---|---|---|---|---|---|---|---|
| Whole mITT |
Step 2 |
Step 3 |
Steps 4–5 |
|||||
| Placebo | IFN-β | Placebo | IFN-β | Placebo | IFN-β | Placebo | IFN-β | |
| N | 56 | 58 | 14 | 16 | 17 | 20 | 25 | 22 |
| Least-squares mean | −5.76 | 13.71 | −3.28 | 2.93 | −7.39 | 9.69 | −6.13 | 25.29 |
| Mean difference (95% CI) | 19.47 (1.62 to 37.31) |
6.21 (−28.99 to 41.42) |
17.08 (−14.62 to 48.78) |
31.42 (3.21 to 59.63) |
||||
| P value | 0.033 | 0.727 | 0.288 | 0.029 | ||||
Definition of abbreviations: AUC = area under the curve; BTS = British Thoracic Society; CI = confidence interval; mITT = modified intention-to-treat; PEF = peak expiratory flow.
Morning PEF was measured daily at home and an area under the curve (AUC) analysis was calculated for change from Day 2 during the treatment period (beginning on Day 2 and ending on Day 14). The AUC data were then divided by the number of days to provide a clinically meaningful outcome (AUC data are shown as L/min/day).
In the Step 2 and 3 subgroups, the active and placebo treatments were not statistically different in respect of either ACQ-6 or PEF. Although the mean rise in ACQ-6 in the Step 2 subgroup receiving IFN-β was greater than 0.5, this did not correspond to the observed changes in PEF, which were in favor of IFN-β (Table 3).
Analysis of the effect of IFN-β treatment on CXCL10 responses in the whole mITT group was also performed on the BTS Step 4-5 subgroup; this showed a treatment-related increase in serum levels of similar significance to those in the entire mITT population (see Figure E2).
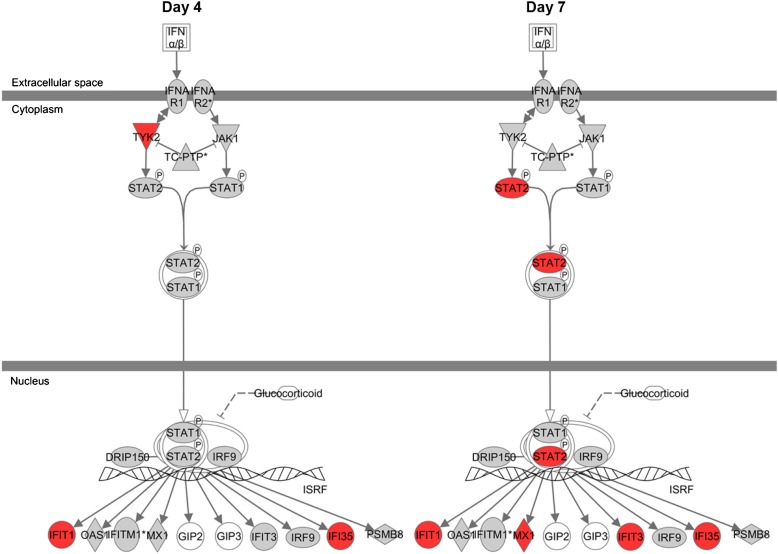
Blood samples collected from patients in the mITT population provided a unique opportunity to assess in a post hoc analysis the effect of treatment on genome-wide gene expression. Good-quality data from the analysis were obtained for 50, 43, and 48 patients from the Step 4-5 mITT group on Days 1, 4, and 7, respectively. Differentially expressed gene lists for IFN-β versus placebo treatment were generated for Days 4 (88 genes) and 7 (94 genes) by selecting probes that were significant at P less than 0.05 and had a fold change of greater than 1.25 following analysis of covariance, including Day 1 as a covariate. Analysis of these data using Ingenuity Pathway Analysis software (Ingenuity Systems [www.ingenuity.com], Mountain View, CA) suggested enhanced type I IFN signaling on both days (Figure 6). After correction for multiple testing using a Benjamini-Hochberg procedure, IFN signaling was the only canonical pathway that was significantly associated with the dataset (P = 0.015, P = 1.02 × 10−5 on Days 4 and 7, respectively). An upstream regulator analysis showed predicted activation of transcriptional factors associated with type I IFN signaling (e.g., IFN regulatory factors and STAT1/ STAT2) (23).
Figure 6.
Up-regulation of the type I IFN canonical pathway by IFN-β treatment. Analysis of circulating blood cell microarray data using Ingenuity Pathway Analysis software (Ingenuity Systems [www.ingenuity.com], Mountain View, CA) showed a significant up-regulation of elements (red) of the IFN-β signaling pathway at both Day 4 and Day 7 after correction for multiple testing using a Benjamini-Hochberg procedure. Data are from patients in the British Thoracic Society Step 4-5 modified intention-to-treat population.
Multiple Regression Analysis
A multiple regression analysis was undertaken to explore whether any of the baseline assessments seemed to be related to subsequent changes in ACQ-6 (a score that reflects asthma symptoms over the previous 7 d) and to any potential treatment effects. The numbers of exacerbations in the last 24 months, screening lung function, BTS step, use of long-acting β-agonists, and baseline cold and asthma symptom scores were all identified as potentially related to the change in ACQ-6 from treatment baseline to Day 8. Based on a subsequent stepwise regression model, the daily asthma symptom score on treatment Day 2 (positive slope; the higher the score the greater the increase in ACQ-6) and the interaction between study treatment and BTS step seemed to have the most influence on the ACQ-6 change. Interestingly, daily cold and asthma symptom scores were worse in the active- compared with the placebo-treated BTS Step 2 patients immediately before the start of treatment. Taking daily asthma symptom scores at the start of treatment (Day 2) into account, the estimated difference in change in ACQ-6 between IFN-β and placebo reduced in both BTS Steps 2 (from 0.41 to 0.3; P = 0.29) and 3 (from 0.19 to 0.09; P = 0.73) but increased in favor of IFN-β in the BTS Step 4-5 group (from −0.63 to −0.76; P < 0.001) (see Table E3).
Safety
Overall, inhaled IFN-β was well tolerated; no patient had to stop treatment and there was little difference between placebo and IFN-β in the frequency of treatment-emergent adverse events with the exception of palpitations experienced by five subjects on IFN-β and none on placebo. All were mild and not considered clinically significant and no other cardiac events were recorded (see Tables E4 and E5).
Discussion
This study is the first demonstration of the ability of a biologic to improve antiviral responses in patients with asthma that is also associated with a beneficial clinical effect. It is, in our view, a good example of translation of observations of enhanced innate immune responses from ex vivo studies (6) into proof of concept clinical studies. The study suggests that, when given at the time of reporting an URTI, inhaled IFN-β can ameliorate the way the airways of patients with asthma respond to viral infection. Although the trial did not show a positive effect on ACQ-6, the primary outcome, treatment with IFN-β, had a positive effect on morning lung function (PEF) and, in parallel, enhanced innate immunity both systemically and in the lungs as assessed by serum CXCL10 concentration and induction of genes for antiviral biomarkers CXCL10, Mx1, and OAS1 in induced sputum. This was associated with reduced proinflammatory cytokines in sputum and a trend toward reduced sputum RV load. Analysis of the moderate to severe people with asthma (Step 4 and 5 according to BTS guidelines) suggested a beneficial clinical effect of treatment, as shown by a mean 0.63 reduction in ACQ-6 score in patients treated with IFN-β (P = 0.004 as compared with those on placebo) that is widely viewed as being clinically significant. Genome-wide gene expression analysis using circulating blood cells from this subset of patients also showed that treatment with nebulized IFN-β enhanced systemic innate immunity.
The lack of effect on the primary outcome is not surprising because the whole mITT population, which also included mild people with asthma, did not sustain an increased ACQ-6 greater than or equal to 0.5 after cold onset during placebo treatment, suggesting that, overall, the exacerbations were not clinically significant in the whole population studied. Furthermore, prespecified analysis (as stated in the statistical analysis plan) of patient subgroups, with asthma severity classified by BTS treatment step criteria (18, 21), showed a significant protective effect of IFN-β in patients from BTS Steps 4-5 (i.e., those patients who require more intensive asthma maintenance treatment). The study, therefore, suggests that IFN-β treatment may be most appropriate for the more difficult to control people with asthma in whom the underlying disease process is likely to be more severe, thus requiring more intensive treatment, and in whom the risks of exacerbations, health impact, and treatment costs are greatest (24).
Asthma exacerbations are a major target for novel therapeutic agents, including such biologics as omalizumab (25) and mepolizumab (26). Although effective at reducing exacerbations, these drugs are given long-term and systemically, and are pharmacodynamically unsuitable for acute administration. ICSs, the mainstay of asthma treatment, also reduce the rate of exacerbations, but doubling their dose at the onset of an exacerbation does not reduce the severity or rate of recovery of the exacerbation (27). Thus, nebulized IFN-β treatment is the first therapy acting on the causal viral pathway that seems able to prevent worsening of asthma symptoms if administered shortly after patients become aware of a cold developing.
IFN-β was selected for clinical development based on abundant evidence of its safety in multiple sclerosis (28–31) and efficacy in preclinical in vitro asthma studies (6, 11). Accordingly, measurement of the biomarker CXCL10 in serum and antiviral biomarkers in sputum cells (OAS1, Mx1, and CXCL10) demonstrated that inhaled IFN-β activated local antiviral defense mechanisms effectively at the dose and frequency selected for this trial while, at the same time, attenuating proinflammatory mediators, as judged by concentrations of CCL4 and CXCL8 measured in airway secretions (sputum). Additionally, IFN-β treatment resulted in a modest, but significant, induction of systemic innate immune responses as judged by the genome-wide gene expression analysis of blood cells, which showed significant differences in genes involved in type I IFN signaling. Together with the sustained and marked elevation of CXCL10 protein levels in blood, this suggests that treatment with IFN-β enhanced innate immunity, which in turn improved the clinical outcome of the cold. The trend toward reduced load of RV in sputum observed with treatment suggests that better antiviral defenses may result in faster clearance of RVs. Further studies, including analysis of other viruses, are required for more definitive proof.
It was noted that BTS Step 2 patients (on low-dose ICS and short-acting β-agonists) receiving IFN-β had a mean increase in ACQ-6 of 0.52. However, this was not significantly different to placebo and analysis of PEF data in this subgroup did not support a deleterious effect of IFN-β, mirroring the lack of IFN-β deficiency observed in vitro in mild patients (12), although this treatment had a positive effect on both ACQ-6 and PEF in patients at BTS Step 4-5. Although the number of patients in the BTS Step 2 group was relatively small (15 placebo; 17 active), we cannot exclude the possibility that IFN-β treatment could have increased asthma symptoms in a small number of patients.
In summary, this study suggests for the first time that administration of IFN-β by inhalation can enhance innate immunity both locally within the lungs and, to a lesser extent, systemically, thereby compensating for the IFN-β deficiency that we have previously demonstrated ex vivo in the epithelium of patients with moderate-severe asthma (6). The possible beneficial clinical effect of treatment seen in patients with moderate-severe asthma that was associated with this enhancement suggests that this treatment may impact favorably on cold-induced asthma exacerbations. The trial was designed and powered on the basis of the entire mITT patient population; therefore, further adequately powered studies focusing on more difficult-to-treat people with asthma are now needed to test the hypothesis that IFN-β is effective in this high-risk patient population.
Footnotes
Supported by Synairgen Research Limited, a University of Southampton spinout company.
Author Contributions: R.D. was the chief investigator of the study and was centrally involved in its design, conduct, and analysis and led the writing of the manuscript. T.H., S.L.J., and H.K.R. were involved in the design, conduct (as PIs in their centers), and analysis of the trial and the writing of the manuscript. P.W., N.C.T., R.N., and D.S. were PIs in their centers and were involved in the writing of the manuscript. F.G. played an important role in the trial design and contributed to the writing of the manuscript. C.B. and S.D. contributed to the laboratory analyses of the measured biomarkers and writing of the manuscript. V.P. undertook the analysis of the whole genome expression analysis. R.M. played a key role in the design and conduct of the study and also critically reviewed the manuscript. S.T.H. and D.E.D. provided strategic input to the trial design and analysis and contributed to the writing of the manuscript. P.M. played a key role in the trial design, conduct of the laboratory analyses and analyses of all the data, and writing of the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.201312-2235OC on June 17, 2014
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Johnston SL, Pattemore PK, Sanderson G, Smith S, Campbell MJ, Josephs LK, Cunningham A, Robinson BS, Myint SH, Ward ME, et al. The relationship between upper respiratory infections and hospital admissions for asthma: a time-trend analysis. Am J Respir Crit Care Med. 1996;154:654–660. doi: 10.1164/ajrccm.154.3.8810601. [DOI] [PubMed] [Google Scholar]
- 2.Corne JM, Marshall C, Smith S, Schreiber J, Sanderson G, Holgate ST, Johnston SL. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet. 2002;359:831–834. doi: 10.1016/S0140-6736(02)07953-9. [DOI] [PubMed] [Google Scholar]
- 3.Papadopoulos NG, Christodoulou I, Rohde G, Agache I, Almqvist C, Bruno A, Bonini S, Bont L, Bossios A, Bousquet J, et al. Viruses and bacteria in acute asthma exacerbations—a GA² LEN-DARE systematic review. Allergy. 2011;66:458–468. doi: 10.1111/j.1398-9995.2010.02505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson DJ, Johnston SL. The role of viruses in acute exacerbations of asthma. J Allergy Clin Immunol. 2010;125:1178–1187, quiz 1188–1189. doi: 10.1016/j.jaci.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, Gibson P, Ohta K, O’Byrne P, Pedersen SE, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143–178. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 6.Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, Holgate ST, Davies DE. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, Bartlett NW, Kebadze T, Mallia P, Stanciu LA, Parker HL, et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12:1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 8.Sykes A, Edwards MR, Macintyre J, del Rosario A, Bakhsoliani E, Trujillo-Torralbo MB, Kon OM, Mallia P, McHale M, Johnston SL. Rhinovirus 16-induced IFN-α and IFN-β are deficient in bronchoalveolar lavage cells in asthmatic patients. J Allergy Clin Immunol. 2012;129:1506–1514, e6. doi: 10.1016/j.jaci.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 9.Bosco A, Ehteshami S, Stern DA, Martinez FD. Decreased activation of inflammatory networks during acute asthma exacerbations is associated with chronic airflow obstruction. Mucosal Immunol. 2010;3:399–409. doi: 10.1038/mi.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards MR, Regamey N, Vareille M, Kieninger E, Gupta A, Shoemark A, Saglani S, Sykes A, Macintyre J, Davies J, et al. Impaired innate interferon induction in severe therapy resistant atopic asthmatic children. Mucosal Immunol. 2013;6:797–806. doi: 10.1038/mi.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cakebread JA, Xu Y, Grainge C, Kehagia V, Howarth PH, Holgate ST, Davies DE. Exogenous IFN-β has antiviral and anti-inflammatory properties in primary bronchial epithelial cells from asthmatic subjects exposed to rhinovirus. J Allergy Clin Immunol. 2011;127:1148–1154, e9. doi: 10.1016/j.jaci.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 12.Lopez-Souza N, Favoreto S, Wong H, Ward T, Yagi S, Schnurr D, Finkbeiner WE, Dolganov GM, Widdicombe JH, Boushey HA, et al. In vitro susceptibility to rhinovirus infection is greater for bronchial than for nasal airway epithelial cells in human subjects. J Allergy Clin Immunol. 2009;123:1384–1390, e2. doi: 10.1016/j.jaci.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sykes A, Macintyre J, Edwards MR, Del Rosario A, Haas J, Gielen V, Kon OM, McHale M, Johnston SL. Rhinovirus-induced interferon production is not deficient in well controlled asthma. Thorax. 2014;69:240–246. doi: 10.1136/thoraxjnl-2012-202909. [DOI] [PubMed] [Google Scholar]
- 14.Boxall C, Dudley S, Beegan R, Tear V, Hrebien S, Lunn K, Monk P. Effect of inhaled sng001 (interferon-beta 1a) on sputum and blood antiviral biomarkers following a respiratory virus infection in asthmatic subjects [abstract] Eur Respir J Suppl. 2013;42:P4369. [Google Scholar]
- 15.Juniper EF, Svensson K, Mörk AC, Ståhl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med. 2005;99:553–558. doi: 10.1016/j.rmed.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Jackson GG, Dowling HF, Spiesman IG, Boand AV. Transmission of the common cold to volunteers under controlled conditions. I. The common cold as a clinical entity. AMA Arch Intern Med. 1958;101:267–278. doi: 10.1001/archinte.1958.00260140099015. [DOI] [PubMed] [Google Scholar]
- 17.Predy GN, Goel V, Lovlin R, Donner A, Stitt L, Basu TK. Efficacy of an extract of North American ginseng containing poly-furanosyl-pyranosyl-saccharides for preventing upper respiratory tract infections: a randomized controlled trial. CMAJ. 2005;173:1043–1048. doi: 10.1503/cmaj.1041470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.British Thoracic Society Scottish Intercollegiate Guidelines Network. British Guideline on the Management of Asthma. Thorax. 2008;63:iv1–iv121. doi: 10.1136/thx.2008.097741. [DOI] [PubMed] [Google Scholar]
- 19.Sorkness RL, Gonzalez-Fernandez G, Billmeyer EE, Evans MD, Gern JE, Jarjour NN. The asthma index: a continuous variable to characterize exacerbations of asthma. J Allergy Clin Immunol. 2008;122:838–840. doi: 10.1016/j.jaci.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 20.Walter MJ, Castro M, Kunselman SJ, Chinchilli VM, Reno M, Ramkumar TP, Avila PC, Boushey HA, Ameredes BT, Bleecker ER, et al. National Heart, Lung and Blood Institute’s Asthma Clinical Research Network. Predicting worsening asthma control following the common cold. Eur Respir J. 2008;32:1548–1554. doi: 10.1183/09031936.00026808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reddel HK, Taylor DR, Bateman ED, Boulet L-P, Boushey HA, Busse WW, Casale TB, Chanez P, Enright PL, Gibson PG, et al. Control obotATSERSTFoA, Exacerbations. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations. Am J Respir Crit Care Med. 2009;180:59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- 22.Kieser M, Friede T. Simple procedures for blinded sample size adjustment that do not affect the type I error rate. Stat Med. 2003;22:3571–3581. doi: 10.1002/sim.1585. [DOI] [PubMed] [Google Scholar]
- 23.Honda K, Takaoka A, Taniguchi T. Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity. 2006;25:349–360. doi: 10.1016/j.immuni.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Serra-Batlles J, Plaza V, Morejón E, Comella A, Brugués J. Costs of asthma according to the degree of severity. Eur Respir J. 1998;12:1322–1326. doi: 10.1183/09031936.98.12061322. [DOI] [PubMed] [Google Scholar]
- 25.Rodrigo GJ, Neffen H, Castro-Rodriguez JA. Efficacy and safety of subcutaneous omalizumab vs placebo as add-on therapy to corticosteroids for children and adults with asthma: a systematic review. Chest. 2011;139:28–35. doi: 10.1378/chest.10-1194. [DOI] [PubMed] [Google Scholar]
- 26.Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, Ortega H, Chanez P. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380:651–659. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 27.Harrison TW, Oborne J, Newton S, Tattersfield AE. Doubling the dose of inhaled corticosteroid to prevent asthma exacerbations: randomised controlled trial. Lancet. 2004;363:271–275. doi: 10.1016/s0140-6736(03)15384-6. [DOI] [PubMed] [Google Scholar]
- 28.Lampl C, You X, Limmroth V. Weekly IM interferon beta-1a in multiple sclerosis patients over 50 years of age. Eur J Neurol. 2012;19:142–148. doi: 10.1111/j.1468-1331.2011.03460.x. [DOI] [PubMed] [Google Scholar]
- 29.De Stefano N, Sormani MP, Stubinski B, Blevins G, Drulovic JS, Issard D, Shotekov P, Gasperini C. Efficacy and safety of subcutaneous interferon β-1a in relapsing-remitting multiple sclerosis: further outcomes from the IMPROVE study. J Neurol Sci. 2012;312:97–101. doi: 10.1016/j.jns.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 30.Kappos L, Traboulsee A, Constantinescu C, Erälinna JP, Forrestal F, Jongen P, Pollard J, Sandberg-Wollheim M, Sindic C, Stubinski B, et al. Long-term subcutaneous interferon beta-1a therapy in patients with relapsing-remitting MS. Neurology. 2006;67:944–953. doi: 10.1212/01.wnl.0000237994.95410.ce. [DOI] [PubMed] [Google Scholar]
- 31.Panitch H, Goodin DS, Francis G, Chang P, Coyle PK, O’Connor P, Monaghan E, Li D, Weinshenker B EVIDENCE Study Group. EVidence of Interferon Dose-response: European North American Compartative Efficacy; University of British Columbia MS/MRI Research Group. Randomized, comparative study of interferon beta-1a treatment regimens in MS: The EVIDENCE Trial. Neurology. 2002;59:1496–1506. doi: 10.1212/01.wnl.0000034080.43681.da. [DOI] [PubMed] [Google Scholar]