Abstract
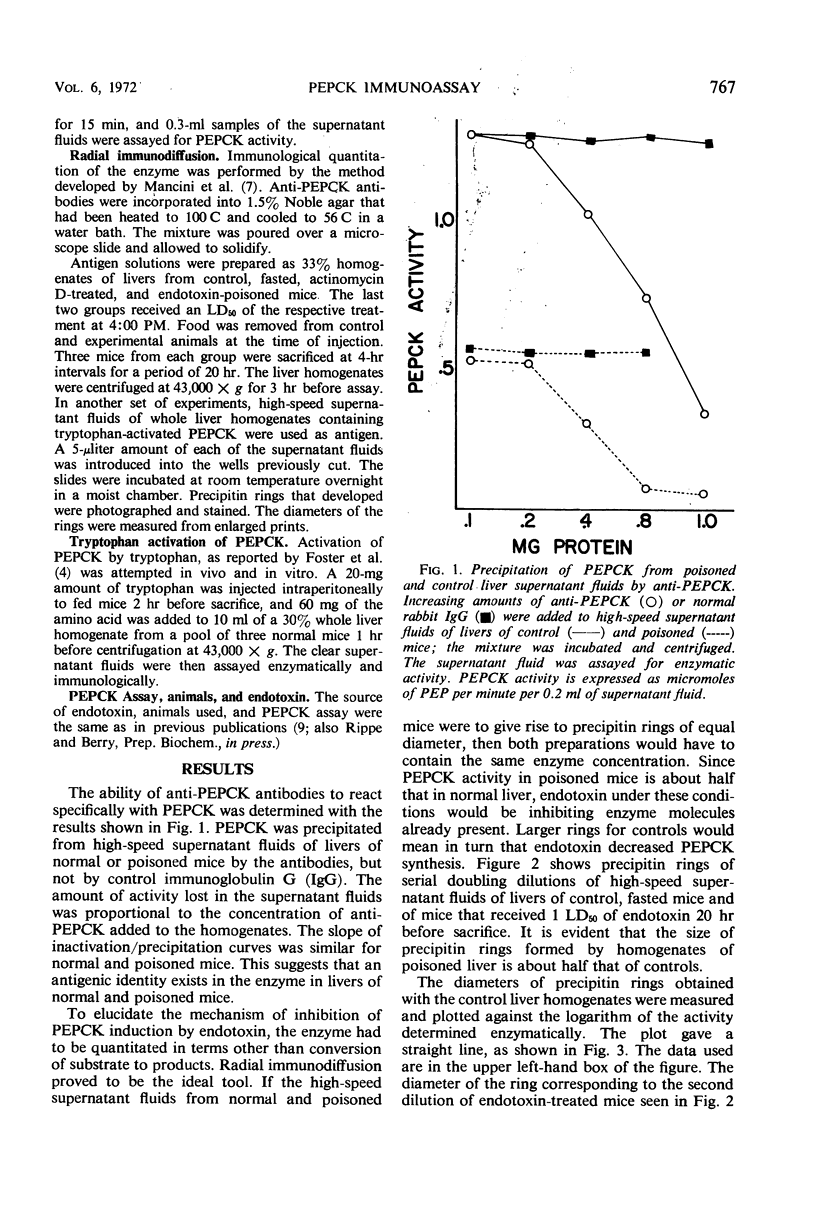
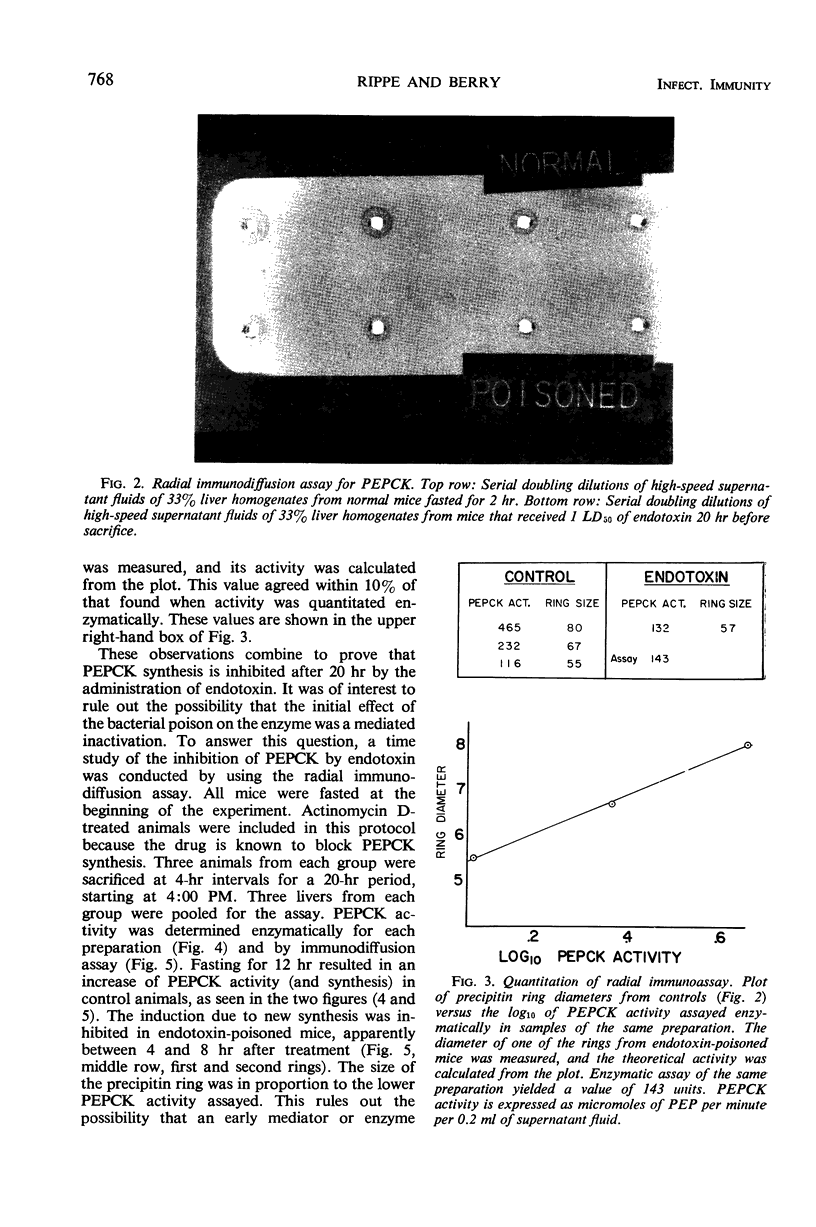
Phosphoenolpyruvate carboxykinase (PEPCK) was precipitated from supernatant fluids of whole mouse liver homogenates by specific antiserum but not by normal rabbit immunoglobulin G. The slopes of inactivation curves were similar for supernatant fluids of control and poisoned mice. It was demonstrated by radial immunodiffusion that PEPCK activity assayed enzymatically was proportional to the diameter of precipitin rings in all cases, except when the enzyme was activated by tryptophan, a process not requiring new enzyme synthesis. Hence, the assay can be used to distinguish between enhanced catalytic activity due to increased enzyme levels or as the result of activation of existing enzyme molecules. Immunological quantitation of PEPCK showed that endotoxin inhibits the induction of the enzyme due to fasting between 4 and 8 hr after administration of the bacterial poison. This result is almost identical to that seen when livers of mice poisoned with actinomycin D were assayed for PEPCK by the radial immunodiffusion technique.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERRY L. J., SMYTHE D. S., YOUNG L. G. Effects of bacterial endotoxin on metabolism. I. Carbohydrate depletion and the protective role of cortisone. J Exp Med. 1959 Sep 1;110:389–405. doi: 10.1084/jem.110.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry L. J., Smythe D. S., Colwell L. S. Inhibition of hepatic enzyme induction as a sensitive assay for endotoxin. J Bacteriol. 1968 Oct;96(4):1191–1199. doi: 10.1128/jb.96.4.1191-1199.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry L. J., Smythe D. S., Colwell L. S. Inhibition of inducible liver enzymes by endotoxin and actinomycin D. J Bacteriol. 1966 Jul;92(1):107–115. doi: 10.1128/jb.92.1.107-115.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D. O., Ray P. D., Lardy H. A. A paradoxical in vivo effect of L-tryptophan on the phosphoenolpyruvate carboxykinase of rat liver. Biochemistry. 1966 Feb;5(2):563–569. doi: 10.1021/bi00866a023. [DOI] [PubMed] [Google Scholar]
- Henning H. V., Stumpf B., Ohly B., Seubert W. On the mechanism of gluconeogenesis and its regulation. 3. The glucogenic capacity and the activities of pyruvate carboxylase and PEP-carboxylase of rat kidney and rat liver after cortisol treatment and starvation. Biochem Z. 1966 Apr 27;344(3):274–288. [PubMed] [Google Scholar]
- Lardy H. A., Foster D. O., Shrago E., Ray P. D. Metabolic and hormonal regulation of phosphopyruvate synthesis. Adv Enzyme Regul. 1964;2:39–47. doi: 10.1016/s0065-2571(64)80004-2. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Phillips L. J., Berry L. J. Hormonal control of mouse liver phosphoenolpyruvate carboxykinase rhythm. Am J Physiol. 1970 Sep;219(3):697–701. doi: 10.1152/ajplegacy.1970.219.3.697. [DOI] [PubMed] [Google Scholar]
- Rippe D. F., Berry L. J. Effect of endotoxin on the activation of phosphoenolpyruvate carboxykinase by tryptophan. Infect Immun. 1972 Jul;6(1):97–98. doi: 10.1128/iai.6.1.97-98.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHRAGO E., LARDY H. A., NORDLIE R. C., FOSTER D. O. METABOLIC AND HORMONAL CONTROL OF PHOSPHOENOLPYRUVATE CARBOXYKINASE AND MALIC ENZYME IN RAT LIVER. J Biol Chem. 1963 Oct;238:3188–3192. [PubMed] [Google Scholar]
- Shtasel T. F., Berry L. J. Effect of endotoxin and cortisone on synthesis of ribonucleic acid and protein in livers of mice. J Bacteriol. 1969 Mar;97(3):1018–1025. doi: 10.1128/jb.97.3.1018-1025.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder I. S., Deters M., Ingle J. Effect of endotoxin on pyruvate kinase activity in mouse liver. Infect Immun. 1971 Aug;4(2):138–142. doi: 10.1128/iai.4.2.138-142.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]






