Abstract
Disrupted sensory processing, characterized by over- or underresponsiveness to environmental stimuli, has been reported in children with a variety of developmental disabilities. This study examined the effects of prenatal stress and moderate-level prenatal alcohol exposure on tactile sensitivity and its relationship to striatal dopamine system function in thirty-eight 5- to 7-year-old rhesus monkeys. The monkeys were from four experimental conditions: (a) prenatal alcohol exposed, (b) prenatal stress, (c) prenatal alcohol exposed + prenatal stress, and (d) sucrose controls. Increased D2 receptor binding in the striatum, evaluated using positron emission tomography neuroimaging, was related to increased withdrawal (aversion) responses to repetitive tactile stimuli and reduced habituation across trials. Moreover, prenatal stress significantly increased overall withdrawal responses to repetitive tactile stimulation compared to no prenatal stress.
Sensory processing disorders, characterized by under- or overresponsiveness to sensory stimulation that most individuals perceive as harmless (Ayres & Robbins, 1979), have been estimated to occur in 5% of the general population (Ahn, Miller, Milberger, & McIntosh, 2004) and seem to pose a unique challenge for people with developmental disabilities (Baranek, 2002), including autism, attention deficit hyperactivity disorder (ADHD), fragile X syndrome, and other developmental disabilities (Ayres & Tickle, 1980; Baranek, 1999; Baranek & Berkson, 1994; Baranek, Foster, & Berkson, 1997; Cermak & Daunhauer, 1997; Grandin, 1992; Kinnealey, 1973; Larson, 1982; Mangeot et al., 2001; Miller et al., 1999). Understanding the neurobiological processes associated with sensory processing disruptions is important to developing appropriate preventative and intervention approaches.
This study examined whether disrupted sensory processing occurs in monkeys as a result of prenatal exposure to alcohol and/or stress, and if so, whether it would be associated with dopamine functioning in the striatum assessed with positron emission tomography (PET) neuroimaging. We employed a primate model for these studies because in human studies causal conclusions are difficult to reach due to confounding variables. Even with the best statistical analyses it is difficult, if not impossible, to separate completely the effects of variables associated with alcohol use, such as psychological stress, tobacco use, or chaotic home life, from the effects of fetal alcohol exposure per se. Nonhuman primates also have the advantage of gestation characteristics and early development similar to the human, and their shorter life span makes longitudinal studies somewhat easier to conduct. Rhesus monkeys were used because of the large amount of available data on their behavior and development in laboratory settings (Harlow & Harlow, 1969; Suomi, 1997)
A. Jean Ayres (1964), an occupational therapist and educational psychologist, coined the term “tactile defensiveness” to characterize the behavioral correlates of a pattern described as “feelings of discomfort and a desire to escape the situation when certain types of tactile stimuli are experienced” (1964, p. 8). Ayres showed that tactile defensiveness or overresponsiveness to tactile stimuli was consistently related to hyperactive and distractible behavior in children (Ayres, 1965, 1966, 1969, 1972). Overresponsiveness to tactile stimuli is easily recognized in children (McIntosh, Miller, Shyu, & Dunn, 1999) and there has been some success in treating it with sensory-based interventions (Ayres & Tickle, 1980; Mailloux, 2001; Miller, Wilbarger, Stackhouse, & Trunnell, 2002; Wilbarger & Wilbarger, 2002).
Ayres (1964) hypothesized that tactile defensiveness could interfere with normal perceptual and cognitive development, as well as motor planning. The core deficit in sensory overresponsiveness is regarded as difficulty suppressing irrelevant sensory stimuli coupled with inappropriately high responsiveness to those stimuli. Little is known about the neurobiological substrates of sensory processing disorders or the developmental precursors. Casey (2001) proposed that disruptions in the basal ganglia thalamocortical circuits underlie poor inhibitory control and viewed disruptions of one or more of these circuits as potential contributors to developmental disorders characterized by poor inhibitory control or difficulty filtering information appropriately. In this study, we examined the dopamine (DA) system in the striatum and its possible relationship to sensory processing function based on several lines of experimental evidence. First, the striatum is part of the basal ganglia. Second, because less than optimal DA functioning has been found to impair learning and inhibitory control in rats and monkeys (Murphy, Arnsten, Goldman-Rakic, & Roth, 1996; Sawaguchi & Goldman-Rakic, 1994; Seamans, Floresco, & Phillips, 1995), we hypothesized that sensory over responsiveness would be associated with altered DA function. Third, studies show that the DA system is vulnerable to both fetal alcohol exposure and prenatal stress. In rats, prenatal alcohol exposure produced a reduction in spontaneous activity in DA neurons, reduced DA uptake and receptor binding sites, and changes in DA receptor-mediated behavior (Druse, Tajuddin, Kuo, & Connerty, 1990; Fulginiti, Minetti, & Virgolini, 1994; Randall & Hannigan, 1999; Sobrian, Jones, James, Kamara, & Holson, 2005). Moreover, prenatal stress has been found to be associated with an increase in DA turnover in the right prefrontal cortex, a decrease in turnover in the left striatum, and an increase in the density of D2 receptors in the nucleus accumbens (Fride & Weinstock, 1989; Henry, Kabbaj, Simon, Le Moal, & Maccari, 1994). Magnetic resonance imaging studies in humans have shown that the basal ganglia, a region rich in dopaminergic neurons, show reductions in volume in individuals with fetal alcohol exposure (Cummings, 1993; Mattson, Goodman, Caine, Delis, & Riley, 1999). All this makes DA in the striatum an important brain region to examine for deficits in sensory processing functions, which are related to inhibitory control.
Little is also known about how sensory processing disorders are influenced by events and conditions such as prenatal exposure to teratogens, early rearing conditions, or genetic influences. Relevant to this study is the finding that fetal alcohol-exposed neonates demonstrated reduced habituation to auditory and visual stimuli 24 – 27 hr after birth (Streissguth, Barr, & Martin, 1983). Similarly, in rodent studies, alcohol-exposed neonates showed a trend for requiring more trials to habituate to olfactory stimuli than controls (Barron & Riley, 1992) as well as reduced habituation of the cardiac orienting reflex to a novel olfactory stimulus (Hunt & Phillips, 2004). Adding to this, small body of scientific evidence is clinical evidence suggesting that fetal alcohol-exposed children demonstrate disruptions in sensory processing (M. Schneider, personal communication).
The goal of the current study was to investigate neurobiological correlates of sensory processing disorder and to examine the effects of prenatal stress and/or prenatal alcohol exposure on tactile withdrawal responses (aversion) and habituation to repeated tactile stimulation. We used an existing cohort of prenatally stressed and/or prenatal alcohol-exposed adult rhesus monkeys. The prenatally stressed monkeys were from mothers that experienced a daily 10-min stressor (10-min removal from home cage and exposure to three random noise blasts) from gestation days 90–145. The prenatal alcohol-exposed monkeys were from mothers that voluntarily consumed 0.6 g/kg alcohol, comparable to drinking two drinks throughout pregnancy. We reasoned that these monkeys would be at risk for sensory processing disorder based on a large body of scientific evidence indicating that certain prenatal perturbations could impact an individual’s developmental trajectory through altered early brain development, inducing long-lasting effects on behavioral regulation (Coles, Platzman, Lynch, & Freides, 2002; Glover & O’Connor, 2002; Jacobson, Jacobson, Sokol, Chiodo, & Corobana, 2004; Mattson et al., 1999; Streissguth, Bookstein, Sampson, & Barr, 1995; Van den Bergh & Marcoen, 2004; Wadhwa et al., 2002; Willford, Richardson, Leech, & Day, 2004).
PET imaging was used in order to examine post-synaptic receptor binding (D2R) and dopamine synthesis (DAsyn) in the striatum (see Roberts et al., 2004, for details). The striatum is rich in dopaminergic innervation and is part of the basal ganglia thalamo-cortical loop involved in behavioral inhibition (Casey, 2001). As part of our longitudinal project, we assessed both DAsyn and D2R binding because it is known that DAsyn and DA receptors have a complementary relationship. Our interdisciplinary team used 6-[18F]-fluoro-m-tyrosine (FMT) as a measure of DAsyn because FMT tracks the final steps of DA synthesis (see DeJesus, 2003, for details). The tracer, [18F]-Fallypride (FAL), an F-18–labeled raclopride analog, was chosen to assess D2 receptor binding as an index of D2 receptor functioning because it has a high affinity for D2 receptors and high brain uptake, almost three times higher compared to [11C] raclopride (Mukherjee et al., 1997; Schneider et al., 2005).
We previously reported the results of the PET DA scans in the monkeys involved in this study (Roberts et al., 2004). We found that the prenatal stress condition increased the ratio of D2 receptor binding to DAsyn compared to controls (Roberts et al., 2004). Moreover, PET measures of DA function in the striatum were negatively correlated with behavioral inhibition—or suppression of irrelevant actions—during cognitive testing (Roberts et al., 2004). In another experiment, we found that the effects of moderate-level prenatal alcohol exposure on DA functioning in the striatum depended on the gestational timing of the exposure (Schneider et al., 2005). Therefore, we hypothesized that DA functioning in the striatum would be related to the pattern of responses to repeated tactile stimulation.
The Present Study
The purpose of the present study was twofold. The first goal was to examine whether the monkeys’ responses to repetitive tactile stimulation would be associated with DA function in the striatum. The second goal was to examine whether prenatal exposure to alcohol, stress, or both would alter withdrawal responses (aversion) to repeated tactile stimulation in rhesus monkeys. Our studies to date indicate that rhesus infants from both prenatal stress- and prenatal alcohol-exposed pregnancies exhibited reduced neonatal orienting and motor maturity (Schneider, 1992; Schneider, Moore, & Becker, 2001; Schneider, Roughton, & Lubach, 1997), increased stress hormone reactivity (Clarke, Wittwer, Abbott, & Schneider, 1994; Schneider, Moore, & Kraemer, 2004), learning deficits (Schneider, Moore, & Kraemer, 2001), and altered striatal DA system function (Roberts et al., 2004; Schneider et al., 2005).
Method
Subjects
The subjects in this study were 38 young adult (5-to 7-year-olds, M 5 6.35 years) rhesus monkeys (Macaca mulatta), 23 females and 15 males. These monkeys are members of an ongoing longitudinal study that investigates the effects of moderate-level prenatal alcohol exposure, alone or in conjunction with prenatal stress, on development and neurobehavioral function. To produce these monkeys, healthy female rhesus monkeys from the breeding colony were identified that consistently and voluntarily consumed an entire 0.6 g/kg, 6% vol/vol alcohol solution sweetened with Nutrasweet (300 mg per 100 ml: Equal Sweetener, Merisant US Inc., Chicago, IL) daily over a 2-week period. Approximately 50% of the females tested fell into this category. This dosage is comparable to an average-sized woman consuming approximately two alcoholic drinks daily, with blood concentrations of 20–50 mg/dl 60 min after consumption (blood samples were obtained prior to pregnancy to avoid stress given that prenatal stress was a variable of interest). Alcohol-consuming females were randomly assigned to one of four groups (control or one of three experimental groups) in a 2 × 2 factorial design with prenatal alcohol and prenatal stress as independent variables.
The pregnant females in the alcohol-only and alcohol + stress groups voluntarily consumed 0.6 g/ kg in a 6% alcohol solution sweetened with Nutrasweet (300 mg per 100 ml) daily throughout gestation at 1600 hr. The treatment was begun 5 days before breeding and ended at parturition. All animals were fed Purina Monkey Chow (St. Louis, MO) daily at 6 a.m. and were given a fresh fruit supplement on Monday, Wednesday, and Friday at 1300 hr. There was no Chow left when the alcohol solution was introduced. Water was available ad libitum, including the time of the day when the alcohol solution was available. The control and prenatal stress mothers consumed a sucrose solution that was designed to be approximately equivolemic and equicaloric (8 g per 100 ml water) to the alcohol solution.
The pregnant females in the prenatal stress and prenatal stress + alcohol-exposure groups were exposed to stress five times per week at approximately 1530 hr. The treatment involved removing the pregnant female from the home cage, placing her in a transport cage and transporting her to a darkened room where three noise bursts were randomly administered over a 10-min period. The noise burst consisted of an alarm horn that produced a 1300-Hz sound of 115-dB intensity at 1 m. With this treatment, plasma cortisol levels increased from 25.2 + 2.2 µg/dl at baseline to 34.8 + 2.4 µg/dl poststress treatment (M = SEM) (Schneider & Moore, 2000). The alcohol + stress treatment group consisted of females who voluntarily consumed the alcohol solution (as described) and were exposed to the prenatal stressor (as described).
The offspring in this study consisted of 13 controls, 10 females and 3 males; 7 prenatal-stressed monkeys, 2 females and 5 males; 9 prenatal alcohol-exposed monkeys, 7 females and 2 males; and 9 prenatal alcohol + stress-exposed monkeys, 4 females and 5 males. The rearing conditions and previous testing of these subjects were described in detail elsewhere (Schneider et al., 1997; Schneider, Moore, & Becker, 2001). Briefly, all infant monkeys were housed with their mothers in individual cages during the first 6 months of life. During the first month of life, they were separated from their mothers weekly and tested for neonatal neurobehavioral function (Schneider et al., 1997). At 6 months, they were separated from their mothers for weaning and then reared in mixed-sex peer groups consisting of 5 – 6 monkeys from similar prenatal conditions (Schneider et al., 2004). At the time of the present study, the animals were pair-housed with same-sex peers from similar treatment groups. They were maintained on a diet of Purina Monkey Chow supplemented three times weekly with fresh fruit. All housing conditions were controlled (16 hr light and 8 hr dark; 21 6 ±.5°C).
When they were 5–7 years old, they were tested with the Sensory Processing Scale for Monkeys (SPS – M) described next. This scale was developed by adapting procedures from sensory processing assessments for children (Baranek & Berkson, 1994; Miller et al., 1999). The monkeys were also assessed for striatal DA system function using the PET methodology as described later.
Sensory Processing Scale for Monkeys
Sensory processing testing was conducted in a 53 × 44 cm testing cage with vertical bars spaced 5.5 cm apart. The size of the cage prevents gross motor locomotion of the monkey but allows some movement and allows experimenter access for administering the tactile stimuli. The cage was situated in a dimly lit and sound-shielded room (62 dB) with a masking white noise of 65 – 70 dB. Each monkey was tested individually by a human experimenter who stood beside the cage and administered the tactile stimulation items through the bars of the cage. A second experimenter videotaped the session for later scoring. Both experimenters were blind to the experimental conditions of the animals. The human experimenters were not known to the animals.
The first tactile stimulus consisted of a 12.5-cm feather that delivered light tactile stimulation. The second stimulus, a 7-cm cotton ball, delivered a soft but slightly firmer tactile stimulation. Finally, the third stimulus, a 15-cm stiff craft brush delivered a scratchy but innocuous tactile stimulation. All stimuli were attached to a 91-cm dowel so the experimenter could maintain a safe distance. Six trials of each stimulus were administered to assess the pattern of responsiveness across trials. Each trial the light feather, soft-cotton ball, and stiff brush were administered in an invariant order, as listed here, as a swipe to the cheek and neck area. Prior to the first presentation of each stimulus, the stimulus was placed in full view and touching range of the monkey and remained there for approximately 3 s. Once the animal looked at the object, the examiner slowly moved the stimulus into the cage and began the series of six trials. Stimuli were applied for approximately 2 s per trial, with an intertrial interval of approximately 2 s and an approximate 4-s pause between each of the textures. The entire testing session lasted for approximately 10 min. Following the completion of the testing, the monkey re-entered the transport cage and was immediately returned to the home cage.
Raters blind to the condition and history of the animals scored the videotapes. The total of 18 trials, 6 trials each with the light feather, soft-cotton ball, and stiff-brush stimuli, were scored for degree of withdrawal from tactile stimuli in 0.25 increments on a 0 to 3 rating scale with the integers labeled as follows: 0 = no withdrawal; 1 = slight withdrawal, such as turning head away from the stimulation; 2 = moderate withdrawal, such as turning full body away from stimulation; 3 = extreme withdrawal, such as moving body away from stimulation. Two raters coded 13 different animals to assess inter-rater reliability. So that all levels of response would be represented in the inter-rater reliability coding, 9 of the animals scored by the second coder were selected from the present data based on the primary coder’s results to represent high, moderate, and low responsiveness. The other 4 animals used in reliability coding were from a different experiment. The ratings of the primary coder were used in all reported data analyses.
Inter-rater reliability as percentage agreement within ±0.25 on the rating scale exceeded 99%. We also calculated inter-rater reliability as estimate variance due to animals divided by error variance (Winer, 1971). This represents an estimate of “true score” variance divided by the sum of true score plus error variance. Reliability exceeded 99% for all three stimuli.
PET Procedure
All PET studies were performed as described previously (Roberts et al., 2004, Schneider et al., 2005). Separate scans on different days were used to assess D2 receptor binding and DAsyn. Briefly, monkeys fasted overnight were anesthetized with ketamine (15 mg/kg) and transported across campus to the PET facility. Anesthesia during the PET scan was maintained with 1.25% to 1.5% isoflurane. Each anesthetized animal was positioned in the ECAT 933 PET scanner with horizontal imaging slices parallel to the orbital-meatus plane. DA synthesis was assessed using FMT as a PET tracer (DeJesus, Endres, Shelton, Nickles, & Holden, 1997). The tracer used to assess D2 receptors was FAL, an F-18–labeled raclopride analog developed by Mukherjee et al. (1997); 5 mCi in 1–5 ml normal saline of either FAL or FMT were administered as an intravenous bolus and a dynamic sequence of images over 90 min, including a total of 13 frames with duration increasing from 2 to 10 min was collected. At the end of scanning, the animals were extubated, allowed to awaken, and then returned to the animal care facility. The protocol used in these studies was approved by the UW Animal Care and Use Committee in compliance with National Institutes of Health regulations on the use of nonhuman primates in research.
PET images were reconstructed from the raw data using the ordered subset estimation method (OSEM; Hudson & Larkin, 1994). Standard regions of interest (ROI) were placed on the occipital cortex (an area known to contain little significant dopaminergic innervation) in order to produce reference region time – activity curves for use as input functions in graphical analysis. Other ROI were placed to cover both left and right caudate and putamen (jointly referred to as the striatum) in the basal ganglia. For the irreversible tracer, FMT, time – activity data for these ROI were analyzed with the standard reference tissue graphical method of Patlak and Blasberg (1985). Influx rate constants (Ki), which measure the rate of irreversible trapping of FMT, reflect DA synthesis and were evaluated in each image voxel.
For the reversible tracer, FAL, time – activity data for the ROI were analyzed with the graphical method of Logan et al. (1996). Ratios of tracer total distribution volumes (DVR) to those in the reference region were evaluated. The method assumes that the unbound components of the tracers are the same in the target regions (striatum) as in the reference region (occipital cortex). The DVR values can then be interpreted directly in terms of fallypride binding potential (BP): Bmax/Kd = DVR-1, where Bmax is the mass-specific concentration of available receptors and Kd the receptor – ligand dissociation constant in that voxel.
Data Analyses
The rated response to tactile stimulation on each trial was used as the DV in a Prenatal Alcohol (2) × Prenatal Stress (2) × Sex (2) × Trials (6) × Texture (3) mixed analysis of variance (ANOVA) with the repeated measures on trials and texture, and polynomial trends on trials. The Huyhn – Feldt adjustment of p levels was used for effects involving repeated measures. This analysis was followed by separate ANOVAs of each texture (feather, cotton ball, stiff brush). To determine whether groups differed in their initial reactions and final reactions to tactile stimulation, ANOVAs were conducted on the first and last trial of each texture. Four animals had missing data for one or more trials. Missing data were imputed separately for each texture using multiple regressions with condition and sex as grouping variables (Dixon, 1988).
In order to examine the relationship of sensory processing to DA function in the striatum, we calculated six scores for each subject. First, each animal’s overall magnitude of response to each stimulus texture was calculated as the average response over the trials. Second, each animal’s linear habituation score to each texture was obtained by multiplying linear trend coefficients (5, 3, 1, −1, −3, −5) by the sensory scores for the six trials of each stimulus (Keppel & Wickens, 2004). Positive values of the linear trend scores represent habituation across trials (higher scores represent a steeper decline across trials), whereas negative linear trend scores represent an increase over trials, or sensitization. We next conducted a factor analysis in order to reduce the number of variables. Specifically, factor analysis with the maximum likelihood method followed by oblique rotation was applied to the six scores (three magnitude scores—Feather Mean, Cotton Mean, and Brush Mean—and three habituation scores—Feather Linear, Cotton Linear, and Brush Linear). To determine whether performance on the SPS – M was related to DA system function, FMT uptake (an index of DA-syn), FAL uptake (indexing D2R binding), and FAL/ FMT ratios were correlated with the sensory scores using Pearson product moment correlations.
Results
Overall Response to Repeated Tactile Stimulation
The ANOVA of Prenatal Alcohol (2) × prenatal stress (2) × sex (2) × trials (6) × texture (3) showed a significant main effect of Prenatal stress, F(1, 30) = 4.21, p < .05, partial η2 = .123, and a marginal effect of prenatal alcohol, F(1, 30) 5 2.93, p < .10, partial η2 = .089. The main effect of prenatal stress indicated that prenatal stress increased the withdrawal response intensity compared to no prenatal stress (prenatal stress M = 1.83, no prenatal stress M = 1.36, SEs = .65 and .55, respectively). A marginal alcohol effect suggested that prenatal alcohol marginally increased the withdrawal response compared to the no prenatal alcohol condition (1.80 vs. 1.40, SEs = .61 and .58, respectively). There was also a significant Prenatal Stress × Trials effect, F(5,150) = 5.17, p = .0003, partial η2 = .147.
There was also a significant main effect of texture, F(2,60) = 5.25, p < .01, partial η2 = .15, and a Texture × Stress interaction, F(2, 20) = 7.71, p < .001, partial η2 = .20.
Texture Effects
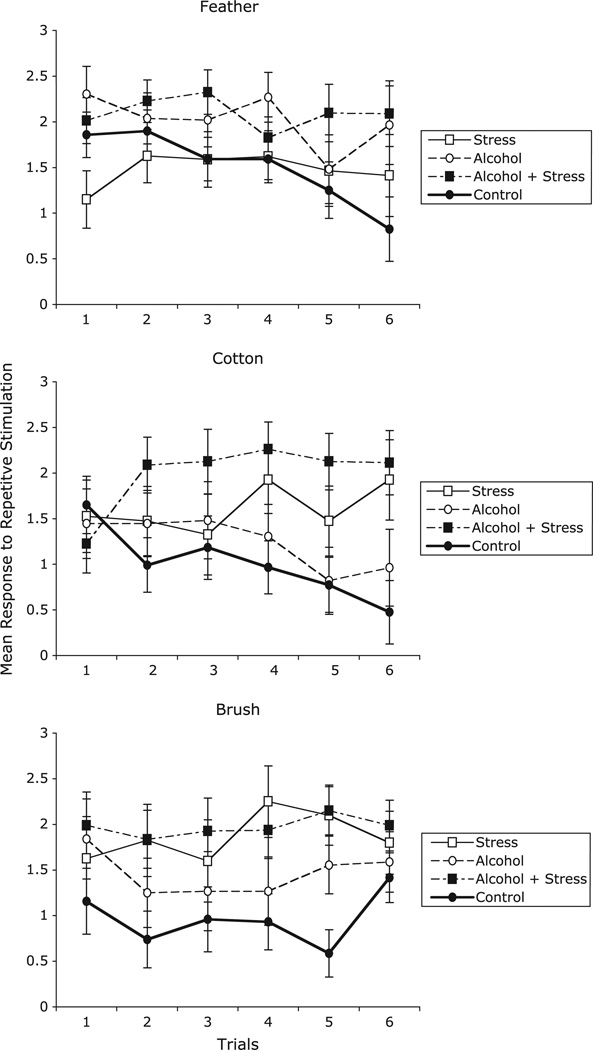
Figure 1 shows the means and standard errors for the withdrawal response scores in the four treatment groups for each texture. Separate ANOVAs for each texture (feather, cotton ball, and brush) showed a significant main effect for prenatal alcohol exposure for the feather stimulus, F(1, 30) = 4.84, p < .04, partial η2 = .139 (see Figure 1). Specifically, the prenatal alcohol condition had a higher withdrawal response to the feather stimulus compared to no prenatal alcohol condition (prenatal alcohol M = 2.05, SE = .183, no prenatal alcohol M = 1.49, SE = .180). A Prenatal Stress × Trials interaction was significant for both the feather, F(5,150) = 3.55, p = .005, partial η2 = .106, and the cotton ball, F(5, 150) = 3.30, p = .008, partial η2 = .099. These interactions indicate that prenatal stress exposure increased withdrawal response intensity across trials, or induced a pattern of sensitization to the feather and cotton ball stimuli, whereas the conditions with no prenatal stress showed a decrease in withdrawal across trials, or a habituation pattern. For the brush stimulus, two main effects were significant: prenatal stress and sex. The prenatal stress exposure conditions had a higher average withdrawal response to the brush compared to the no prenatal stress conditions, F(1, 30) = 7.08, p < .02, partial η2 = .191 (mean response = 1.92, vs. 1.21, SEs = .44 and .37, respectively). Females had higher withdrawal response intensity than males to the brush (1.85 vs. 1.28, respectively, SEs = .36 and .45, respectively), F(1, 30) = 4.65, p = .039, partial η2 = .134. There was also a significant main effect of sex for the cotton ball stimulus, such that females showed higher withdrawal responses than males (1.75 vs. 1.17, respectively, SEs = .33 and .41, respectively), F(1, 30) = 5.73, p = .023, partial η2 = .160, but not for the feather.
Figure 1.
Mean withdrawal responses on a 0 to 3 rating scale for each texture. Higher values indicate higher withdrawal. Bars are ±1 SE.
Initial Response and Response on Last Trial of Sensory Test
The results of comparisons across groups on the initial response and response on the last trial showed a significant main effect of prenatal alcohol exposure for the first and last trials of the feather stimuli: first trial, F(1, 30) = 5.40, p < .03, partial η2 = .15 (prenatal alcohol M = 2.158, SE = .197, and no prenatal alcohol M = 1.50, SE = .20) and last trial, F(1, 30) = 5.15, p = .03, partial η2 = .15 (prenatal alcohol M = 2.03, SE = .28, and no prenatal alcohol M = 1.12, SE = .29). A marginal effect of prenatal stress for the initial response to the feather stimulus was also detected, F(1, 30) = 3.16, p = .086, partial η2 = .10 (prenatal stress M = 1.58, SE = .20, and no prenatal stress M = 2.08, SE = .196). For the cotton stimulus, there was a main effect of prenatal stress for the last trial, F(1, 30) = 10.98, p = .002 partial η2 = .27 (prenatal stress M = 2.01, SE = .28, and no prenatal stress M = 0.72, SE = .27) but not for the first trial. There were no significant effects for the initial or last trials for the brush stimuli.
Relationship of SPS – M Scores to DA Function in the Striatum
The results of the maximum likelihood factor analysis of the animals’ mean responses and the linear trends for each stimulus are presented in Table 1. Two factors accounted for 59% of the variance in the data space. The first factor represents the magnitude of the sensory response across the three stimuli (feather, cotton ball, and brush), and failure to habituate to the first two textures, feather and cotton ball. The second factor represents the degree of habituation to the brush. Based on the factor analysis, we constructed two factor scores.
Table 1.
Rotated Factor Loadings From Maximum Likelihood Factor Analysis of SPS – M Scores
| SPS – M score | Factor 1 | Factor 2 |
|---|---|---|
| Feather mean | .73 | 0.12 |
| Cotton mean | .94 | −0.19 |
| Brush mean | .91 | 0.02 |
| Feather linear | −.42 | 0.05 |
| Cotton linear | −.27 | −0.15 |
| Brush linear | −.02 | 1.00 |
Note N = 38. SPS – M = Sensory Processing Scale for Monkeys.
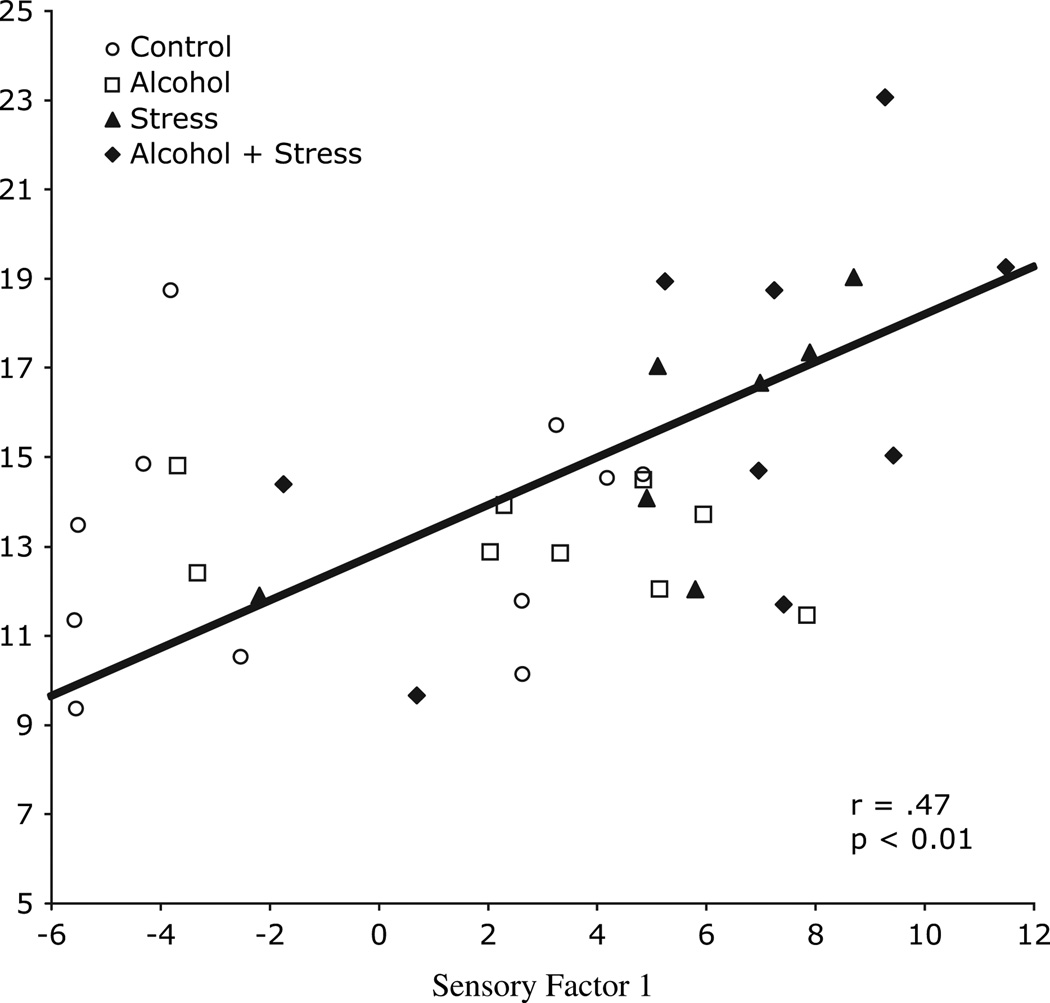
Table 2 presents the correlations of the two SPS – M Sensory Factor scores with the PET measures of striatal DA function, as well as the intercorrelations of the scores. The major finding is that Sensory Factor 1 was positively related to FAL, an index of D2 receptor binding (r = .47, p < 0.01) and to the ratio of FAL to FMT, or D2 receptor binding to DA synthesis, an index of the balance in the dopaminergic system (r =.42, p < .05). Sensory Factor 2 tended to be positively related to FAL, or D2 receptor binding (r = .28, p < .10). Figure 2 presents the scatter plot of Sensory Factor 1 with FAL.
Table 2.
Correlations of Sensory Measures With PET DA Function Measures in the Striatum
| Variable | FALa | FMTb | Ratio FAL/FMTc |
|---|---|---|---|
| Sensory Factor 1 | .47** | −.14 | .42* |
| Sensory Factor 2 | .28† | .03 | .17 |
| Feather linear | −.34* | .22 | −.37* |
| Cotton linear | −.39* | −.10 | −.21 |
| Brush linear | .28† | .03 | .17 |
| Feather mean | .23 | −.13 | .23 |
| Cotton mean | .28 | .23 | .33* |
| Brush mean | .33* | −.20 | .35* |
| Feather trial 1 | .08 | .07 | .01 |
| Cotton trial 1 | −.10 | −.19 | .07 |
| Brush trial 1 | .32† | −.19 | .34* |
| Feather trial 6 | .29† | −.16 | .30 |
| Cotton trial 6 | .28† | −.16 | .31† |
| Brush trial 6 | .14 | −.09 | .16 |
Note N = 36. FAL 5 [18F]-Fallypride; FMT 5 6-[18F]-fluoro-m-tyrosine.
The FAL refers to DA D2R binding,
FMT refers to DA synthesis, and
Ratio FAL/FMT refers to the DA D2R binding to DA synthesis ratio.
p < .10.
p < .05.
p < .01. two tailed.
Figure 2.
Scatter plot of the relationship between [18F]-Fallypride uptake, an index of D2 receptor binding, and Sensory Factor 1 or magnitude of responsiveness and failure to habituate. A significant positive relationship was observed.
Discussion
There were two principal findings of the present study. First, reduced habituation to repeated tactile stimulation and higher average withdrawal response was associated with increased striatal D2R binding and increased ratio of D2R binding to DA synthesis. Second, the pattern of habituation/sensitization to repeated tactile stimuli differed as a function of prenatal treatment. Animals not exposed to prenatal stress showed the expected behavioral pattern of habituation across trials while exposure to prenatal stress induced slight behavioral sensitization. Moreover, compared to no exposure to prenatal alcohol, prenatal alcohol exposure induced a higher overall magnitude of withdrawal response (average across trials) to the feather stimulus.
The first finding was that DA function in the striatum was related to increased withdrawal responses and failure to habituate to repeated tactile stimulation. Sensory Factor 1, which includes the average magnitude or response across all three textures and failure to habituate, was positively related to FAL, an index of D2 receptor binding, and to the ratio of FAL to FMT.
Why would upregulation of D2R binding in the striatum as indexed by FAL and the D2R/DA ratio be related to increased magnitude of withdrawal (aversion) responses and failure to habituate to tactile stimulation? DA is an important neurotransmitter that modulates the activity of many brain regions, signaling both excitatory and inhibitory messages. Nigrostriatal dopaminergic neuron activity is considered a critical component of the process of switching attentional and behavioral selections to unexpected, behaviorally important stimuli. This switching response is viewed as a prerequisite for associative learning, preparing the organism for an appropriate reaction to significant events (Redgrave, Prescott, & Gurney, 1999). The nigrostriatal DA system originates in the substantia nigra and innervates the striatum. Ascending dopaminergic projections from the sub-stantia nigra and ventral tegmental area to the striatum provide critical signals involved in reinforcement learning (Schultz, 1998). The striatum is part of the basal ganglia that mediates a wide range of functions, including inhibitory control (Joti, Kulashekhar, Behari, & Murthy, 2007), perception (Brown, Schneider, & Lidsky, 1997), learning (Gaffan, 1996), and attention (Jackson & Houghton, 1995).
Given that monkeys that consistently responded negatively to repeated tactile stimuli and failed to habituate had the highest D2R binding availability, tactile sensitivity could be linked to altered striatal dopaminergic function, which is itself critical for associative learning and attention switching. High striatal D2R density is considered to reflect a super-sensitive or hypersensitive DA receptor system. For example, in humans, Volkow et al. (2002) found there is an optimal range for striatal D2 receptor stimulation and that too little may be insufficient but too much might be aversive. Perhaps the increased striatal D2R binding in our monkeys, which positively correlated with increased aversive responses and reduced habituation to repetitive tactile stimuli, reflects heightened dopaminergic receptor sensitivity.
Our findings suggest that one contributing factor to sensory processing disorders may be alterations in the functioning of the dopaminergic regulatory systems. Most neural processes in the brain involve delicately tuned feedback mechanisms in that initial responses are either increased or dampened as they are transmitted through the brain. Such feedback systems may be dependent upon developmental processes involving reorganization of interactions between various cortical and subcortical regions. Dopaminergic systems exhibit a remarkable degree of plasticity. For example, after selective destruction of dopaminergic neurons, striatal receptors have been found to become supersensitive to D1 or D2 agonists (LaHoste & Marshall, 1993). Studies have shown that increased D2 sensitivity is observed when D2 receptors are released from the regulatory influence of D1 receptors (LaHoste, Ruskin, & Marshall, 1996). Thus, changes in D1 and D2 receptor density may reflect changes in receptor interaction, or the D1/D2 synergism. Our laboratory has studies underway to assess D1 receptor density in the striatum, prefrontal cortex, and nucleus accumbens in this cohort of monkeys.
The second finding was that prenatal treatment affected the pattern of response to repetitive tactile stimuli. Animals not exposed to prenatal stress showed the expected habituation across trials while those exposed to prenatal stress showed slight behavioral sensitization. That prenatal stress influenced the pattern of withdrawal response to repetitive tactile stimulation is consistent with several studies suggesting that prenatal stress has long-lasting effects on behavioral regulation (Glover & O’Connor, 2002; Van den Bergh & Marcoen, 2004; Wadhwa et al., 2002). In our previous studies of prenatally stressed monkeys, prenatal stress yielded reduced orienting and impaired motor maturity during the neonatal period (Schneider, 1992; Schneider, Roughton, Koehler, & Lubach, 1999) and altered stress hormone responsiveness (Clarke et al., 1994). The prenatally stressed monkeys in this cohort showed increased D2 receptor binding in the striatum at young adulthood (Roberts et al., 2004).
While the mechanisms underlying the developmental sequelae of prenatal stress have not been completely determined, maternal stress hormones cross the placental barrier (Zarrow, Philpott, & Denenberg, 1970). Moreover, in humans there is a positive linear relationship between maternal and prenatal concentrations of cortisol (Gitau, Cameron, Fisk, & Glover, 1998). Rodent studies suggest that prenatal stress affects several areas of the developing brain, including the hippocampus, septum, amygdala, and frontal cortex (Coe et al., 2003; McCormick, Smythe, Sharma, & Meaney, 1995; Uno et al., 1990; Weinstock, 2001). Prenatal stress is also known to affect concentrations of DA, norepinephrine (NE), and serotonin (5-HT) in brain areas of adult rats either chronically stressed or under stress challenge (Fride & Weinstock, 1988; Peters, 1982, 1990; Takahashi, Turner, & Kalin, 1992; Weinstock, 2001).
Because prenatal stress has been shown to alter neurotransmitter concentrations, it is possible that prenatal stress might perturb typical neuronal migration and differentiation. Such perturbations might result in “miswiring,” as well as alterations in synaptic functioning. Indeed, rodent studies found that prenatal stress reduced expression of brain-derived neurotrophic factor (BDNF) in the prefrontal cortex and striatum (Fumagalli, Bedogni, Perez, Racagni, & Riva, 2004). BDNF, a molecule that plays an important role in synaptic plasticity and cellular homeostasis, may also play a role in the etiology of psychiatric disorders (Fumagalli, Racagni, Colombo, & Riva, 2003).
In addition to the prenatal stress effects, prenatal alcohol exposure in the present study yielded a relatively high withdrawal (aversion) response for the feather stimulus. Prenatal alcohol exposure induced group differences on the initial trial as well as the last trial for the feather. The feather stimulus was the first texture presented, so this might account for the strongest effect to the feather. This is the first report, to our knowledge, linking prenatal alcohol exposure to altered responses to repetitive tactile stimulation in monkeys. In rats, hyperresponsiveness to mildly painful stimuli has been found to result from prenatal alcohol exposure (Rogers, Barron, & Littleton, 2004). Alcohol during pregnancy in humans, however, has been shown to contribute to cognitive deficits (Jacobson, Jacobson, Sokol, Martier, & Ager, 1993), slower processing speed (Burden, Jacobson, & Jacobson, 2005), and reduced neonatal habituation (Streissguth et al., 1983). In our previous studies with this cohort of monkeys, we found slower learning of a nonmatch-to-sample task in fetal alcohol-exposed monkeys (Schneider, Moore, & Kreamer, 2001).
There are numerous studies in animals showing the deleterious consequences of in utero alcohol exposure on the developing central nervous system. These include volume reductions in the basal ganglia, cerebrum, and cerebellum, agenesis of the corpus collosum, reduced pyramidal cell density and cell numbers in hippocampal formation (Livy, Miller, Maier, & West, 2003), and reduced numbers of neurons and glia in the somatosensory cortex (Miller & Potempa, 1990). Moreover, prenatal alcohol exposure in rodents has been shown to alter neurotransmitter functioning, including decreased DA uptake, deficits in serotonin reuptake sites, reduced density of NMDA receptor agonist binding sites, altered DA synthesis, and altered behavioral responses to dopaminergic drugs (Druse et al., 1990; Hannigan & Pilati, 1991; Kim & Druse, 1996; Maier, Chen, & West, 1996; Sutherland, McDonald, & Savage, 1997). Our own work has found that prenatal alcohol exposure contributed to altered DA neurotransmitter function in the striatum depending on the gestational timing of the alcohol exposure (Schneider et al., 2005).
Because of the susceptibility of the developing brain to alcohol-induced apoptotic neurodegeneration (Ikonomidou et al., 2000), prenatal alcohol exposure could compromise cortical plasticity and therefore, acquisition of adaptive behavioral responses to environmental events. Both human and animal studies have concluded that prenatal alcohol exposure can disrupt myelination, as well as glial cells, which are critical for neuronal myelin development (Burden et al., 2005; Guerri, Pascual, & Renau-Piqueras, 2001; Sowell et al., 2001). Neuromodulatory circuits in the brain serve important roles in behavioral regulation by amplifying or attenuating signals between various neuronal networks. These neuromodulatory circuits are likely to be sensitive to prenatal perturbations, and can also have cascading effects on later development, possibly contributing to the phenotypic expression of over responsiveness to tactile stimulation.
Limitations
The limitations of the present study are that the DA measurements were made only in the striatum, and not other regions, and only D2-like receptors were assessed. Future studies are planned to evaluate DA function in the prefrontal cortex and nucleus accumbens as well as to assess D1-like receptor binding, DA transporter binding, and serotonin receptor and transporter binding across multiple brain regions. We will then examine the relationships of these other measures of neuronal function to habituation and sensitization to sensory stimuli. Because this is the first study to examine the relationship between tactile sensitivities and dopaminergic function, replication of this effect is needed.
A second limitation is that, as in most nonhuman primate research, the sample size is relatively small. Also, because the mothers were randomly assigned to treatments at breeding, the sex of the offspring could not be controlled. We accommodated the unequal numbers in the cells in the standard way in the ANOVAs by using Type III sums of squares.
Another limitation of the present study concerns the relevance of findings from nonhuman primate studies to the everyday conditions of children and their families. Thus, these results should be regarded as hypotheses generating with respect to children (Gottlieb & Lickliter, 2004). Because animal models provide excellent control of environmental variation, they can also exaggerate causal connections between early events and later measures compared to the causal connections in humans. A variety of intervening variables can either enhance or diminish the relationship between early risk factors and later outcomes in humans (Gottlieb & Lickliter, 2004).
Moreover, in humans, prenatal perturbations, such as prenatal stress and prenatal alcohol exposure, are not linked in a one-to-one fashion with a particular outcome (Gottlieb & Halpern, 2002). Rather, it is the combination or coaction of environmental factors, genetic factors, neural activity, and behavior, and probably timing of their coaction, that leads to certain outcomes. Prenatal stress and prenatal alcohol exposure can be regarded as probabilistically rather than deterministically increasing the likelihood of the expression of tactile sensitivities. Further, just as prenatal stress and prenatal alcohol exposure do not necessarily result in the same outcome in different children, more than one developmental pathway can lead to the same developmental outcome, such as increased tactile sensitivities (Cicchetti & Rogosch, 1996). Further studies are needed to explore other prenatal and postnatal environmental factors, including a range of toxicants and genetic factors as well, that might contribute, in a bidirectional manner, to the expression of sensory processing disorders.
Summary
The present study has found evidence for the existence of a differential phenotypic expression of responsiveness to tactile stimulation in monkeys. Specifically, we have demonstrated that patterns of habituation and the magnitude of withdrawal (aversive) responses to repetitive tactile stimulation in monkeys were modified by prenatal stress and prenatal alcohol exposure. Our data also suggest that measures of specific behavioral processes, such as response inhibition and habituation, may be useful for detecting subtle but measurable deficits from prenatal alcohol exposure and/or prenatal stress and linking them to specific underlying brain mechanisms. We also demonstrated that reduced habituation and overall magnitude of withdrawal response to repetitive tactile stimulation is associated with increased D2R binding in the striatum and increased ratio of D2R binding to DA synthesis. Because of the intimate regulating feedback between the striatum and other brain regions, altered DA system function in the striatum may be one factor influencing less than optimal inhibitory control necessary for appropriate processing of sensory input. Future research is necessary to determine how postnatal factors and genetic vulnerabilities interact with prenatal disturbances to enhance or diminish the likelihood that sensory processing symptoms are expressed. Research is also needed to determine whether helping prenatal-stressed and/or prenatal alcohol-exposed children to reduce tactile sensitivities may be a promising intervention for improving regulatory skills, habits, and adaptive life skills in these vulnerable children.
Acknowledgments
This study was supported by grants from AA10079 and AA12277 from the National Institute of Alcohol Abuse and Alcoholism and the Wallace Research Foundation.
Contributor Information
Mary L. Schneider, University of Wisconsin-Madison
Colleen F. Moore, University of Wisconsin-Madison
Lisa L. Gajewski, University of Wisconsin-Madison
Julie A. Larson, University of Wisconsin-Madison
Andrew D. Roberts, Minnesota State University
Alexander K. Converse, University of Wisconsin-Madison
Onofre T. DeJesus, University of Wisconsin-Madison
References
- Ahn RR, Miller LJ, Milberger S, McIntosh DN. Prevalence of parents’ perceptions of sensory processing disorders among kindergarten children. American Journal of Occupational Therapy. 2004;58:287–293. doi: 10.5014/ajot.58.3.287. [DOI] [PubMed] [Google Scholar]
- Ayres AJ. Tactile functions their relation to hyperactive and perceptual motor behavior. American Journal of Occupational Therapy. 1964;18:6–11. [PubMed] [Google Scholar]
- Ayres AJ. Patterns of perceptual-motor dysfunction in children: A factor analytic study. Perceptual and Motor Skills. 1965;20:335–368. doi: 10.2466/pms.1965.20.2.335. [DOI] [PubMed] [Google Scholar]
- Ayres AJ. Interrelations among perceptual-motor abilities in a group of normal children. American Journal of Occupational Therapy. 1966;20:288–292. [PubMed] [Google Scholar]
- Ayres AJ. Deficits in sensory integration in educationally handicapped children. Journal of Learning Disabilities. 1969;2:13–18. [Google Scholar]
- Ayres AJ. Types of sensory integrative dysfunction among disabled learners. American Journal of Occupational Therapy. 1972;26(1):13–18. [PubMed] [Google Scholar]
- Ayres AJ, Robbins J. Sensory integration and the child. Los Angeles: Western Psychological Services; 1979. [Google Scholar]
- Ayres AJ, Tickle LS. Hyper-responsivity to touch and vestibular stimuli as a predictor of positive response to sensory integration procedures by autistic children. American Journal of Occupational Therapy. 1980;34:375–381. doi: 10.5014/ajot.34.6.375. [DOI] [PubMed] [Google Scholar]
- Baranek GT. Autism during infancy: A retrospective video analysis of sensory-motor gating and social behaviors at 9–12 months of age. Journal of Autism and Developmental Disorders. 1999;29:213–224. doi: 10.1023/a:1023080005650. [DOI] [PubMed] [Google Scholar]
- Baranek GT. Efficacy of sensory and motor interventions for children with autism. Journal of Autism and Developmental Disorders. 2002;32:397–422. doi: 10.1023/a:1020541906063. [DOI] [PubMed] [Google Scholar]
- Baranek GT, Berkson G. Tactile defensiveness in children with developmental disabilities: Responsiveness and habituation. Journal of Autism and Developmental Disorders. 1994;24:457–471. doi: 10.1007/BF02172128. [DOI] [PubMed] [Google Scholar]
- Baranek GT, Foster LG, Berkson G. Tactile defensiveness and stereotyped behaviors. American Journal of Occupational Therapy. 1997;51(2):91–95. doi: 10.5014/ajot.51.2.91. [DOI] [PubMed] [Google Scholar]
- Barron S, Riley EP. The effects of prenatal alcohol exposure on behavioral and neuroanatomical components of olfaction. Neurotoxicology and Teratology. 1992;14:291–297. doi: 10.1016/0892-0362(92)90009-y. [DOI] [PubMed] [Google Scholar]
- Brown LL, Schneider JS, Lidsky TI. Sensory and cognitive functions of the basal ganglia. Current Opinion in Neurobiology. 1997;7(2):157–163. doi: 10.1016/s0959-4388(97)80003-7. [DOI] [PubMed] [Google Scholar]
- Burden MJ, Jacobson SW, Jacobson JL. Relation of prenatal alcohol exposure to cognitive processing speed and efficiency in childhood. Alcoholism Clinical and Experimental Research. 2005;29:1473–1483. doi: 10.1097/01.alc.0000175036.34076.a0. [DOI] [PubMed] [Google Scholar]
- Casey BJ. Disruption of inhibitory control in developmental disorders: A mechanistic model of implicated fronto-striatal circuitry. In: McClelland JL, Siegler RS, editors. Mechanisms of cognitive development: Behavioral and neural perspectives. Mahwah, NJ: Erlbaum; 2001. pp. 327–349. [Google Scholar]
- Cermak SA, Daunhauer LA. Sensory processing in the post institutionalized child. American Journal of Occupational Therapy. 1997;51:500–507. doi: 10.5014/ajot.51.7.500. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Equifinality and multifinality in developmental psychopathology. Development and Psychopathology. 1996;8:597–600. [Google Scholar]
- Clarke AS, Wittwer DJ, Abbott DH, Schneider ML. Long-term effects of prenatal stress on HPA axis activity in juvenile rhesus monkeys. Developmental Psychobiology. 1994;27:257–269. doi: 10.1002/dev.420270502. [DOI] [PubMed] [Google Scholar]
- Coe CL, Kramer M, Czeh B, Gould E, Reeves AJ, Kirschbaum C, et al. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biological Psychiatry. 2003;54:1025–1034. doi: 10.1016/s0006-3223(03)00698-x. [DOI] [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Lynch ME, Freides D. Auditory and visual sustained attention in adolescents prenatally exposed to alcohol. Alcoholism Clinical and Experimental Research. 2002;26:263–271. [PubMed] [Google Scholar]
- Cummings JL. Frontal-subcortical circuits and human behavior. Archives of Neurology. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- DeJesus OT. Positron-labeled DOPA analogs to image dopamine terminals. Drug Development Research. 2003;59:249–260. [Google Scholar]
- DeJesus OT, Endres CJ, Shelton SE, Nickles RJ, Holden JE. Evaluation of fluorinated m-tyro-sine analogs as PET imaging agents of dopamine nerve terminals: Comparison with 6-fluoroDOPA. Journal of Nuclear Medicine. 1997;38:630–636. [PubMed] [Google Scholar]
- Dixon WJ. BMDP statistical software manual. Berkeley: University of California; 1988. [Google Scholar]
- Druse MJ, Tajuddin N, Kuo AP, Connerty M. Effects of in utero ethanol exposure on the developing dopaminergic system in rats. Journal of Neuroscience Research. 1990;27:233–240. doi: 10.1002/jnr.490270214. [DOI] [PubMed] [Google Scholar]
- Fride E, Weinstock M. Prenatal stress increases anxiety-related behavior and alters cerebral lateralization of dopamine activity. Life Sciences. 1988;42:1059–1065. doi: 10.1016/0024-3205(88)90561-9. [DOI] [PubMed] [Google Scholar]
- Fride E, Weinstock M. Alterations in behavioral and striatal dopamine asymmetries induced by prenatal stress. Pharmacology Biochemistry and Behavior. 1989;32:425–430. doi: 10.1016/0091-3057(89)90174-3. [DOI] [PubMed] [Google Scholar]
- Fulginiti S, Minetti SA, Virgolini MB. Effects of acute ethanol intoxication during pregnancy on central dopaminergic system in male rats. Neurotoxicology and Teratology. 1994;16:385–389. doi: 10.1016/0892-0362(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Bedogni F, Perez J, Racagni G, Riva MA. Corticostriatal brain-derived neurotrophic factor dysregulation in adult rats following prenatal stress. European Journal of Neuroscience. 2004;20:1348–1354. doi: 10.1111/j.1460-9568.2004.03592.x. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Racagni G, Colombo E, Riva MA. BDNF gene expression is reduced in the frontal cortex of dopamine transporter knockout mice. Molecular Psychiatry. 2003;8:898–899. doi: 10.1038/sj.mp.4001370. [DOI] [PubMed] [Google Scholar]
- Gaffan D. Memory action and the corpus striatum: Current developments in the memory-habit distinction. Seminars in Neuroscience. 1996;8:33–38. [Google Scholar]
- Gitau R, Cameron A, Fisk NM, Glover V. Fetal exposure to maternal cortisol. Lancet. 1998;352:707–708. doi: 10.1016/S0140-6736(05)60824-0. [DOI] [PubMed] [Google Scholar]
- Glover V, O’Connor TG. Effects of antenatal stress and anxiety: Implications for development and psychiatry. British Journal of Psychiatry. 2002;180:389–391. doi: 10.1192/bjp.180.5.389. [DOI] [PubMed] [Google Scholar]
- Gottlieb G, Halpern CT. A relational view of causality in normal and abnormal development. Development and Psychopathology. 2002;14:421–435. doi: 10.1017/s0954579402003024. [DOI] [PubMed] [Google Scholar]
- Gottlieb G, Lickliter R. The various roles of animal models in understanding human development. Social Development. 2004;13:311–325. [Google Scholar]
- Grandin T. Calming effects of deep touch pressure in patients with autistic disorder, college students, and animals. Journal of Child and Adolescent Psychopharmacology. 1992;2(1):63–72. doi: 10.1089/cap.1992.2.63. [DOI] [PubMed] [Google Scholar]
- Guerri C, Pascual M, Renau-Piqueras J. Glia and fetal alcohol syndrome. Neurotoxicology. 2001;22:593–599. doi: 10.1016/s0161-813x(01)00037-7. [DOI] [PubMed] [Google Scholar]
- Hannigan JH, Pilati ML. The effects of chronic postweaning amphetamine on rats exposed to alcohol in utero: Weight gain and behavior. Neurotoxicology and Teratology. 1991;13:649–656. doi: 10.1016/0892-0362(91)90049-3. [DOI] [PubMed] [Google Scholar]
- Harlow HF, Harlow MK. Effects of various mother-infant relationships on rhesus monkey behaviors. In: Foss BM, editor. Determinants of infant behavior. Vol. 4. New York: Barnes & Noble; 1969. pp. 15–36. [Google Scholar]
- Henry C, Kabbaj M, Simon H, Le Moal M, Maccari S. Prenatal stress increases the hypothalamic-pituitary-adrenal axis response in young and adult rats. Journal of Neuroendocrinology. 1994;6:341–345. doi: 10.1111/j.1365-2826.1994.tb00591.x. [DOI] [PubMed] [Google Scholar]
- Hudson HM, Larkin RS. Accelerated image reconstruction using ordered subsets of projection data. IEEE Transactions on Medical Imaging. 1994;13:601–609. doi: 10.1109/42.363108. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Phillips JS. Postnatal binge ethanol exposure affects habituation of the cardiac orienting response to an olfactory stimulus in preweanling rats. Alcoholism Clinical and Experimental Research. 2004;28(1):123–130. doi: 10.1097/01.ALC.0000108650.02216.1A. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, et al. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Jackson S, Houghton G. Sensorimotor selection and the basal ganglia: A neruonal network model. In: Houk JC, Davis JL, Beiser DG, editors. Models of information processing in the basal ganglia. Cambridge, MA: MIT Press; 1995. pp. 337–369. [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ, Chiodo LM, Corobana R. Maternal age, alcohol abuse history, and quality of parenting as moderators of the effects of prenatal alcohol exposure on 7.5-year intellectual function. Alcoholism Clinical and Experimental Research. 2004;28:1732–1745. doi: 10.1097/01.alc.0000145691.81233.fa. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ, Martier SS, Ager JW. Prenatal alcohol exposure and infant information processing ability. Child Development. 1993;64:1706–1721. [PubMed] [Google Scholar]
- Joti P, Kulashekhar S, Behari M, Murthy A. Impaired inhibitory oculomotor control in patients with Parkinson’s disease. Experimental Brain Research. 2007;177:447–457. doi: 10.1007/s00221-006-0687-0. [DOI] [PubMed] [Google Scholar]
- Keppel G, Wickens TD. Design and analysis: A Researcher’s handbook. 4th ed. Upper Saddle River, NJ: Pearson Prentice Hall; 2004. [Google Scholar]
- Kim JA, Druse MJ. Protective effects of maternal buspirone treatment on serotonin reuptake sites in ethanol-exposed offspring. Brain Research Developmental Brain Research. 1996;92(2):190–198. doi: 10.1016/0165-3806(96)00015-6. [DOI] [PubMed] [Google Scholar]
- Kinnealey M. Aversive and nonaversive responses to sensory stimulation in mentally retarded children. American Journal of Occupational Therapy. 1973;27:464–472. [PubMed] [Google Scholar]
- LaHoste GJ, Marshall JF. New concepts in dopamine receptor plasticity. Annals of the New York Academy of Sciences. 1993;702:183–196. doi: 10.1111/j.1749-6632.1993.tb17248.x. [DOI] [PubMed] [Google Scholar]
- LaHoste GJ, Ruskin DN, Marshall JF. Cerebrocortical fos expression following dopaminergic stimulation: D1/D2 synergism and its breakdown. Brain Research. 1996;728:97–104. [PubMed] [Google Scholar]
- Larson KA. The sensory history of developmen-tally delayed children with and without tactile defensiveness. American Journal of Occupational Therapy. 1982;36:590–596. doi: 10.5014/ajot.36.9.590. [DOI] [PubMed] [Google Scholar]
- Livy DJ, Miller EK, Maier SE, West JR. Fetal alcohol exposure and temporal vulnerability: Effects of binge-like alcohol exposure on the developing rat hippocampus. Neurotoxicology and Teratology. 2003;25:447–458. doi: 10.1016/s0892-0362(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. Journal of Cerebral Blood Flow & Metabolism. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Maier SE, Chen WA, West JR. Prenatal binge-like alcohol exposure alters neurochemical profiles in fetal rat brain. Pharmacology Biochemistry and Behavior. 1996;55:521–529. doi: 10.1016/s0091-3057(96)00282-1. [DOI] [PubMed] [Google Scholar]
- Mailloux Z. Sensory integration principles in intervention with children with autistic disorder. In: Roley SS, Blanche EI, Schaaf RC, editors. Understanding the nature of sensory integration with diverse populations. San Antonio, TX: Harcourt Health Sciences; 2001. pp. 365–384. [Google Scholar]
- Mangeot SD, Miller LJ, McIntosh DN, McGrath-Clarke J, Simon J, Hagerman RJ, et al. Sensory modulation dysfunction in children with attention-deficit-hyperactivity disorder. Developmental Medicine and Child Neurology. 2001;43:399–406. doi: 10.1017/s0012162201000743. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Goodman AM, Caine C, Delis DC, Riley EP. Executive functioning in children with heavy prenatal alcohol exposure. Alcoholism Clinical and Experimental Research. 1999;23:1808–1815. [PubMed] [Google Scholar]
- McCormick CM, Smythe JW, Sharma S, Meaney MJ. Sex-specific effects of prenatal stress on hypothalamic-pituitary-adrenal responses to stress and brain glucocorticoid receptor density in adult rats. Brain Research Developmental Brain Research. 1995;84(1):55–61. doi: 10.1016/0165-3806(94)00153-q. [DOI] [PubMed] [Google Scholar]
- McIntosh DN, Miller LJ, Shyu V, Dunn W. Overview of the short sensory profile (SSP) In: Dunn W, editor. The sensory profile: Examiner’s manual. San Antonio, TX: Psychological Corporation; 1999. pp. 59–73. [Google Scholar]
- Miller LJ, McIntosh DN, McGrath J, Shyu V, Lampe M, Taylor AK, et al. Electrodermal responses to sensory stimuli in individuals with fragile X syndrome: A preliminary report. American Journal of Medical Genetics. 1999;83:268–279. [PubMed] [Google Scholar]
- Miller LJ, Wilbarger JL, Stackhouse TM, Trunnell SL. Use of clinical reasoning in occupational therapy: The STEP-SI model of treatment of sensory modulation dysfunction. In: Bundy AC, Lane SJ, Murray EA, editors. Sensory integration: Theory and practice. 2nd ed. Philadelphia: F.A. Davis; 2002. pp. 435–451. [Google Scholar]
- Miller MW, Potempa G. Numbers of neurons and glia in mature rat somatosensory cortex: Effects of prenatal exposure to ethanol. Journal of Comparative Neurology. 1990;293(1):92–102. doi: 10.1002/cne.902930108. [DOI] [PubMed] [Google Scholar]
- Mukherjee J, Yang ZY, Lew R, Brown T, Kronmal S, Cooper MD, et al. Evaluation of d-amphet-amine effects on the binding of dopamine D-2 receptor radioligand, 18F–fallypride in nonhuman primates using positron emission tomography. Synapse. 1997;27(1):1–13. doi: 10.1002/(SICI)1098-2396(199709)27:1<1::AID-SYN1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Murphy BL, Arnsten AF, Goldman-Rakic PS, Roth RH. Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proceedings of the National Academy of Sciences. 1996;93:1325–1329. doi: 10.1073/pnas.93.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak CS, Blasberg RG. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. Journal of Cerebral Blood Flow & Metabolism. 1985;5:584–590. doi: 10.1038/jcbfm.1985.87. [DOI] [PubMed] [Google Scholar]
- Peters DA. Prenatal stress: Effects of brain biogenic amine and plasma corticosterone levels. Pharmacology Biochemistry and Behavior. 1982;17:721–725. doi: 10.1016/0091-3057(82)90353-7. [DOI] [PubMed] [Google Scholar]
- Peters DA. Maternal stress increases fetal brain and neonatal cerebral cortex 5-hydroxytryptamine synthesis in rats: A possible mechanism by which stress influences brain development. Pharmacology Biochemistry and Behavior. 1990;35:943–947. doi: 10.1016/0091-3057(90)90383-s. [DOI] [PubMed] [Google Scholar]
- Randall S, Hannigan JH. In utero alcohol and postnatal methylphenidate: Locomotion and dopamine receptors. Neurotoxicology and Teratology. 1999;21:587–593. doi: 10.1016/s0892-0362(99)00017-3. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Prescott TJ, Gurney K. The basal ganglia: A vertebrate solution to the selection problem? Neuroscience. 1999;89:1009–1023. doi: 10.1016/s0306-4522(98)00319-4. [DOI] [PubMed] [Google Scholar]
- Roberts AD, Moore CF, DeJesus OT, Barnhart TE, Larson JA, Mukherjee J, et al. Prenatal stress, moderate fetal alcohol, and dopamine system function in rhesus monkeys. Neurotoxicology and Teratology. 2004;26(2):169–178. doi: 10.1016/j.ntt.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Rogers DT, Barron S, Littleton JM. Neonatal ethanol exposure produces a hyperalgesia that extends into adolescence, and is associated with increased analgesic and rewarding properties of nicotine in rats. Psychopharmacology. 2004;171(2):204–211. doi: 10.1007/s00213-003-1574-z. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. The role of D1 dopamine receptor in working memory: Local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed-response task. Journal of Neurophysiology. 1994;71:515–528. doi: 10.1152/jn.1994.71.2.515. [DOI] [PubMed] [Google Scholar]
- Schneider ML. The effect of mild stress during pregnancy on birth weight and neuromotor maturation in rhesus monkey infants (Macaca mulatta) Infant Behavior and Development. 1992;15:389–403. [Google Scholar]
- Schneider ML, Moore CF. Effect of prenatal stress on development: A nonhuman primate model. In: Nelson CA, editor. The effects of early adversity on neuro-behavioral development. Vol. 31. Mahwah, NJ: Erlbaum; 2000. pp. 201–244. [Google Scholar]
- Schneider ML, Moore CF, Barnhart TE, Larson JA, DeJesus OT, Mukherjee J, et al. Moderate-level prenatal alcohol exposure alters striatal dopamine system function in rhesus monkeys. Alcoholism Clinical and Experimental Research. 2005;29:1685–1697. doi: 10.1097/01.alc.0000179409.80370.25. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Becker EF. Timing of moderate alcohol exposure during pregnancy and neonatal outcome in rhesus monkeys (Macaca mulatta) Alcoholism Clinical and Experimental Research. 2001;25:1238–1246. [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Kraemer GW. Moderate alcohol during pregnancy: Learning and behavior in adolescent rhesus monkeys. Alcoholism Clinical and Experimental Research. 2001;25:1–10. [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Kraemer GW. Moderate level alcohol during pregnancy, prenatal stress, or both and limbic-hypothalamic-pituitary-adrenocortical axis response to stress in rhesus monkeys. Child Development. 2004;75:96–109. doi: 10.1111/j.1467-8624.2004.00656.x. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Roughton EC, Koehler AJ, Lubach GR. Growth and development following prenatal stress exposure in primates: An examination of ontogenetic vulnerability. Child Development. 1999;70:263–274. doi: 10.1111/1467-8624.00020. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Roughton EC, Lubach GR. Moderate alcohol consumption and psychological stress during pregnancy induces attention and neuromotor impairments in primate infants. Child Development. 1997;68:747–759. doi: 10.1111/j.1467-8624.1997.tb01959.x. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. Journal of Neurophysiology. 1998;80(1):1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG. Selective impairment of a delayed radial arm task following local administration of a selective D1, but not a D2, antagonist into the prefrontal cortex. Society of Neuroscience Abstracts. 1995;21:1942. [Google Scholar]
- Sobrian SK, Jones BL, James H, Kamara FN, Holson RR. Prenatal ethanol preferentially enhances reactivity of the dopamine D1 but not D2 or D3 receptors in offspring. Neurotoxicology and Teratology. 2005;27(1):73–93. doi: 10.1016/j.ntt.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, et al. Voxel-based morphometric analyses of the brain in children and adolescents prenatally exposed to alcohol. Neuroreport. 2001;12:515–523. doi: 10.1097/00001756-200103050-00018. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Martin DC. Maternal alcohol use and neonatal habituation assessed with the Brazelton scale. Child Development. 1983;54:1109–1118. [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Sampson PD, Barr HM. Attention: Prenatal alcohol and continuities of vigilance and attention problems from 4 through 14 years. Development and Psychopathology. 1995;7:419–446. [Google Scholar]
- Suomi SJ. Early determinants of behaviour: Evidence from primate studies. British Medical Bulletin. 1997;53:170–184. doi: 10.1093/oxfordjournals.bmb.a011598. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, McDonald RJ, Savage DD. Prenatal exposure to moderate levels of ethanol can have long-lasting effects on hippocampal synaptic plasticity in adult offspring. Hippocampus. 1997;7:232–238. doi: 10.1002/(SICI)1098-1063(1997)7:2<232::AID-HIPO9>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Turner JG, Kalin NH. Prenatal stress alters brain catecholaminergic activity and potentiates stress-induced behavior in adult rats. Brain Research. 1992;574(1–2):131–137. doi: 10.1016/0006-8993(92)90809-n. [DOI] [PubMed] [Google Scholar]
- Uno H, Lohmiller L, Thieme C, Kemnitz JW, Engle MJ, Roecker EB, et al. Brain damage induced by prenatal exposure to dexamethasone in fetal rhesus macaques. I Hippocampus. Brain Research Developmental Brain Research. 1990;53(2):157–167. doi: 10.1016/0165-3806(90)90002-g. [DOI] [PubMed] [Google Scholar]
- Van den Bergh BR, Marcoen A. High antenatal maternal anxiety is related to ADHD symptoms, externalizing problems, and anxiety in 8- and 9-year-olds. Child Development. 2004;75:1085–1097. doi: 10.1111/j.1467-8624.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Thanos PP, Logan J, Gatley SJ, et al. Brain DA D2 receptors predict reinforcing effects of stimulants in humans: Replication study. Synapse. 2002;46(2):79–82. doi: 10.1002/syn.10137. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Glynn L, Hobel CJ, Garite TJ, Porto M, Chicz-DeMet A, et al. Behavioral perinatol-ogy: Biobehavioral processes in human fetal development. Regulatory Peptides. 2002;108:149–157. doi: 10.1016/s0167-0115(02)00102-7. [DOI] [PubMed] [Google Scholar]
- Weinstock M. Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Progress in Neurobiology. 2001;65:427–451. doi: 10.1016/s0301-0082(01)00018-1. [DOI] [PubMed] [Google Scholar]
- Wilbarger JL, Wilbarger PL. The Wilbarger approach to treating sensory defensiveness. In: Bundy AC, Lane SJ, Murray EA, editors. Sensory integration theory and practice. Philadelphia: F.A. Davis; 2002. pp. 335–338. [Google Scholar]
- Willford JA, Richardson GA, Leech SL, Day NL. Verbal and visuospatial learning and memory function in children with moderate prenatal alcohol exposure. Alcoholism Clinical and Experimental Research. 2004;28:497–507. doi: 10.1097/01.alc.0000117868.97486.2d. [DOI] [PubMed] [Google Scholar]
- Winer BJ. Statistical principles in experimental design. 2nd ed. New York: McGraw-Hill; 1971. [Google Scholar]
- Zarrow MX, Philpott JE, Denenberg VH. Passage of the 14C-4 corticosterone from the rat mother to the foetus and neonate. Nature. 1970;226:1058–1059. doi: 10.1038/2261058a0. [DOI] [PubMed] [Google Scholar]