Significance
Our observations provide evidence to link epidemiological studies implicating stress-related processes with biological dysfunction in type 2 diabetes. The patterns of cardiovascular, inflammatory, neuroendocrine, and cholesterol responses exemplify the disturbances of reactivity and recovery predicted by the allostatic load model, including prolonged responses to stress due to delayed shutdown of physiological reactivity, and inadequate (blunted) responses resulting in compensatory hyperactivity in other mediating pathways. Chronic allostatic load may be a mechanism through which stress exposures contribute to diabetes risk, while also being implicated in the adverse health consequences of diabetes such as coronary heart disease and cognitive decline.
Keywords: inflammation, neuroendocrine, depression, cardiovascular, metabolic
Abstract
Psychological stress-related processes are thought to contribute to the development and progression of type 2 diabetes, but the biological mechanisms involved are poorly understood. Here, we tested the notion that people with type 2 diabetes experience chronic allostatic load, manifest as dynamic disturbances in reactivity to and recovery from stress across multiple (cardiovascular, neuroendocrine, inflammatory, metabolic) biological systems, coupled with heightened experience of chronic life stress. We carried out an experimental comparison of 140 men and women aged 50–75 y with type 2 diabetes and 280 nondiabetic individuals matched on age, sex, and income. We monitored blood pressure (BP) and heart rate, salivary cortisol, plasma interleukin (IL)-6, and total cholesterol in response to standardized mental stress, and assessed salivary cortisol over the day. People with type 2 diabetes showed impaired poststress recovery in systolic and diastolic BP, heart rate and cholesterol, and blunted stress reactivity in systolic BP, cortisol, cholesterol, and IL-6. Cortisol and IL-6 concentrations were elevated, and cortisol measured over the day was higher in the type 2 diabetes group. Diabetic persons reported greater depressive and hostile symptoms and greater stress experience than did healthy controls. Type 2 diabetes is characterized by disruption of stress-related processes across multiple biological systems and increased exposure to life stress. Chronic allostatic load provides a unifying perspective with implications for etiology and patient management.
Type 2 diabetes has a heterogeneous pathophysiology in which β-cell dysfunction and insulin resistance play pivotal roles (1). Stress-related factors may contribute to risk of type 2 diabetes through their impact on inflammatory, metabolic, cardiovascular, and neuroendocrine regulation (2). Socioeconomic adversity over the life course predicts type 2 diabetes in later life (3), whereas stress at work and more general indicators of perceived stress are associated with future diabetes (4, 5). There appears to be a bidirectional relationship between type 2 diabetes and depressive symptoms (6), and people with type 2 diabetes may report greater social isolation and more limited social support (7).
These diverse associations between stress-related processes and diabetes are only partly accounted for by lifestyle factors such as physical inactivity, alcohol consumption, or adiposity, suggesting that direct psychobiological pathways may be involved. A helpful concept in this regard is allostatic load. Allostasis refers to the dynamic process of adaptation to environmental challenges through adjustments in multiple biological systems, including the hypothalamic–pituitary–adrenocortical (HPA) axis, autonomic nervous system, and metabolic and immune systems (8). Allostasis is essential for maintaining homeostasis, but repeated or sustained stimulation leads to allostatic load, the wear-and-tear that results from dysregulation of mediating processes. Allostatic load is frequently quantified by measuring a range of biomarkers (e.g., blood pressure, cortisol, catecholamines, glycated hemoglobin, waist circumference, cholesterol, inflammatory markers), and allocating individuals scores based on the number of variables on which values are elevated compared with the sample distribution (9, 10). Studies relating allostatic load measures with type 2 diabetes have been inconsistent to date (11, 12). However, another aspect of allostasis is that it is manifest in modifications of dynamic responses to challenge, not only in basal measures. Adaptive biological responses to stress involve brisk increases in activation (stress reactivity) as the person mobilizes for vigorous activity, followed by prompt recovery back to baseline levels. McEwen (8) has noted that high allostatic load disrupts these dynamic biological responses, resulting in changes in the morphology of responses, notably impaired poststress recovery and inadequate biological responses (blunted stress reactivity).
Accordingly, we tested the notion that type 2 diabetes is characterized by high dynamic allostatic load. We hypothesized that in response to standardized mental stress, people with type 2 diabetes would show impaired poststress recovery and blunted stress reactivity in blood pressure (BP), heart rate, cortisol, and serum cholesterol, together with greater inflammation indexed by interleukin (IL)-6. We monitored cortisol output in everyday life by obtaining multiple saliva samples over the day to test whether people with type 2 diabetes have higher cortisol output in naturalistic settings. We also measured relevant psychological variables, conjecturing that people with diabetes would report more emotional distress, greater stress in their lives, and reduced psychosocial resources compared with nondiabetic individuals.
Results
We compared 140 men and women aged 50–75 y with type 2 diabetes with 280 nondiabetic individuals matched by age, sex, and income category. The diabetic participants had better education and were less likely to be married than were controls (Table 1). As expected, glycated hemoglobin (HbA1c), body mass index (BMI), and waist circumference were greater in the diabetes than control groups, and diabetic participants were also more likely to be smokers. The majority of diabetic participants were taking oral medications such as metformin, and hypertensive medications were also common. None of the healthy controls were taking any medications apart from a small proportion of prescribed statins (7.9%).
Table 1.
Characteristics of diabetes and healthy control groups
| Diabetes (n = 140) | Healthy control (n = 280) | Difference (P) | |
| Men/women | 88/52 | 176/104 | 1.00 |
| Age, mean ± SD | 64.0 ± 6.3 | 63.7 ± 7.0 | 0.65 |
| Household income, n (%) | 1.00 | ||
| <£40,000 | 194 (71.6%) | 95 (71.4%) | — |
| ≥£40,000 | 77 (28.4%) | 38 (28.6%) | — |
| Education, n (%) | 0.001 | ||
| Less than high school | 37 (26.8%) | 94 (35.9%) | — |
| High school | 14 (10.0%) | 77 (29.4%) | — |
| College or higher | 87 (63.0%) | 91 (34.7%) | — |
| Status, n (%) | 0.001 | ||
| Married | 70 (50.0%) | 175 (62.9%) | — |
| Single, never married | 31 (22.1%) | 63 (22.7%) | — |
| Divorced, separated, widowed | 39 (27.9%) | 40 (14.4%) | — |
| Current smoker, n (%) | 20 (14.4%) | 18 (6.4%) | 0.011 |
| BMI, kg/m2 mean ± SD | 30.8 ± 5.72 | 25.9 ± 3.82 | 0.001 |
| Waist, cm mean ± SD | 105.5 ± 13.5 | 87.5 ± 12.6 | 0.001 |
| HbA1c, % mean ± SD | 7.25 ± 1.42 | 5.47 ± 0.53 | 0.001 |
| HbA1c, mmol/mol | 56 ± 15.5 | 36 ± 5.8 | — |
| Medication, n (%) | |||
| Statins | 106 (77.9%) | 22 (7.9%) | 0.001 |
| Oral diabetic medication | 109 (80.1%) | — | — |
| Insulin, other diabetic medication | 15 (11.0%) | — | — |
| Aspirin | 48 (35.3%) | — | — |
| Beta blockers | 16 (11.8%) | — | — |
| Other hypertensive medication | 96 (70.6%) | — | — |
Physiological Responses to Mental Stress.
We tested participants individually either in the morning or afternoon, monitoring physiological responses to two standardized mental stress tests. The proportion of laboratory sessions taking place in the morning and afternoon did not differ between groups. The mental stress tasks elicited substantial subjective stress responses, with increases from 1.50 ± (SD) 0.91 to 4.47 ± 1.56 in the diabetes and 1.42 ± 0.81 to 4.06 ± 1.47 in the no diabetes group. The increase in subjective stress during tasks did not differ in the two groups (Table S1). Systolic and diastolic BP and heart rate also rose during stress tasks, returning toward baseline over the poststress recovery period. The diabetes group showed a pattern of cardiovascular response characteristic of high allostatic load (Fig. 1 and Table S1). Notably, systolic BP stress reactivity was blunted in the diabetes compared with control group [adjusted odds of being in the diabetes group per mmHg increase in reactivity = 0.97, 95% confidence interval (CI) 0.95–0.99, P = 0.007], whereas recovery was reduced both at 40–45 min posttress (odds ratio 0.98, 95% CI 0.96–0.99, P = 0.028) and at 70–75 min poststress (odds ratio 0.97, 95% CI 0.95–0.99, P = 0.004). We recorded a similar profile for diastolic BP and heart rate (Fig. 1); thus, for both variables, stress reactivity was lower in the diabetes group, and poststress recovery was impaired (statistical details in Table S1). In addition, the diabetes group had lower diastolic BP but higher heart rate than the healthy control group throughout the stress session (P < 0.001). There were no interactions between sex and diabetic status in any analyses.
Fig. 1.
Mean systolic BP (Upper), diastolic BP (Center), and heart rate (Lower) during baseline, task trials, and 40–45 min and 70–75 min posttasks in the diabetes (red line) and control (black line) groups. BP and heart rate data are adjusted for education, marital status, BMI, smoking status, beta-blocker use, and time of testing. P values indicate group differences in stress reactivity (baseline–task difference) and poststress recovery (task–posttask differences) as detailed in Table S1. Error bars are SEMs.
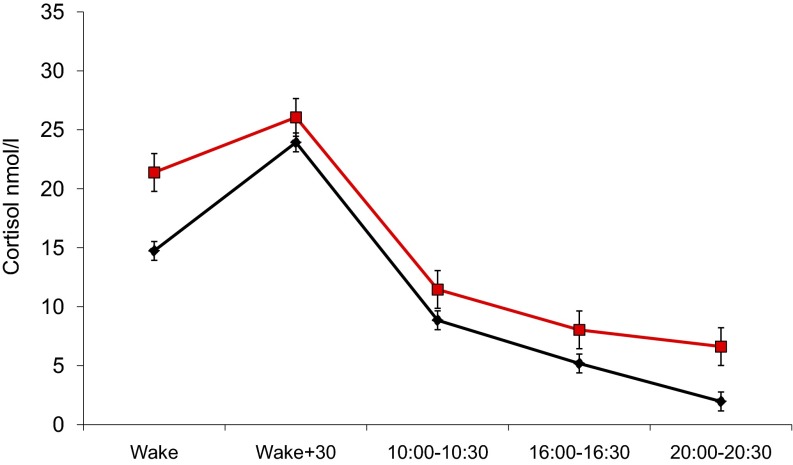
We found that baseline cortisol concentration was substantially greater in diabetic participants, and cortisol level subsequently fell across the task period (Fig. 2). Consequently, the groups differed in cortisol responses to stress, with increases in the control group and decreases in the diabetes group (Table S1; P < 0.001). The groups converged over the posttask recovery period. Plasma IL-6 concentration was higher in the diabetes group at baseline (P = 0.014). We recorded increases in IL-6 following stress in both groups, but the increase was blunted in the diabetes group both at 45 min (P = 0.021) and 75 min (P = 0.028) after tasks. Nonetheless, the concentration of IL-6 remained higher in the diabetes than control groups. The profile of total cholesterol stress responses was again consistent with high allostatic load (Fig. 2), wince the rise in cholesterol with stress was blunted in the diabetes group (adjusted odds of being in the diabetes group per unit mmol increase in cholesterol = 0.02, 95% CI 0.001–0.27, P = 0.004), whereas the recovery following stress was reduced (odds ratio 0.018, 95% CI 0.001–0.88, P = 0.043). Sex did not interact with diabetes status in any of these analyses.
Fig. 2.
Mean salivary cortisol (Upper; geometric means), plasma IL-6 (Center; geometric means), and total cholesterol (Lower) in the diabetes (red line) and control (black line) groups. Cortisol sampled at baseline, immediately after tasks, and 20, 45, and 75 min later. Values are adjusted for education, marital status, BMI, smoking status, use of statins, and time of testing. The P value indicates group differences in stress reactivity. IL-6 was adjusted for education, marital status, BMI, smoking, and time of testing; P values indicate group differences in stress reactivity. Total cholesterol was adjusted for education, marital status, BMI, smoking, aspirin, use of statins, and time of testing. P values indicate group differences in stress reactivity (baseline–task difference) and poststress recovery (task–posttask difference). Error bars are SEM.
Cortisol Output over the Day.
Participants collected five saliva samples over the day. Both groups showed a typical pattern of salivary cortisol output, with high levels on waking, an increase over the first 30 min after waking, and then decreasing levels through the remainder of the day (Fig. 3). Concentrations were an average 36% higher in the diabetes than control groups after adjustment for covariates (P < 0.001), with significant differences in samples on waking, at 1600–1630, and at 2000–2030.
Fig. 3.
Mean salivary cortisol sampled on waking, 30 min after waking, and in the morning (1000–1030), afternoon (1600–1630), and evening (2000–2030) in the diabetes (red line) and control (black line) groups. Values are adjusted for education, marital status, BMI, smoking status, oral diabetic medication, and beta-blocker use. Error bars are SEM.
Stress-Related Psychological Factors.
We measured stress-related psychological factors using standardized questionnaires. Diabetic participants showed a higher stress profile than controls in terms of negative emotional responses and greater reported stress experience (Table 2). They reported more depressive symptoms, greater hostility, more financial strain, less social cohesion in their local neighborhoods, less sense of control over their lives, and less optimism, after adjustment for education and marital status. Diabetic participants were also more likely to be separated, divorced, or widowed than were controls (Table 1). The two groups did not, however, differ in social support, or in the proportion that were caring for aging or disabled family members. We did not observe any interactions between sex and diabetes status in these analyses.
Table 2.
Psychosocial factors in the diabetes and healthy control groups
| Diabetes* | Healthy control* | Odds of diabetes† (95% C.I.) | P | |
| Depression symptoms | 11.86 ± 8.9 | 6.47 ± 6.6 | 1.16 (1.11–1.22) | 0.001 |
| Hostility | 3.78 ± 2.8 | 2.72 ± 2.4 | 1.20 (1.08–1.37) | 0.001 |
| Financial strain | 84 (71.2%) | 122 (43.7%) | 1.38 (1.24–1.53) | 0.001 |
| Social cohesion | 9.83 ± 5.5 | 11.62 ± 4.6 | 0.91 (0.86–0.96) | 0.001 |
| Caregiver | 24 (17.1%) | 37 (13.2%) | 1.37 (0.74–2.56) | 0.32 |
| Sense of control | 3.29 ± 1.6 | 3.92 ± 1.4 | 0.77 (0.65–0.91) | 0.002 |
| Optimism | 14.4 ± 4.3 | 15.5 ± 3.8 | 0.91 (0.86–0.96) | 0.002 |
| Social support | 25.7 ± 7.1 | 26.3 ± 5.4 | 0.99 (0.95–1.04) | 0.72 |
Mean ± SD, or n (%).
Odds of being in the diabetes group per unit change in the independent variable, adjusted for education and marital status.
Intercorrelation Between Responses in Different Systems.
There were consistent associations between responses in the different systems monitored in this study. Thus, cardiovascular (BP and heart rate) reactions to tasks were negatively correlated with cortisol output over the day (P < 0.05) and with baseline IL-6 concentration (P < 0.01), while being positively related to cortisol (P < 0.01) and cholesterol (P < 0.001) responses to mental stress. Measures of poststress recovery showed similar patterns. Interrelationships between biological responses and psychological characteristics were also present. For example, systolic BP reactivity was negatively correlated with depressive symptoms, financial strain, and hostility, and positively associated with social cohesion, social support, control over life, and optimism, after adjustment for group status (all P < 0.05). Baseline IL-6 concentration was positively related to depressive symptoms and financial strain (P < 0.05), and negatively with social support (P = 0.024). Cortisol responses to acute stress were positively related to social cohesion (P < 0.05) and social support (P = 0.009), whereas individuals with greater cortisol output over the day were low in optimism (P < 0.001).
Discussion
This study explored the hypothesis that people with type 2 diabetes experience chronic allostatic load, manifest in alterations in dynamic physiological responses to standardized mental stress, higher cortisol output over the day, and greater psychological distress and experience of chronic life stress, in comparison with age, sex, and income matched controls. We found that poststress recovery was attenuated in the diabetes group in systolic and diastolic BP, heart rate, and total cholesterol, together with blunted stress reactivity in BP, heart rate, cortisol, and cholesterol concentration. These effects were independent of covariates including medication and were evident in both men and women. The acute increases in cholesterol concentration are likely to be due in part to reductions in blood volume following stress, leading to greater hemoconcentration. Plasma IL-6 concentration was higher in people with type 2 diabetes, so that although the increases following mental stress were smaller than those of controls, absolute levels remained higher. The type 2 diabetes group had higher baseline cortisol than controls in the laboratory, together with heightened cortisol output throughout the day. We also found that people with type 2 diabetes had more depressive and hostile symptoms, and reported greater chronic stress in terms of financial and neighborhood strain.
The multisystem measures of allostatic load described in the literature typically involve measures taken under resting conditions and include several components of the metabolic syndrome such as elevated BP, triglycerides, and waist circumference (13), so it is surprising that the concept has not been successfully applied to type 2 diabetes. One study of middle-aged Puerto Ricans living in Boston showed that greater allostatic load was associated with increased risk of diabetes and other chronic conditions (12), but another investigation focusing specifically on diabetes failed to find that the components of allostatic load clustered reliably in people with diabetes (11).
Our results suggest that attention to the dynamic aspects of allostatic load may be fruitful. Our systematic review of published literature found no previous studies that have examined the dynamic responses to mental stress across multiple biological systems in type 2 diabetes. One small study showed that systolic BP responses to a mental arithmetic task were greater in diabetes than healthy groups, but it involved an inappropriate comparison group and did not use continuous measures of BP (14). Three studies have compared diurnal salivary cortisol profiles in people with and without type 2 diabetes: Two reported smaller cortisol responses to waking in diabetic participants (15, 16) and one a flatter slope over the day (17), but differences in cortisol output over the day have been inconsistent. We observed striking elevations in cortisol at baseline and in everyday life in people with type 2 diabetes, and elevated cortisol has been implicated in the development of the metabolic syndrome (18). Heightened cortisol output is also thought to be involved in the development of obesity, but we controlled statistically for BMI in these analyses. Blunted cortisol stress reactivity may have a permissive effect on IL-6 in type 2 diabetes. The high IL-6 concentration is likely related to the role of inflammation in type 2 diabetes, promoting insulin resistance and dyslipidemia by inhibiting enzymes involved in fatty acid oxidation, down-regulating the expression of genes involved in insulin-stimulated glucose transport and lipid uptake in adipocytes (19, 20).
Apart from heightened dynamic allostatic load, one alternative explanation for our results is that people with diabetes were less stressed by the behavioral challenges, so they produced smaller biological responses; however, the subjective ratings indicate that the diabetes and control groups were stressed to the same extent by the tasks. A second possibility is that effects were confounded by the multiple medications used to control diabetes. These medications may have contributed to the low baseline levels of diastolic BP and total cholesterol observed in the diabetes group (Figs. 1 and 2), but we took account statistically of medications that had associations with biological stress responses. Third, it is conceivable that the differences in BP and heart rate were early manifestations of neuropathy in people with diabetes, although none had clinical signs of cardiac autonomic neuropathy (21).
Our observation that depressive symptoms were elevated in people with type 2 diabetes replicates previous research (6) and is important in light of the evidence that depression in diabetic populations is associated with accelerated cognitive decline (22) and increased mortality risk (23). It is interesting that all of the psychosocial measures except for caregiving indicated greater stress among people with diabetes than controls. The result is a profile of psychosocial adversity in the type 2 diabetes group likely to promote heightened allostatic load.
Measures of allostatic load have been criticized for bringing together an arbitrary set of biomarkers, assuming that extreme values load on an underlying unitary construct. However, recent factor analytic studies indicate that a single common factor underlies variation across autonomic, neuroendocrine, inflammatory, and metabolic processes (13, 24). The intercorrelations between responses in the different biological systems, and between biological responses and psychosocial factors, provide further support for the value of this approach.
The study was cross-sectional, so no causal conclusions can be drawn. It is possible that heightened allostatic load precedes the development of type 2 diabetes and is a mechanism through which psychosocial factors contribute to diabetes risk. There are direct effects of inflammation on β cells (1), and heightened inflammation predicts diabetes onset (25). Autonomic and glucocorticoid regulation are also implicated in central fat deposition and the metabolic syndrome (26, 27), so they may contribute to the development of diabetes. Studies of heightened dynamic allostatic load in people with insulin resistance but no diabetes would throw light of this pathway. The alternative is that allostatic load is a manifestation of diabetes that is secondary to the abnormalities of glucose metabolism. The cardiovascular, neuroendocrine, and inflammatory responses we observed may be significant for the broader health consequences of diabetes. Disturbances of cortisol regulation are apparent in coronary heart disease and depression (28, 29), and it has been argued that glucocorticoids may contribute to the development of cognitive impairment in people with diabetes (30). Chronic systemic inflammation is also involved in cardiovascular disease, dementia, and depression (31, 32). Our results are consistent with the possibility that some of the comorbidities of type 2 diabetes arise from the disruption of the multiple systems involved in allostatic load rather than being direct consequences of impaired glucose regulation.
The dynamic aspects of multisystem dysregulation in allostatic load have not been studied prospectively in relation to long-term health outcomes. However, other measures of allostatic load predict future mortality and functional decline in older people (9). Individual components such as reduced cardiovascular poststress recovery, blunted stress reactivity, and increased IL-6 during acute stress are also associated with future adverse health outcomes (33).
In addition to the cross-sectional design, other limitations of this study are that the diabetes group was more ethnically diverse than the control group, and that the assessment of stress responses was carried out over a single session. Further, we did not measure glucose and insulin responses across the mental stress session. Nonetheless, our observations provide fresh evidence to link epidemiological studies implicating stress-related processes with biological dysfunction in type 2 diabetes. The patterns of cardiovascular and cholesterol responses exemplify the disturbances of reactivity and recovery noted in McEwen’s model of allostatic load (8). It has been posited that high allostatic load leads to prolonged responses due to delayed shutdown of physiological reactivity, so poststress recovery is impeded. Blunted reactivity may also occur, resulting in compensatory hyperactivity in other mediating pathways. The allostatic load concept synthesizes diverse findings concerning stress-related dysregulation across cardiovascular, neuroendocrine, metabolic, and inflammatory systems. Our findings highlight the importance of moving beyond glucose regulation to address a range of disturbances across multiple systems. Although individual pathways can be targeted by pharmacotherapy, the allostatic approach implies that systems interact in a dynamic fashion. Interventions that affect both brain and body are likely to be particularly beneficial. Two promising candidates are physical activity and social integration, both of which are implicated in diabetes (34). Development of the allostatic approach to type 2 diabetes may open new avenues for pharmacological and social-behavioral approaches to management and prevention.
Materials and Methods
Participants.
One hundred and forty individuals with type 2 diabetes were recruited from diabetic outpatient and primary care clinics in the London area with an invitation to take part in an investigation of how people with type 2 diabetes respond to stress. We limited enrollment to patients without a history or previous diagnosis of coronary heart disease (CHD), inflammatory diseases, allergies or mood disorders, and no evidence of autonomic neuropathy. Respondents included 88 men and 52 women aged 50–75 y.
The healthy controls in this analysis came from the Heart Scan Study, a subsample of the Whitehall II epidemiological cohort recruited between 2006 and 2008 to investigate socioeconomic and psychosocial factors, physiological stress responsivity, and subclinical coronary artery disease (35). Volunteers were invited to take part in a study of how people respond to stress, and a sample of 543 men and women of white European origin with no history or objective signs of CHD, no previous diagnosis or treatment for hypertension, diabetes, inflammatory diseases, or allergies was recruited. The absence of diabetes was confirmed by low HbA1c levels (≤6.5%, or 48 mmol/mol) and negative oral glucose tolerance tests over the previous 20 y. We matched every person with diabetes as closely as possible by age, sex, and income category with two healthy controls, so the 140 diabetic respondents were compared with 280 nondiabetic individuals. All participants gave full informed consent, and ethical approval was obtained from the National Research Ethics Service.
Mental Stress Testing.
We tested participants individually either in the morning or afternoon. BP and heart rate were monitored continuously by using a Finometer, and a venous cannula was inserted for blood collection. Baseline blood and saliva samples were collected, and subjective stress ratings were obtained on a seven-point scale from 1 = no stress to 7 = maximum stress. We then administered two 5-min behavioral tasks in random order. These were a computerized version of the Stroop color-word interference task and a mirror drawing task as described (35). These tasks were originally designed as tests of cognitive function, but were adapted to provide moderately stressful behavioral challenges. Subjective stress, blood, and saliva samples were taken immediately after tasks. The posttask recovery period lasted 75 min, with further saliva samples at 20, 45, and 75 min and additional blood samples and stress ratings at 45 and 75 min. Blood samples were centrifuged immediately at 400 × g for 10 min at room temperature and plasma was separated and stored at −80 °C until analysis.
Cortisol Output over the Day.
Participants collected five saliva samples over a single day by using Salivettes (Sarstedt), at waking, 30 min later, and then within three 30-min time windows in the morning (1000–1030), afternoon (1600–1630) and evening (2000–2030). They were told not to eat, drink tea or coffee, or smoke in the 30 min before each sample was collected. Violations of the protocol and sample timing were recorded in a log. Satisfactory data were obtained from 119 diabetic and 236 nondiabetic participants.
Biological Measures.
Plasma IL-6 was assayed by using a Quantikine high sensitivity two-site ELISA (R&D Systems). The sensitivity of the assay ranged from 0.016 to 0.110 pg/mL, and the intraassay and interassay coefficient of variations was 7.3% and 7.7% respectively. Cortisol was assessed from saliva samples by using a time resolved immunoassay with fluorescence detection at the University of Dresden, Germany. Total cholesterol was measured in a centrifugal analyzer by enzymatic colorimetric methods. Plasma IL-6 was assayed from all four blood samples, HbA1c from the baseline sample alone, and total cholesterol from the baseline, task, and 45 min posttask samples.
Psychological Measures.
We measured two stress-related psychological characteristics, depressive symptoms and hostility, using the Center for Epidemiologic Studies Depression Scale and the 10-item Cook–Medley cynical hostility scale, respectively. Stress exposure was assessed in terms of financial strain and neighborhood social cohesion with a measure of collective efficacy (36). We also noted whether the participant was a caregiver for an elderly or disabled relative or friend. Participants rated their sense of control over their lives in general by using a six-point scale from 1 = very low to 6 = very high, whereas social support and optimism were assessed with standardized measures (37, 38). The Cronbach α was satisfactory for all psychological measures (>0.75).
Other Measures.
Participants reported household income in bands that were subsequently reduced to lower (<£40,000) and higher (≥£40,000) income categories. Education was categorized into less than high school, high school or equivalent, and college or higher. Medication in the diabetes group was categorized into oral diabetic medication (metformin), insulin and other injected diabetic medication, aspirin, beta blockers, other hypertensive medication (ACE inhibitors, calcium channel blockers), and statins.
Statistical Analysis.
We computed mean systolic BP, diastolic BP, and heart rate for five periods: baseline (the last 5 min of the 30-min baseline period), the two task trials combined, and poststress recovery minutes 40–45 and 70–75. We conducted repeated measures analysis of variance to assess patterns of change across the session, with group and sex as between-person factors and trial as the within-person factor. Because this study was a matched case-control design, analysis of variance and general estimating equation models were not appropriate for comparisons between groups. Instead, we analyzed differences between groups in stress reactivity and stress recovery by using conditional logistic regression, which takes account of the matched case-control design (39). We computed difference scores between tasks and baseline (for stress reactivity) and differences between tasks and recovery measures (for recovery analyses), and entered these along with covariates into regressions on group (diabetes or control) membership. Results are presented as adjusted odds of being in the diabetes as opposed to the control group per unit change in the predictor variable, with 95% confidence intervals (95% CI). A value >1 indicates that larger values of the predictor are associated with increased odds of being in the diabetes group, while values <1 indicate that larger values are associated with reduced odds of being in the diabetes group. Stress responses in IL-6 are delayed in comparison with other measures, so we assessed reactivity as differences between values recorded at 45 min and 75 min compared with baseline. Poststress recovery in cardiovascular measures, cortisol, and cholesterol was measured by using difference scores between task and recovery period means. We log transformed cortisol and IL-6 before analysis because of positive skews in the distribution, and geometric means are presented in Results. Cortisol output over the day was quantified as area under the curve (40). There were 28 (20%) ethnic minority participants in the diabetes group, but adding ethnicity as a factor to the analyses did not alter the pattern of results, so it is not included in the models described here.
All analyses of physiological data were adjusted for BMI and smoking status, because these variables are known to influence stress responses. Time of day of stress testing was included as a covariate, because profiles of response to stress may vary across the day. We also adjusted for education and marital status because of differences between groups, because these factors might affect stress responsivity. We selected medication covariates by testing for associations between medication status and stress responses within the diabetes group. Thus, BP and HR analyses were additionally adjusted for use of beta blockers in addition to education, marital status, BMI, and smoking; cortisol analyses for statins; and cholesterol responses for use of statins and aspirin. Psychological differences between groups were analyzed by using conditional logistic regression adjusted for education and marital status. We also explored interrelationships between cardiovascular and other biological responses, and between biological responses and psychological factors, by computing product–moment correlations.
Supplementary Material
Acknowledgments
This study was supported by the British Heart Foundation Grant RG/10/05/28296.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1410401111/-/DCSupplemental.
References
- 1.Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: Perspectives on the past, present, and future. Lancet. 2014;383(9922):1068–1083. doi: 10.1016/S0140-6736(13)62154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5(7):374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- 3.Stringhini S, et al. Health behaviours, socioeconomic status, and mortality: Further analyses of the British Whitehall II and the French GAZEL prospective cohorts. PLoS Med. 2011;8(2):e1000419. doi: 10.1371/journal.pmed.1000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Novak M, et al. Perceived stress and incidence of Type 2 diabetes: A 35-year follow-up study of middle-aged Swedish men. Diabet Med. 2013;30(1):e8–e16. doi: 10.1111/dme.12037. [DOI] [PubMed] [Google Scholar]
- 5.Nyberg ST, et al. IPD-Work Consortium Job strain as a risk factor for type 2 diabetes: A pooled analysis of 124,808 men and women. Diabetes Care. 2014;37(8):2268–2275. doi: 10.2337/dc13-2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golden SH, et al. Examining a bidirectional association between depressive symptoms and diabetes. JAMA. 2008;299(23):2751–2759. doi: 10.1001/jama.299.23.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hempler NF, Ekholm O, Willaing I. Differences in social relations between persons with type 2 diabetes and the general population. Scand J Public Health. 2013;41(4):340–343. doi: 10.1177/1403494813482535. [DOI] [PubMed] [Google Scholar]
- 8.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338(3):171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 9.Gruenewald TL, Seeman TE, Ryff CD, Karlamangla AS, Singer BH. Combinations of biomarkers predictive of later life mortality. Proc Natl Acad Sci USA. 2006;103(38):14158–14163. doi: 10.1073/pnas.0606215103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci USA. 2001;98(8):4770–4775. doi: 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlsson AC, Nixon Andreasson A, Wändell PE. Poor self-rated health is not associated with a high total allostatic load in type 2 diabetic patients—but high blood pressure is. Diabetes Metab. 2011;37(5):446–451. doi: 10.1016/j.diabet.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Mattei J, Demissie S, Falcon LM, Ordovas JM, Tucker K. Allostatic load is associated with chronic conditions in the Boston Puerto Rican Health Study. Soc Sci Med. 2010;70(12):1988–1996. doi: 10.1016/j.socscimed.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCaffery JM, Marsland AL, Strohacker K, Muldoon MF, Manuck SB. Factor structure underlying components of allostatic load. PLoS ONE. 2012;7(10):e47246. doi: 10.1371/journal.pone.0047246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deepak A, Deepak AN, Nallulwar S, Khode V. Time domain measures of heart rate variability during acute mental stress in Type 2 diabetics- A case control study. Nat J Physiol Pharmacy Pharmacol. 2014;4(1):34–38. [Google Scholar]
- 15.Bruehl H, Wolf OT, Convit A. A blunted cortisol awakening response and hippocampal atrophy in type 2 diabetes mellitus. Psychoneuroendocrinology. 2009;34(6):815–821. doi: 10.1016/j.psyneuen.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Champaneri S, et al. Diurnal salivary cortisol and urinary catecholamines are associated with diabetes mellitus: The Multi-Ethnic Study of Atherosclerosis. Metabolism. 2012;61(7):986–995. doi: 10.1016/j.metabol.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lederbogen F, et al. Flattened circadian cortisol rhythm in type 2 diabetes. Exp Clin Endocrinol Diabetes. 2011;119(9):573–575. doi: 10.1055/s-0031-1275288. [DOI] [PubMed] [Google Scholar]
- 18.Anagnostis P, Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP. Clinical review: The pathogenetic role of cortisol in the metabolic syndrome: A hypothesis. J Clin Endocrinol Metab. 2009;94(8):2692–2701. doi: 10.1210/jc.2009-0370. [DOI] [PubMed] [Google Scholar]
- 19.Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27(3):813–823. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- 20.Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol Med. 2008;14(3-4):222–231. doi: 10.2119/2007-00119.Tilg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pop-Busui R. Cardiac autonomic neuropathy in diabetes: A clinical perspective. Diabetes Care. 2010;33(2):434–441. doi: 10.2337/dc09-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sullivan MD, et al. Association of depression with accelerated cognitive decline among patients with type 2 diabetes in the ACCORD-MIND trial. JAMA Psychiatry. 2013;70(10):1041–1047. doi: 10.1001/jamapsychiatry.2013.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park M, Katon WJ, Wolf FM. Depression and risk of mortality in individuals with diabetes: A meta-analysis and systematic review. Gen Hosp Psychiatry. 2013;35(3):217–225. doi: 10.1016/j.genhosppsych.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Booth T, Starr JM, Deary I. Modeling multisystem biological risk in later life: Allostatic load in the Lothian birth cohort study 1936. Am J Hum Biol. 2013;25(4):538–543. doi: 10.1002/ajhb.22406. [DOI] [PubMed] [Google Scholar]
- 25.Carstensen M, et al. Accelerated increase in serum interleukin-1 receptor antagonist starts 6 years before diagnosis of type 2 diabetes: Whitehall II prospective cohort study. Diabetes. 2010;59(5):1222–1227. doi: 10.2337/db09-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asensio C, Muzzin P, Rohner-Jeanrenaud F. Role of glucocorticoids in the physiopathology of excessive fat deposition and insulin resistance. Int J Obes Relat Metab Disord. 2004;28(Suppl 4):S45–S52. doi: 10.1038/sj.ijo.0802856. [DOI] [PubMed] [Google Scholar]
- 27.Licht CM, et al. Increased sympathetic and decreased parasympathetic activity rather than changes in hypothalamic-pituitary-adrenal axis activity is associated with metabolic abnormalities. J Clin Endocrinol Metab. 2010;95(5):2458–2466. doi: 10.1210/jc.2009-2801. [DOI] [PubMed] [Google Scholar]
- 28.Brotman DJ, Golden SH, Wittstein IS. The cardiovascular toll of stress. Lancet. 2007;370(9592):1089–1100. doi: 10.1016/S0140-6736(07)61305-1. [DOI] [PubMed] [Google Scholar]
- 29.Stetler C, Miller GE. Depression and hypothalamic-pituitary-adrenal activation: A quantitative summary of four decades of research. Psychosom Med. 2011;73(2):114–126. doi: 10.1097/PSY.0b013e31820ad12b. [DOI] [PubMed] [Google Scholar]
- 30.Strachan MW, Reynolds RM, Frier BM, Mitchell RJ, Price JF. The role of metabolic derangements and glucocorticoid excess in the aetiology of cognitive impairment in type 2 diabetes. Implications for future therapeutic strategies. Diabetes Obes Metab. 2009;11(5):407–414. doi: 10.1111/j.1463-1326.2008.00963.x. [DOI] [PubMed] [Google Scholar]
- 31.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12(3):204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 32.Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychol Bull. 2014;140(3):774–815. doi: 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: A meta-analysis of prospective evidence. Hypertension. 2010;55(4):1026–1032. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- 34.Sluik D, et al. Physical activity and mortality in individuals with diabetes mellitus: A prospective study and meta-analysis. Arch Intern Med. 2012;172(17):1285–1295. doi: 10.1001/archinternmed.2012.3130. [DOI] [PubMed] [Google Scholar]
- 35.Hamer M, O’Donnell K, Lahiri A, Steptoe A. Salivary cortisol responses to mental stress are associated with coronary artery calcification in healthy men and women. Eur Heart J. 2010;31(4):424–429. doi: 10.1093/eurheartj/ehp386. [DOI] [PubMed] [Google Scholar]
- 36.Sampson RJ, Raudenbush SW, Earls F. Neighborhoods and violent crime: A multilevel study of collective efficacy. Science. 1997;277(5328):918–924. doi: 10.1126/science.277.5328.918. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell PH, et al. A short social support measure for patients recovering from myocardial infarction: The ENRICHD Social Support Inventory. J Cardiopulm Rehabil. 2003;23(6):398–403. doi: 10.1097/00008483-200311000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): A reevaluation of the Life Orientation Test. J Pers Soc Psychol. 1994;67(6):1063–1078. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- 39.Elwood M. Critical Appraisal of Epidemiological Studies and Clinical Trials. 3rd Ed Oxford Univ Press; Oxford: 2007. [Google Scholar]
- 40.Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.