Significance
Understanding and accurately predicting how global terrestrial primary production responds to rising atmospheric CO2 concentrations is a prerequisite for reliably assessing the long-term climate impact of anthropogenic fossil CO2 emissions. Here we demonstrate that current carbon cycle models underestimate the long-term responsiveness of global terrestrial productivity to CO2 fertilization. This underestimation of CO2 fertilization is caused by an inherent model structural deficiency related to lack of explicit representation of CO2 diffusion inside leaves, which results in an overestimation of CO2 available at the carboxylation site. The magnitude of CO2 fertilization underestimation matches the long-term positive growth bias in the historical atmospheric CO2 predicted by Earth system models. Our study will lead to improved understanding and modeling of carbon–climate feedbacks.
Keywords: mesophyll conductance, CO2 fertilization, carbon cycle, gross primary production, photosynthetic model
Abstract
In C3 plants, CO2 concentrations drop considerably along mesophyll diffusion pathways from substomatal cavities to chloroplasts where CO2 assimilation occurs. Global carbon cycle models have not explicitly represented this internal drawdown and therefore overestimate CO2 available for carboxylation and underestimate photosynthetic responsiveness to atmospheric CO2. An explicit consideration of mesophyll diffusion increases the modeled cumulative CO2 fertilization effect (CFE) for global gross primary production (GPP) from 915 to 1,057 PgC for the period of 1901–2010. This increase represents a 16% correction, which is large enough to explain the persistent overestimation of growth rates of historical atmospheric CO2 by Earth system models. Without this correction, the CFE for global GPP is underestimated by 0.05 PgC/y/ppm. This finding implies that the contemporary terrestrial biosphere is more CO2 limited than previously thought.
To reach Rubisco, the carboxylating enzyme of the Calvin cycle, CO2 molecules must diffuse through two consecutive segments of a continuous pathway in leaves of C3 plant species. The first segment connects leaf intercellular air space with ambient air and is controlled by stomata; the second one consists of mesophyll layers from intercellular air space to stroma of chloroplasts where Rubisco resides (1, 2). These two stages differ in the media through which CO2 moves. Diffusion in the first segment (stomatal diffusion) is through gases only; that in the second segment (mesophyll diffusion) occurs in a variety of media including liquids and lipids, i.e., cell walls, plasmalemma, cytosol, chloroplast envelope membranes, and stroma. The path length of this mesophyll diffusion is generally shorter than that of stomatal diffusion (2). However, diffusion of CO2 through liquids is several orders of magnitude slower than it is through gases; diffusion through lipids in membranes is even slower than it is through liquid water (3), although it may be facilitated by aquaporin-like channels (4). Consequently, mesophyll layers constitute a major barrier for CO2 movement inside leaves (5–9).
However, the importance of this mesophyll diffusion limitation for photosynthesis has yet to be reflected in carbon cycle modeling. Current large-scale carbon cycle models (10, 11) have explicitly considered stomatal diffusion but not mesophyll diffusion. Most carbon cycle models use some form of the biochemical model of Farquhar, von Caemmerer, and Berry (FvCB) for modeling photosynthesis (12). In theory, the FvCB model should use the CO2 concentration at the site of carboxylation inside the chloroplast (Cc). Nevertheless, most modelers have knowingly or unknowingly applied it directly to the CO2 concentration inside the substomatal cavity (Ci). Since Cc can be much smaller than Ci, because of the mesophyll resistance to CO2 diffusion, a compensating adjustment is needed to correct for this overestimate of CO2 available for carboxylation, an adjustment that has been provided by the use of phenomenological, rather than actual, values of fundamental photosynthetic parameters. These parameters include the maximum carboxylation rate (Vcmax), maximum electron transport rate (Jmax), and triose phosphate utilization rate (TPU) and have typically been estimated from leaf gas exchange measurements commonly known as A/Ci curves obtained under carefully controlled environmental conditions (13, 14). The parameter estimation procedures used in such efforts have treated mesophyll conductance (gm) as if it were infinitely large, even though laboratory studies indicate that it is finite and that the mesosphyll diffusion limitation on photosynthesis can be substantial (1–9, 15, 16).
Without explicit consideration of mesophyll diffusion, fundamental photosynthetic parameters inferred from A/Ci curves are significantly underestimated (7, 15, 17). Vcmax is particularly sensitive to gm and is underestimated by as much as 75% if gm is assumed infinite (15). Therefore, the phenomenological parameters used in current carbon cycle models substantially undervalue the actual biochemical capacities of the photosynthetic machinery.
Will this underestimation of actual biochemical capacities of photosynthetic apparatus compensate for the overestimation of CO2 available for carboxylation in determining the long-term terrestrial fertilization effect of anthropogenic CO2 emissions estimated by carbon cycle models? Photosynthesis is more sensitive to changes in CO2 at low CO2 than at high CO2 concentrations because the photosynthetic response to CO2 as an enzyme-catalyzed reaction is a saturating curve. Consequently an overestimation of CO2 at the carboxylation site leads to an underestimation of photosynthetic sensitivity to variations in ambient CO2 concentration. The degree of this underestimation is not constant; rather, it will dynamically vary with all environmental factors that affect photosynthesis. As a result, it may be difficult for this dynamically varying bias to be compensated for by the use of a few phenomenological parameters tuned to a limited number of measurements made under narrow environmental conditions. If so, lacking explicit consideration of gm represents an inherent structual deficiency that may prevent carbon cycle models from adequately simulating the long-term responses of global photosynthesis to historial and future changes in atmospheric CO2 concentration due to anthropogenic emissions.
To evaluate the consequence of this deficiency, we examine the simulated responses of global annual gross primary production (GPP) to the increase in atmospheric CO2 concentration since the beginning of the last century. We focus on GPP because it is the first step of the terrestrial carbon cycle and is affected directly by mesophyll diffusion of CO2. Our interest is in the long-term trend of global GPP, rather than in its absolute magnitude for a particular year or the mean GPP over a period. For short-term applications, the model structural deficiency can be easily compensated for by a tuning of model parameters, i.e., Vcmax and/or Jmax can be adjusted so that a carbon cycle model lacking gm will give the same GPP as estimated by a model including gm, e.g., for the first year of a 100-year period alone, or for that matter, for the last year alone; however, it is considerably more difficult to match these two models all the way from the first to last year for atmospheric CO2 that keeps rising during this 100-year period. Thus, focusing on the long-term trend is an effective way to quantify the effects of model structural deficiencies and their potential consequences.
We developed an empirical global gm model for C3 plant species based on a synthesis of data in the literature (SI Text) and implemented it into the state-of-the-art Community Land Model 4.5 (CLM4.5) (18, 19). This implementation allows us to contrast simulations that either consider or omit the mesophyll diffusion limitation. We refer to these simulations as the gm-including and gm-lacking simulations, respectively.
To enable a correct comparison between the gm-including and gm-lacking simulations, a matching correspondence must be established between the original phenomenological photosynthetic parameters in CLM4.5 (denoted thereafter as the gm-lacking parameters) and the fundamental photosynthetic parameters that reflect the actual capacities of the photosynthetic machinery (denoted thereafter as the gm-including parameters). This matching correspondence was achieved via a parameter conversion function that was developed from a global leaf gas exchange dataset collected by LeafWeb (leafweb.ornl.gov) (15, 20). The development of this conversion function was based on the CLM4.5 formulation of the FvCB model (SI Text). Other measures have also been taken to ensure that any difference in the trend of GPP between simulations can be attributed unambiguously to the mesophyll diffusion treatments (SI Text).
We ran CLM4.5 including or lacking gm from 1901 to 2010 with historical climate in conjunction with either observed or constant atmospheric CO2 concentrations (SI Text). In a given year t, the CO2 fertilization effect [CFE, in units of petagram (1015g) carbon (PgC) per year] on GPP of the historical anthropogenic carbon emissions was quantified relative to a baseline reference (GPPref), set to be the average of annual GPP of 1901–1910 from simulations with constant CO2 (296 ppm)
| [1] |
We examined the impact of accounting for mesophyll diffusion via the difference in CFE (ΔCFE) between the gm-including and gm-lacking simulations. If both models were to predict the CO2 fertilization effect equally well, there would be no long-term trend in ΔCFE.
We also applied the so-called Keeling’s β factor to measure divergence in the degree of CO2 fertilization between the gm-including and gm-lacking simulations. The β factors for these two types of simulations were compared through their ratio R
| [2] |
where the subscripts I and L denote the gm-including and gm-lacking simulations, respectively. The denominators of βI and βL share a common logarithmic CO2 term, which cancels in the expression of R. Because R is the ratio of two β factors, its dynamic behavior (e.g., in response to changes in ambient CO2) will be different from those of the β factors themselves. If models including and lacking gm were to predict the same effect of CO2 fertilization on GPP, then R should be close to 1. A value R > 1 indicates that the gm-lacking model underestimates the CO2 fertilization effect compared with the gm-including model; R < 1 indicates the opposite.
Results and Discussion
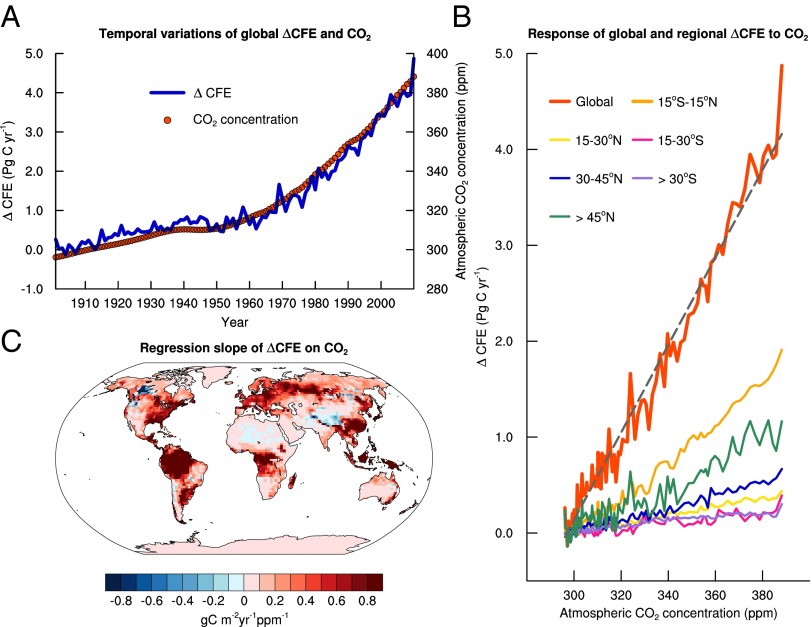
The ΔCFE for global GPP between the gm-including and gm-lacking simulations increases from 1901 to 2010 (Fig. 1A). A relatively gentle pre-1950 increase in ΔCFE is followed by an upsurge after 1950. This pattern closely matches that of the rising atmospheric CO2 over the same period (Fig. 1A) such that there is a strong positive linear relationship between ΔCFE and atmospheric CO2 (Fig. 1B). These results indicate that the global GPP modeled without explicit consideration of mesophyll diffusion substantially underestimates the long-term fertilization effect of anthropogenic CO2 emissions on global photosynthesis. Globally, this underestimation is 0.05 PgC/y/ppm (Fig. 1B).
Fig. 1.
Temporal and spatial variations of the difference in the CO2 fertilization effect (ΔCFE, PgC/y) on annual gross primary production simulated with CLM4.5 between including and lacking explicit consideration of mesophyll conductance (gm). (A) Historical trends in ΔCFE (blue curve, left ordinate) and in atmospheric CO2 concentration (ppm, red dots, right ordinate) from 1901 to 2010. (B) The variation of the global and latitudinal ΔCFE with atmospheric CO2 concentration. The global curve (red) is fitted with a line (y = −13.55 + 0.05x, r2 = 0.98). (C) The spatial variation in the slope (gC/m2/y/ppm) of the linear regression of the grid-based ΔCFE as a function of atmospheric CO2 concentration. The increase of ΔCFE with time and atmospheric CO2 concentration demonstrates that carbon cycle models without explicit representation of mesophyll diffusion underestimate CO2 fertilization effect.
From 1901 to 2010, the fertilization of the anthropogenic fossil CO2 emissions stimulates a cumulative total of 915 PgC (the time integration of Eq. 1) to the global GPP of the entire period in the gm-lacking simulations. In the gm-including simulations, however, the cumulative anthropogenic stimulation is estimated to be 1,057 PgC. Thus, with the baseline reference CO2 of 296 ppm, an explicit consideration of mesophyll diffusion increases the modeled cumulative CFE on global GPP by 16% by 2010.
Climate variability affects the magnitude of underestimation of the CO2 fertilization effect by the gm-lacking simulations, which is seen as interannual variations in ΔCFE (Fig. 1 A and B). The relationship between ΔCFE and CO2 also varies spatially (Fig. 1 B and C). The global trend is mostly contributed by the tropics (15°S to 15°N) and the boreal and arctic regions (>45°N). However, essentially all regions with vegetation activity have a positive relationship between ΔCFE and CO2, suggesting that the need for representing mesophyll diffusion to correctly model the CO2 fertilization effect is universal.
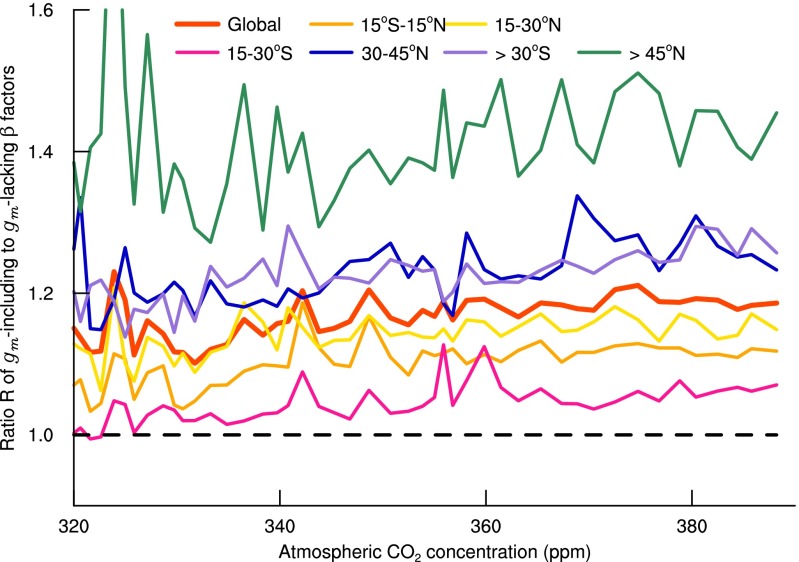
The results based on the analysis of R likewise indicate that models lacking gm underestimate the degree of CO2 fertilization. R is consistently larger than 1 for the globe as well as for all latitudinal bands in northern and southern hemispheres (Fig. 2). It is larger for the boreal and arctic regions, indicating that in a relative sense, the simulated CO2 fertilization effect in high latitudinal regions is more sensitive to the consideration of mesophyll diffusion than in lower latitudinal regions. This latitudinal trend in R can be explained by the generally lower temperatures and increased presence of needleleaf evergreen trees in higher latitudes, both factors leading to smaller mesophyll conductance (SI Text and Fig. S1) and therefore larger sensitivity of modeled CO2 fertilization to the representation of mesophyll diffusion.
Fig. 2.
Changes with atmospheric CO2 concentration of the global and latitudinal ratio (R) of the β factors calculated with CLM4.5 between including and lacking explicit consideration of mesophyll conductance (gm). R fluctuates erratically for the first half of the 20th century when the atmospheric CO2 is close to the baseline reference of 296 ppm in 1901, causing the denominator of R to vary around zero. Therefore, values of R when the atmospheric CO2 is less than 320 ppm are not shown. The dashed line at R = 1 separates the underestimation (R > 1) from the overestimation (R < 1) of the CO2 fertilization effect on gross primary production by CLM4.5 lacking gm compared with CLM4.5 including gm.
This identification of elevated importance of mesophyll diffusion in the tropics and the boreal and arctic regions over the midlatitudes highlights the necessity of using both ΔCFE and R in our evaluation. ΔCFE provides an absolute measure of the impact of mesophyll diffusion on estimated land CO2 fertilization in carbon flux units. Consequently, it scales with vegetation productivity and total baseline GPP. In contrast, R is calculated from relative changes in GPP. As a result, it is suited for sensitivity comparison across climate regions and vegetation types which may differ in productivity. Thus, ΔCFE and R complement each other by revealing different aspects of the importance of mesophyll diffusion for modeling long-term global and regional CO2 fertilization effects. The importance of representing mesophyll diffusion for modeling tropical photosynthesis lies in the region’s high productivity and large contribution to global GPP, whereas for the boreal and arctic regions, the importance comes from the increased photosynthetic sensitivity associated with their relatively high mesophyll diffusion limitation.
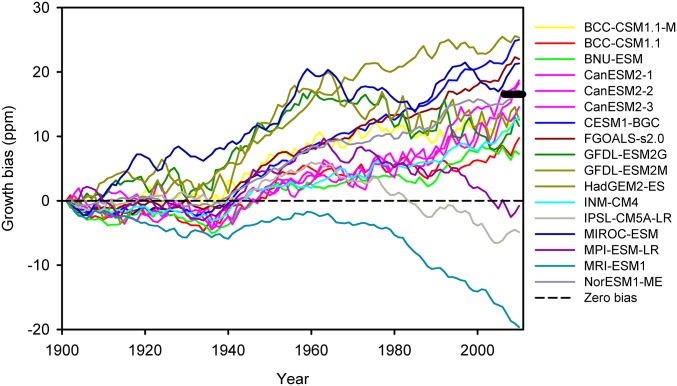
Our results imply that Earth system models (ESMs) will overpredict the long-term growth rate of atmospheric CO2 concentrations due to anthropogenic carbon emissions when their terrestrial carbon cycle modeling components do not consider mesophyll diffusion explicitly. Most ESMs investigated by the fifth phase of the Coupled Model Intercomparison Project (CMIP5) show persistent high bias in their predictions of historical atmospheric CO2, and it has been suggested that weak ocean uptake could contribute to this high bias (21, 22). We analyzed the outputs of CMIP5 for our simulation period of 1901–2010 and found that in 14 of 17 ESMs, the prognostically computed atmospheric CO2 grows too fast compared with the observations (Fig. 3). Relative to the 1901 value, the overpredictions of these 14 ESMs range from 10 to 25 ppm by 2010.
Fig. 3.
Growth biases of atmospheric CO2 concentrations prognostically computed by emission-driven ESMs in the fifth phase of CMIP5. The growth bias in a given year t is calculated as Cm(t) − Cm(1901) – [Co(t) – Co(1901)], where C is atmospheric CO2 concentration and the subscripts m and o denote model and observation, respectively. The reference baseline year is 1901 for which the growth bias is forced to be zero, allowing a focus on the long-term trend. The thick black bar indicates our estimated bias (∼17 ppm) caused by lacking explicit representation of mesophyll diffusion. Details about these ESMs and CMIP5 can be found elsewhere (21, 22).
By how much can their lack of explicit representation of mesophyll diffusion help explain the overpredictions of historical atmospheric CO2 growth by ESMs? To answer this question simply, we assume that net primary production is half of GPP (23) and half of the CO2 released into the atmosphere stays in the atmosphere (24). With these two empirically supported assumptions, we estimate that a quarter of the underestimated cumulative CO2 fertilization effect by models lacking explicit representation of mesophyll diffusion will be reflected in an overpredicted growth of the atmospheric CO2 concentration. For the period from 1901 to 2010, the underestimated cumulative CFE is 1,057 − 915 = 142 PgC, which corresponds to an overprediction of 17 ppm in atmospheric CO2 by 2010 [142/(4 × 2.123) ∼ 17 ppm; 1 ppm = 2.123 PgC], a value right in the middle of the range of overpredictions by current ESMs. This 17-ppm bias is significant because it almost equals the observed increase in recent decades from intensive fossil CO2 emissions and occurs over a period with a 100-ppm increase in atmospheric CO2 (i.e., a 17% overestimation). The lack in the ESMs of an explicit representation of mesophyll diffusion provides a plausible explanation to their atmospheric CO2 prediction bias, as an alternative to weak ocean uptake.
A rigorous evaluation of the question posted above would be much more complicated and require detailed global carbon budget accounting and expensive simulations in a costly ESM framework for carbon-climate feedbacks. Biomass carbon residence time will need to be considered as not all net primary production once fixed remains with vegetation for very long (25). Also, recent studies found that plant respiratory CO2 may be transported upward via a transpiration stream in xylem and then be refixed (26), reducing direct dependence of carboxylation on ambient CO2. However, as all carbon in plant organs must ultimately originate from CO2 moving through mesophyll, the refixation of xylem-transported respiratory CO2 will unlikely substantially diminish the impact of the mesophyll diffusion limitation on photosynthesis.
Other model deficiencies besides lack of explicit representation of mesophyll diffusion, for example, inadequate consideration of nitrogen deposition and land use and land cover change (27), could also explain much of the overprediction of the growth rate of historical atmospheric CO2 by current ESMs, assuming the cause of their overprediction does reside over land. However, because mesophyll diffusion affects the first step of terrestrial carbon cycle, it is important for its effect to be accounted for so that impacts of other factors can be evaluated more reliably.
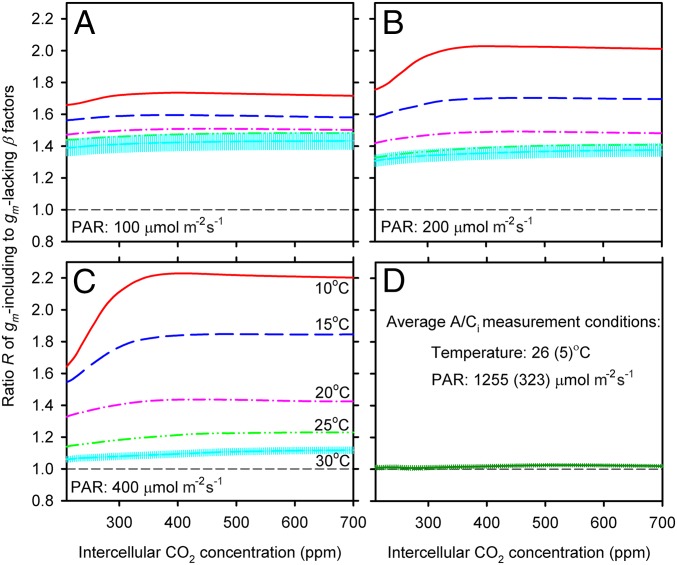
Although our gm model is based on empirical data from a large number of species (SI Text), its global application, as in any such effort, is bound to have uncertainties. We therefore conducted a leaf-scale uncertainty analysis with the global LeafWeb database of leaf gas exchange measurements. In this leaf-scale analysis, no gm model was needed; instead, actual parameters optimized from leaf gas exchange measurements were used directly. We calculated leaf-scale R (Eq. 2) for about 130 C3 species covering all major plant functional types of the world from herbaceous temperate plants to woody tropical species (15). This obtained leaf scale R is consistent with our global analyses with CLM4.5 (Fig. 4). The results show that under nonsaturating levels of photosynthetic photon flux density (typically PPFD < 1,000 µmol/m2/s; Fig. 4 A–C), the leaf scale R, which is averaged across all species, is significantly larger than 1 across a wide range of intercellular CO2 for all temperature levels examined (intercellular instead of ambient CO2 was used in this leaf-scale calculation to avoid uncertainties associated with modeling stomatal conductance). For a given nonsaturating PPFD level, the averaged leaf scale R tends to increase with decrease in temperatures, consistent with the trend of latitudinal R in the northern hemisphere simulated by CLM4.5. Only under conditions similar to the measurement conditions of the original A/Ci curves (i.e., saturating light levels and high temperatures) is the averaged leaf-scale R close to 1 (Fig. 4D).
Fig. 4.
Changes with intercellular CO2 concentration of the averaged ratio (R) of the β factors for leaf net photosynthetic rates calculated with a leaf photosynthetic model between including and lacking explicit consideration of mesophyll conductance (gm). Each curve in each plot represents an average of >1,000 R ratio curves. For each nonsaturating level of photosynthetic photon flux density (PPFD = 100, 200, and 400 μmol/m2/s, respectively, for A, B, and C), five levels of temperature (10, 15, 20, 25, and 30 °C) are used. Calculations are also done at the original leaf gas exchange (A/Ci) measurement conditions which vary somewhat from measurement to measurement (D). The averaged PPFD for the leaf gas exchange measurements is 1,255 ± 323 μmol/m2/s and the averaged temperature is 26 ± 5 °C. The 95% confidence interval is shown for the mean R ratio curve at 30 °C (A–C) and at the measurement conditions (D; although barely seen due to a large volume of samples). This figure demonstrates that, although photosynthetic models with and without gm show similar leaf-level CO2 fertilization effects for the conditions used in measurements, this similarity degrades with increasing distances from the measurement conditions.
Why do carbon cycle models without explicit representation of mesophyll diffusion underestimate the degree of CO2 fertilization, even when their key photosynthetic parameters have been fitted against A/Ci curves? Why does R vary so much with environmental conditions? The answers are not straightforward but can be most clearly understood through demonstrations with the Excel spreadsheet-based Tool for Evaluating Mesophyll Impact on Predicting Photosynthesis (TEMIPP), which is provided as part of SI Text.
The key to understanding these two questions lies in a combination of two factors: (i) the way A/Ci curves are measured and (ii) the unique structure of the highly nonlinear FvCB model. A/Ci curves are generally made under a saturating level of PPFD (typically >1,000 µmol/m2/s) and fixed temperature (e.g., 25 °C) and thus represent measurements in one dimension (A vs. CO2 concentrations). This strict control of measurement conditions at saturating PPFD and fixed temperature is necessary for obtaining data about key biochemical processes of photosynthesis (13, 14). In contrast, carbon cycle models have to run for natural environmental conditions in a 3D space with PPFD, temperature, and CO2 concentrations all varying simultaneously. Thus, model applications fall outside the ranges of conditions used for parameter calibration. This mismatch is of concern because the highly nonlinear FvCB model consists of three distinct submodels (Rubisco-, RuBP regeneration-, and TPU-limited carboxylation rates) whose respective applicable domains vary dynamically with environmental conditions that affect photosynthesis. When mesophyll diffusion is not considered explicitly, the condition mismatch discussed above will cause the submodel domains to be incorrectly identified, which results in carboxylation rates being determined by wrong submodels. Hence, even if A/Ci curves were fit apparently well, predictions under naturally varying conditions will be still problematic.
Fig. S7 demonstrates the points made above with two measured and one simulated examples of leaf photosynthetic response to changes in intercellular CO2 concentration. An unlimited number of cases can be generated with TEMIPP. In Fig. S7, the data were obtained over a range of CO2 concentrations but at a constant saturating PPFD and a fixed temperature, following the common practices in A/Ci curve measurements that are used for tuning model photosynthetic parameters. On a first look, both the gm-lacking and gm-including models fit the original data very well. Such an apparent good fit to data even by gm-lacking models is very common in the literature of A/Ci curve analyses and probably has contributed to the underappreciation by carbon cycle modelers of the importance of mesophyll diffusion for modeling photosynthesis. However, the residual plots (the Insets in Fig. S7) reveal biases easy to miss in visual examination: there are always systematic differences in the predicted photosynthetic rate between the two models, depending on CO2 concentrations. More importantly, when the fitted parameters are used to predict photosynthetic rates at other values of PPFD and temperatures, the difference between them enlarges. The magnitude of this enlargement depends on levels of CO2 concentration. At extremely high values of CO2 that saturate photosynthesis, the limiting effect of mesophyll diffusion diminishes and the gm-lacking and gm-including curves tend to merge, regardless of specific values of PPFD and temperature. However, within the intermediate range of CO2, the gm-lacking curves are always above the corresponding gm-including curves, indicating that the gm-lacking model approaches photosynthetic saturation faster and at a lower CO2 than does the gm-including model.
The reason for this faster approach to photosynthetic saturation by the gm-lacking model is that it overestimates CO2 concentrations available at the site of carboxylation and therefore underestimates photosynthetic sensitivity to variations in ambient CO2 concentration. Although this underestimation of sensitivity to CO2 is minimized when model parameters are tuned against measurements, the effectiveness of the tuned parameters is limited to the narrow PPFD and temperature conditions under which the measurements for parameter tuning are made. When the natural environmental conditions deviate from these parameter tuning conditions, the tuned parameters become less effective as the applicable domains of the highly nonlinear FvCB submodels are misidentified and wrong submodels are applied to calculate carboxylation rates. Thus, a lack of explicit consideration of mesophyll diffusion represents an inherent structural deficiency for carbon cycle models, a structural deficiency that cannot be compensated for by the use of phenomenologically obtained photosynthetic parameters.
In addition to providing a potential explanation for the cause of the overprediction of historical atmospheric CO2 growth by ESMs, our study has identified a common mechanism that could help resolve several other important issues. A recent inventory and field observation-based report showed that, contrary to expectation, the world’s forests have continued to serve as a large persistent carbon sink (28), consistent with our finding that terrestrial ecosystems may have responded to historical anthropogenic CO2 emissions more strongly than models have indicated. Also, carbon cycle models generally underestimate the long-term trends in the seasonal amplitude of atmospheric CO2 (29) and in forest ecosystem water use efficiencies (30). These underestimations could be explained by the underestimation by carbon cycle models of the long-term response of net ecosystem productivity to the increase in atmospheric CO2 concentration, which in turn could be explained by our finding that models lacking explicit consideration of mesophyll diffusion underestimate the CO2 fertilization effect. Furthermore, in the northern hemisphere, the increase in the seasonal amplitude of atmospheric CO2 has been larger in high latitudes than in low latitudes and substantially larger than simulated by carbon cycle models (31), which agrees with our finding that the estimated CO2 fertilization effect in the regions of >45°N is particularly sensitive to the consideration of mesophyll diffusion (Fig. 2).
Our results at the global scale are a logical extension of what has long been known at the leaf scale by plant physiologists. Numerous studies have reported that mesophyll and stomatal conductances have similar magnitudes and are equally important in controlling CO2 concentrations available for photosynthesis (1–9, 15–17, 20). The drawdown of CO2 from substomatal cavities to chloroplasts may reduce photosynthesis by 25–75%, depending on species (32). Differences in gm may even have the potential to alter the balance in species competitiveness as plant communities respond to rising atmospheric CO2 because an atmosphere enriched in CO2 may favor species with lower gm such as needleleaf evergreen trees and others (2, 6). Thus, mesophyll diffusion can play a crucial role in our understanding and predicting photosynthetic responses to the increase in atmospheric CO2 concentration from leaf to global scales.
An uncertainty in estimating the long-term impact of mesophyll diffusion on global land CO2 fertilization is the acclimation of biochemical capacities of photosynthetic machinery to elevated atmospheric CO2 concentrations. Photosynthetic acclimation has been observed in many free-air CO2 enrichment (FACE) experiments of C3 plant species (33). Typical acclimation involves down-regulation of Vcmax and Jmax to balance resource allocation to reactions controlling photosynthesis. Our leaf-level analysis indicates that mesophyll diffusion plays similar roles in controlling CO2 availability at the site of carboxylation regardless of leaf productivity, which can be seen by comparing Fig. S7 A and B with C. Thus, it is unlikely that photosynthetic acclimation to elevated CO2 will qualitatively change the findings reported here.
A related issue is how limited availability of nutrients, particularly nitrogen and phosphorous, interacts with the impact of mesophyll diffusion on the CO2 fertilization effect. Model simulations have shown that explicit representations of nitrogen and phosphorus limitations generally result in reduced response of the terrestrial carbon sink to historical increases in atmospheric CO2 concentrations (27, 34, 35). Thus, nutrient limitations may reduce the impact of mesophyll diffusion on estimated CO2 fertilization effect. However, if the main effect of nutrient limitations is to reduce Vcmax and Jmax, then our argument made above on photosynthetic acclimation also applies to nutrient limitation. Furthermore, it is possible that an explicit consideration of mesophyll diffusion would influence model evaluations of nutrient limitations on terrestrial carbon cycle. Given the unequivocal fact that mesophyll diffusion limits CO2 availability at the site of carboxylation, an interesting question is as follows: will explicit representation of mesophyll diffusion delay the development of nutrient limitation in the terrestrial carbon cycle in response to increase in atmospheric CO2 concentrations? With improved representations of nutrient limitation and mesophyll diffusion, this question may be answerable in the near future.
In summary, the terrestrial biosphere may be more CO2 limited and therefore absorb more carbon per unit increase of atmospheric CO2 than previously thought. Over the period investigated in this study (1901–2010), atmospheric CO2 only started to rise rapidly after the 1950s. If the current trend of increasing atmospheric CO2 continues, the underestimation of the CO2 fertilization effect by models lacking explicit representation of mesophyll diffusion should grow to well above the 16% determined for the period of 1901–2010. Although mesophyll conductance increases with warming, this increase does not keep pace with the increased carboxylation capacity of Rubisco and thus mesophyll diffusion may become even more limiting for photosynthesis at high temperatures (i.e., as measured by ΔCFE) (4). To adequately predict long-term effects of anthropogenic emissions and carbon–climate interactions, carbon cycle models should explicitly consider mesophyll resistance to CO2 diffusion. As we demonstrated with our global mesophyll conductance model (SI Text), this consideration does not add substantial computational burden or excessive new parameterization. Carbon cycle models that lack explicit representation of mesophyll diffusion will underestimate historical and future terrestrial carbon uptake. Consequently, they will overestimate historical and future growth rates of atmospheric CO2 concentration due to fossil fuel emissions, with ramifications for predicted climate change.
Methods
The global gm model was developed by synthesizing the latest advances in plant physiological literature. Large-scale carbon cycle models generally use the concept of plant functional types (PFTs) to simulate carbon, water, and energy fluxes in terrestrial ecosystems. Our global gm model is likewise based on the PFT concept so that it is consistent with current large-scale modeling philosophy and applicable broadly to different vegetation types, rather than to particular ecosystems. In the gm model, leaf structures, which differ among PFTs, determine the maximum attainable gm, whereas temperature and moisture stress factors and within-canopy environmental gradients modify this maximum value (SI Text). The global gm model and the conversion function that establishes the gm-lacking to gm-including parameter correspondence are all of the structural elements that we have added to CLM4.5. A series of global simulations were conducted to verify the internal consistency and validity of the modified CLM4.5 (SI Text). All simulations are offline experiments driven by the CRU/NCEP/NCAR reanalysis (www.cru.uea.ac.uk/cru/data/ncep/) and transient land use. The historical atmospheric CO2 concentrations, which are used in all simulations except in constant CO2 runs, are derived from ice cores and atmospheric observations. A detailed description on methods is available in SI Text.
Supplementary Material
Acknowledgments
We thank Drs. Paul Hanson, Stan Wullschleger, Anthony Walker, and David Weston for comments and suggestions. This material is based upon work supported by the US Department of Energy, Office of Science, Biological and Environmental Research Program Grants DE-FG02-01ER64746 to University of Texas at Austin and DE-AC05-00OR22725 to Oak Ridge National Laboratory (ORNL). The ORNL's Laboratory Directed Research and Development (LDRD) program also partially supported the research. ORNL is managed by University of Tennessee-Battelle, LLC.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1418075111/-/DCSupplemental.
References
- 1.Evans JR, Sharkey TD, Berry JA, Farquhar GD. Carbon isotope discrimination measured concurrently with gas-exchange to investigate CO2 diffusion in leaves of higher-plants. Aust J Plant Physiol. 1986;13(2):281–292. [Google Scholar]
- 2.Niinemets U, Flexas J, Peñuelas J. Evergreens favored by higher responsiveness to increased CO₂. Trends Ecol Evol. 2011;26(3):136–142. doi: 10.1016/j.tree.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Evans JR, Kaldenhoff R, Genty B, Terashima I. Resistances along the CO2 diffusion pathway inside leaves. J Exp Bot. 2009;60(8):2235–2248. doi: 10.1093/jxb/erp117. [DOI] [PubMed] [Google Scholar]
- 4.Bernacchi CJ, Portis AR, Nakano H, von Caemmerer S, Long SP. Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo. Plant Physiol. 2002;130(4):1992–1998. doi: 10.1104/pp.008250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warren CR. Stand aside stomata, another actor deserves centre stage: The forgotten role of the internal conductance to CO2 transfer. J Exp Bot. 2008;59(7):1475–1487. doi: 10.1093/jxb/erm245. [DOI] [PubMed] [Google Scholar]
- 6.Niinemets U, Díaz-Espejo A, Flexas J, Galmés J, Warren CR. Role of mesophyll diffusion conductance in constraining potential photosynthetic productivity in the field. J Exp Bot. 2009a;60(8):2249–2270. doi: 10.1093/jxb/erp036. [DOI] [PubMed] [Google Scholar]
- 7.Niinemets U, Díaz-Espejo A, Flexas J, Galmés J, Warren CR. Importance of mesophyll diffusion conductance in estimation of plant photosynthesis in the field. J Exp Bot. 2009b;60(8):2271–2282. doi: 10.1093/jxb/erp063. [DOI] [PubMed] [Google Scholar]
- 8.Flexas J, et al. Mesophyll diffusion conductance to CO2: An unappreciated central player in photosynthesis. Plant Sci. 2012;193-194:70–84. doi: 10.1016/j.plantsci.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Sharkey TD. Virtual special issue on [corrected] mesophyll conductance: Constraint on carbon acquisition by C3 plants. Plant Cell Environ. 2012;35(11):1881–1883. doi: 10.1111/pce.12012. [DOI] [PubMed] [Google Scholar]
- 10.Taylor KE, Meehl GA, Stouffer RJ. An overview of CMIP5 and the experiment design. Bull Am Meteorol Soc. 2012;93(4):485–498. [Google Scholar]
- 11.Piao S, et al. Evaluation of terrestrial carbon cycle models for their response to climate variability and to CO2 trends. Glob Change Biol. 2013;19(7):2117–2132. doi: 10.1111/gcb.12187. [DOI] [PubMed] [Google Scholar]
- 12.Farquhar GD, von Caemmerer S, Berry JA. A biochemical model of photosynthetic CO2 assimilation in leaves of C 3 species. Planta. 1980;149(1):78–90. doi: 10.1007/BF00386231. [DOI] [PubMed] [Google Scholar]
- 13.Long SP, Bernacchi CJ. Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J Exp Bot. 2003;54(392):2393–2401. doi: 10.1093/jxb/erg262. [DOI] [PubMed] [Google Scholar]
- 14.Sharkey TD, Bernacchi CJ, Farquhar GD, Singsaas EL. Fitting photosynthetic carbon dioxide response curves for C(3) leaves. Plant Cell Environ. 2007;30(9):1035–1040. doi: 10.1111/j.1365-3040.2007.01710.x. [DOI] [PubMed] [Google Scholar]
- 15.Sun Y, et al. Asymmetrical effects of mesophyll conductance on fundamental photosynthetic parameters and their relationships estimated from leaf gas exchange measurements. Plant Cell Environ. 2014;37(4):978–994. doi: 10.1111/pce.12213. [DOI] [PubMed] [Google Scholar]
- 16.Evans JR, von Caemmerer S. Temperature response of carbon isotope discrimination and mesophyll conductance in tobacco. Plant Cell Environ. 2013;36(4):745–756. doi: 10.1111/j.1365-3040.2012.02591.x. [DOI] [PubMed] [Google Scholar]
- 17.Ethier GJ, Livingston NJ. On the need to incorporate sensitivity to CO2 transfer conductance into the Farquhar-von Caemmerer-Berry leaf photosynthesis model. Plant Cell Environ. 2004;27(2):137–153. [Google Scholar]
- 18.Bonan GB, et al. Improving canopy processes in the Community Land Model version 4 (CLM4) using global flux fields empirically inferred from FLUXNET data. J Geophys Res. 2011;116(G2):G02014. [Google Scholar]
- 19.Oleson KW, et al. 2013 Technical description of version 4.5 of the community land model (CLM). NCAR Technical Note. Available at www.cesm.ucar.edu/models/cesm1.2/clm/. Accessed October 2, 2013.
- 20.Gu L, Pallardy SG, Tu K, Law BE, Wullschleger SD. Reliable estimation of biochemical parameters from C₃ leaf photosynthesis-intercellular carbon dioxide response curves. Plant Cell Environ. 2010;33(11):1852–1874. doi: 10.1111/j.1365-3040.2010.02192.x. [DOI] [PubMed] [Google Scholar]
- 21.Friedlingstein P, et al. Uncertainties in CMIP5 climate projections due to carbon cycle feedbacks. J Clim. 2014;27(2):511–526. [Google Scholar]
- 22.Hoffman FM, et al. Causes and implications of persistent atmospheric carbon dioxide biases in Earth System Models. J Geophys Res Biogeosci. 2014;119(2):141–162. [Google Scholar]
- 23.Waring RH, Landsberg JJ, Williams M. Net primary production of forests: A constant fraction of gross primary production? Tree Physiol. 1998;18(2):129–134. doi: 10.1093/treephys/18.2.129. [DOI] [PubMed] [Google Scholar]
- 24.Keeling CD, Whorf TP, Wahlen M, van der Plichtt J. Interannual extremes in the rate of rise of atmospheric carbon dioxide since 1980. Nature. 1995;375(6533):666–670. [Google Scholar]
- 25.Friend AD, et al. Carbon residence time dominates uncertainty in terrestrial vegetation responses to future climate and atmospheric CO2. Proc Natl Acad Sci USA. 2014;111(9):3280–3285. doi: 10.1073/pnas.1222477110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trumbore SE, Angert A, Kunert N, Muhr J, Chambers JQ. What’s the flux? Unraveling how CO₂ fluxes from trees reflect underlying physiological processes. New Phytol. 2013;197(2):353–355. doi: 10.1111/nph.12065. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Q, Pitman AJ, Wang YP, Dai YJ, Lawrence PJ. The impact of nitrogen and phosphorous limitation on the estimated terrestrial carbon balance and warming of land use change over the last 156 yr. Earth System Dynamics. 2013;4(2):333–345. [Google Scholar]
- 28.Pan Y, et al. A large and persistent carbon sink in the world’s forests. Science. 2011;333(6045):988–993. doi: 10.1126/science.1201609. [DOI] [PubMed] [Google Scholar]
- 29.McGuire AD, et al. Carbon balance of the terrestrial biosphere in the twentieth century: Analyses of CO2, climate and land use effects with four process-based models. Global Biogeochem Cycles. 2001;15(1):183–206. [Google Scholar]
- 30.Keenan TF, et al. Increase in forest water-use efficiency as atmospheric carbon dioxide concentrations rise. Nature. 2013;499(7458):324–327. doi: 10.1038/nature12291. [DOI] [PubMed] [Google Scholar]
- 31.Graven HD, et al. Enhanced seasonal exchange of CO2 by northern ecosystems since 1960. Science. 2013;341(6150):1085–1089. doi: 10.1126/science.1239207. [DOI] [PubMed] [Google Scholar]
- 32.Terashima I, Hanba YT, Tazoe Y, Vyas P, Yano S. Irradiance and phenotype: Comparative eco-development of sun and shade leaves in relation to photosynthetic CO2 diffusion. J Exp Bot. 2006;57(2):343–354. doi: 10.1093/jxb/erj014. [DOI] [PubMed] [Google Scholar]
- 33.Ainsworth EA, Long SP. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005;165(2):351–371. doi: 10.1111/j.1469-8137.2004.01224.x. [DOI] [PubMed] [Google Scholar]
- 34.Wang YP, Polglase PJ. Carbon balance in the tundra, boreal forest and humid tropical forest during climate change: Scaling up from leaf physiology and soil carbon dynamics. Plant Cell Environ. 1995;18(10):1226–1244. [Google Scholar]
- 35.Zhang Q, Wang YP, Pitman AJ, Dai YJ. Limitations of nitrogen and phosphorous on the terrestrial carbon uptake in the 20th century. Geophys Res Lett. 2011;38(22):L22701. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.