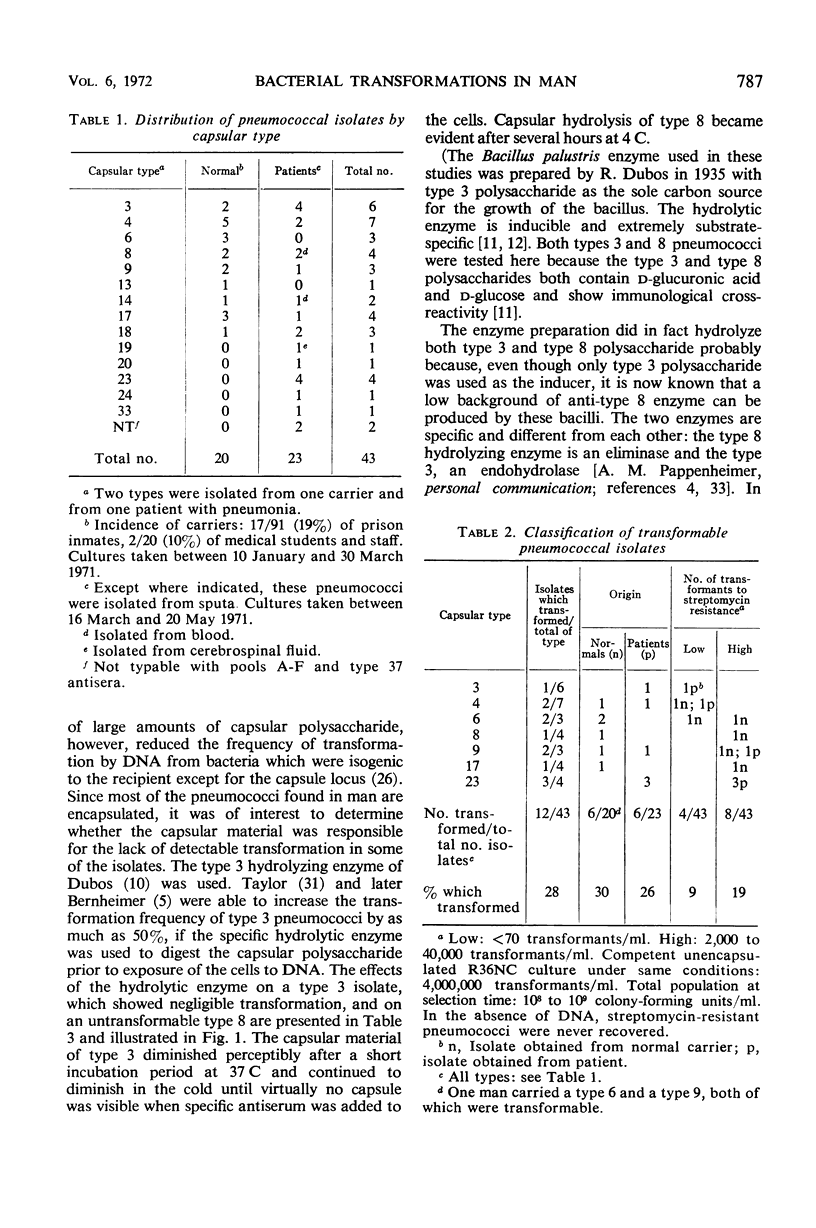
Abstract
A survey of pneumococci isolated from 19 healthy carriers and from 23 patients with pneumococcal disease showed that, for both groups, 25 to 30% of the isolates were competent for transformation by soluble deoxyribonucleic acid (DNA) in vitro. Untransformable type 3 and type 8 pneumococci, whose capsules had been hydrolyzed by the Bacillus palustris enzymes prior to exposure to DNA, remained untransformable. Thus, at least for these isolates, it was not the presence of capsule that prevented transformation. Type 9 pneumococci in a healthy human carrier were transformed by DNA released from living unencapsulated pneumococci sprayed onto the pharynx. The donor bacteria were resistant to 1,000 μg of streptomycin/ml. Two types of streptomycin-resistant bacteria were recovered from the carrier's pharynx: a type 9 pneumococcus and an alpha-hemolytic streptococcus. No streptomycin-resistant, gram-positive cocci were isolated from this individual prior to inoculation of the pharynx with the resistant organisms. It seems possible that transformations can occur in the natural environment of some gram-positive cocci.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUSTRIAN R. Observations on the possible role of nucleic acid exchange reactions in pneumococcal capsular type transformation; a preliminary note. Bull Johns Hopkins Hosp. 1952 Feb;90(2):170–174. [PubMed] [Google Scholar]
- Auerbach-Rubin F., Ottolenghi-Nightingale E. Effect of ethanol on the clearance of airborne pneumococci and the rate of pneumococcal transformations in the lung. Infect Immun. 1971 May;3(5):688–693. doi: 10.1128/iai.3.5.688-693.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRACCO R. M., KRAUSS M. R., ROE A. S., MACLEOD C. M. Transformation reactions between Pneumococcus and three strains of Streptococci. J Exp Med. 1957 Aug 1;106(2):247–259. doi: 10.1084/jem.106.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker G. E., Pappenheimer A. M., Jr Lyase activity of inducible S8-depolymerases from Bacillus palustris. Biochim Biophys Acta. 1966 Jun 29;121(2):343–348. doi: 10.1016/0304-4165(66)90123-1. [DOI] [PubMed] [Google Scholar]
- Bernheimer H. P., Wermundsen I. E. Unstable binary capsulated transformants in pneumococcus. J Bacteriol. 1969 Jun;98(3):1073–1079. doi: 10.1128/jb.98.3.1073-1079.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CATLIN B. W. Transformation of Neisseria meningitidis by deoxyribonucleates from cells and from culture slime. J Bacteriol. 1960 Apr;79:579–590. doi: 10.1128/jb.79.4.579-590.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conant J. E., Sawyer W. D. Transformation during mixed pneumococcal infection of mice. J Bacteriol. 1967 Jun;93(6):1869–1875. doi: 10.1128/jb.93.6.1869-1875.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. N., Sheehe P. R., Feldman H. A. Pharyngeal pneumococcal acquisitions in "normal" families: a longitudinal study. J Infect Dis. 1971 Jul;124(1):9–17. doi: 10.1093/infdis/124.1.9. [DOI] [PubMed] [Google Scholar]
- Ephrati-Elizur E. Spontaneous transformation in Bacillus subtilis. Genet Res. 1968 Feb;11(1):83–96. doi: 10.1017/s0016672300011216. [DOI] [PubMed] [Google Scholar]
- HOTCHKISS R. D. Transfer of penicillin resistance in pneumococci by the desoxyribonucleate derived from resistant cultures. Cold Spring Harb Symp Quant Biol. 1951;16:457–461. doi: 10.1101/sqb.1951.016.01.032. [DOI] [PubMed] [Google Scholar]
- Johanson W. G., Jr, Blackstock R., Pierce A. K., Sanford J. P. The role of bacterial antagonism in pneumococcal colonization of the human pharynx. J Lab Clin Med. 1970 Jun;75(6):946–952. [PubMed] [Google Scholar]
- OTTOLENGHI E., HOTCHKISS R. D. Appearance of genetic transforming activity in pneumococcal cultures. Science. 1960 Oct 28;132(3435):1257–1258. [PubMed] [Google Scholar]
- OTTOLENGHI E., HOTCHKISS R. D. Release of genetic transforming agent from pneumococcal cultures during growth and disintegration. J Exp Med. 1962 Oct 1;116:491–519. doi: 10.1084/jem.116.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OTTOLENGHI E., MACLEOD C. M. GENETIC TRANSFORMATION AMONG LIVING PNEUMOCOCCI IN THE MOUSE. Proc Natl Acad Sci U S A. 1963 Sep;50:417–419. doi: 10.1073/pnas.50.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottolenghi-Nightingale E. Spontaneously occurring bacterial transformations in mice. J Bacteriol. 1969 Oct;100(1):445–452. doi: 10.1128/jb.100.1.445-452.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAVIN A. W. Reciprocal capsular transformations of pneumococci. J Bacteriol. 1959 Mar;77(3):296–309. doi: 10.1128/jb.77.3.296-309.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROLFE R., EPHRUSSI-TAYLOR H. Density differences between genetic markers in Pneumococcal transforming principle. Proc Natl Acad Sci U S A. 1961 Sep 15;47:1450–1461. doi: 10.1073/pnas.47.9.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. P., Iyer V. N. Competence for genetic transformation and the release of DNA from Bacillus subtilis. Biochim Biophys Acta. 1971 Feb 25;232(1):61–71. doi: 10.1016/0005-2787(71)90491-6. [DOI] [PubMed] [Google Scholar]
- Suhs R. H., Feldman H. A. Pneumococcal types detected in throat cultures from a population of "normal" families. Am J Med Sci. 1965 Oct;250(4):424–427. doi: 10.1097/00000441-196510000-00008. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI I. Genetic transformation of Bacillus subtilis by extracellular DNA. Biochem Biophys Res Commun. 1962 Jun 4;7:467–470. doi: 10.1016/0006-291x(62)90337-6. [DOI] [PubMed] [Google Scholar]
- TORRIANI A., PAPPENHEIMER A. M., Jr Inducible polysaccharide depolymerases of Bacillus palustris. J Biol Chem. 1962 Jan;237:3–13. [PubMed] [Google Scholar]