Abstract
The quest for a prophylactic AIDS vaccine is ongoing, but it is now clear that the successful vaccine must elicit protective antibody responses. Accordingly, intense efforts are underway to identify immunogens that elicit these responses. Regardless of the mechanism of antibody-mediated protection, be it neutralization, Fc-mediated effector function, or both, antibody persistence and appropriate T-cell help are significant problems confronting the development of a successful AIDS vaccine. Here, we discuss the evidence illustrating the poor persistence of antibody responses to Env, the envelope glycoprotein of HIV-1, and the related problem of CD4+ T-cell responses that compromise vaccine efficacy by creating excess cellular targets of HIV-1 infection. Finally, we propose solutions to both problems that are applicable to all Env-based AIDS vaccines regardless of the mechanism of antibody-mediated protection.
Keywords: HIV, vaccine, antibody persistence, AIDS, T cell
HIV vaccine development presents unprecedented challenges on multiple levels, a reality, often overlooked, that cannot be overstated. The chief challenge is that HIV is a human retrovirus that replicates by irreversibly inserting its genes into the host genome. Thus, HIV infection is established permanently in a matter of days or perhaps even hours (1–6), and it cannot be cleared by primary or anamnestic responses that occur after exposure. In addition to integrating into the host genome, a second unique challenge is that HIV replicates in CD4+ T cells that are key players in protective immunity not only to HIV itself (7–9) but also to many other pathogens (cf. ref. 10). These central features distinguish the path to an HIV vaccine from the traditional design principles that led to successful vaccines against other infectious agents. The inability of these principles to yield an HIV vaccine became abundantly clear in six large HIV vaccine trials, where efficacy was not observed (11–16) (Table 1). Strikingly, vaccination increased the risk of infection in two of these studies that selectively targeted T-cell immunity (13–15), providing a stark contrast between the development of conventional and HIV vaccines. Against this backdrop, what will it take to develop the first protective vaccine against a human retrovirus?
Table 1.
HIV vaccine efficacy trials
| Vaccine concept | Trial no. | Study population | Outcome | Ref(s). |
| Clade B gp120 subunit/alum | VAX 003 | IVDU, Thailand, high risk | No efficacy | (10) |
| Clade B gp120 subunit/alum | VAX 004 | MSM, heterosexual; North America, The Netherlands; high risk | No efficacy | (9) |
| Clade B, gag, pol, nef Ad5 (T-cell vaccine) | HVTN-504 (Step) | MSM; Americas, Caribbean, Australia; high risk | No efficacy, increased transmission | (11) |
| Clade B, gag, pol, nef Ad5 (T-cell vaccine) | HVTN-503 (Phambili) | Heterosexual, South Africa, high risk | No efficacy, increased transmission | (12) |
| Clade A/E gp120, gag, pol Canarypox (ALVAC) prime and three boosts; two additional boosts with ALVAC plus clade B/E gp120s in alum | RV144 | Heterosexual, some MSM/IVDU; Thailand; community-based risk; 47.5% low risk, 28.4% moderate risk, 24.1% high risk | 31.2% overall efficacy, 59.9% early efficacy, waning to background by study end | (17) |
| Clade A, B, and C env plus clade B gag, pol DNA-prime; clade A, B, and C env plus clade B gag, pol Ad5 boost (antibody and T-cell vaccine) | HVTN-505 | MSM, USA, high risk | No efficacy | (14) |
IVDU, intravenous drug users; MSM, men who have sex with men.
The nature of HIV infection argues strongly that an effective vaccine must block infection such that it never becomes established in vaccinated individuals (i.e., sterilizing protection) (17). Critically, this protection must persist because there will not be time for a recall response to block infection (1–6, 17). A large body of data points toward a role for antibody responses to the HIV envelope glycoprotein (Env) in sterilizing protection against HIV. A beneficial role for CD8+ T cells in blocking acquisition is less apparent, although this possibility cannot be dismissed. Intriguing studies suggest that CD8+ T-cell responses elicited by a replicating viral vector vaccine can clear a nascent simian immunodeficiency virus (SIV) infection in roughly half of vaccinated nonhuman primates (18). These responses alone did not block acquisition but could contribute to such an effect if combined with certain humoral responses. These responses could be neutralizing, Fc receptor-dependent, or both. This viewpoint is driven by several considerations: an expanding body of evidence linking antibody responses with protection (reviewed in ref. 19); increasingly detailed structural information regarding anti-Env mAbs (cf. ref. 20); and the modest success of the RV144 trial, where an antibody response correlated with reduced infection risk (21) (Table 1). Paradoxically, this realization introduces another major challenge: Antibody responses to gp120-based vaccines persist poorly in humans or other mammalian species (reviewed in ref. 22). The point is moot whether this problem is unique to HIV gp120 or extends to responses against other viral vaccines, as suggested for the influenza virus hemagglutinin (23); it must be solved for an effective HIV vaccine. The importance of this issue is immediately apparent in HIV vaccine trials involving Env. As shown in Table 1, there have been six HIV vaccine efficacy trials of four vaccine concepts; only the RV144 trial exhibited efficacy. The protection was modest, with an overall of efficacy 31.2% (21) in one of three subgroup analyses. Specifically, this subgroup analysis was the modified intention-to-treat analysis, which excluded seven volunteers in the trial who were found to have HIV infection before vaccination began (additional statistical considerations supporting vaccine efficacy in the RV144 trial are discussed in ref. 24). Protection correlated with antibodies against the V1/V2 region of gp120 (25), particularly those antibodies of the IgG3 subclass that mediate potent antibody-dependent cellular cytotoxicity (ADCC) (26), in addition to other aspects of anti-gp120 humoral immunity (25–29). Importantly, efficacy in the RV144 trial was temporally dependent, peaking at ∼59.9% early in the study and waning to background by the end of the 3-y postvaccination follow-up, thus producing an overall efficacy of 31.2% (30). Why did efficacy decline in this study?
The most probable answer is that the anti-gp120 responses linked with efficacy did not persist, as shown by Yates et al. (26). In this light, had the antibody titers responsible for 59.9% protection in the RV144 trial been maintained, the vaccine could be licensable theoretically, at least for use in Thailand. In this context, it should be noted that the RV144 study population was enrolled without regard to risk of HIV infection (i.e., community risk) and was composed of low-risk (47.5%), moderate-risk (28.4%), and high-risk (24.1%) subjects (21). By contrast, the five ineffective trials in Table 1 recruited high-risk study populations and the degree to which the different study populations affected outcomes is not known. Even with this caveat, the RV144 vaccine regimen appeared to be perfectly capable of eliciting a protective antibody response; the problem is that the protective antibody response did not persist. Unless this problem is solved, no Env-based vaccine is likely to be effective, even if it elicits protective antibodies in the short term. Below, we discuss this problem in detail and offer potential solutions. This reality abuts the second major issue confronting HIV vaccine development: HIV thrives in an activated immune system, particularly in follicular CD4+ T cells (Tfhs) (31–33). Tfhs are essential to help germinal center B cells undergo somatic hypermutation and affinity selection (34, 35). It is noteworthy that the frequencies of total circulating “Tfh-like” cells correlate with the production of broadly neutralizing antibodies (34), which are typically highly mutated (36).
In this light, improving the strength and persistence of antibody responses to an HIV vaccine can be attacked in a number of ways, but it is difficult to avoid the prospect that improved persistence will require significant help by CD4+ Tfhs, which are also reservoirs of HIV infection (31–33). Curiously, although CD4+ Tfhs are infected by HIV, they lack the principal HIV coreceptor, C-C chemokine receptor type 5 (CCR5), that is used by transmitted founder viruses (37). It is likely that they are infected at an earlier, CCR5+, stage in Tfh development, as indicated by Xu et al. (32). Recent studies imply that Tfhs arise epigenetically from multiple CD4+ T-cell lineages, including T helper 1 (Th1), Th2, Th17, and Th120 cells and regulatory T cells (38 and discussed in refs. 39–41). Of these lineages, only Th17 cells are readily infected with R5 HIV (42, 43), and they are the major CD4+ T-cell lineage at mucosal sites (reviewed in ref. 44). CD4+ T-cell lineage commitment is determined by the cytokine composition of the microenvironment in which the CD4+ T cells are primed and boosted via transcriptional regulation of lineage-specific cytokine gene families (38, 39, 45). This observation leads to the sobering possibility that CD4+ CCR5+ T cells (i.e., Th17 cells) responding to an HIV vaccine designed to elicit a persistent and protective antibody response could blunt protection or, worse, increase susceptibility to infection, as suggested in the adenovirus serotype 5 (Ad5) vaccine trials (13–16). This finding was presaged by an earlier study in nonhuman primates vaccinated with an attenuated varicella zoster vaccine (VZV) encoding SIV gp120 (46). This vaccine increased antigen-specific CD4+ T-cell responses, leading to a marked increase in virus replication and pathogenesis compared with controls (46). More recent studies suggest that the number of CD4+ CCR5+ T cells at the site of mucosal HIV exposure is a key determinant of transmission. For example, mother-to-infant SIV transmission is attenuated in the natural SIV host, sooty mangabeys, due to a dearth of CD4+ CCR5+ T cells in secondary lymphoid tissue of the infants (47). By contrast, mother-to-infant transmission of SIV occurs more readily in rhesus macaques and correlates with the increased frequencies and proliferative potential of these cells in secondary lymphoid tissue of the infants (47).
The increased transmission associated with unappreciated immune activation in Ad5-HIV efficacy trials is abundantly apparent in the follow-up to the HIV Vaccine Trials Network 503 (HVTN-503; Phambili) (14, 15) and HVTN-504 (Step) (13, 48) trials, in which vaccinees experienced a higher and sustained increased risk of infection compared with placebo recipients (15, 48). Because the vaccines tested in these trials did not include the HIV envelope, any impact of T-cell activation would be relatively unbridled. Based on the above observations, it is highly likely that some aspect of immune activation related to the use of Ad5 vectors in the vaccine led to the increased risk of infection. There is evidence that prevaccination anti-Ad5 titers correlated with altered early innate responses to the vaccine (49). Further, there is evidence that Ad5 immunization itself expands memory CD4+ T cells with a mucosal homing phenotype and that such CD4+ T cells are highly susceptible to HIV infection (16, 50), which raises the possibility that vaccine-elicited CD4+ T cells provide an increased target population favoring transmission. There was no statistically significant increased transmission observed in the HVTN-505 trial (16), although there was an apparent trend toward increased acquisition up to the time that the trial was unblinded and the volunteers were informed of their vaccination status. The absence of increased risk could have been mitigated, in part, by a study design that controlled for population factors that were thought to play a role in the two earlier trials (16). The HVTN-505 vaccine also used Ad5 vectors but included HIV envelope antigens in the design and raised anti-Env humoral responses. Thus, it has been speculated that the HVTN-505 trial represents a case where any potential benefit from humoral responses was countered by T-cell activation. Results were presented in a very recent workshop showing that the Ad5-HIV vaccine used in the HVTN-505 trial elicited strong Ad5-specific CD4+ CCR5+ T cells in the rectal mucosa, consistent with this hypothesis. The challenge for HIV vaccines arises from the recognition that the problems of protective antibody persistence and vaccine-induced activation of CD4+ CCR5+ T cells at the site of infection are heavily intertwined, such that it will be difficult to attenuate one safely without careful consideration of the other. The two phenomena are almost certainly causally related in that a vaccine should elicit sufficient T-cell help to promote antibody durability without eliciting frequencies of CD4+ CCR5+ T cells that favor transmission over protection. We will refer to these obstacles to AIDS vaccine development collectively as the persistence and balance problem. In the sections to follow, we will provide further evidence that the problem is general for all Env-based vaccines, discuss possible mechanisms for the problem, and provide possible solutions to overcome it that might avoid the T-cell activation risk.
Strategies to Elicit Continuous Protection Against HIV by Vaccination: Repeat Boost Model
If a protective level of vaccine-elicited antibody can be established, repeated immunizations can keep it above that level even if the response decays rapidly. Influenza vaccination is an example of this strategy, where antigenic drifts of circulating H3N2 and H1N1 assortants often require formulation of new vaccines and repeated yearly boosting (cf. ref. 51). Although boosting is designed to counter antigenic drift, there is also evidence that it is required for cross-reactive protection (52). This approach requires constant and near–real-time monitoring of circulating viral variants and the rapid formulation and evaluation of a new vaccine to protect against them. In principle, this approach could also work for an HIV vaccine, where the Vax003 and Vax004 gp120 efficacy trials (11, 12) are examples of the repeat boost approach. Volunteers in both trials were immunized seven times at 6-mo intervals to maintain the antibody responses at the highest levels possible throughout the trial, but no protection was observed. By contrast, modest efficacy of 31.2% was observed in the RV144 trial, where volunteers were immunized four times (at weeks 0, 4, 12, and 24), followed by evaluation every 6 mo over a 3-y period (21). It is important to note that vaccine efficacy was as high as 59.9% within the first year, waning to background over the course of the study (30). This finding leads to the striking possibility that if regular boosting could keep protection at the peak, the RV144 vaccine combination would be potentially licensable. For comparison, a recent CDC assessment of current influenza vaccine efficacy was in this range (53). A follow-up clinical trial, RV305, is designed to boost responses in RV144-uninfected vaccinees as a prelude to determining if repeat boosting maintains protective immunity over time (clinicaltrials.gov/ct2/show/NCT01435135?term=RV305&rank=1).
Although the repeat boost approach might be feasible for an effective HIV vaccine, it is more likely that the follow-up studies of this strategy will provide proof-of-principle data that maintenance of antibody responses at protective levels is possible but will not result in vaccine licensure. The logistics of this approach are likely to be prohibitory in resource-poor settings, where an AIDS vaccine is most needed. This problem is due to the need for extensive tracking of vaccinees, cold chain problems, and the need for ongoing surveillance for emerging variants and the rapid modification of the vaccine to counter them. In addition, there is evidence that continued boosting with the gp120 vaccines in the VAX003 trial altered the functional properties of anti-Env antibodies over time (54). Finally, there is the distinct possibility that long-term repeat boosting might increase the number of activated CD4+ T cells that attenuate protection, as discussed below.
Strategies to Elicit Continuous Protection Against HIV by Vaccination: Long-Term Persistence Model
Aside from the influenza vaccines discussed above, the long-term persistence of antibody responses elicited by licensed vaccines sets the stage for what is likely to be necessary for an effective Env-based AIDS vaccine. Although persistence of vaccine-elicited antibody responses has been known for many years (discussed in refs. 55, 56), it was investigated, to our knowledge, for the first time using modern immunological methods in two studies of immunity to vaccinia (57, 58), where antibody responses were found to persist for decades after immunization. This observation was confirmed and extended in a comprehensive analysis of antibody persistence to common vaccines and infections (55). Table 2 (modified from table 1 in ref. 56) lists the t1/2s of circulating antibody responses to these immunogens, as well as more estimates of antibody t1/2s to hepatitis A (HepA) (59) and human papillomavirus (HPV) (60–62) vaccines from more recent cohort studies. It is apparent from Table 2 that antibody responses to the currently approved vaccines for tetanus, diphtheria, vaccinia, HPV, HepA, mumps, measles, and rubella persist for many years. The least durable responses are to tetanus and diphtheria, with antibody t1/2s of 11 and 19 y, respectively. Although these responses are quite durable, those responses elicited by the other vaccines and infections persist for most, if not all, of the human life span, particularly those responses elicited by replication-competent viral vaccines. Thus, long-term antibody persistence is a common property shared among these vaccines and viral infections. A key distinction between the repeat boost and long-term persistence models of vaccine development is that the latter only requires a short immunization course, often only a single inoculation, to elicit protective immunity that persists for decades. It is important to consider how antibody persistence is elicited and evaluated for the licensed vaccines listed in Table 2.
Table 2.
t1/2s of antibody responses to vaccines and selected viral infections
Strategies to Elicit Continuous Protection Against HIV by Vaccination: Mechanism and Evaluation of Long-Term Antibody Persistence
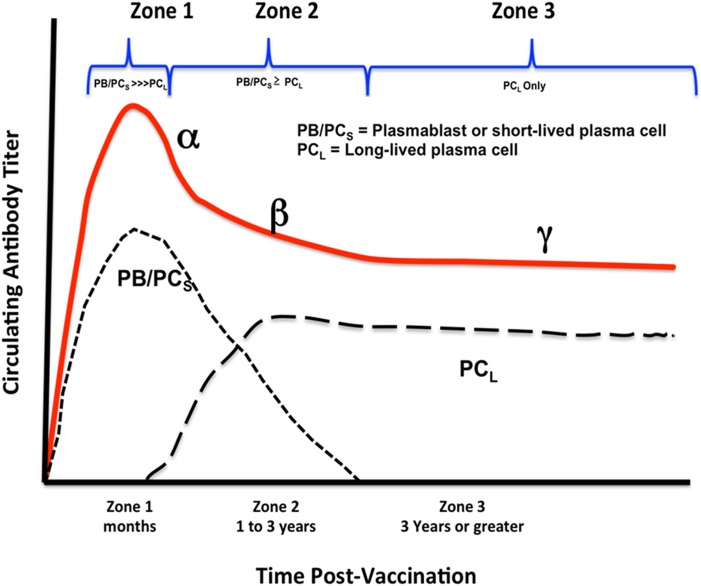
The stylized dynamics of persistent antibody responses elicited by vaccination with the licensed vaccines in Table 2 are illustrated in Fig. 1 (adapted from figure 3 in ref. 56). Three distinct zones of plasma antibody concentration are apparent after vaccination. Zone 1 spans the initial peak of antibody formation after vaccination, where the response is thought to be largely due to short-lived plasmablasts in secondary lymphoid tissues with t1/2s measured in days (63–66). This phase lasts from weeks to months after immunization in humans (56) and represents a rapid exponential expansion of antibody-secreting, short-lived plasmablasts, a steady state period, followed by a rapid exponential decline (α-slope) of these cells and concomitant circulating antibody. Zone 2 is characterized by a more relaxed decay (β-slope) of circulating antibody that persists from 1 to 3 y in humans, depending on the immunogen (56). The antibody sources during this time are not well established, but the antibody is postulated to derive from short-term plasmablasts generated from memory B cells activated by residual antigen, as well as long-lived plasma cells (56). As antigen decays, the plasmablast response diminishes to background, leading to zone 3, true persistent antibody responses mediated by long-lived bone marrow plasma cells. There is continuing decay (γ-slope) during this period that varies with the vaccine (Table 2); however, even the shortest antibody t1/2s are measured in decades (i.e., tetanus, diphtheria toxoids; Table 2). As originally discussed by Amanna and Slifka (56), this time frame imposes significant constraints on measuring the true persistence of antibody responses. If a vaccine trial is not carried into zone 3, estimates of true persistence will be underestimated because there is greater decay during zones 1 and 2 (Fig. 1). During vaccine development, it is uncommon for trials to extend well into zone 3, which can begin as late as 3 y postvaccination and can persist for many years thereafter. As will be discussed below, this problem is a serious concern in HIV vaccine trials. Fortunately, models have been developed that predict long-term zone 3 decay curves based on data obtained in zones 1 and 2 and in early zone 3. Examples of the mathematical models and their predictions can be found elsewhere (59–61). A comparative evaluation of the three most current mathematical models for single datasets can be found in a study by Andraud et al. (67). The key message is that it is possible to predict zone 3 antibody persistence, within limits, using datasets from zones 1 and 2 and early in zone 3, as shown in Fig. 1. Nevertheless, this prediction still requires 4 to 6 y of data postvaccination (cf. ref. 60). Thus, there is an acute need for mathematical methods that enable long-term predictions based on data obtained during late zone 1 and zone 2, where long-lived plasma cells are clearly moving to the bone marrow (cf. ref. 68). It is possible that sequential sampling of antigen-specific plasma cells in the bone marrow during zones 1 and 2 will enable predictions of long-term antibody production in zone 3. Until such methods are developed, the only way to determine antibody persistence is to follow it into zone 3 and use the mathematical methods referred to above.
Fig. 1.
Dynamics of a persistent antibody response. The stylized dynamics of persistent antibody responses elicited by vaccination with the licensed vaccines in Table 2 are illustrated (adapted from figure 3 in ref. 56). Three zones of increasing antibody persistence characterized by three decay constants, α, β, and γ, are shown, along with the relative frequencies (in arbitrary units) of the short-lived plasmablasts (PB), short-lived plasma cells (PCS), and long-lived plasma cells (PCL) responsible for circulating antibody titers over time.
One solution to this problem is to use animal models, but it is essential to calibrate the timing of the three zones in Fig. 1 proportionally to the maximum life span of humans, which is 122 y (69). An excellent example of the temporal appearance of short-lived plasmablasts in secondary lymphoid tissue and long-lived plasma cells in the bone marrow can be found in a study by Taillardet et al. (68). Although this study uses mice, we have calculated that one mouse month is equivalent to ∼2.6 human years [based on a 4-y maximum life span (70)]; thus, the relative appearances of the three phases of circulating antibody and concomitant emergence of plasmablasts and plasma cells are in reasonable agreement between the two species. Rhesus macaques are most often used for HIV vaccine studies, and we have calculated that one macaque month is equivalent to three human months [based on a maximum life span of 40 y (71)]; thus, phase 3 is predicted to occur between 4 mo and 2 y in this species. In this regard, it is useful to compare time courses of antibody production and, where possible, protection elicited by Env-based vaccines in humans and rhesus macaques.
An example of poor persistence of human antibody responses elicited by gp120 in potent adjuvants is provided in a study by McCormack et al. (72). In that study, healthy human volunteers were immunized with gp120 formulated in alum, QS21 plus 3-deacyl-monophosporyl-lipid A, or water in oil emulsion of QS21 plus 3-deacyl-monophosporyl-lipid A. Vaccine was administered on weeks 0, 8, and 24, and the volunteers were followed for various anti-gp120 antibody responses over an 84-wk (1.6-y) period. The data are striking in that with the exception of the gp120 binding antibody response, all measured responses were at background by week 84, and often by week 68, of the study. Further, the gp120 binding antibody responses at 84 wk were only marginally above background in some volunteers. Thus, only zone 1 and zone 2 antibody responses were observed without ever entering zone 3. This finding is a common observation in Env-based vaccine trials. It should be noted that the VAX003 and VAX004 gp120 efficacy trials required repeated boosting at 6-mo intervals to keep antibody titers in a range where they were predicted to be protective (11, 12, 73). The sawtooth behavior of the antibody decay curves in a study by Gilbert et al. (11) are reminiscent of those antibody decay curves observed in the study by McCormack et al. (72), where responses were only present during zones 1 and 2, strongly indicating a persistence problem. The best example of this problem is the RV144 efficacy trial, in which modest protection was observed (21).
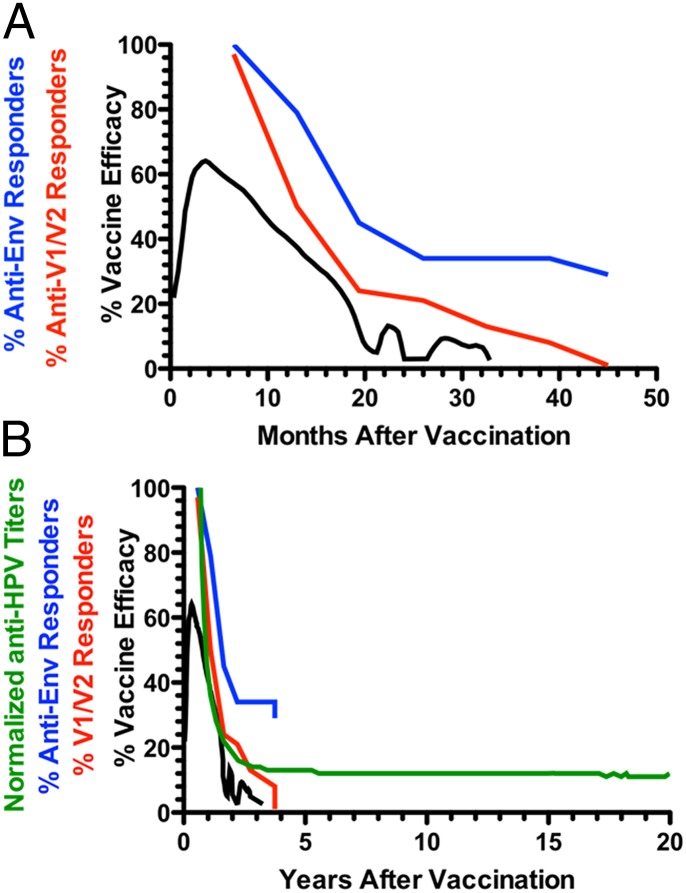
The RV144 trial used the same gp120 immunogens (AIDSVAX B/E) used in the VAX003/VAX004 trials, with the addition of a recombinant canarypox vector vaccine (ALVAC-HIV [vCP1521]) encoding a membrane-anchored gp120 (21). Extensive analyses have been carried out seeking correlations between immune responses and reduced risk of infection (25, 26, 28, 29, 54, 74–76). The strongest correlation with reduced risk of infection was binding antibodies to the V1/V2 region of gp120 (25, 26, 29, 76). Interestingly, both protection (30) and anti-V1/V2 antibody responses (26) decline after vaccination. This finding is shown in Fig. 2A, where data from Yates et al. (26) and Robb et al. (30) are replotted in a single graph. Protection reached levels as high as ∼60% early but declined to undetectable levels by 18 mo. Strikingly, protection and IgG anti-V1/V2 seropositivity were at their peak around 10 mo but decayed in parallel, becoming undetectable at ∼20 mo and 40 mo, respectively.
Fig. 2.
Temporal decay of protection and antibody responses in the RV144 trial on two time scales: months (A) and years (B). In A, the decay of vaccine efficacy (black line), anti-V1/V2 seropositivity (red line), and anti-gp120 (blue line) are depicted. The protection data are from a study by Robb et al. (30), and the seropositivity data are from a study by Yates et al. (26). In B, the same data are plotted on a time scale more representative of durable antibody responses elicited by the licensed vaccines in Table 2. The green line represents data for the persistence of anti-HPV antibody titers modeled by a modified power law in a study by David et al. (60). The data in A and B were plotted from tables in the study by Yates et al. (26) or digitized from the original publications (23, 30, 60) using the commercially available software DigitizeIt (www.digitizeit.de). Accordingly, the digitized data should be viewed as approximate, but this procedure should not affect the conclusions drawn from their analysis in this report.
IgG anti-gp120 binding titers showed a slightly delayed decay, with ∼30% of volunteers remaining seropositive by the end of the study. Fig. 2B shows the same data replotted on a time scale of years required to determine whether the responses entered zone 2. For comparison, a decay curve is shown for the anti-HPV antibody response in a study by David et al. (60) normalized to 100 for graphical continuity. It is apparent that both protection and anti-V1/V2 antibody seropositivity decay in parallel and to background within the first 4 y of the RV144 trial, with no sign of antibody persistence characteristic of zone 2 in Fig. 1. By contrast, anti-HPV binding titers clearly enter zone 2 and are predicted to persist for decades. The contrast between the two vaccines is even more striking when it is considered that the RV144 V1/V2 curve is seropositivity, whereas the anti-HPV curve is titers, where the vast majority of vaccinees remain seropositive for decades (60). The t1/2 estimate for the putatively protective IgG anti-V1/V2 response in the RV144 trial is estimated to be from 11.7 to 23.7 wk depending on the assay antigen (26). This estimate stands in stark contrast to the t1/2s of licensed vaccines listed in Table 1, which range from nearly 10 y to more than 100 y, particularly for replication-competent vectors and particle-based vaccines with potent adjuvants (i.e., the HPV vaccine). Taken together, these results lead to the startling suggestion that the vaccines used in the RV144 trial would be licensable if persistence of the antibody response could be increased to persistence of the antibody response of the standard tetanus and diphtheria vaccines.
In summary, the studies cited above show clearly that anti-Env antibody responses do not persist at levels typical of licensed vaccines designed to protect over long periods of time. Further, none of the HIV vaccine trials have extended well into zone 3 such that antibody persistence could be established. Ironically, the RV144 trial is the only trial that approached this zone, but the protection disappeared by approximately 20 mo and seropositivity for the IgG anti-V1/V2 correlate disappeared shortly thereafter; thus, the relevant responses never extended into zone 3. This persistence problem must be solved for any Env-based vaccine to be effective, but how? There are two approaches. The first approach is empirical, where a series of studies are carried out screening antigen/adjuvant combinations for eliciting persistent protective responses. Such studies are feasible in rhesus macaques, but it is critical that attention be paid to evaluating persistence into zone 3, which we calculate to be from 6 mo to 2 y in that species. This approach suffers from empiricism, and it is possible that an effective immunogen/adjuvant combination cannot be found. The second approach is to define the basis for poor persistence of anti-gp120 antibody responses and develop an approach to solve the problem. This goal can also be approached in rhesus macaques, but it requires both persistent and nonpersistent responses to the same Env-based immunogen. Fortunately, this approach is now possible using a DNA/protein coimmunization protocol, which elicits the most persistent anti-gp120 antibody responses to date in rhesus macaques (77). Using this approach, it should be possible to dissect the important components, define the cellular and molecular bases of their contributions to persistence, and develop a simplified approach to improve the persistence of antibody responses for all Env-based vaccines.
Vaccine-Elicited CD4+ T-Cell Responses Tip the Balance Between Infection and Protection: Hard Lessons from Ad5-HIV Vaccine Efficacy Trials
The failure of an Ad5-HIV “T-cell” vaccine in the Step (13, 48) and Phambili (14, 15) trials was a turning point in HIV vaccine development, and not for the better. Not only was efficacy absent but vaccinees were put at increased risk of infection (reviewed in ref. 78). The increased risk correlated with prevaccination antibody titers to Ad5 in uncircumcised men who have sex with men (13–15, 48). Subsequently, the HVTN-505 trial was designed to control for these factors, and the vaccine also included Env to enable the production of potentially protective antibodies (16). HVTN-505 failed the futility analysis, where it was clear that efficacy was not achieved, and the trial was unblinded (16). There was no statistically significant evidence of increased acquisition, although the vaccines appeared to have a trend toward increased acquisition before unblinding. It is not clear whether there was truly no increased risk of acquisition or whether risk-taking behavior changed after unblinding. Further studies of the volunteers should clarify this issue.
In this regard, the Phambili trial might be informative. It was also unblinded due to lack of efficacy, and there was a trend toward increased acquisition that was not statistically significant at the time of unblinding, but the trend became statistically significant during follow-up studies of the volunteers (15). A metaanalysis of the Step, Phambili, and HVTN-505 trials clearly demonstrated that Ad-5 immunity correlated with increased infection, where most of the risk was afforded by the Merck Ad-5 T-cell vaccine (78, 79). The HVTN-505 trial ruled out preexisting Ad5 titers and the lack of circumcision in men who have sex with men as mitigating factors for the lack of efficacy (16), both of which had been offered as explanations for the increased acquisition seen in the Step and Phambili trials. A previous study in humans predicted that Ad5 would elicit mucosal homing CD4+ T cells that are highly susceptible to HIV infection (50), but this finding was not observed in an initial nonhuman primate study (80). However, a very recent study clearly demonstrated that adenovirus vector immunization increases the frequencies of activated CD4+ T cells in the rectal mucosa (81). Fortunately, mucosal specimens were collected in the HVTN-505 trial, making it possible to evaluate CD4+ T-cell responses in the area of transmission. Strikingly, Ad-5–specific CD4+ T cells with increased levels of CCR5 were present in rectal and colonic biopsies, whereas HIV-specific CD4+ T cells were absent, although they were readily detected in blood (79). The selective presence of Ad5-specific CD4+ T cells at mucosal sites suggests that they provided a fertile field for planting an HIV infection in the vaccinees that attenuates protection (if any) afforded by the vaccine. This phenomenon is illustrated in Fig. S1.
Increased infection risk was not apparent in the HVTN-505 trial, whereas it occurred in the Step and Phambili trials. Because efficacy was absent in all three studies, it is likely that the mitigating CD4+ T-cell responses produce a spectrum of outcomes depending on whether potentially protective responses are also extant at the time of exposure. The Ad5-HIV immunogen used in the Step and Phambili trials lacked Env, whereas that used in the HVTN-505 trial had Env in addition to Gag and Pol, which were present in both vaccines. It is possible that the anti-Env antibody responses blunted the increased risk of infection but that there were still sufficient Ad-5–specific CD4+ T cells at the sites of exposure to attenuate an overall vaccine effect. We make this speculation on our recent vaccine studies in rhesus macaques (epostersonline.com/aidsvax2013/?q=node/639 and http://epostersonline.com/aidsvax2013/?q=node/645&posterview=true) using a conformationally constrained gp120 subunit immunogen (82, 83). Taken together, the three ineffective Ad5-HIV trials strongly suggest the presence of CD4+ CCR5+ T cells at the area of transmission. This balance will be quantitative in nature, and new models need to be developed to measure it accurately.
Vaccine-Elicited CD4+ T-Cell Responses Tip the Balance Between Infection and Protection: Positive Lessons from the RV144 Trial
Although efficacy in the R144 trial was modest, it stands in stark contrast to the Ad5-HIV trials, where the vaccines were not only ineffective but at least one of them increased acquisition. Against this backdrop, it is useful to consider the nature of T-cell immunity in the RV144 trial. As pointed out above, the correlates analysis of the RV144 trial identified anti-V1/V2 antibody titers as an inverse correlate with infection risk and IgA antibodies to the C1 region of gp120 as a direct correlate of infection risk (25). Secondary statistical analyses showed significant interactions between IgA antibodies to the C1 region of gp120, ADCC, neutralization, and CD4+ T-cell responses (25). A follow-up study (84) characterized the CD4+ and CD8+ T-cell responses in detail, confirming the original observation of modest CD4+ T-cell responses and weak to nonexistent CD8+ T cells in the RV144 trial (25). These responses were roughly comparable in magnitude to those responses observed in the Ad5 vaccine trials (84), suggesting that they were sufficient to provide T-cell help but that they were not sufficient to attenuate the protection. It is possible that qualitative differences in the CD4+ T-cell response, such as mucosal homing, effector phenotype, or both, rather than magnitude determine, whether the protection afforded by antibodies is diminished or not. Thus, comparisons of the Ad5-HIV and RV144 trials illustrate a second fundamental challenge of HIV-1 vaccine development: how to induce CD4+ T-cell responses required for B-cell help without creating CD4+ CCR5+ target cells sufficient to reduce the vaccine effect. This challenge requires careful measurement of these responses in all future vaccine studies, where nonhuman primate models will be key tools in identifying vaccine strategies that favor protection over infection.
Vaccine-Elicited CD4+ T-Cell Responses Tip the Balance Between Infection and Protection: Monkeys Do Often Tell the Truth
The lack of efficacy and increased transmission in the Step and Phambili trials sparked significant debate about the usefulness of nonhuman primate models in HIV vaccine development (85–93). Despite the obvious limitations of any animal model of human disease, nonhuman primate studies provided at least one overlooked clue that presaged the likelihood of increased risk in the Step and Phambili trials. A 2004 study reported increased SIV replication and progression to AIDS in rhesus macaques that had been immunized with a VZV-SIV-Env immunogen designed to prime CD4+ T-cell responses (46). This study was the first unambiguous demonstration, to our knowledge, that an AIDS vaccine elicits CD4+ T-cell responses that increase viral replication and pathogenesis. By contrast, nonhuman primate studies using DNA/Ad5-SIV immunogens carried out before and during the Step and Phambili trials showed postinfection control and no evidence of increased viral replication or pathogenesis (94–96), leading to doubts about using nonhuman primates for AIDS vaccine research. However, post hoc studies in nonhuman primates designed to model preexisting Ad5 immunity recapitulated the Step and Phambili trial results (97). Thus, nonhuman primate studies clearly show the possibility that vaccination can increase infection (46, 97, 98). The fact that the signal was missed in the nonhuman primate studies supporting the Step and Phambili trials (94, 95, 99) was more indicative of a problem with the study design than with the animal model itself because those studies did not take into account the possibility that preexisting Ad5 immunity could increase infection. When it was taken into account, the nonhuman primate model recapitulated the Step and Phambili trials.
There is another setting where the design of nonhuman primate studies overlooks the problem of concomitant CD4+ T-cell responses and protection. Passive immunization studies using neutralizing mAbs in nonhuman primates have established unequivocally that antibodies can block infection with simian human immunodeficiency viruses (SHIVs) (100–103). Surprisingly, aside from homologous challenges where Env vaccines and SHIV Env proteins are matched (104, 105), it has been difficult to correlate comparable vaccine-elicited neutralizing antibody titers with protection against SHIV where the Env protein is heterologous to the vaccine (82, 106). The principal difference between the two approaches is that passive immunization is carried out against a “clean” background, devoid of ongoing host responses elicited by vaccination. Based on the considerations above, passive immunization might significantly overestimate the potency of a comparable vaccine-elicited antibody to protect. This potential complication will be an important consideration for future active and passive immunization studies.
In summary, the evidence is clear that vaccine-elicited CD4+ T-cell responses are a double-edged sword. On one hand, they are intimately associated with protection through the provision of T-cell help for antibody production (34) and possibly direct cytolytic activity (9, 25, 107–109). On the other hand, as predicted by Staprans et al. (46) and confirmed elsewhere (97, 98) in nonhuman primate studies, they can attenuate protection and lead to increased acquisition. The problem is now in focus, but its solution is intimately connected with the solution of antibody persistence.
Approach to Solving the Antibody Persistence and T-Cell Balance Problems for HIV Vaccine Design
As detailed above, manipulating the replicative environment (i.e., the innate and adaptive immune systems) of HIV for the purpose of preventing infection is a tricky business. Approaches that are intuitively rational based on other viral systems might produce more harm than good for an HIV vaccine, as evinced by the Ad5-HIV T-cell vaccine trials (13–15). Fortunately, recent data from human clinical trials and experimental vaccine models highlight the problem and provide insights for moving forward.
Although most HIV vaccine studies in nonhuman primates or humans have examined both humoral and T-cell responses in a search for efficacy, each study emphasized the major response that the vaccines were designed to elicit in the hope that a single correlate of protection emerges. For example, the Ad5-HIV T-cell vaccine trials focused largely on CD8+ T-cell response as a potential correlate of protection. Based on the studies cited above, we advocate an alternative analytical approach that systematically identifies multiple variables that interact for an optimal window of protective immunity against HIV. The RV144 correlate studies approached this strategy, albeit in a post hoc, empirical fashion (25–30, 54, 76). The emergence of high ADCC titers in low to intermediate anti-C1 IgA responders as a correlate of decreased infection risk is one such example (25). Similarly, post hoc analyses of SHIV/SIV challenge studies using our conformationally constrained gp120 vaccine (82, 83) in nonhuman primates revealed that humoral responses being equal, protection from acquisition was lowest or abrogated in rhesus macaques with the highest vaccine-elicited anti-Env T-cell responses (epostersonline.com/aidsvax2013/?q=node/639 and http://epostersonline.com/aidsvax2013/?q=node/645&posterview=true). Thus, both the RV144 trial and nonhuman primate protection studies suggest that multiple variables interact to create a window of protection. As noted above for nonhuman primate challenge studies and shown clearly in the RV144 trial, not only the magnitude of protection but also its durability is an issue (26, 30). Based on these considerations, future efficacy studies of Env-based HIV vaccines must consider, a priori, the prospect that antibody persistence at protective levels and potential attenuation of CD4+ T-cell responses are major hurdles to developing a vaccine that protects subjects at risk for HIV exposure.
This consideration tempers standard approaches used previously to develop successful vaccines against other viral pathogens. For example, a conventional (and viable) approach might be to perform small phase I trials to screen immunogen/adjuvant combinations that increase antibody persistence. Without concomitant nonhuman primate challenge studies, this approach leaves “blind” the issue of whether an immunogen/adjuvant combination increases the CD4+ T-cell response to levels such that it abrogates protection or actually increases acquisition. At the moment in nonhuman primates and humans, we have no surrogate markers for antibody persistence and, as indicated above, we also lack a mathematically precise definition of antibody persistence. This deficit is a major issue because there is no general agreement on the length of an HIV vaccine trial required to determine antibody persistence. In addition, we do not know the origins or detailed phenotypes of vaccine-elicited CD4+ T cells that attenuate protection, save for the strong likelihood that their phenotype will include CD4+ CCR5+ T cells (78, 79). With these two issues in mind, what, then, is a workable path forward?
In our view, the solution to the problem of durable protection against HIV by a vaccine lies in a detailed understanding of the immunological basis of poor anti-Env antibody persistence, regardless of whether it is unique to HIV, and how it couples with the nature of CD4+ T-cell subsets that favor HIV replication while providing the requisite help for protective antibody production.
Fortunately, the tools are in hand such that each problem can be studied independently in nonhuman primates and the mechanistic information translated into the design of small phase 1b trials to determine whether an immunogen/adjuvant combination elicits responses that should provide a durable window of protection in phase 2b/phase 3 efficacy trials. In all instances, in addition to having a vaccine in hand that elicits the desired antibody responses, the issues of antibody persistence and T-cell balance must be considered as essential variables in trial design to maximize the likelihood that a vaccine candidate elicits broad, durable protection against HIV.
Supplementary Material
Acknowledgments
We thank our colleagues at the Institute of Human Virology for outstanding support of the studies leading to the ideas presented above. Similarly, we thank Dr. Tim Fouts (Profectus Biosciences) for his unwavering collaboration in our quest for an HIV vaccine. Finally, we thank Drs. Chris Wilson and Chris Karp of the Bill and Melinda Gates Foundation for stimulating discussions that significantly expanded our view of the problem of durable protection against HIV. This work was supported by Grants OPP1017606 and OPP1033109 from The Bill and Melinda Gates Foundation (to R.C.G. and G.K.L., respectively). It was also supported by National Cancer Institute, NIH Grant RC2CA148473 (to R.C.G.) and by National Institute of Allergy and Infectious Diseases, NIH Grant R01AI087181 (to G.K.L.).
Footnotes
Conflict of interest statement: The authors own stock in Profectus Biosciences.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1413550111/-/DCSupplemental.
References
- 1.Finzi D, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278(5341):1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 2.Chun TW, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387(6629):183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 3.Tsai CC, et al. Effectiveness of postinoculation (R)-9-(2-phosphonylmethoxypropyl) adenine treatment for prevention of persistent simian immunodeficiency virus SIVmne infection depends critically on timing of initiation and duration of treatment. J Virol. 1998;72(5):4265–4273. doi: 10.1128/jvi.72.5.4265-4273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishimura Y, et al. Transfer of neutralizing IgG to macaques 6 h but not 24 h after SHIV infection confers sterilizing protection: Implications for HIV-1 vaccine development. Proc Natl Acad Sci USA. 2003;100(25):15131–15136. doi: 10.1073/pnas.2436476100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrantelli F, et al. Time dependence of protective post-exposure prophylaxis with human monoclonal antibodies against pathogenic SHIV challenge in newborn macaques. Virology. 2007;358(1):69–78. doi: 10.1016/j.virol.2006.07.056. [DOI] [PubMed] [Google Scholar]
- 6.Foresman L, et al. Neutralizing antibodies administered before, but not after, virulent SHIV prevent infection in macaques. AIDS Res Hum Retroviruses. 1998;14(12):1035–1043. doi: 10.1089/aid.1998.14.1035. [DOI] [PubMed] [Google Scholar]
- 7.Thèze J, Chakrabarti LA, Vingert B, Porichis F, Kaufmann DE. HIV controllers: A multifactorial phenotype of spontaneous viral suppression. Clin Immunol. 2011;141(1):15–30. doi: 10.1016/j.clim.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ranasinghe S, et al. HIV-specific CD4 T cell responses to different viral proteins have discordant associations with viral load and clinical outcome. J Virol. 2012;86(1):277–283. doi: 10.1128/JVI.05577-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soghoian DZ, et al. HIV-specific cytolytic CD4 T cell responses during acute HIV infection predict disease outcome. Sci Transl Med. 2012;4(123):123ra125. doi: 10.1126/scitranslmed.3003165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanley SK, et al. Effect of immunization with a common recall antigen on viral expression in patients infected with human immunodeficiency virus type 1. N Engl J Med. 1996;334(19):1222–1230. doi: 10.1056/NEJM199605093341903. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert PB, et al. Correlation between immunologic responses to a recombinant glycoprotein 120 vaccine and incidence of HIV-1 infection in a phase 3 HIV-1 preventive vaccine trial. J Infect Dis. 2005;191(5):666–677. doi: 10.1086/428405. [DOI] [PubMed] [Google Scholar]
- 12.Pitisuttithum P, et al. Bangkok Vaccine Evaluation Group Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis. 2006;194(12):1661–1671. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- 13.Buchbinder SP, et al. Step Study Protocol Team Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): A double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372(9653):1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray GE, et al. HVTN 503/Phambili study team Safety and efficacy of the HVTN 503/Phambili study of a clade-B-based HIV-1 vaccine in South Africa: A double-blind, randomised, placebo-controlled test-of-concept phase 2b study. Lancet Infect Dis. 2011;11(7):507–515. doi: 10.1016/S1473-3099(11)70098-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray GE, et al. Recombinant adenovirus type 5 HIV gag/pol/nef vaccine in South Africa: Unblinded, long-term follow-up of the phase 2b HVTN 503/Phambili study. Lancet Infect Dis. 2014;14(5):388–396. doi: 10.1016/S1473-3099(14)70020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammer SM, et al. HVTN 505 Study Team Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N Engl J Med. 2013;369(22):2083–2092. doi: 10.1056/NEJMoa1310566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallo RC. The end or the beginning of the drive to an HIV-preventive vaccine: A view from over 20 years. Lancet. 2005;366(9500):1894–1898. doi: 10.1016/S0140-6736(05)67395-3. [DOI] [PubMed] [Google Scholar]
- 18.Hansen SG, et al. Immune clearance of highly pathogenic SIV infection. Nature. 2013;502(7469):100–104. doi: 10.1038/nature12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koff WC, et al. Accelerating next-generation vaccine development for global disease prevention. Science. 2013;340(6136):1232910. doi: 10.1126/science.1232910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doria-Rose NA, et al. NISC Comparative Sequencing Program Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature. 2014;509(7498):55–62. doi: 10.1038/nature13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rerks-Ngarm S, et al. MOPH-TAVEG Investigators Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361(23):2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 22.Klasse PJ, Sanders RW, Cerutti A, Moore JP. How can HIV-type-1-Env immunogenicity be improved to facilitate antibody-based vaccine development? AIDS Res Hum Retroviruses. 2012;28(1):1–15. doi: 10.1089/aid.2011.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sundling C, et al. Immunization of macaques with soluble HIV type 1 and influenza virus envelope glycoproteins results in a similarly rapid contraction of peripheral B-cell responses after boosting. J Infect Dis. 2013;207(3):426–431. doi: 10.1093/infdis/jis696. [DOI] [PubMed] [Google Scholar]
- 24.Gilbert PB, Shepherd BE, Hudgens MG. Sensitivity Analysis of Per-Protocol Time-to-Event Treatment Efficacy in Randomized Clinical Trials. J Am Stat Assoc. 2013;108(503):789–800. doi: 10.1080/01621459.2013.786649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haynes BF, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366(14):1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yates NL, et al. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci Transl Med. 2014;6(228):228ra239. doi: 10.1126/scitranslmed.3007730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gottardo R, et al. Plasma IgG to linear epitopes in the V2 and V3 regions of HIV-1 gp120 correlate with a reduced risk of infection in the RV144 vaccine efficacy trial. PLoS ONE. 2013;8(9):e75665. doi: 10.1371/journal.pone.0075665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomaras GD, et al. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc Natl Acad Sci USA. 2013;110(22):9019–9024. doi: 10.1073/pnas.1301456110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zolla-Pazner S, et al. Vaccine-induced IgG antibodies to V1V2 regions of multiple HIV-1 subtypes correlate with decreased risk of HIV-1 infection. PLoS ONE. 2014;9(2):e87572. doi: 10.1371/journal.pone.0087572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robb ML, et al. Risk behaviour and time as covariates for efficacy of the HIV vaccine regimen ALVAC-HIV (vCP1521) and AIDSVAX B/E: A post-hoc analysis of the Thai phase 3 efficacy trial RV 144. Lancet Infect Dis. 2012;12(7):531–537. doi: 10.1016/S1473-3099(12)70088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perreau M, et al. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med. 2013;210(1):143–156. doi: 10.1084/jem.20121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu Y, et al. Simian immunodeficiency virus infects follicular helper CD4 T cells in lymphoid tissues during pathogenic infection of pigtail macaques. J Virol. 2013;87(7):3760–3773. doi: 10.1128/JVI.02497-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brenchley JM, et al. Differential infection patterns of CD4+ T cells and lymphoid tissue viral burden distinguish progressive and nonprogressive lentiviral infections. Blood. 2012;120(20):4172–4181. doi: 10.1182/blood-2012-06-437608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Locci M, et al. International AIDS Vaccine Initiative Protocol C Principal Investigators Human circulating PD-1+CXCR3-CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity. 2013;39(4):758–769. doi: 10.1016/j.immuni.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gitlin AD, Shulman Z, Nussenzweig MC. Clonal selection in the germinal centre by regulated proliferation and hypermutation. Nature. 2014;509(7502):637–640. doi: 10.1038/nature13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klein F, et al. Somatic mutations of the immunoglobulin framework are generally required for broad and potent HIV-1 neutralization. Cell. 2013;153(1):126–138. doi: 10.1016/j.cell.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keele BF, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci USA. 2008;105(21):7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu KT, et al. Functional and epigenetic studies reveal multistep differentiation and plasticity of in vitro-generated and in vivo-derived follicular T helper cells. Immunity. 2011;35(4):622–632. doi: 10.1016/j.immuni.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cannons JL, Lu KT, Schwartzberg PL. T follicular helper cell diversity and plasticity. Trends Immunol. 2013;34(5):200–207. doi: 10.1016/j.it.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 42.Bernier A, et al. Transcriptional profiling reveals molecular signatures associated with HIV permissiveness in Th1Th17 cells and identifies peroxisome proliferator-activated receptor gamma as an intrinsic negative regulator of viral replication. Retrovirology. 2013;10:160. doi: 10.1186/1742-4690-10-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gosselin A, et al. Peripheral blood CCR4+CCR6+ and CXCR3+CCR6+CD4+ T cells are highly permissive to HIV-1 infection. J Immunol. 2010;184(3):1604–1616. doi: 10.4049/jimmunol.0903058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weaver CT, Elson CO, Fouser LA, Kolls JK. The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annu Rev Pathol. 2013;8:477–512. doi: 10.1146/annurev-pathol-011110-130318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Evans CM, Jenner RG. Transcription factor interplay in T helper cell differentiation. Brief Funct Genomics. 2013;12(6):499–511. doi: 10.1093/bfgp/elt025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Staprans SI, et al. Enhanced SIV replication and accelerated progression to AIDS in macaques primed to mount a CD4 T cell response to the SIV envelope protein. Proc Natl Acad Sci USA. 2004;101(35):13026–13031. doi: 10.1073/pnas.0404739101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chahroudi A, et al. Target cell availability, rather than breast milk factors, dictates mother-to-infant transmission of SIV in sooty mangabeys and rhesus macaques. PLoS Pathog. 2014;10(3):e1003958. doi: 10.1371/journal.ppat.1003958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duerr A, et al. Step/HVTN 504 Study Team Extended follow-up confirms early vaccine-enhanced risk of HIV acquisition and demonstrates waning effect over time among participants in a randomized trial of recombinant adenovirus HIV vaccine (Step Study) J Infect Dis. 2012;206(2):258–266. doi: 10.1093/infdis/jis342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zak DE, et al. Merck Ad5/HIV induces broad innate immune activation that predicts CD8+ T-cell responses but is attenuated by preexisting Ad5 immunity. Proc Natl Acad Sci USA. 2012;109(50):E3503–E3512. doi: 10.1073/pnas.1208972109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benlahrech A, et al. Adenovirus vector vaccination induces expansion of memory CD4 T cells with a mucosal homing phenotype that are readily susceptible to HIV-1. Proc Natl Acad Sci USA. 2009;106(47):19940–19945. doi: 10.1073/pnas.0907898106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uyeki TM. Preventing and controlling influenza with available interventions. N Engl J Med. 2014;370(9):789–791. doi: 10.1056/NEJMp1400034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tricco AC, et al. Comparing influenza vaccine efficacy against mismatched and matched strains: A systematic review and meta-analysis. BMC Med. 2013;11:153. doi: 10.1186/1741-7015-11-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flannery B, et al. Centers for Disease Control and Prevention (CDC) Interim estimates of 2013-14 seasonal influenza vaccine effectiveness—United States, February 2014. MMWR Morb Mortal Wkly Rep. 2014;63(7):137–142. [PMC free article] [PubMed] [Google Scholar]
- 54.Chung AW, et al. Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Sci Transl Med. 2014;6(228):228ra238. doi: 10.1126/scitranslmed.3007736. [DOI] [PubMed] [Google Scholar]
- 55.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357(19):1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 56.Amanna IJ, Slifka MK. Mechanisms that determine plasma cell lifespan and the duration of humoral immunity. Immunol Rev. 2010;236:125–138. doi: 10.1111/j.1600-065X.2010.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hammarlund E, et al. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9(9):1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 58.Crotty S, et al. Cutting edge: Long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171(10):4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 59.Hens N, Habteab Ghebretinsae A, Hardt K, Van Damme P, Van Herck K. Model based estimates of long-term persistence of inactivated hepatitis A vaccine-induced antibodies in adults. Vaccine. 2014;32(13):1507–1513. doi: 10.1016/j.vaccine.2013.10.088. [DOI] [PubMed] [Google Scholar]
- 60.David MP, et al. Long-term persistence of anti-HPV-16 and -18 antibodies induced by vaccination with the AS04-adjuvanted cervical cancer vaccine: Modeling of sustained antibody responses. Gynecol Oncol. 2009;115(3) Suppl:S1–S6. doi: 10.1016/j.ygyno.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 61.Fraser C, et al. Modeling the long-term antibody response of a human papillomavirus (HPV) virus-like particle (VLP) type 16 prophylactic vaccine. Vaccine. 2007;25(21):4324–4333. doi: 10.1016/j.vaccine.2007.02.069. [DOI] [PubMed] [Google Scholar]
- 62.Ault KA. Long-term efficacy of human papillomavirus vaccination. Gynecol Oncol. 2007;107(2) Suppl 1:S27–S30. doi: 10.1016/j.ygyno.2007.07.078. [DOI] [PubMed] [Google Scholar]
- 63.Nossal GJ, Makela O. Autoradiographic studies on the immune response. I. The kinetics of plasma cell proliferation. J Exp Med. 1962;115:209–230. doi: 10.1084/jem.115.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Makela O, Nossal GJ. Autoradiographic studies on the immune response. II. DNA synthesis amongst single antibody-producing cells. J Exp Med. 1962;115:231–244. doi: 10.1084/jem.115.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cooper EH. Production of lymphocytes and plasma cells in the rat following immunization with human serum albumin. Immunology. 1961;4:219–231. [PMC free article] [PubMed] [Google Scholar]
- 66.Schooley JC. Autoradiographic observations of plasma cell formation. J Immunol. 1961;86:331–337. [PubMed] [Google Scholar]
- 67.Andraud M, et al. Living on three time scales: The dynamics of plasma cell and antibody populations illustrated for hepatitis a virus. PLOS Comput Biol. 2012;8(3):e1002418. doi: 10.1371/journal.pcbi.1002418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taillardet M, et al. The thymus-independent immunity conferred by a pneumococcal polysaccharide is mediated by long-lived plasma cells. Blood. 2009;114(20):4432–4440. doi: 10.1182/blood-2009-01-200014. [DOI] [PubMed] [Google Scholar]
- 69.Allard M, Lebre V, Robine J-M, Calment J, Coupland B. Jeanne Calment: From Van Gog’s Time to Ours. Freeman; New York: 1998. [Google Scholar]
- 70.Miller RA, Harper JM, Dysko RC, Durkee SJ, Austad SN. Longer life spans and delayed maturation in wild-derived mice. Exp Biol Med (Maywood) 2002;227(7):500–508. doi: 10.1177/153537020222700715. [DOI] [PubMed] [Google Scholar]
- 71.Mattison JA, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489(7415):318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McCormack S, et al. A phase I trial in HIV negative healthy volunteers evaluating the effect of potent adjuvants on immunogenicity of a recombinant gp120W61D derived from dual tropic R5X4 HIV-1ACH320. Vaccine. 2000;18(13):1166–1177. doi: 10.1016/s0264-410x(99)00388-6. [DOI] [PubMed] [Google Scholar]
- 73.Francis DP, et al. Advancing AIDSVAX to phase 3. Safety, immunogenicity, and plans for phase 3. AIDS Res Hum Retroviruses. 1998;14(Suppl 3):S325–S331. [PubMed] [Google Scholar]
- 74.Liu P, et al. Infectious virion capture by HIV-1 gp120-specific IgG from RV144 vaccinees. J Virol. 2013;87(14):7828–7836. doi: 10.1128/JVI.02737-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pegu P, et al. Antibodies with high avidity to the gp120 envelope protein in protection from simian immunodeficiency virus SIV(mac251) acquisition in an immunization regimen that mimics the RV-144 Thai trial. J Virol. 2013;87(3):1708–1719. doi: 10.1128/JVI.02544-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zolla-Pazner S, et al. Analysis of V2 antibody responses induced in vaccinees in the ALVAC/AIDSVAX HIV-1 vaccine efficacy trial. PLoS ONE. 2013;8(1):e53629. doi: 10.1371/journal.pone.0053629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jalah R, et al. DNA and protein co-immunization improves the magnitude and longevity of humoral immune responses in macaques. PLoS ONE. 2014;9(3):e91550. doi: 10.1371/journal.pone.0091550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fauci AS, Marovich MA, Dieffenbach CW, Hunter E, Buchbinder SP. Immunology. Immune activation with HIV vaccines. Science. 2014;344(6179):49–51. doi: 10.1126/science.1250672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.National Institute of Allergy and Infectious Disease, National Institutes of Health . Mini-Summit on Adenovirus Platforms for HIV Vaccines. NIH; Bethesda, MD: 2013. [Google Scholar]
- 80.Masek-Hammerman K, et al. Mucosal trafficking of vector-specific CD4+ T lymphocytes following vaccination of rhesus monkeys with adenovirus serotype 5. J Virol. 2010;84(19):9810–9816. doi: 10.1128/JVI.01157-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bukh I, et al. Increased mucosal CD4+ T cell activation in rhesus macaques following vaccination with an adenoviral vector. J Virol. 2014;88(15):8468–8478. doi: 10.1128/JVI.03850-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.DeVico A, et al. Antibodies to CD4-induced sites in HIV gp120 correlate with the control of SHIV challenge in macaques vaccinated with subunit immunogens. Proc Natl Acad Sci USA. 2007;104(44):17477–17482. doi: 10.1073/pnas.0707399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fouts TR, et al. Expression and characterization of a single-chain polypeptide analogue of the human immunodeficiency virus type 1 gp120-CD4 receptor complex. J Virol. 2000;74(24):11427–11436. doi: 10.1128/jvi.74.24.11427-11436.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.de Souza MS, et al. Ministry of Public Health–Thai AIDS Vaccine Evaluation Group Collaborators The Thai phase III trial (RV144) vaccine regimen induces T cell responses that preferentially target epitopes within the V2 region of HIV-1 envelope. J Immunol. 2012;188(10):5166–5176. doi: 10.4049/jimmunol.1102756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Corey L, et al. HIV-1 vaccines and adaptive trial designs. Sci Transl Med. 2011;3(79):79ps13. doi: 10.1126/scitranslmed.3001863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Watkins DI, Burton DR, Kallas EG, Moore JP, Koff WC. Nonhuman primate models and the failure of the Merck HIV-1 vaccine in humans. Nat Med. 2008;14(6):617–621. doi: 10.1038/nm.f.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morgan C, et al. The use of nonhuman primate models in HIV vaccine development. PLoS Med. 2008;5(8):e173. doi: 10.1371/journal.pmed.0050173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Genescà M, Miller CJ. Use of nonhuman primate models to develop mucosal AIDS vaccines. Curr HIV/AIDS Rep. 2010;7(1):19–27. doi: 10.1007/s11904-009-0035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Staprans SI, Feinberg MB, Shiver JW, Casimiro DR. Role of nonhuman primates in the evaluation of candidate AIDS vaccines: an industry perspective. Curr Opin HIV AIDS. 2010;5(5):377–385. doi: 10.1097/COH.0b013e32833d2e19. [DOI] [PubMed] [Google Scholar]
- 90.Lifson JD, Haigwood NL. Lessons in nonhuman primate models for AIDS vaccine research: From minefields to milestones. Cold Spring Harb Perspect Med. 2012;2(6):a007310. doi: 10.1101/cshperspect.a007310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Van Rompay KK. The use of nonhuman primate models of HIV infection for the evaluation of antiviral strategies. AIDS Res Hum Retroviruses. 2012;28(1):16–35. doi: 10.1089/aid.2011.0234. [DOI] [PubMed] [Google Scholar]
- 92.Evans DT, Silvestri G. Nonhuman primate models in AIDS research. Curr Opin HIV AIDS. 2013;8(4):255–261. doi: 10.1097/COH.0b013e328361cee8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McChesney MB, Miller CJ. New directions for HIV vaccine development from animal models. Curr Opin HIV AIDS. 2013;8(5):376–381. doi: 10.1097/COH.0b013e328363d3a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shiver JW, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415(6869):331–335. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- 95.Casimiro DR, et al. Attenuation of simian immunodeficiency virus SIVmac239 infection by prophylactic immunization with dna and recombinant adenoviral vaccine vectors expressing Gag. J Virol. 2005;79(24):15547–15555. doi: 10.1128/JVI.79.24.15547-15555.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wilson NA, et al. Vaccine-induced cellular responses control simian immunodeficiency virus replication after heterologous challenge. J Virol. 2009;83(13):6508–6521. doi: 10.1128/JVI.00272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qureshi H, et al. Low-dose penile SIVmac251 exposure of rhesus macaques infected with adenovirus type 5 (Ad5) and then immunized with a replication-defective Ad5-based SIV gag/pol/nef vaccine recapitulates the results of the phase IIb step trial of a similar HIV-1 vaccine. J Virol. 2012;86(4):2239–2250. doi: 10.1128/JVI.06175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tenbusch M, et al. Risk of immunodeficiency virus infection may increase with vaccine-induced immune response. J Virol. 2012;86(19):10533–10539. doi: 10.1128/JVI.00796-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wilson NA, et al. Vaccine-induced cellular immune responses reduce plasma viral concentrations after repeated low-dose challenge with pathogenic simian immunodeficiency virus SIVmac239. J Virol. 2006;80(12):5875–5885. doi: 10.1128/JVI.00171-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mascola JR, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6(2):207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 101.Mascola JR, et al. Protection of Macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol. 1999;73(5):4009–4018. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Baba TW, et al. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6(2):200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 103.Parren PW, et al. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J Virol. 2001;75(17):8340–8347. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Barnett SW, et al. Protection of macaques against vaginal SHIV challenge by systemic or mucosal and systemic vaccinations with HIV-envelope. AIDS. 2008;22(3):339–348. doi: 10.1097/QAD.0b013e3282f3ca57. [DOI] [PubMed] [Google Scholar]
- 105.Barnett SW, et al. Antibody-mediated protection against mucosal simian-human immunodeficiency virus challenge of macaques immunized with alphavirus replicon particles and boosted with trimeric envelope glycoprotein in MF59 adjuvant. J Virol. 2010;84(12):5975–5985. doi: 10.1128/JVI.02533-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xu R, et al. Immunization with HIV-1 SF162-derived Envelope gp140 proteins does not protect macaques from heterologous simian-human immunodeficiency virus SHIV89.6P infection. Virology. 2006;349(2):276–289. doi: 10.1016/j.virol.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 107.Siliciano RF, et al. Analysis of host-virus interactions in AIDS with anti-gp120 T cell clones: Effect of HIV sequence variation and a mechanism for CD4+ cell depletion. Cell. 1988;54(4):561–575. doi: 10.1016/0092-8674(88)90078-5. [DOI] [PubMed] [Google Scholar]
- 108.Norris PJ, et al. Beyond help: Direct effector functions of human immunodeficiency virus type 1-specific CD4(+) T cells. J Virol. 2004;78(16):8844–8851. doi: 10.1128/JVI.78.16.8844-8851.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schieffer M, et al. Induction of Gag-specific CD4 T cell responses during acute HIV infection is associated with improved viral control. J Virol. 2014;88(13):7357–66. doi: 10.1128/JVI.00728-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.