Significance
How our brains capture and store new information is heavily influenced by what we already know. While prior work demonstrates that existing memories are spontaneously reactivated and strengthened in the brain during passive rest periods, the prospective benefits of spontaneous offline reactivation for future learning remain unknown. Here, we use functional MRI to interrogate how reactivation and interregional coupling support the ability to learn related content in later situations. We find that offline processing of prior memories is associated with better subsequent learning. Our results provide a mechanistic account of the circumstances under which prior knowledge can come to facilitate—as opposed to interfere with—new learning, serving as a strong foundation upon which new content is encoded.
Keywords: episodic memory, hippocampus, memory integration, interference, inference
Abstract
Although a number of studies have highlighted the importance of offline processes for memory, how these mechanisms influence future learning remains unknown. Participants with established memories for a set of initial face–object associations were scanned during passive rest and during encoding of new related and unrelated pairs of objects. Spontaneous reactivation of established memories and enhanced hippocampal–neocortical functional connectivity during rest was related to better subsequent learning, specifically of related content. Moreover, the degree of functional coupling during rest was predictive of neural engagement during the new learning experience itself. These results suggest that through rest-phase reactivation and hippocampal–neocortical interactions, existing memories may come to facilitate encoding during subsequent related episodes.
Numerous empirical studies (1–4) and theoretical accounts (5, 6) highlight the importance of offline processes—such as reinstatement of recent experience and enhanced interregional communication—for episodic memory. It has been proposed that through hippocampal (HPC)–neocortical interactions (6, 7), memories are reactivated during periods of sleep and awake rest. Such reactivation (or “replay”) is thought to support the strengthening and transfer of memory traces from the HPC to neocortical regions for long-term storage, a process termed “consolidation.” The functional significance of reactivation of recent experience for memory has been demonstrated during awake rest using neurophysiological techniques in rodents (2) and, more recently, in humans using pattern information analysis of functional magnetic resonance imaging (fMRI) data (1, 3). For instance, more delay period reactivation has been observed for stimuli that were remembered, relative to those that were forgotten in a subsequent test (3). Moreover, studies have shown that the degree of HPC–neocortical functional coupling during rest periods following learning relates to later memory for the learned content (4).
This existing body of work demonstrates that rest-phase neural signatures relate to memory for prior experiences. However, one important quality of memory is that it is inherently prospective (8); that is, memories are formed for maximal utility in future situations. Whereas research shows that rest-phase reactivation impacts memory for the reactivated content itself (1, 3), how this mechanism might be prospectively advantageous remains unknown. In the present study, we turn our attention to this question: How does spontaneous reactivation of established memories and enhanced HPC–neocortical connectivity during rest affect learning during subsequent related episodes?
A number of theories underscore the highly interactive nature of episodic memories (9, 10). One prominent view, “interference theory,” highlights that existing knowledge may impair learning of related content. A host of studies confirm this intuition; that is, people often have worse memory for information that is related to their existing memories relative to unrelated information, a phenomenon termed “proactive interference” (11–13). However, this impairment is not universally observed, even in the classic literature; on the contrary, prior knowledge can also be beneficial to new learning under some circumstances (14). For example, one study showed a memory advantage for new responses paired with well-learned old stimuli (i.e., stimuli previously learned with a different response), a phenomenon known as “associative facilitation” (11). Such facilitation may also extend to novel judgments that require the simultaneous consideration of multiple memories (e.g., inferences).
Whereas these data and others (15) suggest that strong prior knowledge may facilitate new learning, the neural mechanisms supporting such associative facilitation are not well understood. One possible explanation stems from a perspective known as “integrative encoding,” which describes how new memories are created in relation to existing knowledge (16, 17). Mechanistically, it has been proposed that when newly encountered content overlaps with one’s stored memory representations, the neural patterns associated with that preexisting knowledge may be reactivated in the brain during new learning (18–20). New episodes may then be encoded in the context of these internally generated representations, connecting these related memories. A recent fMRI study suggests that reactivation of existing knowledge during encoding of new, overlapping events may strengthen preexisting memory traces, making the prior knowledge itself less susceptible to interference (18). Reactivation during learning has also been shown to support novel judgments that span experiences (20), consistent with the notion that this mechanism enables the linking of related memories. However, the potential impact of encoding-phase reactivation on the new learning itself has not been addressed. That is, although reactivation has been shown to strengthen both established memories and the connections among discrete experiences, it is as yet undetermined whether this process also facilitates memory formation for the new, related events through integration.
We propose that the degree to which memory processes are engaged during offline periods influences whether prior knowledge interferes with or facilitates new encoding. Importantly, interference theory and integrative encoding make opposing predictions for the impact of rest-phase processes on subsequent learning of related events. Both perspectives might predict that memories are strengthened during offline periods; and that stronger memories are more likely to be reactivated during learning of new, related events. However, these perspectives diverge in their predictions for the consequences of that reactivation on new learning. Although interference theory would suggest that rest-phase strengthening of the initially acquired information might lead to more “competition” and thus worse memory for new, related content (21), integrative encoding predicts the opposite. Because stronger memories are more readily reinstated, they are also more likely to be “updated” with new information during subsequent experiences. For this reason, more engagement of rest-phase memory processing might facilitate both the later encoding of related events and novel judgments that span episodes. We sought to adjudicate between these perspectives by investigating the impact of offline reactivation and functional coupling on subsequent encoding of distinct but related experiences.
We used a classic interference paradigm (11, 13) in which adult human participants with prior knowledge encoded new, overlapping pairs. We first trained participants (n = 35) on a set of face–object associations (hereafter AB pairs, where “AB” denotes a studied Aface–Bobject association) across four study–test repetitions (Fig. 1A, Experimental Procedures, and SI Methods and Results, Memory Task). We then collected fMRI data while participants engaged in passive rest and encoding of both new overlapping (BC) object–object pairs and nonoverlapping (i.e., unrelated; XY) object–object pairs in a single exposure. Importantly, the order of BC and XY learning was counterbalanced across participants. After scanning, participants completed a cued recall test for studied associations (BC and XY) and a surprise test of inferential (AC) relationships. The AC inference test required participants to recall the Aface item that was indirectly related to the Cobject cue through their common association with Bobject, indexing each individual’s ability to combine remembered associations across episodes. This paradigm enables investigation of the neural mechanisms that modulate how existing memories (AB) impact future learning (BC) and inference (AC), thus improving our fundamental understanding of the interactive nature of real-world memory.
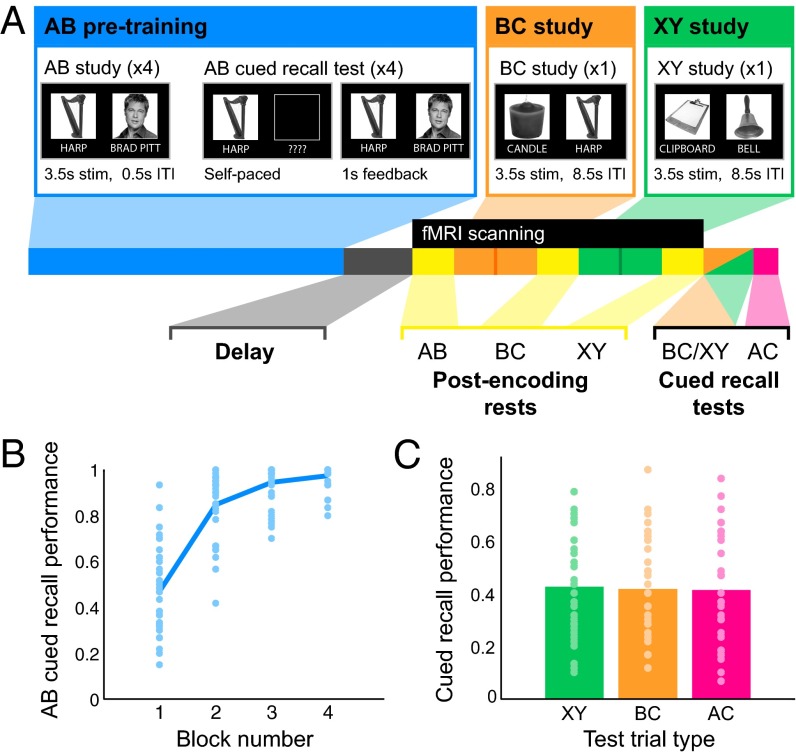
Fig. 1.
Experimental procedure and performance. (A) Participants encoded AB pairs in four alternating study–test repetitions during the pretraining phase (blue). Participants then studied new overlapping (BC; orange) and nonoverlapping (XY; green) pairs during fMRI scanning. BC and XY study blocks were interleaved with rest scans (yellow); the encoding order of BC vs. XY was counterbalanced across participants. After scanning, memory for BC and XY pairs (intermixed; orange/green) and AC inferences (pink) was tested using cued recall. (B) AB memory performance as proportion correct on each test block. Line represents the group mean; points show individual participants. (C) Performance for nonoverlapping XY pairs (green), overlapping BC pairs (orange), and AC inferences (pink). Bar heights represent group means; points show individual participants. See also Fig. S1.
Results
Behavioral Performance.
As intended, AB pairs were well learned by the fourth test block (mean ± SEM: 97.3 ± 0.9% correct recall; Fig. 1B). We define proactive interference as performance on overlapping BC relative to nonoverlapping XY pairs (i.e., XY − BC accuracy), with higher values indicating more interference of AB pair knowledge on new BC encoding. Importantly, BC (11.7–86.7%, 41.5 ± 3.3% correct) and XY (10–78.3%, 42.4 ± 3.4% correct) were matched in terms of both content type and number of presentations, allowing us to directly compare performance in these two conditions. Interestingly, we observed neither proactive interference nor facilitation across the group (XY vs. BC performance: t34 = 0.40, P = 0.693; Fig. 1C); rather, we found that the degree of proactive interference was highly variable across individuals (Fig. S1A). This variability enabled us to investigate how rest-phase processes following initial AB learning modulate encoding of overlapping BC relative to control XY pairs. We also found that performance on AC inferences (6.7–83.3%; 41 ± 3.5% correct)—which notably, require retrieval of the initially learned Aface item—paralleled BC memory, further demonstrating the strong nature of the AB memories at the end of the experiment (SI Methods and Results, Analysis of Behavioral Data). We also investigated how the strength of the initially acquired AB pairs impacted later BC learning and AC inference. We found that both within (Fig. S1B) and across individuals, superior AB memory was associated with better performance on overlapping BC pairs and AC inference judgments (SI Methods and Results, Analysis of Behavioral Data).
Face Reactivation During Rest.
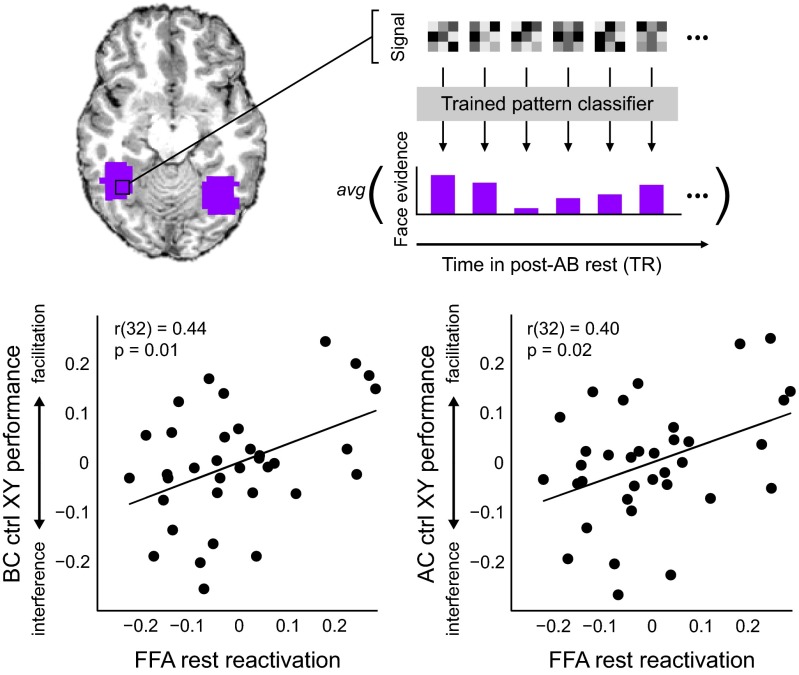
We examined the impact of neural engagement during the post-AB rest period on encoding of related BC information and AC inferences. Here, we focus on reactivation of face information in face-sensitive regions of visual cortex (e.g., fusiform face area, FFA). To measure spontaneous reactivation during the rest period, we trained a pattern classifier to distinguish between different types of visual content on the basis of activation patterns in each participant’s functionally defined FFA. Importantly, the classifier was trained on independent visual localizer data. The trained classifier was then applied to each volume of the post-AB rest period (Fig. 2, Upper; Experimental Procedures; and SI Methods and Results, Reactivation: Pattern Classification Analysis).
Fig. 2.
Reactivation following initial learning predicts subsequent encoding of related content. (Upper) Depiction of rest-phase pattern classification analysis. A pattern classifier was trained to discriminate FFA (purple) activation patterns associated with faces, objects, scrambled objects, and fixation baseline (not depicted). The trained classifier was then applied to each time point of the rest data (grayscale matrices). (Lower) Relationship between FFA reactivation and BC (Left) and AC (Right) performance, plotted as residuals after regressing each on XY performance. See also Figs. S2 and S3.
We first examined the relationship between reactivation and performance over time using a 60-volume (2 min) window swept across the rest scan. For each window, we calculated a reactivation index (defined as the mean classifier evidence for faces) for each participant. We then related this reactivation index to BC learning and AC inference using two approaches. As our primary approach, we quantified the degree of facilitation for BC encoding and AC inference by performing across-participant partial correlations. [We used partial correlation to index the degree to which prior memories facilitate versus interfere with the acquisition of new, related knowledge. Because general associative memory ability (i.e., XY performance) was highly related to both BC memory and AC inference across participants, we needed to statistically control for these differences to answer our central question—how prior knowledge specifically impacts overlapping encoding, relative to one’s general associative encoding ability. Mathematically, this is accomplished by performing a correlation on the residuals after regressing both reactivation and BC or AC performance on the controlling variable, XY performance. This analysis approach mirrors other studies that control for various factors such as age (22, 23), sex (23), general cognitive ability (20, 24), or neural measures (25, 26).] Specifically, we interrogated the relationships between (i) reactivation and BC memory performance and (ii) reactivation and AC inference performance, after statistically controlling for the effects of XY performance (our metric of general associative memory ability). This analysis was performed to index the unique relationship between memory reactivation and later encoding of related information. As a secondary approach, we also investigated the individual relationships between reactivation and performance on related BC pairs, AC inferences, and unrelated XY pairs using Pearson’s correlation (SI Methods and Results, Reactivation: Pattern Classification Analysis). However, we note that due to the high correlation between XY pair memory and performance on both BC pairs (r33 = 0.80, P < 1 × 10−8) and AC inferences (r33 = 0.81, P < 1 × 10−8), these relationships are heavily influenced by general associative memory and thus do not specifically reflect the impact of prior knowledge on subsequent encoding of related information.
We found a significant relationship between face reactivation and BC performance controlling for XY that was unique to the beginning of the rest period (partial correlation during first 2-min window; r32 = 0.44, P = 0.010; Fig. 2, Lower Left and Fig. S2, Upper Left) and that was not observed for other classes of visual content (Fig. S2, Upper Right). Reactivation during the first 2-min window also tracked AC performance after controlling for XY memory (r32 = 0.40, P = 0.019; Fig. 2, Lower Right). That is, participants who showed more face reactivation following initial AB learning also showed superior memory for related BC associations and AC inferences after controlling for general associative memory ability. This finding can also be conceptualized as a negative association between face reactivation and proactive interference, i.e., less reactivation was observed for participants who showed more proactive interference. Importantly, neither the degree of reactivation nor its relationship to performance was significantly impacted by differences in lag duration or encoding order across participants (SI Methods and Results, Delay and Encoding Order Analyses). Moreover, the relationship between reactivation and performance was specific to the post-AB rest scan. Reactivation during the post-XY rest period did not relate to XY performance or to BC or AC performance after controlling for general associative memory (SI Methods and Results, Reactivation: Pattern Classification Analysis).
Next, we repeated the same analysis using an expanded region of interest (ROI) encompassing the entire posterior fusiform gyrus to further validate our findings. We found significant relationships between reactivation in posterior fusiform and BC performance (r32 = 0.38, P = 0.028) as well as AC inference (r32 = 0.37, P = 0.032) when controlling for general associative memory. Moreover, we observed significant individual correlations of BC performance and AC inference with reactivation that were not observed in the smaller FFA ROI (SI Methods and Results, Reactivation: Pattern Classification Analysis; Fig. S3). Importantly, reactivation during the post-AB scan was not related to XY performance for either ROI (SI Methods and Results, Reactivation: Pattern Classification Analysis; Fig. S3).
We also performed a control analysis to determine whether our findings could be attributed to individual differences in baseline levels of face reactivation. Because the post-XY encoding rest scan was the most removed from face-related encoding, we reasoned that this scan should be the least likely to contain face-related neural signatures that would support memory. Thus, we subtracted each participant’s post-XY encoding reactivation index from their post-AB reactivation index (4). The resulting difference scores reflecting the degree to which post-AB reactivation deviated from baseline (as indexed by the post-XY scan) were then related to performance as described above. For both FFA and posterior fusiform gyrus, we observed significant relationships between post-AB reactivation with BC and AC performance controlling for general associative memory using partial correlation. Moreover, in the posterior fusiform gyrus ROI, individual relationships between reactivation and performance were significant for BC and AC, but not XY (SI Methods and Results, Reactivation: Pattern Classification Analysis).
FFA Functional Connectivity During Rest.
Next, we sought to determine how FFA connectivity with medial temporal lobe (MTL) regions predicted subsequent learning of object–object pairs. We used two approaches: first, a timeseries correlation approach within anatomically and functionally defined ROIs; and second, a voxelwise regression approach using FFA as a seed region. Both analyses were performed with consideration of the entire rest scan, as prior reports have shown the importance of sufficiently long timeseries for extracting stable measures of functional connectivity (27).
Timeseries correlation approach.
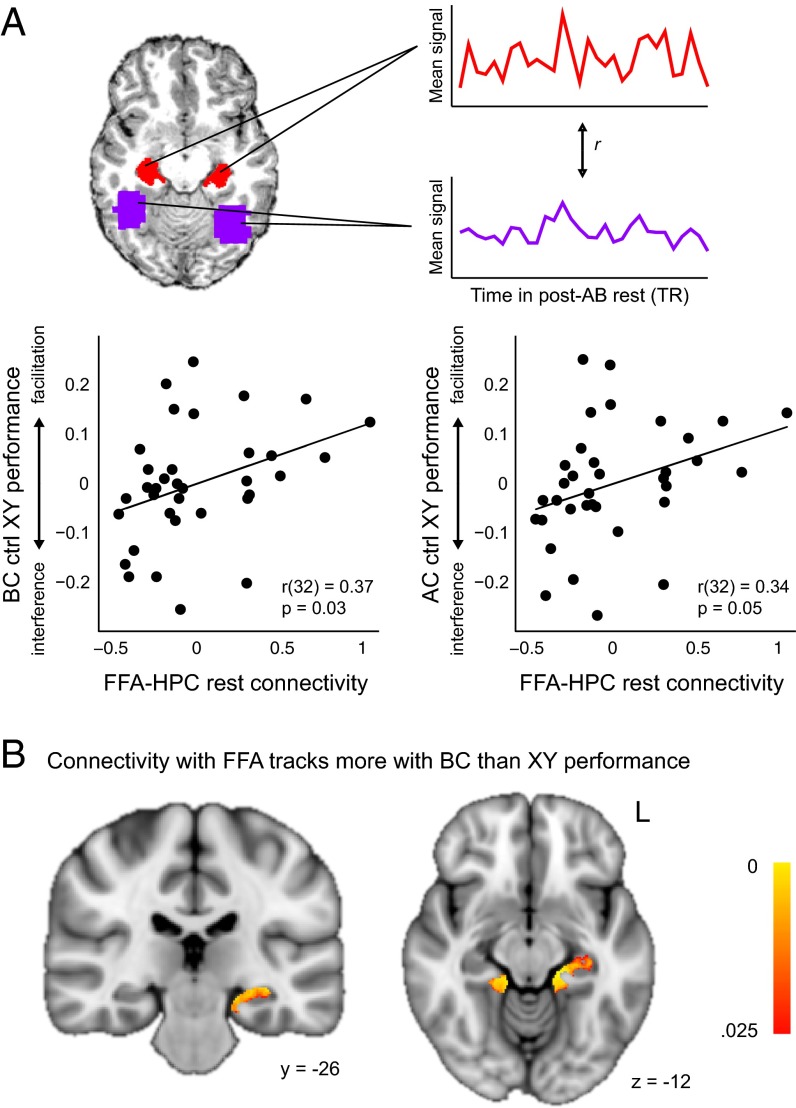
Our ROIs included functionally defined FFA and anatomically defined HPC, perirhinal, entorhinal, and parahippocampal cortices (all bilateral). We extracted the first eigenvariate across all voxels in each ROI from the high-pass filtered post-AB rest data. We then correlated the FFA timeseries with the timeseries from each of the four MTL ROIs (Fig. 3A, Upper and SI Methods and Results, Timeseries correlation analysis). As in the rest-phase reactivation analysis, functional coupling during post-AB rest was significantly related to BC performance after controlling for XY performance (r32 = 0.37, P = 0.033; Fig. 3A, Lower Left). A similar relationship was observed with AC performance (r32 = 0.34, P = 0.049; Fig. 3A, Lower Right). That is, participants showing enhanced FFA–HPC functional coupling following AB encoding also showed an advantage specific to learning of the overlapping BC associations and inferring the AC relationships. Connectivity between FFA and all other MTL regions showed no significant relationship to BC or AC performance after controlling for XY (all |r32| < 0.18, P > 0.312; see also SI Methods and Results, Timeseries correlation analysis for individual correlations with performance). Moreover, neither the degree of connectivity itself nor its relationship to performance was modulated by lag duration or encoding order (SI Methods and Results, Delay and Encoding Order Analyses). Importantly, the relationship between connectivity and performance was also specific to the post-AB rest; there was no correlation between FFA–HPC connectivity during the post-XY rest period and XY performance or with BC or AC performance controlling for XY performance (SI Methods and Results, Timeseries correlation analysis). When various nuisance sources (signal from white matter and ventricular ROIs and motion-related regressors) were regressed out from the post-AB rest data, the pattern of results was similar but slightly weaker (SI Methods and Results, Timeseries correlation analysis).
Fig. 3.
FFA–HPC connectivity following initial learning predicts subsequent encoding of related content. (A, Upper) depiction of timeseries correlation analysis. For each participant, FFA (purple) and HPC (red) timecourses from the post-AB rest scan were correlated to quantify the degree to which FFA and HPC exhibit similar activations over time (Upper Right). (Lower) Relationship between FFA–HPC connectivity and BC (Left) and AC (Right) performance, controlling for XY performance. Data are displayed as in Fig. 2. (B) HPC showed connectivity with FFA during rest that was significantly more predictive of BC than XY performance (displayed on the 1-mm MNI template brain). Color bar indicates uncorrected voxelwise P value. Coordinates are in millimeters. See also Figs. S4 and S5.
As with the reactivation results, we performed a control analysis to account for individual differences in baseline connectivity by subtracting the degree of post-XY FFA–HPC connectivity from each participant’s post-AB connectivity measure of interest. We found that our results held, with significant correlations between connectivity and BC learning (as measured by both individual and partial correlations), as well as AC inference. There was no relationship between FFA–HPC connectivity during post-AB rest and XY performance (SI Methods and Results, Timeseries correlation analysis).
Seed-based approach.
We also used a more sensitive seed-based regression approach to identify specific MTL voxels for which connectivity with FFA tracked subsequent BC performance. We regressed each participant’s MTL data on their FFA timeseries from the post-AB encoding rest scan, resulting in a statistics image representing the degree of correspondence between each MTL voxel and FFA activation over time. These results were combined across participants in a group level general linear model (SI Methods and Results, Seed-based analysis). We found two regions for which FFA connectivity tracked more with BC than XY performance: one in left [Montreal Neurological Institute (MNI) template coordinates (in millimeters) x, y, z = −16, −30, −19] and one in right (14, −33, −11) HPC, extending into parahippocampal cortex (PHC) (Fig. 3B and Fig. S4). An overlapping cluster (−18, −29, −13) in the left hemisphere was found to predict BC performance (Fig. S5).
Multiple Regression.
To investigate the degree to which reactivation and connectivity independently explained variance in subsequent learning, we next performed two multiple linear regression analyses with indices of reactivation, connectivity, and XY performance as independent variables and BC and AC performance, respectively, as the dependent variables. The BC model fit was significant (F3,31 = 38.90, P < 0.0001), accounting for 77.0% of the variance in BC performance (adjusted R2). Moreover, all three independent variables showed a significant positive relationship to BC performance (reactivation: β = 0.33, P = 0.0004; connectivity: β = 0.30, P = 0.001; XY performance: β = 0.87, P < 0.0001; all statistics reflect standardized β), demonstrating the unique contributions of rest-phase reactivation and FFA–HPC connectivity to subsequent learning of related experiences. Similar results were found in the regression model predicting AC performance (F3,31 = 36.67, P < 0.0001; adjusted R2 = 0.759; reactivation: β = 0.30, P = 0.002; connectivity: β = 0.27, P = 0.004; XY performance: β = 0.87, P < 0.0001).
Univariate Encoding Activation.
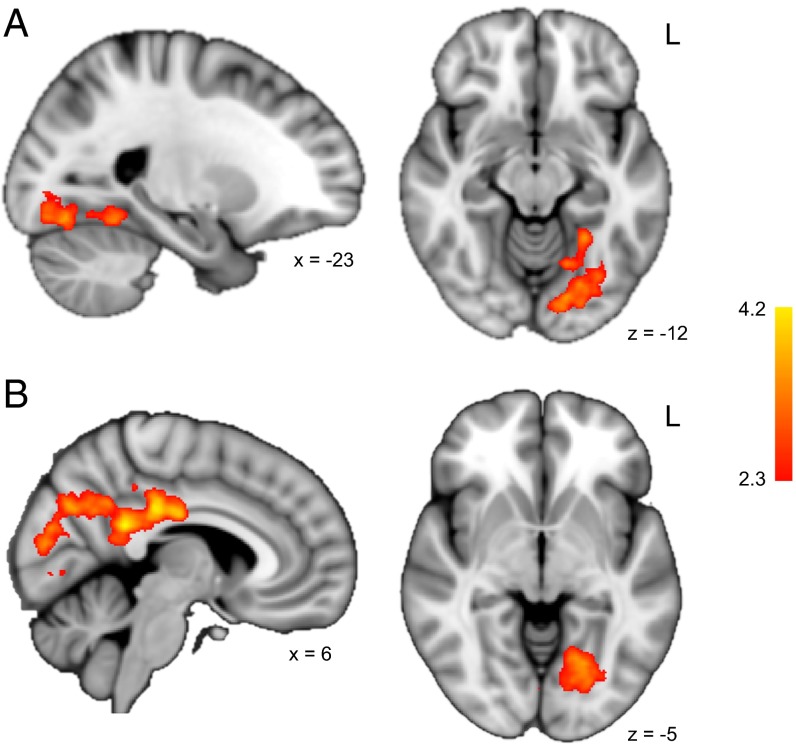
We next investigated neural engagement during encoding of new object–object associations. We were specifically interested in regions demonstrating a subsequent memory effect for BC (i.e., more engagement during study of BC pairs that were subsequently remembered vs. forgotten) but not XY pairs. As the above results showed that certain rest-phase processes can facilitate BC encoding, we hypothesized that (i) face-sensitive regions of visual cortex would be engaged during BC trials, indicative of reinstatement of previously learned Aface stimuli and that (ii) such engagement would support encoding of the new BC object–object pairs. A whole-brain analysis confirmed our predictions, revealing a significant subsequent recall by condition interaction (correct > incorrect × BC > XY) in left fusiform gyrus (−20, −75, −15; Fig. 4A and Fig. S6).
Fig. 4.
Regions showing a significant subsequent recall by condition interaction during encoding. (A) Left fusiform gyrus was the only region to show greater subsequent recall effects for BC relative to XY pairs. (B) The interaction term was significantly modulated by the degree of FFA–HPC connectivity during the post-AB rest scan in portions of medial parietal and occipital cortex, including the fusiform gyrus and posterior cingulate cortex. Color bar indicates z score. Coordinates are in millimeters. See also Fig. S6.
Relationship Between Univariate Encoding Activation and Neural Measures at Rest.
We then considered how individual differences in rest-phase reactivation and FFA–HPC connectivity related to neural engagement during subsequent learning. We created two general linear models at the group level that included each participant’s FFA reactivation and FFA–HPC connectivity indices, respectively, as covariates. We hypothesized that greater reactivation and connectivity during rest would be associated with more reactivation of Aface stimuli during encoding. No region showed a significant relationship between the interaction term and FFA reactivation. We did, however, find activation in medial parietal and occipital cortex (centered on −5, −39, 22), including fusiform gyrus, for which the interaction term tracked positively with the degree of FFA–HPC connectivity following AB encoding (Fig. 4B).
Discussion
We used a combination of methods—multivariate pattern classification, functional connectivity approaches, and task-based univariate analyses—to provide empirical evidence that offline neural processes may mediate the relationship between prior knowledge and new encoding. Our findings converge to suggest that rest-phase reactivation may benefit future learning by promoting subsequent integration during encoding. Interestingly, this benefit was observed even despite a long delay of approximately 1 h between overlapping event encoding and test. Moreover, we suggest that the rest-phase reactivation observed in the present study occurred spontaneously. As participants were unaware of the overlap between the pretraining phase (AB learning) and the scanned portion of the experiment (BC and XY learning) at the time of post-AB rest (SI Methods and Results, Memory Task), it is unlikely that our results reflect intentional rehearsal of AB associations during that period.
Whereas the benefits conferred by offline processes on prior memories have been shown previously, the present work is, to our knowledge, the first to demonstrate how such benefits might also be prospectively advantageous. That is, rest-phase reactivation and connectivity serve to make our memories better suited for new learning in future situations, providing a foundational knowledge base upon which new experiences can be encoded. Importantly, because our classifier was trained on data from an independent localizer task consisting of a different stimulus set and task, our results suggest that reinstatement of episodic content (i.e., face information from learned AB face–object pairs)—rather than reinstatement of a learning-related state or context—supports the formation of memories for related information.
However, we note that like the vast majority of studies on this topic, our data do not provide evidence for processing of specific AB memories during the post-AB rest period. Thus, one possible alternative explanation of our findings is that participants reactivated task-irrelevant face information, and that doing so supported their later ability to encode BC content, as AB memories became less likely to interfere with new learning. However, we feel this possibility is unlikely given our results. For example, we found that greater reactivation and connectivity were associated with superior AC inference performance, which requires retrieval of the Aface learned during pretraining. Moreover, both BC memory and AC inference were better for the AB pair memories acquired earlier in the pretraining phase, suggesting that strong AB knowledge promotes BC encoding. Thus, we suggest that the most likely interpretation of the data presented here is that AB memories were spontaneously reinstated during the post-AB rest period. Through this process, they became stronger or more readily accessible (1–3) and therefore easier to reactivate and integrate during BC study.
We would also note that associative facilitation was not observed across the entire group of participants; rather, we observed large individual differences in the degree to which participants showed facilitation vs. interference. In fact, approximately half of our participants did not show evidence of facilitation at all, but rather showed interference (Fig. S1A). Thus, we would not conclude that proactive interference does not occur, but rather that there exists a tradeoff between interference and facilitation; and that this tradeoff may be mediated by offline processes. In other words, participants with a greater degree of post-AB reactivation and connectivity may show associative facilitation through an integrative encoding mechanism, whereas participants showing a lesser degree may tend to exhibit interference.
More broadly, we suggest that one factor that may determine whether prior knowledge facilitates or interferes with the acquisition of new information is the strength of the initial memory, with strong prior knowledge being predominantly facilitative. This may occur not only during overt encoding or rehearsal, but also spontaneously during periods of passive rest. Notably, prior work suggests that low to moderate levels of memory reactivation may weaken traces, whereas high reactivation serves to strengthen memories (28). In the present study, lesser offline reactivation may thus be associated with weaker AB memories. Such weak traces may fail to be reactivated at all or may be weakly reactivated during learning, perhaps being forgotten when integration fails (29) or interfering with new encoding. In contrast, greater reactivation during rest strengthens AB memories, which can then later support BC learning through learning-phase retrieval and integration. Future work may characterize differences in item-level reactivation within participants to address how strengthening of individual memories impacts the balance between facilitation and interference.
One interesting aspect of our data is that the observed relationships between reactivation and both BC learning and AC inference were specific to early in the post-AB rest scan. Importantly, this finding cannot be explained by differences in the amount of time between the encoding and rest scan across participants; delay duration did not predict the degree of face reactivation. Thus, interpreted in the context of the converging rest- and task-based neural measures provided here, we believe this reactivation measure serves as a valid index of neural processes that mediate the interactions between prior memories, new learning, and subsequent inference. Whereas a mechanistic explanation for the temporal dependence of this signature is unclear, we believe our measure of reactivation provides additional insight into how processing of prior memories during offline periods shape later learning experiences.
Our results converge across multiple measures to demonstrate the important relationship between postencoding reactivation, functional connectivity, and episodic memory, consistent with a host of findings from rodent (2) and human (1, 3, 4) studies. In addition, our data provide previously unidentified evidence that the mnemonic advantage conferred by offline processes extends beyond the initial memories themselves to influence the subsequent encoding of related content. Our data suggest a specific mechanism through which offline reactivation and HPC–neocortical connectivity leads to the strengthening of memory traces, thereby supporting later learning of related content via integrative encoding (9). Consistent with this interpretation, we found greater engagement of face-sensitive regions (i.e., fusiform gyrus) during encoding of object–object pairs that related to prior face knowledge as a function of FFA–HPC connectivity at rest. This extends prior work demonstrating the benefits of learning-phase reactivation for the reactivated memories themselves (18) and for linking experiences across time (20). We suggest that memory strengthening during rest facilitates retrieval of related content during subsequent learning experiences, thereby supporting new encoding by enabling linking of related memories (17).
Experimental Procedures
Subjects and Procedures.
Forty-eight volunteers participated in this study; a total of 13 participants were excluded due to hardware malfunction (n = 5), handedness concerns (n = 1), and low memory performance for the directly learned associations (n = 7). Data from the remaining 35 participants were analyzed. Participants first learned a set of face–object associations (AB pairs) outside of the scanner. These pairs were encoded across four alternating study and test blocks, ensuring participants had extensive experience with these pairs. Tests were cued recall format and included feedback. Participants were then transferred to the scanner and fMRI data were acquired during an initial (post-AB encoding) rest period. They were told to remain awake and keep their eyes open but to think about whatever they like. We then presented participants with overlapping (BC) and nonoverlapping (XY) object–object pairs in separate scans. Each pair type was followed by a postencoding rest scan, and the order of BC vs. XY was counterbalanced across participants. After the final rest scan, participants were removed from the scanner and completed a cued recall test for the associations studied in the scanner (BC and XY) and a surprise test of inferential (AC) relationships. For the AC test, participants were shown the Cobject and were asked to produce the indirectly associated Aface. No feedback was provided for either test. Following the memory task, participants were transferred back into the scanner to complete a one-back functional localizer task comprising faces, objects, scrambled objects, and fixation baseline. These data were used to obtain neural patterns associated with viewing different types of visual stimuli, which were then used to define face-sensitive ROIs and for training the neural pattern classifier (see below). Full procedures and MRI data acquisition and processing details are described in SI Methods and Results.
Pattern Classification Analysis.
Multivoxel pattern analysis (MVPA) (30, 31) was performed using sparse multinomial logistic regression (SMLR) implemented in PyMVPA (32). For each participant, a classifier was trained to differentiate viewing of face, object, scrambled object, and passive fixation stimuli on the basis of activation patterns in face-sensitive regions (i.e., FFA and posterior fusiform gyrus). The trained classifier was then applied to each volume of the post-AB encoding rest scan. Face reactivation indices were defined as the mean classifier face evidence over time and related to performance across participants using partial correlation and Pearson’s correlation. For more details, see SI Methods and Results, Reactivation: Pattern Classification Analysis.
Functional Connectivity Analysis.
MTL functional connectivity with FFA was assessed using both timeseries correlation and voxelwise regression. The first eigenvariate of the post-AB rest signal over time was extracted from bilateral HPC, perirhinal, entorhinal, and parahippocampal cortices; and bilateral FFA. MTL timeseries were then correlated with the FFA timeseries; the resulting correlation statistics were Fisher transformed and related to performance using partial correlation and correlation. For the voxelwise regression approach, we constructed general linear models that included the timeseries from the FFA seed as a regressor for each participant. The resulting statistics images were warped to the MNI template using ANTS (33) and combined across the group. We were interested specifically in those voxels whose connectivity with FFA tracked with BC performance more than XY performance; thus, BC and XY performance were added to the group level model as covariates. For full details, see SI Methods and Results, Functional Connectivity.
Supplementary Material
Acknowledgments
The authors thank Jackson Liang, Michael Mack, Tammy Tran, Amelia Wattenberger, and Dagmar Zeithamova for assistance with data collection and helpful discussions, and Jarrod Lewis-Peacock for insightful comments on an earlier version of the manuscript. This work was supported by the National Institute of Mental Health of the National Institutes of Health Grant R01 MH100121 (to A.R.P.), the National Science Foundation CAREER Award 1056019 (to A.R.P.), and the Department of Defense through the National Defense Science and Engineering Graduate Fellowship Program (M.L.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1404396111/-/DCSupplemental.
References
- 1.Deuker L, et al. Memory consolidation by replay of stimulus-specific neural activity. J Neurosci. 2013;33(49):19373–19383. doi: 10.1523/JNEUROSCI.0414-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jadhav SP, Kemere C, German PW, Frank LM. Awake hippocampal sharp-wave ripples support spatial memory. Science. 2012;336(6087):1454–1458. doi: 10.1126/science.1217230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staresina BP, Alink A, Kriegeskorte N, Henson RN. Awake reactivation predicts memory in humans. Proc Natl Acad Sci USA. 2013;110(52):21159–21164. doi: 10.1073/pnas.1311989110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tambini A, Ketz N, Davachi L. Enhanced brain correlations during rest are related to memory for recent experiences. Neuron. 2010;65(2):280–290. doi: 10.1016/j.neuron.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marr D. A theory for cerebral neocortex. Proc R Soc Lond B Biol Sci. 1970;176(1043):161–234. doi: 10.1098/rspb.1970.0040. [DOI] [PubMed] [Google Scholar]
- 6.McClelland JL, McNaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102(3):419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- 7.Nadel L, Samsonovich A, Ryan L, Moscovitch M. Multiple trace theory of human memory: Computational, neuroimaging, and neuropsychological results. Hippocampus. 2000;10(4):352–368. doi: 10.1002/1098-1063(2000)10:4<352::AID-HIPO2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 8.Buckner RL. The role of the hippocampus in prediction and imagination. Annu Rev Psychol. 2010;61:27–48. doi: 10.1146/annurev.psych.60.110707.163508. [DOI] [PubMed] [Google Scholar]
- 9.Preston AR, Eichenbaum H. Interplay of hippocampus and prefrontal cortex in memory. Curr Biol. 2013;23(17):R764–R773. doi: 10.1016/j.cub.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Kesteren MTR, Ruiter DJ, Fernández G, Henson RN. How schema and novelty augment memory formation. Trends Neurosci. 2012;35(4):211–219. doi: 10.1016/j.tins.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Underwood BJ. Proactive inhibition as a function of time and degree of prior learning. J Exp Psychol. 1949;39(1):24–34. doi: 10.1037/h0059550. [DOI] [PubMed] [Google Scholar]
- 12.Whitely P. The dependence of learning and recall upon prior intellectual activities. J Exp Psychol. 1927;10(6):489–508. [Google Scholar]
- 13.Postman L. Transfer of training as a function of experimental paradigm and degree of first-list learning. J Verbal Learn Verbal Behav. 1962;1:109–118. [Google Scholar]
- 14.Bransford JD, Johnson MK. Contextual prerequisites for understanding: Some investigations of comprehension and recall. J Verbal Learn Verbal Behav. 1972;11:717–726. [Google Scholar]
- 15.Tse D, et al. Schemas and memory consolidation. Science. 2007;316(5821):76–82. doi: 10.1126/science.1135935. [DOI] [PubMed] [Google Scholar]
- 16.Shohamy D, Wagner AD. Integrating memories in the human brain: Hippocampal-midbrain encoding of overlapping events. Neuron. 2008;60(2):378–389. doi: 10.1016/j.neuron.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlichting ML, Preston AR. Memory integration: Neural mechanisms and implications for behavior. Curr Opin Behav Sci. 2015;1:1–8. doi: 10.1016/j.cobeha.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhl BA, Shah AT, DuBrow S, Wagner AD. Resistance to forgetting associated with hippocampus-mediated reactivation during new learning. Nat Neurosci. 2010;13(4):501–506. doi: 10.1038/nn.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wimmer GE, Shohamy D. Preference by association: How memory mechanisms in the hippocampus bias decisions. Science. 2012;338(6104):270–273. doi: 10.1126/science.1223252. [DOI] [PubMed] [Google Scholar]
- 20.Zeithamova D, Dominick AL, Preston AR. Hippocampal and ventral medial prefrontal activation during retrieval-mediated learning supports novel inference. Neuron. 2012;75(1):168–179. doi: 10.1016/j.neuron.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ratcliff R, Clark SE, Shiffrin RM. List-strength effect: I. Data and discussion. J Exp Psychol Learn Mem Cogn. 1990;16(2):163–178. [PubMed] [Google Scholar]
- 22.Feldman HM, Lee ES, Yeatman JD, Yeom KW. Language and reading skills in school-aged children and adolescents born preterm are associated with white matter properties on diffusion tensor imaging. Neuropsychologia. 2012;50(14):3348–3362. doi: 10.1016/j.neuropsychologia.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki H, et al. Structural-functional correlations between hippocampal volume and cortico-limbic emotional responses in depressed children. Cogn Affect Behav Neurosci. 2013;13(1):135–151. doi: 10.3758/s13415-012-0121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiu C-YP, Schmithorst VJ, Brown RD, Holland SK, Dunn S. Making memories: A cross-sectional investigation of episodic memory encoding in childhood using FMRI. Dev Neuropsychol. 2006;29(2):321–340. doi: 10.1207/s15326942dn2902_3. [DOI] [PubMed] [Google Scholar]
- 25.Barron HC, Dolan RJ, Behrens TEJ. Online evaluation of novel choices by simultaneous representation of multiple memories. Nat Neurosci. 2013;16(10):1492–1498. doi: 10.1038/nn.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Supekar K, et al. Development of functional and structural connectivity within the default mode network in young children. Neuroimage. 2010;52(1):290–301. doi: 10.1016/j.neuroimage.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Dijk KRA, et al. Intrinsic functional connectivity as a tool for human connectomics: Theory, properties, and optimization. J Neurophysiol. 2010;103(1):297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Detre GJ, Natarajan A, Gershman SJ, Norman KA. Moderate levels of activation lead to forgetting in the think/no-think paradigm. Neuropsychologia. 2013;51(12):2371–2388. doi: 10.1016/j.neuropsychologia.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson MC, McCulloch KC. Integration as a general boundary condition on retrieval-induced forgetting. J Exp Psychol Learn Mem Cogn. 1999;25:608–629. [Google Scholar]
- 30.Polyn SM, Natu VS, Cohen JD, Norman KA. Category-specific cortical activity precedes retrieval during memory search. Science. 2005;310(5756):1963–1966. doi: 10.1126/science.1117645. [DOI] [PubMed] [Google Scholar]
- 31.Norman KA, Polyn SM, Detre GJ, Haxby JV. Beyond mind-reading: Multi-voxel pattern analysis of fMRI data. Trends Cogn Sci. 2006;10(9):424–430. doi: 10.1016/j.tics.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Hanke M, et al. PyMVPA: A python toolbox for multivariate pattern analysis of fMRI data. Neuroinformatics. 2009;7(1):37–53. doi: 10.1007/s12021-008-9041-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avants BB, et al. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54(3):2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.