To the Editor:
Of the three major particulate matter (PM) size fractions (ultrafine, fine, and coarse), coarse PM (PM2.5–10) has been the least examined in terms of its health effects on susceptible populations, this despite having characteristics that make it particularly likely to affect those with airway diseases such as asthma. For example, PM2.5–10 preferentially deposits in the bronchial airways, a site proximal to asthma pathology (1), and contains biological agents such as endotoxin and allergens that are primary triggers associated with asthma exacerbation (2). We have reported that endotoxin inhalation challenge in subjects with allergic asthma enhances airway inflammation, a key underlying pathophysiological feature of asthma, and modifies airway phagocyte function 4–6 hours after exposure (3). We have also shown that subjects with late-phase allergen-responsive asthma demonstrate enhanced bronchial airway deposition of inhaled particles and slowed clearance of those particles from the central airways 4 hours after particle inhalation, a time coinciding with enhanced inflammation from endotoxin inhalation (4). Hence, specific characteristics of PM2.5–10, together with the fact that individuals with asthma compared with those without asthma have greater sensitivity to air pollutants in general (5), make it likely that individuals with asthma will demonstrate deleterious pulmonary responses after exposure to PM2.5–10. These responses, however, remain largely speculative because they have been described only in healthy individuals. Indeed, we have previously shown that healthy individuals exposed to coarse size (PM2.5–10) concentrated ambient particles (CAPs) demonstrated only modest increases in pulmonary neutrophil levels with no increase in inflammatory mediators (6). The assumption that individuals with asthma will demonstrate a comparatively more robust inflammatory response than those without asthma must be verified by a proof-of-concept study. We undertook a proof-of-concept study to determine whether exposure to coarse size (PM2.5–10) CAPs, at a concentration previously shown to induce only mild changes in healthy subjects (6), would induce robust pulmonary inflammatory and innate immune alterations in subjects with allergic asthma.
The experimental design and details of the study replicate those of our previously published coarse CAP study in healthy subjects without asthma (Graff and colleagues, 2009 [6]). Specifically, the urban PM exposure source, exposure months, mechanism of PM concentration, concentrator type, and exposure chamber used in this study were identical to those used in the Graff and colleagues study (6). Furthermore, the PM concentration and calculated dose compared closely between the two studies.
This study was approved by the institutional review board at the University of North Carolina (Chapel Hill, NC). In brief, this study was a single-blind crossover study of 10 subjects with mild to moderate allergic asthma, in which each subject was studied on two occasions (2-h exposure to CAPs or filtered air [FA] from ambient Chapel Hill, NC) at least 4 weeks apart. The concentration of coarse particles suspended in the particulate exposure chamber at the U.S. Environmental Protection Agency (EPA) Human Studies facility in North Carolina was measured on a continuous scale and varied from subject to subject depending on the outdoor particle concentration that day. There was a mean overall total particle concentration of 101.8 ± 18.0 μg/m3 and a PM2.5–10 concentration of 86.9 ± 17.4 μg/m3 on CAP days (FA days had a mean total particle concentration of 1.2 μg/m3). The mean coarse PM concentration (101.8 ± 18.0 μg/m3) measured in this study was not unrealistically high and can be found in many areas throughout the world, including locations in the U.S. Southwest (7). Individual and overall coarse PM exposure data are shown in Table E1 (see the online supplement). Lung cells and fluid-phase components were obtained by bronchoalveolar lavage (BAL) and bronchial wash (BW, the first 30 ml of BAL recovered) 24 hours postexposure. Differential leukocytes and fluid-phase components were examined as previously described (6). Flow cytometry was performed on BAL leukocytes for assessment of cell surface phenotypes associated with innate host defense (CD11b/CR3, mCD14, CD64/FcγRI [Fc γ receptor type I]), antigen presentation/T-cell interaction (CD23/low-affinity IgE, HLA-DR, CD86/B7.2, CD80/B7.1, CD40), and inflammation (CD16/FcγRIII). The detailed flow methodology appears in the online supplement and in our review (8). Informed consent was obtained before study and all volunteers with asthma (n = 10; age, 18–45 yr) were nonsmokers with mild to moderate disease severity. Inclusion criteria, baseline medical assessment, and study procedures are detailed in the online supplement. Parametric and nonparametric paired analyses were used to compare all study end points 20 hours after filtered air and CAP exposures, and a linear mixed-effects model was used to compare with the data of Graff and colleagues (6). Significance was set at α = 0.05.
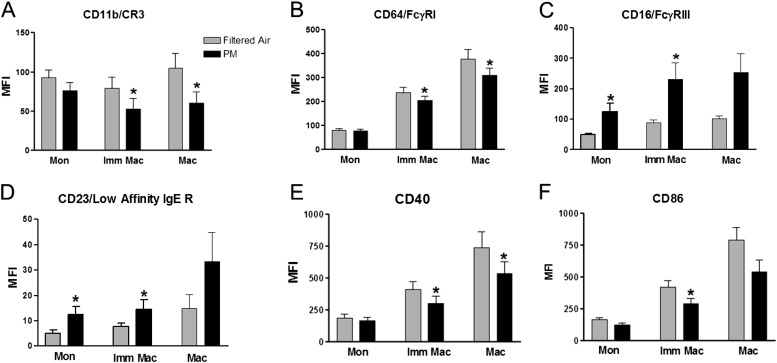
As shown in Table 1, we observed a robust increase in BW polymorphonuclear neutrophils after CAP exposure (8 vs. 13%), an effect significantly (P < 0.05) different when compared with our earlier study in subjects without asthma (6). Furthermore, we demonstrated significantly elevated levels of IL-1β and IL-8 in both BW and BAL. Although BAL IL-6 was not significantly different after exposure to CAPs, it was significantly negatively associated with PM dose (R = –0.65) and PM concentration (R = –0.62). These negative IL-6 associations were unexpected because IL-6 is typically positively associated with increased ambient PM levels (9). However, one explanation may be that IL-6–producing alveolar macrophages have become tolerant from preexisting ambient PM exposure and elevated airway inflammation, an underlying feature of asthma, thereby producing the negative association observed here after an acute exposure to PM2.5–10. No change in lung function (FEV1, FVC) was reported after CAP exposure (Table E2), and with the exception of decreased blood IL-6 after CAP exposure (3.255 ± 1.068 vs. 1.740 ± 0.2914 pg/ml), no markers of systemic inflammation were modified by CAPs (IL-8, tumor necrosis factor-α [TNF-α], CD40 ligand [CD40L], E-selectin, soluble vascular cell adhesion molecule-1 [sVCAM1], plasminogen, fibrin, C-reactive protein [CRP], fibrinogen, soluble intercellular adhesion molecule-1 [sICAM-1], myeloperoxidase [MPO]). Immunophenotyping of immature and mature macrophages revealed decreased cell surface expression (mean fluorescence intensity [MFI]) of innate immune receptors (CD11b/CR3, CD64/FcγRI) (Figures 1A and 1B) and antigen presentation receptors (CD40, CD86/B7.2) (Figures 1E and 1F); with increased expression of inflammatory receptors CD16/FcγRIII and the low-affinity IgE receptor (CD23) (Figures 1C and 1D) after CAP exposure. It is intriguing that we have found similar inflammatory responses and cell surface phenotype changes in subjects with asthma exposed to ozone (e.g., elevated IL-1β and IL-8 and increased expression of low-affinity IgE receptor/CD23) (10), and that subjects with asthma have enhanced response to allergen after challenge with both ozone and PM component endotoxin (11). Like endotoxin and ozone, coarse PM induced a neutrophil response albeit at a comparatively reduced magnitude. This component of the overall PM response may be nonspecific and in common with xenobiotics in general. However, downstream effects of coarse PM appear to differ from those of ozone and, depending on the dose, may be similar or different from those of endotoxin. Because CAP endotoxin levels were not measured in this study or in the study by Graff and colleagues (6), we were unable to assess or compare the impact of endotoxin as a driver of observed cell responses. However, our previous mechanistic coarse PM studies (2, 12) clearly point to endotoxin as an important driver of immune cell responses after coarse PM exposure. We also note that the up-regulation of the CD23/IgE receptor reported here suggests an asthma-specific pathway induced by coarse PM not typically observed with other xenobiotics, such as ozone or endotoxin. The observations reported here, namely significant CAP-induced pulmonary inflammation, altered innate host defense response, and potentially enhanced IgE signaling, lead us to hypothesize that coarse-mode CAP exposure increases the responsiveness of individuals with allergic asthma to inhaled allergens and therefore enhances the risk of exacerbation.
Table 1.
Airway Neutrophil Proportion and Inflammatory Cytokine Levels
| BW |
BAL |
||||||
|---|---|---|---|---|---|---|---|
| 20 h after FA | 20 h after CAPs | Mean Individual Change from FA (%) | 20 h after FA | 20 h after CAPs | Mean Individual Change from FA (%) | ||
| PMNs, % | 8 (3) | 13 (3)* | 1 (0.2) | 2 (0.3) | |||
| IL-1β, pg/ml | 448 (164) | 680 (117) | 351 (139)† | 109 (18) | 206 (39)* | 155 (53)† | |
| IL-6, pg/ml | 716 (292) | 801 (110) | 206 (115) | 513 (82) | 610 (108) | 34 (22) | |
| IL-8, pg/ml | 20,660 (2,778) | 38,000 (6,184)* | 136 (69)† | 8,315 (1,207) | 11,300 (1,487)* | 51 (20) | |
| TNF-α, pg/ml | 205 (45) | 271 (29) | 168 (65) | 253 (43) | 276 (47) | 5 (16) | |
Definition of abbreviations: BAL = bronchoalveolar lavage; BW = bronchial wash; CAPs = concentrated ambient particles; FA = filtered air; PMNs = polymorphonuclear neutrophils; TNF-α = tumor necrosis factor-α.
Data are presented as means (±SEM).
P < 0.05 versus post-FA.
P < 0.05 versus 0% change from FA.
Figure 1.
Cell surface marker expression (MFI) on BAL inflammatory cells after filtered air (FA) and particulate matter (PM) exposure. BAL = bronchoalveolar lavage; IgE R = IgE receptor; Imm Mac = immature macrophages; Mac = macrophages; MFI = mean fluorescence intensity; Mon = monocytes. *P < 0.05 versus FA.
This proof-of-concept study confirms the assumption that coarse-size PM, like other pollutants, can initiate deleterious responses in individuals with asthma at concentrations not observed in healthy individuals without asthma. These responses include increased airway inflammation and alterations in immune cell phenotype expression. Our data suggest that individuals with asthma have increased susceptibility to coarse-size PM exposure compared with healthy individuals without asthma, and interventions focused on these responses may be useful approaches to mitigate the impact of PM air pollution in those with asthma.
Footnotes
Supported by NIEHS grant R01ES012706 and US EPA cooperative agreement CR 83346301.
Author Contributions: Conception and design: N.E.A., Y.C.T.H., H.K., A.G.R., R.D., and D.B.P.; analysis and interpretation: N.E.A., A.G.R., R.D., and D.B.P.; drafting of the manuscript: N.E.A., R.D., and D.B.P.
Although the research described in this letter has been funded wholly or in part by the U.S. Environmental Protection Agency through cooperative agreement CR 83346301 with the Center for Environmental Medicine and Lung Biology at the University of North Carolina at Chapel Hill, it has not been subjected to the Agency’s required peer and policy review, and therefore does not necessarily reflect the views of the Agency and no official endorsement should be inferred.
This letter has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Kim CS, Hu SC. Regional deposition of inhaled particles in human lungs: comparison between men and women. J Appl Physiol. 1998;84:1834–1844. doi: 10.1152/jappl.1998.84.6.1834. [DOI] [PubMed] [Google Scholar]
- 2.Alexis NE, Lay JC, Zeman K, Bennett WE, Peden DB, Soukup JM, Devlin RB, Becker S. Biological material on inhaled coarse fraction particulate matter activates airway phagocytes in vivo in healthy volunteers. J Allergy Clin Immunol. 2006;117:1396–1403. doi: 10.1016/j.jaci.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 3.Alexis NE, Eldridge MW, Peden DB. Effect of inhaled endotoxin on airway and circulating inflammatory cell phagocytosis and CD11b expression in atopic asthmatic subjects. J Allergy Clin Immunol. 2003;112:353–361. doi: 10.1067/mai.2003.1651. [DOI] [PubMed] [Google Scholar]
- 4.Bennett WD, Herbst M, Alexis NE, Zeman KL, Wu J, Hernandez ML, Peden DB. Effect of inhaled dust mite allergen on regional particle deposition and mucociliary clearance in allergic asthmatics. Clin Exp Allergy. 2011;41:1719–1728. doi: 10.1111/j.1365-2222.2011.03814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holgate ST, Sandström T, Frew AJ, Stenfors N, Nördenhall C, Salvi S, Blomberg A, Helleday R, Söderberg M. Health effects of acute exposure to air pollution. I. Healthy and asthmatic subjects exposed to diesel exhaust. Res Rep Health Eff Inst. 2003;112:1–30, discussion 51–67. [PubMed] [Google Scholar]
- 6.Graff DW, Cascio WE, Rappold A, Zhou H, Huang YC, Devlin RB. Exposure to concentrated coarse air pollution particles causes mild cardiopulmonary effects in healthy young adults. Environ Health Perspect. 2009;117:1089–1094. doi: 10.1289/ehp0900558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson WE, Suh HH. Fine particles and coarse particles: concentration relationships relevant to epidemiologic studies. J Air Waste Manag Assoc. 1997;47:1238–1249. doi: 10.1080/10473289.1997.10464074. [DOI] [PubMed] [Google Scholar]
- 8.Lay JC, Peden DB, Alexis NE. Flow cytometry of sputum: assessing inflammation and immune response elements in the bronchial airways. Inhal Toxicol. 2011;23:392–406. doi: 10.3109/08958378.2011.575568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubowsky SD, Suh H, Schwartz J, Coull BA, Gold DR. Diabetes, obesity, and hypertension may enhance associations between air pollution and markers of systemic inflammation. Environ Health Perspect. 2006;114:992–998. doi: 10.1289/ehp.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hernandez ML, Lay JC, Harris B, Esther CR, Jr, Brickey WJ, Bromberg PA, Diaz-Sanchez D, Devlin RB, Kleeberger SR, Alexis NE, et al. Atopic asthmatic subjects but not atopic subjects without asthma have enhanced inflammatory response to ozone. J Allergy Clin Immunol. 2010;126:537–544, e1. doi: 10.1016/j.jaci.2010.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernstein JA, Alexis N, Barnes C, Bernstein IL, Bernstein JA, Nel A, Peden D, Diaz-Sanchez D, Tarlo SM, Williams PB. Health effects of air pollution. J Allergy Clin Immunol. 2004;114:1116–1123. doi: 10.1016/j.jaci.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 12.Becker S, Soukup JM, Sioutas C, Cassee FR. Response of human alveolar macrophages to ultrafine, fine, and coarse urban air pollution particles. Exp Lung Res. 2003;29:29–44. doi: 10.1080/01902140303762. [DOI] [PubMed] [Google Scholar]