Abstract
Rationale: Pulmonary hypertension (PH) associated with fibrotic idiopathic interstitial pneumonia (IIP; idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia) confers important additional morbidity and mortality.
Objectives: To evaluate the safety and clinical efficacy of the dual endothelin-1 receptor antagonist bosentan in this patient group.
Methods: In a randomized, double-blind, placebo-controlled study, 60 patients with fibrotic IIP and right heart catheter confirmed PH were randomized 2:1 to bosentan (n = 40) or placebo (n = 20). The primary study endpoint was a fall from baseline pulmonary vascular resistance index (PVRi) of 20% or more over 16 weeks.
Measurements and Main Results: Sixty patients (42 men; mean age, 66.6 ± 9.2 yr), with a mean pulmonary artery pressure of 36.0 (± 8.9) mm Hg, PVRi 13.0 (± 6.7) Wood Units/m2 and reduced cardiac index of 2.21 (± 0.5) L/min/m2 were recruited to the study. Accounting for deaths and withdrawals, paired right heart catheter data were available for analysis in 39 patients (bosentan = 25, placebo = 14). No difference in the primary outcome was detected, with seven (28.0%) patients receiving bosentan, and four (28.6%) receiving placebo achieving a reduction in PVRi of greater than or equal to 20% (P = 0.97) at 16 weeks. There was no change in functional capacity or symptoms between the two groups at 16 weeks, nor any difference in rates of serious adverse events or deaths (three deaths in each group).
Conclusions: This study shows no difference in invasive pulmonary hemodynamics, functional capacity, or symptoms between the bosentan and placebo groups over 16 weeks. Our data do not support the use of the dual endothelin-1 receptor antagonist, bosentan, in patients with PH and fibrotic IIP.
Clinical trial registered with www.clinicaltrials.gov (NCT 00637065).
Keywords: hypertension, pulmonary, interstitial lung diseases, clinical trial
At a Glance Summary
Scientific Knowledge on the Subject
Pulmonary hypertension (PH) complicating interstitial lung disease is associated with considerable morbidity and mortality. At present there are very few randomized trials published reporting outcome data in patients with interstitial lung disease treated with pulmonary arterial hypertension–specific therapies, despite recommendations from the last two PH World Symposia (Dana Point and Nice).
What This Study Adds to the Field
This is the first randomized, double-blind, placebo-controlled study evaluating the pulmonary arterial hypertension–specific therapy bosentan, an endothelin receptor antagonist, in PH associated with fibrotic idiopathic interstitial pneumonia. This study shows no difference in invasive pulmonary hemodynamics, functional capacity, or symptoms between the bosentan- and placebo-treated groups over 16 weeks. Our data do not support the use of bosentan in patients with PH and fibrotic idiopathic interstitial pneumonia.
In fibrotic idiopathic interstitial pneumonia (IIP), including idiopathic pulmonary fibrosis (IPF) and nonspecific interstitial pneumonia (NSIP), the presence of pulmonary hypertension (PH) has a major adverse impact on morbidity and mortality (1, 2). Despite significant therapeutic advances in many forms of pulmonary arterial hypertension (PAH), lack of robust, controlled data has limited treatment recommendations for PH in this patient group.
PH associated with advanced IIP is relatively common; the prevalence of PH in patients with IPF referred for lung transplantation is estimated between 46% and 85% (3, 4). Assessment of pulmonary hemodynamics (including pulmonary vascular resistance [PVR] and right ventricular [RV] function) carries prognostic importance (5, 6). However, the safety and efficacy of PH-specific therapies in IIP-PH have been evaluated in only a handful of studies (7–10). None of these, however, have had the rigor of a placebo-controlled, blinded, randomized design.
Endothelin (ET)-1 is a potent vasoconstrictor, which also mediates cell proliferation, fibrosis, and inflammation (11, 12). Bosentan (Actelion Pharmaceuticals, Allschwil, Switzerland), a dual ET-1 receptor antagonist (ERA), has been shown to improve pulmonary hemodynamics and functional capacity in various conditions associated with PAH (13–15), and has been linked to improved outcomes in uncontrolled studies in interstitial lung disease (ILD) with associated PH (10, 16).
This is the first and only randomized controlled study evaluating the safety and efficacy of PAH-specific therapy in patients with PH associated with fibrotic IIP. Some of these results have previously been reported in the form of an abstract (17).
Methods
Study Design and Patient Selection
The BPHIT study was conducted as a 16-week multicenter, randomized (2:1 bosentan: placebo), double-blind, placebo-controlled trial designed to evaluate the safety and efficacy of bosentan in patients with fibrotic IIP and right heart catheter (RHC)-confirmed PH. Study participants were recruited between September 2008 and March 2012. Patients aged 18–80 years with a diagnosis of IPF or idiopathic fibrotic NSIP (according to multidisciplinary consensus at a specialist ILD referral center and in concordance with the American Thoracic Society/European Respiratory Society Multidisciplinary Consensus Statement [2002]) and PH were eligible for study enrollment. All patients received supplemental oxygen for resting, nocturnal, and/or exercise hypoxemia as appropriate, and according to our unit protocol during the course of the study.
PH was defined on RHC as a mean pulmonary artery pressure (mPAP) of greater than or equal to 25 mm Hg with either left ventricular end diastolic pressure or pulmonary capillary wedge pressure of less than or equal to 15 mm Hg. PVR index (PVRi) was calculated as follows: (mPAP-left atrial pressure)/cardiac index (CI). Cardiac output (CO) was calculated by the Fick equation: CO = (oxygen consumption)/(arterial oxygen saturation-mixed venous oxygen saturation) using standardized reference tables to estimate oxygen consumption. This value was then indexed according to calculated body surface area.
Important exclusion criteria included significant hepatic or renal impairment, a greater extent of emphysema compared with interstitial change on high-resolution computed tomography, or clinically overt ischemic heart disease. No PAH-specific treatments (prostanoids, phosphodiesterase type V inhibitors, or other ERAs) were permitted for at least 3 months before enrollment. All patients who completed the randomized trial were offered bosentan on an open-label basis, with masking of their treatment allocation during the randomized phase preserved until completion of the study. The study was performed in accordance with the Good Clinical Practice guidelines, and the guiding principles detailed in the Declaration of Helsinki.
Ethical approval was obtained from the nationally approved Research Ethics Committee and each center obtained local specific R&D approval. All patients gave fully informed written consent to participate. The study complied with regulatory requirements of the European Union. Adverse events were reviewed by experienced staff masked to treatment allocation to verify classification and potential association with treatment. An independent data safety monitoring board reviewed safety data at regular intervals throughout the study.
Study Drug
Study drug (bosentan or placebo) was administered orally twice daily for 16 weeks. The initial dose was 62.5 mg twice daily, with medically supervised up titration as tolerated to 125 mg twice daily after 1 month.
Study Endpoints
The primary study endpoint was a fall from baseline PVRi of 20% or more over 16 weeks. After initial steering committee review, PVRi was chosen as the primary endpoint partly in relation to work performed by our group showing the importance of PVR in predicting outcome in the 12 months following right heart catheterization in this patient group (6). A 20% change was considered a clinically meaningful change and the largest change that allowed the study to be powered. Secondary study endpoints included changes from baseline in pulmonary hemodynamics (mPAP, right atrial pressure, CI, absolute PVRi), exercise capacity (as measured by the 6-minute-walk test [6MWT]), World Health Organization functional class, quality of life (as measured by the Cambridge Pulmonary Hypertension Outcome Review [CAMPHOR] questionnaire), lung function (as determined by diffusing capacity of carbon monoxide [DlCO], transfer factor, and composite physiologic index [CPI] [18]), oxygen saturation at rest, plasma brain natriuretic peptide (BNP) concentrations, echocardiographic parameters (RV systolic pressure, tricuspid annular plane excursion, and RV inlet size), and disease progression (defined as a >15% fall in DlCO, death, or transplantation).
Statistical Analysis
The primary endpoint is a categorical endpoint: a fall from baseline PVRi of 20% over 16 weeks. Assuming 1% of the control group and 30% of the treatment group would achieve this threshold, a group sample size of 48 (2:1, bosentan/placebo) would achieve 81% power to detect a difference between the group proportions of 0.29 (using the two-sided z test with pooled variance; a = 0.05, b = 0.19). Allowing for a 20% withdrawal from follow-up rate (for whatever reason) the sample size was adjusted to 60 patients in total.
Statistical analyses were performed using R version 3·0·0 (R: A Language and Environment for Statistical Computing, http://www.R-project.org/). Descriptive statistics were presented as numbers (proportion) for categorical and mean ± SD for continuous variables. The primary outcome was analyzed using Fisher exact test. Patients who died or withdrew from the study before the final cardiac catheter were by default excluded from the analysis. No substitution rules were used for missing data in our predefined analysis. As a sensitivity, post hoc analysis, the primary outcome was also analyzed using substitution: all patients who died or withdrew during the study and had no final catheter were assigned the worst value for the primary endpoint (i.e., no improvement in PVRi).
The primary endpoint was also assessed using Fisher test in predefined subgroups, according to age, sex, 6MWT distance, mPAP, PVRi, PVR, DlCO, and CPI at baseline. For graphical representation of the subgroup analysis, the odds ratio (and 95% confidence intervals) of the primary endpoint in the two randomization groups was also calculated for each of the above subgroups, using logistic regression. Secondary endpoints were assessed using Wilcoxon signed rank test or Fisher test as appropriate. Safety outcomes were reported as rates in the overall and randomization groups and comparisons were made using Fisher exact test. A two-sided P value less than 0.05 was considered indicative of statistical significance.
Results
Patient Disposition
A total of 60 patients were recruited to the study from eight hospitals across the United Kingdom. Paired RHC data were available for analysis in 39 patients (bosentan = 25, placebo = 14). Six patients died during the study period (bosentan = 3, placebo = 3), whereas six patients were withdrawn from the study following serious adverse events (SAE; bosentan = 5, placebo = 1; described later in the safety section). Four patients were withdrawn after randomization but before commencing the study drug because of protocol violations (pulmonary emboli discovered on computerized tomography pulmonary angiogram in two patients, newly diagnosed lung malignancy in one patient, and severe obstructive sleep apnea in one patient), one patient was withdrawn because of progression of underlying fibrotic lung disease, and three patients withdrew consent for other reasons. Finally, the follow-up catheter in one patient did not include an estimation of left atrial pressure, for technical reasons, and therefore PVRi was not calculated. Patient randomization and retention is summarized in Figure 1.
Figure 1.
CONSORT diagram. Flow diagram demonstrating the outcome of all 60 patients who consented to participate in the BPHIT trial. RHC = right heart catheter; SAE = serious adverse event.
Baseline Demographics and Clinical Characteristics
Sixty patients (42 men) with a mean age 66.6 (±9.2) years were recruited to the study. Forty-six patients had a diagnosis of IPF, with fibrotic NSIP in 14. Baseline assessment revealed significant PH with mPAP of 36.0 (±8.9) mm Hg, PVRi 13.0 (±6.7) Wood units/m2, and reduced CI of 2.21 (±0.5) L/min/m2. Patients had significant functional impairment, with World Health Organization functional Class III in 26 (43.3%) and Class IV in 30 (50%) patients. Mean FVC of 54.2% (±21.2) and mean CPI of 67.8 (±8.3) indicated advanced fibrotic IIP. The two randomized treatment groups were well matched with respect to baseline patient characteristics (Table 1). Table E1 in the online supplement summarizes the specific background ILD therapy for the patients enrolled into the study.
Table 1.
Demographic and Clinical Information of the Patients at Enrollment
| Bosentan (n = 40) | Placebo (n = 20) | P Value | |
|---|---|---|---|
| Age | 66.4 (9.2) | 66.9 (9.3) | 0.77 |
| Male | 27 (67.5%) | 15 (75%) | NA |
| WHO FC II/III/IV | 2/17/21 | 2/9/9 | NA |
| 6MWD, m | 149.3 (99.6) | 170.7 (97.0) | 0.39 |
| mPAP, mm Hg | 37.2 (9.9) | 33.5 (6.1) | 0.30 |
| mRAP, mm Hg | 7.4 (5.4) | 6.1 (5.4) | 0.26 |
| PVR, Wood units | 7.4 (4.0) | 6.0 (2.4) | 0.33 |
| PVR index, Wood Units/m2 | 13.9 (7.5) | 11.4 (4.5) | 0.31 |
| Cardiac index, L/min/m2 | 2.2 (0.5) | 2.2 (0.5) | 0.68 |
| DlCO, % predicted | 21.3 (9.6) | 21.2 (7.5) | 0.88 |
| Kco, % predicted | 45.1 (21.8) | 48.8 (20.5) | 0.43 |
| FEV1, % predicted | 58.8 (20.8) | 49.8 (19.8) | 0.10 |
| FVC%, % predicted | 55.7 (19.8) | 51.1 (24.0) | 0.28 |
| CPI | 67.5 (8.6) | 68.2 (7.9) | 0.79 |
Definition of abbreviations: 6MWD = 6-minute-walk distance; CPI = composite physiologic index; DlCO = diffusing capacity of carbon monoxide; Kco = transfer factor; mPAP = mean pulmonary artery pressure; mRAP = mean right atrial pressure; PVR = pulmonary vascular resistance; WHO FC = World Health Organization functional class.
All values are presented as mean (standard deviation), with the exception of sex and WHO functional class, which are presented as absolute numbers.
Efficacy
Primary endpoint analysis.
No difference in the primary outcome measure was detected between the active treatment and the placebo groups. In the bosentan arm, 7 of 25 (28.0%) patients achieved a reduction in PVRi of greater than or equal to 20%, compared with 4 of 14 (28.6%) in the placebo arm (P = 0.97; Fisher exact test) (Figure 2). In our post hoc analysis using substitution for missing data in patients who died or withdrew before the final RHC, no significant difference was again detected between the two allocation arms (P = 1.0). In addition, 26.7% of patients in the IPF group reached the primary PVRi endpoint versus 33.3% in the NSIP group (P = 0.69). Within the NSIP and IPF subgroups, there was no significant difference in the number of patients reaching the primary endpoint between placebo and bosentan patients (data not shown).
Figure 2.
Proportion of patients with a change in pulmonary vascular resistance index of 20% from baseline. After 16 weeks, there was no difference in the percentage of patients who achieved a greater than or equal to 20% reduction in pulmonary vascular resistance index between the bosentan- and placebo-treated groups.
Secondary endpoint analyses.
Outcome results are summarized in Table 2.
Table 2.
Change in Prespecified Outcomes at 16 Weeks
| Bosentan (n = 25) | Placebo (n = 14) | P Value | |
|---|---|---|---|
| ∆ PVR index | −1.1 (3.9) | 0.8 (4.2) | 0.19 |
| ∆ mPAP, mm Hg | −1.3 (5.6) | 0.2 (7.4) | 0.43 |
| ∆ mRAP, mm Hg | −1.7 (5.5) | −0.8 (5.2) | 0.74 |
| ∆ Cardiac index, L/min/m2 | 0.1 (0.5) | −0.1 (0.4) | 0.31 |
| ∆ SpO2, % | −0.76 (4.0) | −0.57 (3.9) | 0.74 |
| ∆ 6MWD, m | −25.9 (56.7) | −53.1 (66.9) | 0.42 |
| ∆ 6MWT, dyspnea pre | 0.8 (1.4) | 1.1 (2.0) | 0.98 |
| ∆ 6MWT, dyspnea post | −0.04 (2.3) | 0.0 (1.2) | 0.51 |
| ∆ 6MWT, fatigue pre | 0.4 (2.9) | 0.4 (1.7) | 0.71 |
| ∆ 6MWT, fatigue post | 0.2 (3.7) | −1.8 (1.8) | 0.12 |
| ∆ CAMPHOR, symptom score | 0.0 (4.5) | 0.4 (3.5) | 0.92 |
| ∆ CAMPHOR, activities score | 1.2 (3.8) | 0.9 (4.5) | 0.94 |
| ∆ CAMPHOR, QOL score | 0.2 (4.3) | 0.3 (3.8) | 0.96 |
| ∆ DlCO, % predicted | −3.2 (6.9) | −2.1 (4.9) | 0.96 |
| ∆ Kco, % predicted | −5.8 (8.9) | −0.2 (14.5) | 0.54 |
| ∆ FVC, % predicted | −0.9 (6.8) | −2.6 (23.2) | 0.96 |
| ∆ CPI | 2.0 (5.2) | 0.4 (6.0) | 0.95 |
| ∆ BNP, pg/ml | 13.0 (90.5) | 21.0 (50.4) | 0.32 |
| ∆ TAPSE, mm | 1.8 (4.4) | 1.4 (4.7) | 0.56 |
| ∆ RV inlet size, mm | 0.4 (0.8) | −0.1 (0.6) | 0.12 |
Definition of abbreviations: 6MWD = 6-minute-walk distance; 6MWT = 6-minute-walk test; BNP = brain natriuretic peptide; CAMPHOR = Cambridge Pulmonary Hypertension Outcome Review; CPI = composite physiologic index; DlCO = diffusing capacity of carbon monoxide; Kco = transfer factor; mPAP = mean pulmonary artery pressure; mRAP = mean right atrial pressure; PVR = pulmonary vascular resistance; QOL = quality of life; RV = right ventricle; Spo2 = oxygen saturation; TAPSE = tricuspid annular plane systolic excursion.
All values are presented as mean (standard deviation).
Functional capacity and quality of life.
The mean 6-minute-walk distance (6MWD) decreased by 25.9 (±56.7) m in patients treated with bosentan, compared with a decline of 53.1 (±66.9) m in those patients treated with placebo (P = 0.42). Pre- and post-6MWT Borg scores for fatigue and dyspnea did not differ between patients receiving bosentan or placebo. Change in total CAMPHOR score did not differ between the bosentan- and placebo-treated groups (1.41 ± 9.28 vs. 1.57 ± 8.46; P = 0.69). Similarly, changes in CAMPHOR scores for symptoms (0.0 ± 4.51 vs. 0.43 ± 3.50; P = 0.92), activity (1.18 ± 3.80 vs. 0.86 ± 4.49; P = 0.94), and quality of life (0.23 ± 4.32 vs. 0.29 ± 3.77; P = 0.96) did not differ between the two groups.
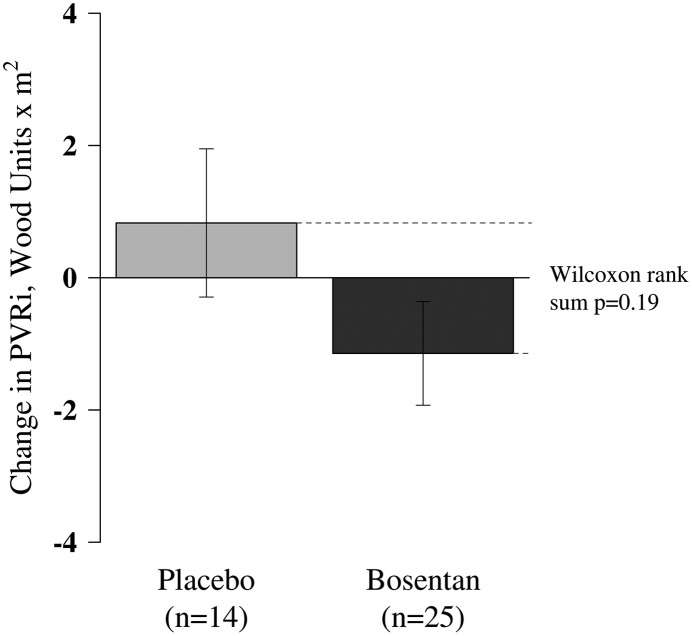
Hemodynamic variables.
Treatment with bosentan did not result in significant changes in hemodynamic parameters. In the bosentan-treated group, there was a mean reduction in PVRi of 1.14 (±3.92) Wood units/m2 compared with a 0.83 (±4.19) Wood units/m2 increase in the placebo group (P = 0.19) (Figure 3). Mean PAP declined by 1.31 (±5.55) mm Hg in the bosentan group, compared with an increase of 0.21 (±7.40) mm Hg in the placebo group (P = 0.43), whereas mean right atrial pressure declined by 1.74 (±5.50) mm Hg in the bosentan group, compared with a decline of 0.77 (±5.15) mm Hg in the placebo group (P = 0.74).
Figure 3.
Absolute change in pulmonary vascular resistance index (PVRi) after 16 weeks in patients treated with bosentan or placebo. After 16 weeks, there was no difference in the absolute change in PVRi between the bosentan- and placebo-treated groups.
Echocardiographic parameters (including RV systolic pressure) did not change significantly following treatment. In particular, tricuspid annular plane excursion, a measure of RV function, increased by 1.76 (±4.38) mm in the bosentan group and 1.44 (±4.71) mm in the placebo group (P = 0.56). RV inlet size increased by 0.36 (±0.78) mm in the bosentan group and declined by 0.08 (±0.64) mm in the placebo group (P = 0.12). In keeping with these results, there was no significant change in BNP concentration following treatment (increase of 13.0 [±90.5] pg/ml in the bosentan group and increase of 21.0 [±50.4] pg/ml in the placebo group).
Oxygen saturation and requirement.
Resting arterial oxygen saturation (Spo2) was assessed at baseline and every 4 weeks during the course of the study (Figure 4). There was no significant difference in Spo2 between the bosentan- and placebo-treated groups over the 16-week study period (−0.76 ± 3.97% vs. −0.57 ± 3.9%; P = 0.79) (Table 2). At baseline RHC, 21 patients were on O2 with a median requirement of 4 L/min (interquartile range [IQR], 2–6). At the follow-up RHC 30 patients were on O2 with a median requirement of 3.5 L/min (IQR, 2–15). The median change in O2 requirement from baseline to final follow-up visit for those patients receiving oxygen at baseline and/or at follow-up was 2 L/min (IQR, 0–4). There was no significant difference in the change (from baseline RHC to follow-up RHC) in O2 requirement between placebo and bosentan groups (placebo, 1.5 L/min [IQR, 0.25–2] vs. bosentan, 2 L/min [IQR 0.5–4]; P = 0.08).
Figure 4.
Change in arterial oxygen saturations, measured every 4 weeks, from baseline to Week 16. There was no difference in arterial oxygen saturations between the bosentan- or placebo-treated groups during the randomized phase. Bars = standard errors; solid lines = means.
Disease progression.
Disease progression (defined as a >15% fall in DlCO, death, or transplantation) was observed in 8 (13.3%) of the 60 patients recruited; 4 (10.0%) in the bosentan group and 4 (20.0%) in the placebo group (P = 0.47). There were three deaths in each group, with one patient demonstrating a greater than 15% fall in DlCO in the bosentan-treated group, and one patient transplanted in the placebo-treated group. In addition, using change in FVC as a surrogate for ILD progression, we found no difference in the overall group over the trial period (P = 0.42) and, in particular, no change between placebo and bosentan groups (−2.6 ± 23.18% in placebo vs. −0.92 ± 6.82% in bosentan group; P = 0.96).
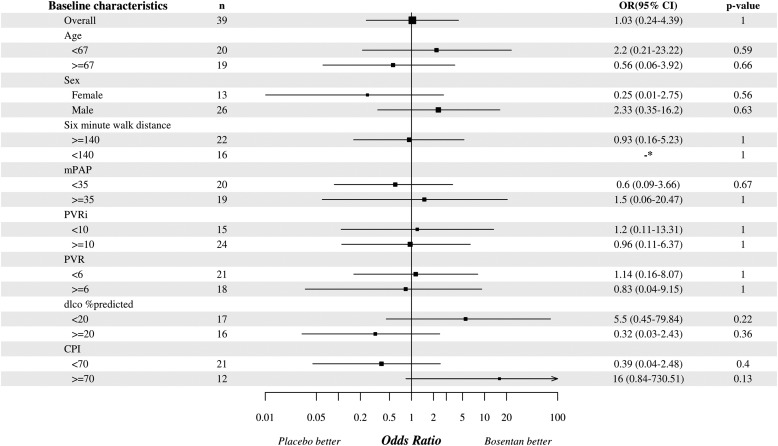
Subgroup analyses.
The primary outcome was analyzed in predefined patient subgroups, including age, sex, 6MWD, mPAP, PVR, DlCO, and CPI. Subgroup analysis thresholds were based on the median values, except for sex. No subgroup was identified in which a statistically significant change in the primary outcome was observed (Figure 5).
Figure 5.
Analysis of response to bosentan or placebo in prespecified subgroups. Except for sex, thresholds for subgroup analyses were selected as the median value for each variable. Following study withdrawals and deaths, paired right heart catheter data were available for subgroup analysis in 39 participants (although 6-minute-walk test and DlCO data were not available in all patients because of inability to perform the test). CI = confidence interval; CPI = composite physiologic index; DlCO = diffusing capacity of carbon monoxide; mPAP = mean pulmonary artery pressure; OR = odds ratio; PVR = pulmonary vascular resistance; PVRi = pulmonary vascular resistance index.
Safety and Tolerability
Fifty-six patients received at least one dose of the study medication and were valid for safety analysis (four patients were withdrawn because of protocol violations before receiving the study drug) (Table 3). Eighteen patients (45%) receiving bosentan and 10 patients (50%) receiving placebo experienced at least one SAE, with the most frequent SAEs including chest infection (n = 8) and heart failure (n = 3) (Table 4). Hepatic dysfunction was encountered in two (5%) of the bosentan group and one patient (5%) in the placebo group (P = 0.7). Six patients died during the study (bosentan = 3, placebo = 3; with five deaths attributable to disease progression), and one patient receiving placebo underwent successful lung transplantation.
Table 3.
Timing and Reasons for Withdrawal from Treatment, Including Deaths
| Reason for Withdrawal | Active (n = 40) [n (%)] | Placebo (n = 20) [n (%)] | P Value |
|---|---|---|---|
| Withdrawal after baseline | 8 (20) | 1 (5) | 0.25 |
| SAE | 2 | 0 | 0.55 |
| Withdrew consent | 1 | 0 | 1 |
| Protocol violation: pulmonary embolism | 2 | 0 | 0.55 |
| Protocol violation: severe obstructive sleep apnea | 1 | 0 | 1 |
| Protocol violation: bronchogenic carcinoma | 0 | 1 | 1 |
| Death | 2 | 0 | 0.55 |
| Withdrawal after Week 4 | 1 (2.5) | 2 (10) | 1 |
| Withdrew consent | 1 | 1 | 1 |
| Death | 0 | 1 | 1 |
| Withdrawal after Week 8 | 1 (2.5) | 3 (15) | 0.10 |
| SAE | 1 | 0 | 1 |
| Lung transplant | 0 | 1 | 0.33 |
| Death | 0 | 2 | 0.11 |
| Withdrawal after Week 12 | 4 (10) | 0 (0) | 0.29 |
| SAE | 2 | 0 | 0.55 |
| Progression of underlying fibrotic lung disease | 1 | 0 | 1 |
| Death | 1 | 0 | 1 |
| Total | 14 (35) | 6 (30) |
Definition of abbreviation: SAE = serious adverse event.
Table 4.
Incidence of the Most Frequent Serious Adverse Events and Death in the Bosentan and Placebo Groups during the Randomized Phase
| Event | Active (n = 40) [n (%)] | Placebo (n = 20) [n (%)] | P Value |
|---|---|---|---|
| Total number of patients with at least one SAE | 18 (45) | 10 (50) | |
| Respiratory SAE | 8 (20) | 8 (40) | 0.13 |
| Chest infection | 5 | 3 | 1 |
| Progression of underlying fibrotic lung disease | 1 | 3 | 0.10 |
| Hypercapnea | 1 | 0 | 1 |
| Upper respiratory tract infection | 1 | 0 | 1 |
| Lung transplant | 0 | 1 | 0.33 |
| Bronchogenic carcinoma | 0 | 1 | 0.33 |
| Hepatic impairment SAE | 2 (5) | 1 (5) | 1 |
| Heart failure | 2 (5) | 1 (5) | 1 |
| Other SAE | 5 (12.5) | 3 (15) | 1 |
| Supraventricular tachycardia (before first dose of study drug administered) | 1 | 0 | 1 |
| Anxiety | 1 | 0 | 1 |
| Cellulitis | 1 | 0 | 1 |
| Peripheral edema, headache, flushing, side effects | 1 | 0 | 1 |
| Bilateral deafness | 1 | 0 | 1 |
| Thrombophlebitis in right arm post catheter procedure | 0 | 1 | 0.33 |
| Urinary tract infection | 0 | 2 | 0.11 |
| Death | 3 (7.5) | 3 (15) | 0.39 |
| Death caused by progression of disease | 3 | 2 | 1 |
| Other respiratory cause of death | 0 | 1 | 0.33 |
| Total | 20 (50) | 16 (80) |
Definition of abbreviation: SAE = serious adverse event.
There was no difference in the rates of SAEs between the two groups.
Discussion
According to the most recent world PH congress meeting, held in Nice, France, patients with ILD and PH should not receive PAH-specific therapy until appropriate trials report evidence suggesting a benefit. In this first, randomized, double-blind, placebo-controlled study evaluating PAH-specific therapy (the ERA bosentan) in fibrotic IIP associated with PH, we found no significant difference in invasive or noninvasive pulmonary hemodynamics, functional capacity, and symptoms between active drug and placebo subgroups over the 16-week randomization period. On subgroup analysis, no difference was seen between treatment and placebo groups in patients with limited or severe interstitial disease, nor in patients with early versus advanced pulmonary vascular disease. The results of the current study imply that patients with IIP with PH do not benefit from treatment with bosentan, and that an “off-label therapeutic trial” is not warranted.
In fibrotic IIP, and even within defined disease entities, such as IPF, there is a wide variation in the natural history of disease progression. In IPF, disease severity and decline in pulmonary function tests are independent predictors of disease progression (19), but clinical deterioration is not always linked to worsening of the ILD. We have previously shown that pulmonary vascular disease is predictive of increased mortality and have suggested that pulmonary vasculopathy may contribute to a final common pathway across the IIP population (9, 20). In support of this, we have demonstrated that in ILD mortality is strongly linked to parameters of pulmonary vascular disease including increased PVR and serum BNP levels (9, 20).
The prevalence of PH in IPF populations varies from 10% at diagnosis up to 85% immediately before transplantation (2, 3, 21). PH is associated with a higher morbidity and mortality in these patients (2). Although PH is more common in the context of severe lung fibrosis, it can occur at any stage of the ILD (22). When PH is present in the context of severe fibrosis, it is considered to be “secondary to” or “associated with” the lung disease (Group 3, Nice PH Classification) (23). In a recent randomized placebo-controlled trial of patients with sarcoidosis-associated PH, Baughman and coworkers (24) reported improvements in mPAP and PVR with bosentan, including patients with evidence of fibrosis on chest radiograph. However, current PH guidelines discourage the use of PH-specific therapies in the ILD population, recommending reversal of hypoxemia and control of the underlying IIP, where possible, and the enrollment of these patients in randomized, double-blind, placebo-controlled trials (25).
ET-1 is a potent vasoconstrictor, which also mediates cell proliferation, fibrosis, and inflammation (11, 12). As such, ET-1 has been implicated in both the pathogenesis of PH and lung fibrosis. Bosentan has been shown to improve pulmonary hemodynamics and functional capacity in patients with PAH (13), and is now a cornerstone of treatment. Unfortunately, treatment of lung fibrosis has been more disappointing. Indeed, the Bosentan Use in Interstitial Lung Disease (BUILD) 1 and 3 studies demonstrated no benefit of bosentan over placebo in patients with IPF, although it should be made clear that these patients were not reported to have coexistent PH (26, 27). In the recently published ARTEMIS Study, ambrisentan, a selective type A ERA, has been associated with worse outcomes in patients with IPF (21). We show for the first time in patients with IIP with PH, that the dual ERA bosentan is not associated with hemodynamic, clinical, or physiologic benefit over placebo at 16 weeks. However, unlike ambrisentan, there did not seem to be any adverse outcome associated with the use of bosentan in this population. In fact, this is also in keeping with a small uncontrolled pilot study published by Gunther and coworkers (10).
There is more recent information to suggest that other classes of PH-specific therapy may be of benefit in patients with IIP. Limited and uncontrolled evidence suggests that the phosphodiesterase-5 inhibitor sildenafil is associated with improved hemodynamics and gas exchange acutely, and exercise tolerance at 3–6 months (7, 28). A placebo-controlled, 12-week study of the use of sildenafil in advanced IPF (STEP Study) was negative in terms of its primary outcome, 6MWT improvement of 20% versus placebo (29). Although patients with possible coexistent PH were not formally identified, the inclusion of patients with a DlCO of less than 35% makes pulmonary vascular disease very likely. There were significant improvements in secondary endpoints, such as DlCO and Pao2, which may be considered surrogates of pulmonary vascular disease, and no effect on FVC. Although this is suggestive of a pulmonary vascular effect, more convincing evidence for an effect on cardiopulmonary hemodynamics is provided in the post hoc analysis of patients who had RV dysfunction measured by echocardiography. In this subgroup there was an improvement in exercise capacity and quality of life with sildenafil compared with placebo (9). Furthermore, a recent uncontrolled study has indicated a benefit of riociguat, a guanylate cyclase stimulator, in a 12-week, open-label study of 22 patients with ILD with PH (8). A small increase in 6MWD was observed at 12 weeks, and in those who had follow-up RHC, there was an increase in mean CO and a fall in PVR and Pao2. Clearly, further well-designed studies are necessary to determine the role of sildenafil and riociguat in this patient population.
Bosentan was well tolerated in our patient population, with similar rates of SAEs in the active and placebo groups. Importantly, hepatic impairment, respiratory decompensation, and oxygen desaturation were not more frequent in patients receiving bosentan. This last finding is in keeping with the results of the BUILD studies and disproves the traditional belief of a potential risk of worsened gas exchange and hypoxemia associated with ERAs in this patient group. Indeed, as mentioned previously, DlCO and Pao2 were improved by the addition of sildenafil in the STEP study (29). It must be clear, however, that these conclusions may only apply to patients with lung fibrosis, because there is compelling evidence that pulmonary vasodilators, including bosentan, may worsen gas exchange in patients with chronic obstructive pulmonary disease (30, 31).
Despite the relatively small size of the trial, we were interested in assessing predefined exploratory subgroups to establish whether any particular subgroups may benefit more from therapy. In particular we looked at those patients with less severe fibrosis, defined by DlCO and CPI, and worse PH defined by mPAP and PVR. All such subgroup analyses were negative but underpowered for this purpose. We submit, therefore, that the results of our study do not dismiss future studies examining the effect of PH-specific therapies in patients with mild lung disease and severe PH (the so called “out of proportion PH”).
The result of our study is limited by the relatively high drop-out and mortality rates. Traditionally this patient population is extremely difficult to study in a controlled fashion. Study enrollment is slow, because this is a relatively rare condition, evidenced in our study, where 60 patients were recruited in a consecutive fashion across eight centers in the United Kingdom over a period of 43 months. Additionally, as previously discussed, patients with fibrotic IIP and PH have an extremely poor prognosis, with high short-term mortality. In this study we had six deaths (10%) over only 16 weeks (three in both study arms). A further limitation of our study was our a priori decision to only compare the results from paired RHC and not to allow for missing data. However, when on post hoc analysis we used a substitution rule for dead and withdrawn patients, there was no difference in the primary outcome between placebo and bosentan groups.
The optimal endpoint in PH therapeutic trials (whether hemodynamic, functional, survival, or a combination thereof) remains a conundrum. In IIP-associated PH, we have demonstrated the prognostic importance of PVR over other variables (including mPAP, DlCO, and Pao2) (6), and we hypothesized that a reduction in PVR with therapy would represent a clinically meaningful and hemodynamically significant change. However, we accept the use of PVR as the primary endpoint has limitations; the magnitude of change in PVR that is clinically and hemodynamically significant is not known, and there are uncertainties regarding reproducibility of PVR. To minimize these limitations, we selected a relatively high PVR threshold for change of 20%. Although such an arbitrary categorical threshold carries methodologic risk, analysis of PVR as a continuous variable yielded the same result: no significant change between bosentan and placebo groups.
Conclusions
PH is associated with increased morbidity and mortality in the patient with fibrotic IIP. This study evaluates the safety and efficacy of the dual ERA bosentan in this patient group, and is the first randomized, double-blind, placebo-controlled study evaluating PAH-specific therapies in PH associated with fibrotic IIP. This study shows no difference in invasive pulmonary hemodynamics, functional capacity, or symptoms between the bosentan and placebo groups over 16 weeks. Although bosentan was well tolerated, based on the data generated by this trial we cannot recommend its use in patients with fibrotic IIP and PH. Further studies specifically addressing the role of PH therapies in patients with mild lung disease and severe PH are necessary to determine whether there is a therapeutic role for these agents in this context.
Footnotes
Supported by an educational grant from Actelion Pharmaceuticals Ltd (Allschwil, Switzerland), and supported by the Respiratory Biomedical Research Unit at Royal Brompton and Harefield NHS Foundation Trust. Actelion Pharmaceuticals Ltd also supplied bosentan and placebo used in this study.
Author Contributions: T.J.C., G.J.K., K.D., L.H., P.A.C., A.U.W., and S.J.W. had substantial involvement in the design of the study, and critically revising the paper for important intellectual content. T.J.C., G.J.K., A.U.W., and S.J.W. had substantial involvement in the drafting of the paper. All authors had substantial involvement in either acquisition of data, analysis of data, or interpretation of data. All authors critically revised the paper for important intellectual content. All authors gave approval of this final version of the paper.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201403-0446OC on June 17, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Minai OA, Santacruz JF, Alster JM, Budev MM, McCarthy K. Impact of pulmonary hemodynamics on 6-min walk test in idiopathic pulmonary fibrosis. Respir Med. 2012;106:1613–1621. doi: 10.1016/j.rmed.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Lettieri CJ, Nathan SD, Barnett SD, Ahmad S, Shorr AF. Prevalence and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary fibrosis. Chest. 2006;129:746–752. doi: 10.1378/chest.129.3.746. [DOI] [PubMed] [Google Scholar]
- 3.Shorr AF, Wainright JL, Cors CS, Lettieri CJ, Nathan SD. Pulmonary hypertension in patients with pulmonary fibrosis awaiting lung transplant. Eur Respir J. 2007;30:715–721. doi: 10.1183/09031936.00107206. [DOI] [PubMed] [Google Scholar]
- 4.Yang SJ, Hoffman C, Mulligan K.Pulmonary arterial hypertension in patients with idiopathic pulmonary fibrosis when listed for lung transplantation Proc Am Thorac Soc 20063:A369 [Google Scholar]
- 5.Rivera-Lebron BN, Forfia PR, Kreider M, Lee JC, Holmes JH, Kawut SM. Echocardiographic and hemodynamic predictors of mortality in idiopathic pulmonary fibrosis. Chest. 2013;144:564–570. doi: 10.1378/chest.12-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corte TJ, Wort SJ, Gatzoulis MA, Macdonald P, Hansell DM, Wells AU. Pulmonary vascular resistance predicts early mortality in patients with diffuse fibrotic lung disease and suspected pulmonary hypertension. Thorax. 2009;64:883–888. doi: 10.1136/thx.2008.112847. [DOI] [PubMed] [Google Scholar]
- 7.Collard HR, Anstrom KJ, Schwarz MI, Zisman DA. Sildenafil improves walk distance in idiopathic pulmonary fibrosis. Chest. 2007;131:897–899. doi: 10.1378/chest.06-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoeper MM, Halank M, Wilkens H, Günther A, Weimann G, Gebert I, Leuchte HH, Behr J. Riociguat for interstitial lung disease and pulmonary hypertension: a pilot trial. Eur Respir J. 2013;41:853–860. doi: 10.1183/09031936.00213911. [DOI] [PubMed] [Google Scholar]
- 9.Han MK, Bach DS, Hagan PG, Yow E, Flaherty KR, Toews GB, Anstrom KJ, Martinez FJ IPFnet Investigators. Sildenafil preserves exercise capacity in patients with idiopathic pulmonary fibrosis and right-sided ventricular dysfunction. Chest. 2013;143:1699–1708. doi: 10.1378/chest.12-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Günther A, Enke B, Markart P, Hammerl P, Morr H, Behr J, Stähler G, Seeger W, Grimminger F, Leconte I, et al. Safety and tolerability of bosentan in idiopathic pulmonary fibrosis: an open label study. Eur Respir J. 2007;29:713–719. doi: 10.1183/09031936.00149205. [DOI] [PubMed] [Google Scholar]
- 11.Giaid A, Yanagisawa M, Langleben D, Michel RP, Levy R, Shennib H, Kimura S, Masaki T, Duguid WP, Stewart DJ. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993;328:1732–1739. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- 12.Park SH, Saleh D, Giaid A, Michel RP. Increased endothelin-1 in bleomycin-induced pulmonary fibrosis and the effect of an endothelin receptor antagonist. Am J Respir Crit Care Med. 1997;156:600–608. doi: 10.1164/ajrccm.156.2.9607123. [DOI] [PubMed] [Google Scholar]
- 13.Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, Pulido T, Frost A, Roux S, Leconte I, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 14.Galiè N, Rubin Lj, Hoeper M, Jansa P, Al-Hiti H, Meyer G, Chiossi E, Kusic-Pajic A, Simonneau G. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trial. Lancet. 2008;371:2093–2100. doi: 10.1016/S0140-6736(08)60919-8. [DOI] [PubMed] [Google Scholar]
- 15.Galiè N, Beghetti M, Gatzoulis MA, Granton J, Berger RM, Lauer A, Chiossi E, Landzberg M Bosentan Randomized Trial of Endothelin Antagonist Therapy-5 (BREATHE-5) Investigators. Bosentan therapy in patients with Eisenmenger syndrome: a multicenter, double-blind, randomized, placebo-controlled study. Circulation. 2006;114:48–54. doi: 10.1161/CIRCULATIONAHA.106.630715. [DOI] [PubMed] [Google Scholar]
- 16.Ahmadi-Simab K, Hellmich B, Gross WL. Bosentan for severe pulmonary arterial hypertension related to systemic sclerosis with interstitial lung disease. Eur J Clin Invest. 2006;36:44–48. doi: 10.1111/j.1365-2362.2006.01695.x. [DOI] [PubMed] [Google Scholar]
- 17.Keir GJ, Corte TJ, Parfitt L, Maher T, Marino P, Renzoni E, Dimopoulos K, Gatzoulis M, Madden B, Howard L, et al. Bosentan in pulmonary hypertension associated with fibrotic idiopathic interstitial pneumonia: a randomized, double-blind, placebo-controlled study. Eur Respir J. 2013;42:A1779. [Google Scholar]
- 18.Wells AU, Desai SR, Rubens MB, Goh NS, Cramer D, Nicholson AG, Colby TV, du Bois RM, Hansell DM. Idiopathic pulmonary fibrosis: a composite physiologic index derived from disease extent observed by computed tomography. Am J Respir Crit Care Med. 2003;167:962–969. doi: 10.1164/rccm.2111053. [DOI] [PubMed] [Google Scholar]
- 19.Collard HR, King TE, Jr, Bartelson BB, Vourlekis JS, Schwarz MI, Brown KK. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2003;168:538–542. doi: 10.1164/rccm.200211-1311OC. [DOI] [PubMed] [Google Scholar]
- 20.Corte TJ, Wort SJ, Gatzoulis MA, Engel R, Giannakoulas G, Macdonald PM, Wells AU. Elevated brain natriuretic peptide predicts mortality in interstitial lung disease. Eur Respir J. 2010;36:819–825. doi: 10.1183/09031936.00173509. [DOI] [PubMed] [Google Scholar]
- 21.Raghu G, Behr J, Brown KK, Egan JJ, Kawut SM, Flaherty KR, Martinez FJ, Nathan SD, Wells AU, Collard HR, et al. ARTEMIS-IPF Investigators*. Treatment of idiopathic pulmonary fibrosis with ambrisentan: a parallel, randomized trial. Ann Intern Med. 2013;158:641–649. doi: 10.7326/0003-4819-158-9-201305070-00003. [DOI] [PubMed] [Google Scholar]
- 22.Nathan SD, Shlobin OA, Ahmad S, Urbanek S, Barnett SD. Pulmonary hypertension and pulmonary function testing in idiopathic pulmonary fibrosis. Chest. 2007;131:657–663. doi: 10.1378/chest.06-2485. [DOI] [PubMed] [Google Scholar]
- 23.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25, Suppl):D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 24.Baughman RP, Culver DA, Cordorva FC, Padilla M, Gibson KF, Lower EL, Engel PJ. Bosentan for sarcoidosis associated pulmonary hypertension: a double-blind placebo controlled randomized trial. Chest. 2014;145:810–817. doi: 10.1378/chest.13-1766. [DOI] [PubMed] [Google Scholar]
- 25.Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, et al. Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC); European Respiratory Society (ERS); International Society of Heart and Lung Transplantation (ISHLT) Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34:1219–1263. doi: 10.1183/09031936.00139009. [DOI] [PubMed] [Google Scholar]
- 26.King TE, Jr, Behr J, Brown KK, du Bois RM, Lancaster L, de Andrade JA, Stähler G, Leconte I, Roux S, Raghu G. BUILD-1: a randomized placebo-controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177:75–81. doi: 10.1164/rccm.200705-732OC. [DOI] [PubMed] [Google Scholar]
- 27.King TE, Jr, Brown KK, Raghu G, du Bois RM, Lynch DA, Martinez F, Valeyre D, Leconte I, Morganti A, Roux S, et al. BUILD-3: a randomized, controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184:92–99. doi: 10.1164/rccm.201011-1874OC. [DOI] [PubMed] [Google Scholar]
- 28.Ghofrani HA, Wiedemann R, Rose F, Schermuly RT, Olschewski H, Weissmann N, Gunther A, Walmrath D, Seeger W, Grimminger F. Sildenafil for treatment of lung fibrosis and pulmonary hypertension: a randomised controlled trial. Lancet. 2002;360:895–900. doi: 10.1016/S0140-6736(02)11024-5. [DOI] [PubMed] [Google Scholar]
- 29.Zisman DA, Schwarz M, Anstrom KJ, Collard HR, Flaherty KR, Hunninghake GW Idiopathic Pulmonary Fibrosis Clinical Research Network. A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. N Engl J Med. 2010;363:620–628. doi: 10.1056/NEJMoa1002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lederer DJ, Bartels MN, Schluger NW, Brogan F, Jellen P, Thomashow BM, Kawut SM. Sildenafil for chronic obstructive pulmonary disease: a randomized crossover trial. COPD. 2012;9:268–275. doi: 10.3109/15412555.2011.651180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stolz D, Rasch H, Linka A, Di Valentino M, Meyer A, Brutsche M, Tamm M. A randomised, controlled trial of bosentan in severe COPD. Eur Respir J. 2008;32:619–628. doi: 10.1183/09031936.00011308. [DOI] [PubMed] [Google Scholar]