Significance
The work reported in this paper describes a previously unknown signaling pathway in skeletal muscle acting through G protein-coupled receptor 56-Galpha12/13. This discovery elucidates a previously unknown mechanism of muscle anabolism and gives another target of investigation for therapies against the loss of muscle mass seen with aging and various wasting diseases.
Keywords: GPR56, mTOR, Gα12/13, muscle hypertrophy, overload
Abstract
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha 4 (PGC-1α4) is a protein isoform derived by alternative splicing of the PGC1α mRNA and has been shown to promote muscle hypertrophy. We show here that G protein-coupled receptor 56 (GPR56) is a transcriptional target of PGC-1α4 and is induced in humans by resistance exercise. Furthermore, the anabolic effects of PGC-1α4 in cultured murine muscle cells are dependent on GPR56 signaling, because knockdown of GPR56 attenuates PGC-1α4–induced muscle hypertrophy in vitro. Forced expression of GPR56 results in myotube hypertrophy through the expression of insulin-like growth factor 1, which is dependent on Gα12/13 signaling. A murine model of overload-induced muscle hypertrophy is associated with increased expression of both GPR56 and its ligand collagen type III, whereas genetic ablation of GPR56 expression attenuates overload-induced muscle hypertrophy and associated anabolic signaling. These data illustrate a signaling pathway through GPR56 which regulates muscle hypertrophy associated with resistance/loading-type exercise.
Skeletal muscle is an extremely plastic tissue capable of responding to multiple stimuli. Mechanical tension is a potent regulator of muscle mass. Extensive unloading caused by immobilization or space flight results in rapid muscle atrophy that typically is reversible upon reloading (1). In contrast, mechanical overloading can result in muscle hypertrophy commonly observed in human resistance-type training (2) or in rodent models of chronic mechanical overload (3). Furthermore, resistance training is effective in preserving muscle mass in aged and diseased individuals in whom muscle atrophy is common (4). Therefore, understanding the molecular mechanisms that regulate the anabolic effects of mechanical load-induced hypertrophy has great translational importance.
Our laboratory recently has discovered a splice isoform of the peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) gene, referred to as “PGC-1α4,” whose encoded protein induces muscle hypertrophy through the induction of insulin-like growth factor 1 (IGF1) and suppression of myostatin (5). Because of the potent hypertrophic effects of PGC-1α4, we aimed to investigate mediators of muscle hypertrophy revealed by the PGC-1α4 program. Here we report that the G protein-coupled receptor 56 (GPR56) is a transcriptional target of PGC-1α4 and regulates muscle anabolism. GPR56 is a member of the adhesion G protein-coupled receptor (GPCR) family which is characterized by a large extracellular N terminus and a GPCR proteolysis-inducing (GAIN) domain (6, 7). Most adhesion GPCRs undergo GAIN domain-mediated autoproteolytic process at the GPCR proteolysis site to generate an N- and a C-terminal fragment (6, 7). In addition, GPR56 has been shown to signal through Gα12/13 and to activate Rho (8, 9). Gα13 signaling has been shown to regulate pressure overload-induced cardiac hypertrophy (10). However, its role as a skeletal muscle mechanoreceptor is currently unknown.
Molecular signaling events during mechanical transduction are just starting to be understood. The mammalian target of rapamycin (mTOR) appears to have a central role in regulating overload-induced muscle hypertrophy (1). Mechanical overload in both mouse (11) and humans (12) increases mTOR phosphorylation and its downstream target p70S6K. The upstream activators of mTOR during mechanical overload are currently unclear. IGF1 expression during mechanical overload was a natural candidate for the increased PI3K/mTOR signaling; however, muscles lacking the IGF1 receptor are able to hypertrophy following mechanical overload (13). The link between GPCRs and mTOR activation in skeletal muscle is currently unknown and could elucidate novel mechanisms of signal transduction. Although Gαi signaling in muscle recently has been shown to regulate hypertrophy (14, 15), the association between Gα12/13 signaling and mTOR activation has yet to be investigated, especially as a mechanosensitive pathway.
Although mechanical overload is a well-established hypertrophic stimulus, the understanding of associated molecular signaling is still unclear. Here we describe the role of GPR56 in skeletal muscle mechanotransduction. GPR56 appears to be a mediator of the hypertrophic effects of PGC-1α4 and can regulate muscle anabolism that is independent of PGC-1α4.
Results
GPR56 Is a Transcriptional Target of the PGC-1α4 Isoform.
Gene expression array analysis was done previously to determine gene transcripts with possible regulatory functions driven by the PGC-1α4 isoform (5). In particular, we noticed mRNA for an adhesion GPCR, referred to as “GPR56,” as being induced by PGC-1α4. Quantitative PCR (qPCR) analyses of mRNA from primary myotubes showed that GPR56 is increased roughly twofold by forced PGC-1α4 expression; interestingly, forced expression of the canonical full-length PGC-1α1 did not induce GPR56 mRNA (Fig. 1A). In addition, a similar change in protein expression of GPR56 was observed with forced PGC-1α4 expression in primary myotubes (Fig. 1B and Fig. S1A).
Fig. 1.
PGC-1α4 drives expression of GPR56 and Col3a1 in primary myotubes. (A and B) GPR56 mRNA (A) and protein (B) expression with adenoviral expression of GFP, PGC-1α1, or PGC-1α4 in primary myotubes. (C and D) GPR56 mRNA (C) and protein (D) expression in muscle from wild-type, Myo PGC-1α4 (−/+), and Myo PGC-1α4 (+/+) mice. (E) Col3a1 mRNA expression with adenoviral expression of GFP, PGC-1α1, or PGC-1α4 in primary myotubes. (F) Peptide expression of collagen III peptides in the media of primary myotubes expressing GFP or PGC-1α4. (G) GPR56 and Col3a1 mRNA expression after intramuscular injection of PGC-1α4 adenovirus or LacZ control. *Denotes significant difference from GFP or wild-type mice. #Denotes significant difference from Myo PGC-1α4 (−/+) mice. Data are presented as mean + SE (n = 3 or 4 per group).
We previously generated a muscle-specific PGC-1α4 transgenic mouse (Myo PGC-1α4) (5). Gene dosage can be controlled by mating to produce a graded expression of muscle PGC-1α4 in these mice (Fig. S1B). This graded expression of muscle PGC-1α4 mRNA results in a 91% increase in GPR56 mRNA in Myo-PGC-1α4 heterozygotes and a 276% increase in Myo-PGC-1α4 homozygotes (Fig. 1C). The increase in GPR56 mRNA in Myo PGC-1α4 mice also was reflected at the level of protein expression (Fig. 1D and Fig. S1C). Collagen III, recognized as a ligand of GPR56 (8), also is increased roughly twofold, specifically by the PGC-1α4 isoform (Fig. 1E). Moreover, secreted proteins in the medium of primary myotubes expressing PGC-1α4 were examined by mass spectrometry; we found a fourfold increase in secreted collagen type III (collagen III) protein as compared with cells expressing a GFP control mRNA (Fig. 1F). To investigate acute overexpression of PGC-1α4 in vivo, adenoviral vectors expressing PGC-1α4 were injected into the quadriceps muscle and incubated for 3 d. This procedure resulted in a nearly twofold increase in GPR56 mRNA and a nearly eightfold increase in the mRNA for its ligand, collagen type III alpha 1 (Col3a1) (Fig. 1G).
GPR56 Signaling Is Required for the Full PGC-1α4–Induced Hypertrophic Response in Primary Myotubes.
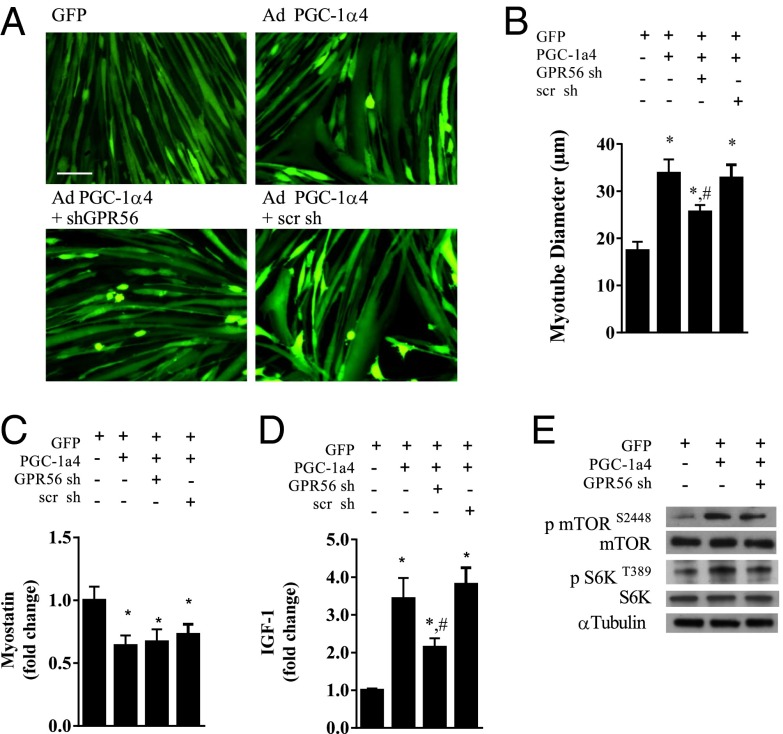
PGC-1α4 expression in primary myotubes results in myotube hypertrophy that is associated with an increased expression of IGF1 and a decreased expression of myostatin (5). To investigate the role of GPR56 in the PGC-1α4–induced hypertrophy, we generated an shRNA against GPR56 to suppress its expression. The shRNA against GPR56 was effective in suppressing mRNA (Fig. S2A) and protein expression (Fig. S2B) by roughly 80%, whereas a nonspecific scramble shRNA did not affect GPR56 mRNA expression. Furthermore, knockdown of GPR56 did not affect forced expression of PGC-1α4 or expression of collagen III (Fig. S2C). We show in Fig. 2 A and B that PGC-1α4 expression increased myotube diameter by 100% as compared with the GFP control myotubes. Knockdown of GPR56 during forced PGC-1α4 expression reduced the hypertrophic response by more than half, leading to only a 41% increase (Fig. 2 A and B). PGC-1α4–induced myotube hypertrophy was not affected by simultaneous expression of the scramble shRNA control (Fig. 2 A and B). Although knockdown of GPR56 during forced PGC-1α4 expression did not affect the suppression of myostatin mRNA (Fig. 2C), it did attenuate IGF1 mRNA expression by 37% (Fig. 2D). Interestingly, phosphorylation of mTOR and S6K, a potent anabolic signaling pathway downstream of the IGF1 receptor, was increased by roughly fourfold with the forced PGC-1α4 expression. Knockdown of GPR56 reduced PGC-1a4-induced mTOR and S6K phosphorylation by roughly 50% of what was observed with a nonspecific scramble sh (Fig. 2E and Fig. S2 D and E).
Fig. 2.
GPR56 knockdown attenuates PGC-1α4–induced hypertrophy. (A) Myotube morphology with adenoviral (Ad) expression of PGC-1α4 with or without an shRNA against GPR56. (Scale bar, 50 μm.) scr sh, scramble short hairpin. (B–D) Myotube diameter (B), myostatin mRNA (C), and IGF1 mRNA (D) expression in myotubes with adenoviral forced expression of PGC-1α4 with or without a short hairpin to GPR56. (E) Representative Western blot of total and phosphorylated mTOR and S6K protein expression. *Denotes significant difference from GFP control myotubes. #Denotes significant difference from myotubes with forced expression of PGC-1α4. Data are presented as mean + SE (n = 3 per group).
GPR56 Signaling Drives Muscle Hypertrophy Downstream of the Gα12/13 Subunit.
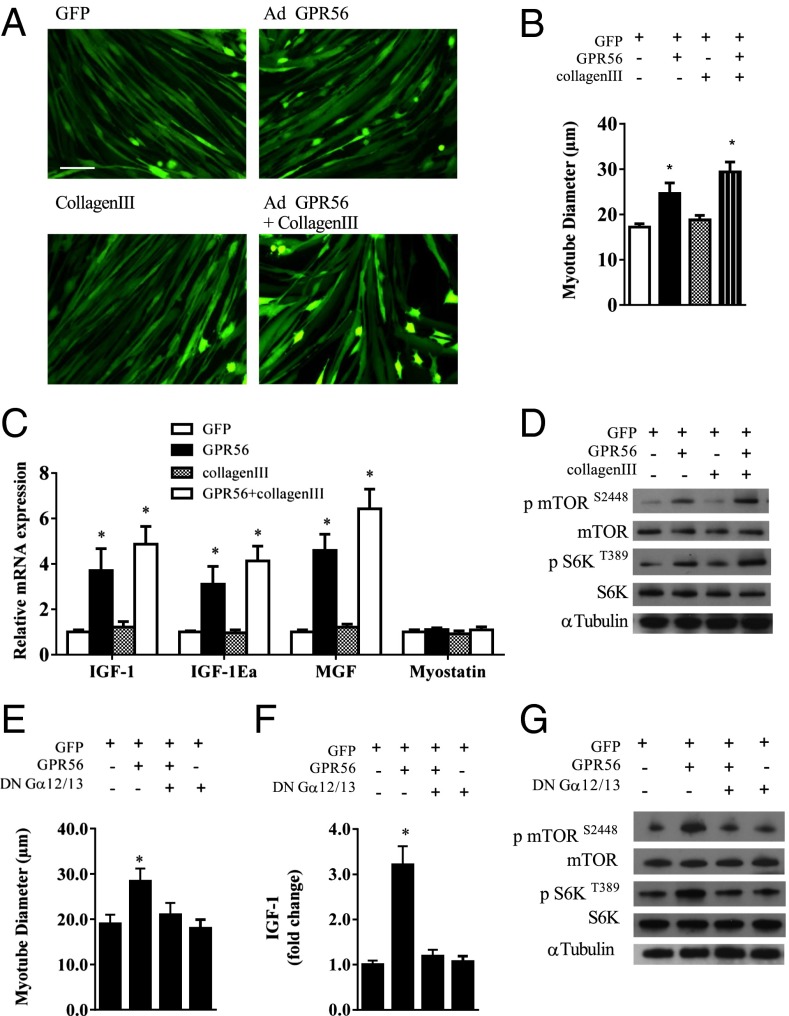
To investigate the biological function of GPR56 in skeletal muscle, adenoviral-mediated expression of GPR56 was used in primary myotubes with or without collagen III. Upon GPR56 expression there was a 43% change in myotube diameter as compared with the GFP control (Fig. 3 A and B). Collagen III treatment of GFP-expressing myotubes had no effect on myotube diameter. Forced expression of GPR56 along with collagen III treatment resulted in a 71% increase in myotube diameter as compared with myotubes infected with a GFP-expressing vector. No additive effect was seen when myotubes were treated with collagen III along with adenoviral expression of GPR56 (Fig. 3 A and B). Coinciding with the observed myotube hypertrophy, forced GPR56 expression induced an increase in mRNAs encoding IGF1, IGF-1Ea, and mechano growth factor (MGF) (Fig. 3C). Treatment with collagen III did not increase the expression of IGF1 or other IGF1 isoforms. The combination of collagen III with GPR56 expression resulted in no additional increase in IGF1 expression. mTOR and S6K phosphorylation were increased fourfold with GPR56 expression (Fig. 3D and Fig. S3 A and B). Although treatment with collagen III alone did not increase mTOR or S6K phosphorylation, the combination of collagen III treatment with GPR56 expression resulted in a synergistic increase in mTOR and S6K phosphorylation, i.e, a nearly 4.5-fold increase over the control GFP myotubes (Fig. 3D and Fig. S3 A and B).
Fig. 3.
Primary mouse myotubes expressing GPR56 show cellular hypertrophy. (A) Primary mouse myotubes infected with recombinant adenovirus expressing GFP alone with or without collagen III or expressing GPR56 with or without collagen III. (Scale bar, 50 μm.) (B and C) Myotube diameter (B) and IGF1 isoform and myostatin mRNA expression (C) in myotubes expressing GFP or GPR56 with or without collagen III. (D) Representative Western blots of phosphorylated and total mTOR and S6K protein expression in myotubes expressing GFP or GPR56 with or without collagen III. (E and F) Myotube diameter (E) and IGF1 mRNA (F) of myotubes expressing GFP and GPR56 with or without expression of a DN Gα12/13 subunit. (G) Representative Western blots of phosphorylated and total mTOR and S6K protein expression in myotubes expressing GFP and GPR56 with or without expression of a DN Gα12/13 subunit. *Denotes significant difference from GFP control myotubes. Data are presented as mean + SE (n = 3 per group).
GPR56 has been shown to signal through the Gα12/13–Rho pathway in neuronal tissue (8, 9). Currently, this pathway has not been described in skeletal muscle. We expressed a previously described dominant negative (DN) version of the Gα12/13 subunit (8) to inactivate this pathway during GPR56-induced myotube hypertrophy. Forced expression of GPR56 induced a 49% increase in myotube diameter, whereas the coexpression of the DN Gα12/13 completely prevented GPR56-induced myotube hypertrophy (Fig. 3E) and the induction of IGF1 (Fig. 3F). Furthermore, the DN Gα12/13 prevented the increase in mTOR and S6K phosphorylation (Fig. 3G and Fig. S3 C and D) associated with forced expression of GPR56. These data strongly suggest that Ga12/13 is involved in mediating the effects of GPR56 in this in vitro model of muscle hypertrophy.
GPR56-Induced Hypertrophy Is Independent of PGC-1α4 Expression.
PGC-1α4 is sufficient to drive expression of GPR56. We next investigated whether PGC-1α4 is necessary for GPR56-induced hypertrophy. Myotube hypertrophy was induced with both forced GPR56 expression and collagen III treatment in the presence of an shRNA against PGC-1α4 or its scramble shRNA control (Fig. S3 E and F). Equal myotube hypertrophy was evident during infection with either the shRNA against PGC-1α4 or the scramble shRNA. Moreover, the GPR56-induced IGF1 expression was not affected by the knockdown of PGC-1α4 (Fig. S3G). This finding suggests that, although GPR56 expression can be driven by PGC1α4, GPR56-induced hypertrophy can occur independently of PGC-1α4 expression.
GPR56 Expression Is Increased During Models of Muscle Hypertrophy.
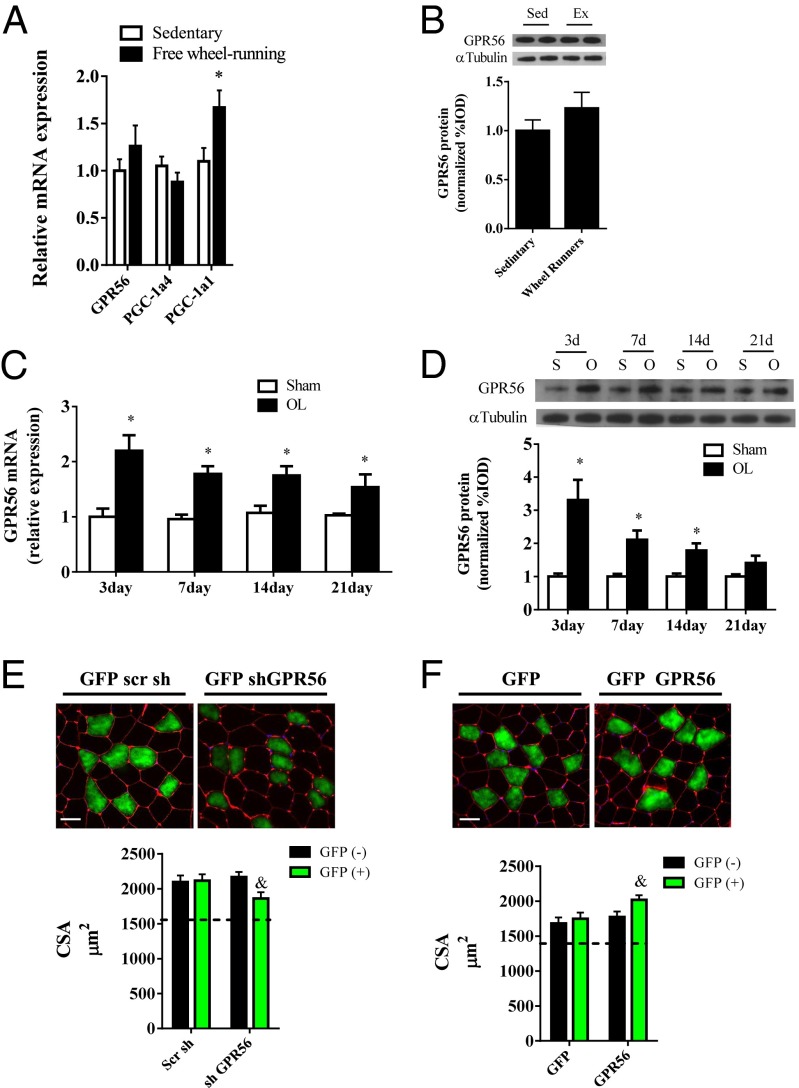
We next explored the role of GPR56 in vivo in physiological models of exercise and muscle hypertrophy. Thirty days of voluntary wheel running had no effect on GPR56 or PGC-1α4 expression but did increase PGC-1α1 by 67% (Fig. 4A). As with mRNA expression, there was no difference in GPR56 protein expression with wheel running (Fig. 4B). We then measured GPR56 expression in the synergistic ablation model, an established model of muscle hypertrophy. The ablation procedure resulted in muscle hypertrophy through the 21-d time course of compensatory muscle overload (Fig. S4A). In addition, we observed an increase in muscle IGF1 mRNA, whereas PGC-1α4 mRNA was reduced during the early weeks of overload (Fig. S4 B and C). In contrast to the wheel running, the synergistic ablation model of hypertrophy increased GPR56 (Fig. 4C) and Col3a1 mRNA (Fig. S4D) expression throughout the time course of overload. In addition, mRNA expression of the Gα12 and Gα13 subunits was increased 50% at day 7 of overload (Fig. S4 E and F). GPR56 protein expression increased throughout the first 2 wk of overload (Fig. 4D). We then used electroporation-based gene transfer to introduce an shRNA against GPR56 into the plantaris muscle to determine whether the knockdown of GPR56 during the 21 d of overload would affect the hypertrophic response. After 21 d of overload, myofibers positive for the shGPR56 (GFP+) showed a 14% reduction in myofiber cross-sectional area as compared with nontransfected (GFP−) myofibers. The cross-sectional area of the myofibers taking up the scramble shRNA was not different from that of the nontransfected fibers (Fig. 4E). In a different experiment using the ablation model, we electroporated a GFP-labeled plasmid expressing GPR56 into the plantaris muscle. For this experiment the muscle was overloaded for 10 d to observe the muscle in a rapid growth phase. GFP+ myofibers showed a 13% increase in myofiber cross-sectional area as compared with nontransfected myofibers, suggesting an enhanced rate of hypertrophy with GPR56 gain of function (Fig. 4F). Transfection of the GFP control vector did not affect myofiber cross-sectional area in overloaded muscle. As a reference point, the mean cross-sectional area from unloaded control plantaris muscle is represented by a dotted line in Fig. 4 E and F.
Fig. 4.
Muscle GPR56 expression is increased in models of muscle hypertrophy. (A) GPR56, PGC-1α1, and PGC-1α4 mRNA expression in mouse muscle after 30 d of free wheel running or sedentary control. (B) Muscle protein expression of GPR56 after 30 d of wheel running (Ex) or sedentary control. (C and D) GPR56 mRNA (C) and protein (D) expression throughout a time course of functional overload (OL). *Denotes significant difference from sedentary or sham controls. (E, Upper) Representative immunohistochemistry of muscle cross-sections stained with wheat germ agglutinin (red) and GFP (green) after 21 d of functional overload. DAPI staining is shown in blue. (Lower) Cross-sectional area of GFP+ and GFP− myofibers. (F, Upper) Representative Immunohistochemistry of muscle cross-sectional area stained with wheat germ agglutinin and GFP after 10 d of functional overload. (Lower) Cross-sectional area of GFP+ and GFP− myofibers. &Denotes significant difference from GFP− myofibers. Data are presented as mean + SE (n = 5–7 per group). The dashed lines across the graphs represent the mean cross-sectional area (CSA) of unloaded sham control muscle for the given experiment. (Scale bars, 50 μm.)
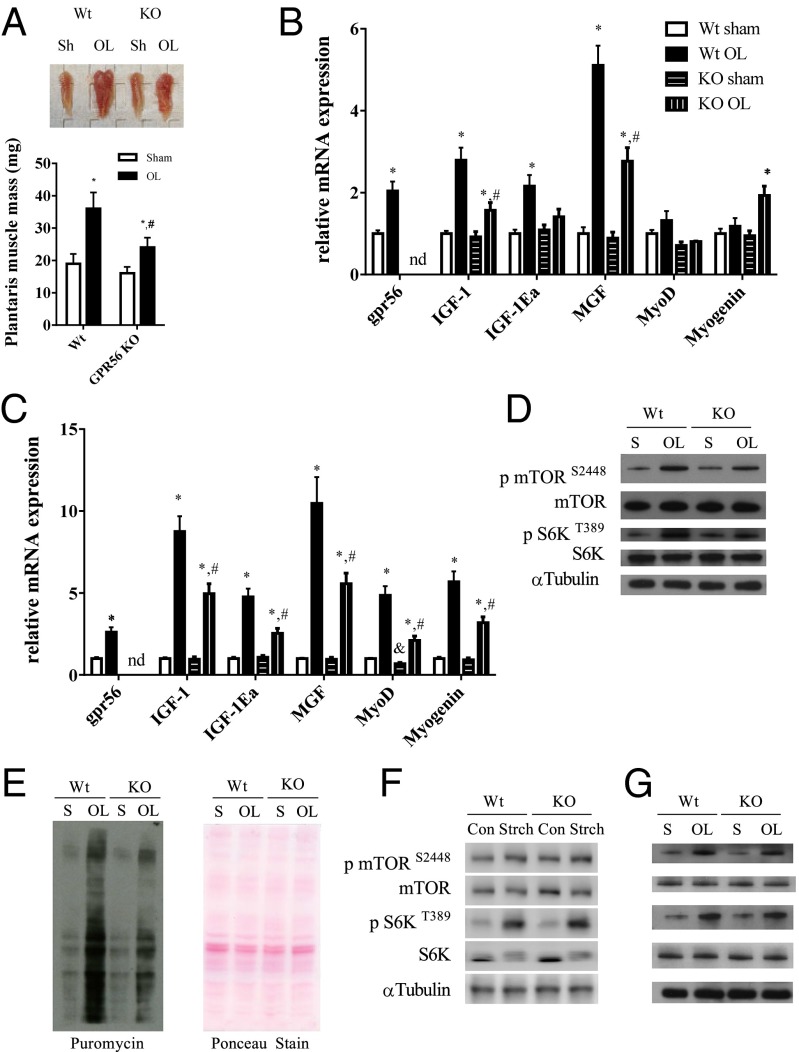
Gpr56-KO Mice Have Normal Muscle Mass and Function but Show Attenuation in Responding to Overload-Induced Muscle Hypertrophy.
A Gpr56 constitutive KO mouse has been generated previously (8, 16). Body weight and muscle mass are similar in wild-type and Gpr56-KO mice (Fig. S5 A and B). Similarly, muscle morphology and cross-sectional area are similar in Gpr56-KO and wild-type mice (Fig. S5 C and D). In addition, no changes were observed in grip strength measurements (Fig. S5E). Wild-type and KO mice were subjected to the synergistic ablation procedure for 21 d to determine if the loss of Gpr56 would have an effect on overload-induced hypertrophy. The mass of the plantaris muscle increased by 89% in the wild-type mice but by only 48% in KO mice (Fig. 5A). In addition to the reduction in muscle mass, cross-sectional area in the KO mouse was reduced as compared to wild-type mice (Fig. S5F). Gene expression at 21 d of overload showed a twofold induction in GPR56 with overload in the wild-type mice, whereas no GPR56 was detectable in the KO mice (Fig. 5B). Muscle IGF1 and the IGF1 isoforms MGF and IGF-1Ea were increased roughly three-, two-, and fivefold, respectively, in the wild-type mice, but the expression of the IGF1 gene family was attenuated in Gpr56-KO mice, in which IGF1, IGF-1Ea, and MGF were increased by only 50%, 40%, and 2.5-fold, respectively, compared with the sham-operated control. At 21 d of overload, the MyoD level did not differ in wild-type and Gpr56-KO muscle, whereas myogenin expression was increased twofold (Fig. 5B). At 2 d of overload (an established time point of active myogenesis), GPR56 was increased 2.6-fold, whereas IGF1, IGF-1Ea, and MGF were increased between 5- and 10-fold over sham controls (Fig. 5C). In the KO mouse, IGF1, IGF-1Ea, and MGF expression were reduced to 3-, 2.5-, and 5.5-fold, respectively. Relative to sham-operated muscle, the myogenic markers MyoD and myogenin were increased five- and sevenfold, respectively, in overloaded wild-type mice. The KO muscle showed an attenuated myogenic response with the induction of MyoD and myogenin reduced by two- and threefold, respectively, over the KO sham-operated group (Fig. 5C).
Fig. 5.
Loss of GPR56 attenuates overload-induced hypertrophy and anabolic signaling. (A, Upper) Representative plantaris muscles from wild-type and Gpr56-KO mice subjected to either sham treatment or overload. (Lower) Quantified muscle wet weights. (B and C) Plantaris mRNA expression at 21 (B) and 2 (C) d of functional overload. nd, not determined. (D) Representative Western blots of phosphorylated and total mTOR and S6K for wild-type and Gpr56-KO mice subjected to 7 d of overload. (E, Left) Representative Western blot of puromycin-incorporated proteins and (Right) Ponceau staining. (F) Representative Western blots of phosphorylated and total mTOR and S6K of wild-type and Gpr56-KO muscle after 90 min of acute stretching. Con, control; Strch, stretching. (G) Representative Western blots of phosphorylated and total mTOR and S6K of wild-type and Gpr56-KO muscle after 90 min of overload. *Denotes significant difference from sham control. #Denotes significant difference from wild-type overloaded muscle. Data are presented as mean + SE (n = 6 or 7 per group).
Anabolic Signaling and Protein Synthesis Are Suppressed During Functional Overload in Gpr56-KO Mice.
Muscle protein content, anabolic signaling through mTOR, and subsequent protein synthesis rates have been shown to increase during mechanical overload (1, 17). In the overloaded wild-type muscle, mTOR and S6K phosphorylation increased three- and fourfold, respectively (Fig. 5D and Fig. S5 G and H). In KO mice mTOR phosphorylation increased by roughly 2.5-fold with overload, and S6K phosphorylation increased by approximately threefold. We next used the SUnSET method to determine the rate of de novo protein synthesis in overloaded wild-type and KO muscle (18). Protein synthesis increased by fourfold in the muscle of wild-type mice but by only twofold in overloaded KO muscle (Fig. 5E). To determine if the mTOR and S6K phosphorylation induced by acute stretching would be attenuated in Gpr56-KO mice, isolated extensor digitorum longus muscles from both wild-type and Gpr56-KO mice were subjected to acute intermittent stretching for 90 min. Unlike the findings in the chronic mechanical overload model, mTOR and S6K phosphorylation caused by acute stretching were not affected by the loss of GPR56 (Fig. 5F and Fig. S5 I and J). To determine if this result was a phenomenon of the stretching model or a result of acute versus chronic overload, we ablated wild-type and Gpr56-KO mice for an acute 90-min period of overload. Although the acute period of overload was sufficient to increase mTOR and S6K phosphorylation, we observed no differences between wild-type and Gpr56-KO mice (Fig. 5G and Fig. S5 K and L). These results suggest that GPR56 signaling does not regulate the acute activation of mTOR during mechanical strain/overload.
GPR56 and Downstream Gα12/13 Subunits Increase with Resistance Exercise.
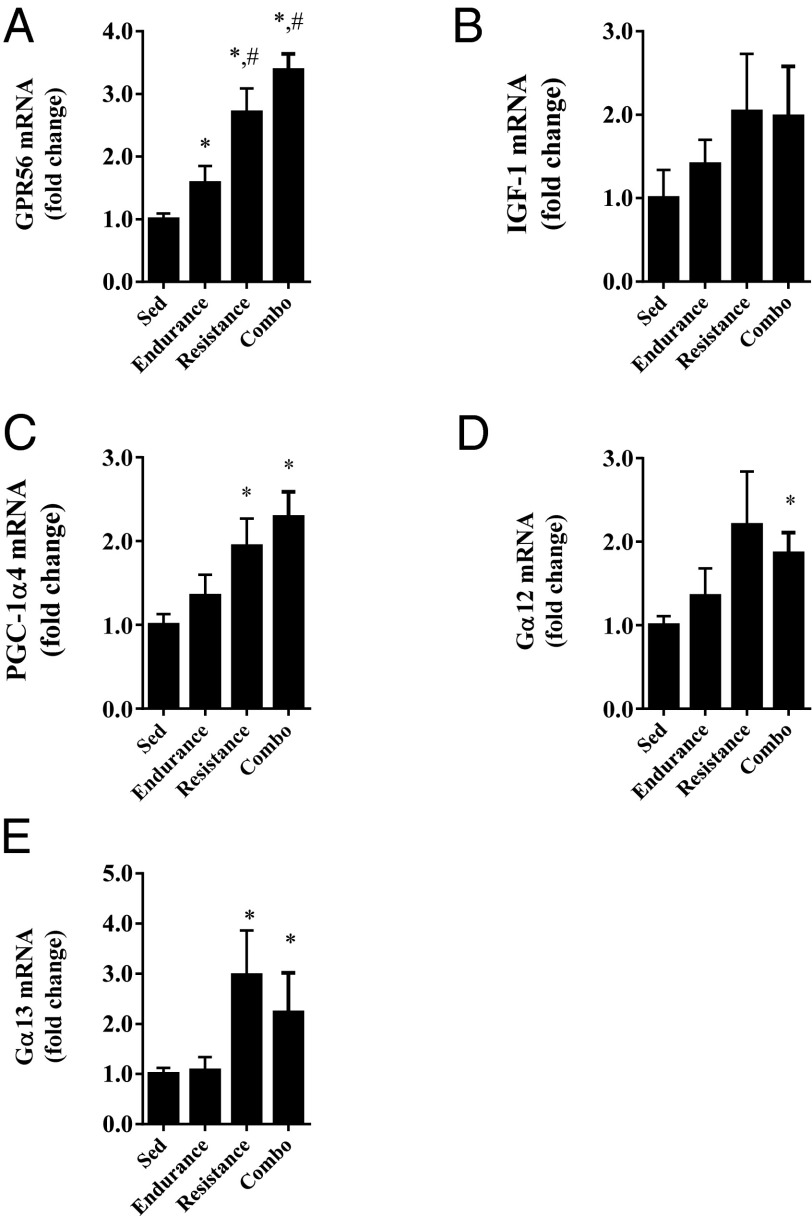
GPR56 mRNA was first measured in muscle from a human exercise cohort in which humans were subjected to a 12-wk training protocol consisting of endurance, resistance, or a combination of endurance and resistance training. Compared with the sedentary controls, GPR56 mRNA expression in muscle increased by 58% in the endurance exercise group and by 2.7- and 3.3-fold, respectively, in the resistance and resistance/endurance training groups (Fig. 6A). IGF1 mRNA did not differ among groups (Fig. 6B), but PGC-1α4 mRNA was increased in the resistance training groups (Fig. 6C). mRNA expression of the Gα12 and Gα13 subunits increased in groups performing resistance training (Fig. 6 D and E).
Fig. 6.
GPR56 and Gα12/13 subunit mRNA are increased in human muscle with resistance-based exercise. GPR56 (A), IGF1 (B), PGC-1a4 (C), Ga12 (D), and Ga13 (E) mRNA expression in human muscle after a 12-wk exercise training in groups undergoing endurance training, resistance training, or a combination of both endurance and resistance training, and in sedentary controls. *Denotes significant difference from sedentary control. #Denotes significant difference from endurance-trained subjects. Data are presented as mean + SE (n = 5–10 per group).
Discussion
The recent discovery of PGC-1α4 has provided an interesting tool for identifying previously unidentified mediators of muscle hypertrophy. Unlike PGC1α1, PGC1α4 does not appear to regulate a broad program of mitochondrial gene expression; rather, its function seems to be focused on muscle hypertrophy and strength. Here we show that the adhesion GPCR GPR56 and its ligand collagen III are transcriptional targets of PGC-1α4 in muscle cells. We have not discerned whether PGC-1α4 regulates GPR56 expression in a direct or indirect manner; however, GPR56 signaling appears to play a role in PGC-1α4–induced hypertrophy through induction of IGF1 expression and related mTOR signaling. In fact, previous studies of PGC-1α4 showed that IGF1 signaling is necessary for the process of PGC-1α4–induced hypertrophy (5). Interestingly, the relationship between PGC-1α4 and GPR56 in muscle hypertrophy is not symmetric: Although PGC1α4 requires the function of GPR56, PGC-1α4 is not necessary for the induction of GPR56-induced hypertrophy. This lack of a requirement for PGC-1α4 expression in certain murine models of muscle hypertrophy has been observed here and in other studies (19).
Signaling through GPR56 has been best described in the brain. Upon binding its ligand collagen III, GPR56 signals through the Gα12/13 subunit to activate downstream Rho kinases (8, 9). Here we show that forced expression of GPR56 in myotubes results in myotube hypertrophy, and this muscle cell growth shows strong dependence on Gα12/13 signaling. Although GPR56 expression is associated with hypertrophy in myotubes, others have shown that GPR56 loss of function does not affect myotube size, at least in the unprovoked state (20). Taken together, these data support the notion that GPR56 signaling is a part of the stimulus-provoked hypertrophic process but has a minimal role in basal, early-stage myogenesis or myotube maturation. Although downstream Rho expression has been shown to increase during mechanical overload and other conditions of skeletal muscle hypertrophy (21), the scope of Gα12/13 signaling in skeletal muscle is still unknown. Downstream signaling through Gα13/Rho has been associated with muscle hypertrophy in pressure-overloaded cardiac muscle (10), supporting the concept that Gα12/13 is a mechanosensitive anabolic pathway. Moreover, Gα12 signaling has been shown to activate the PI3K/Akt pathway in 3T3 cells (22), supporting our findings and confirming the Gα12/13 signaling as a regulatory pathway for mTOR. The loss of GPR56 could limit activation of Gα12/13 signaling during overload and prevent full activation of mTOR, similar to our observations in overloaded Gpr56-KO muscle (see model in Fig. 7).
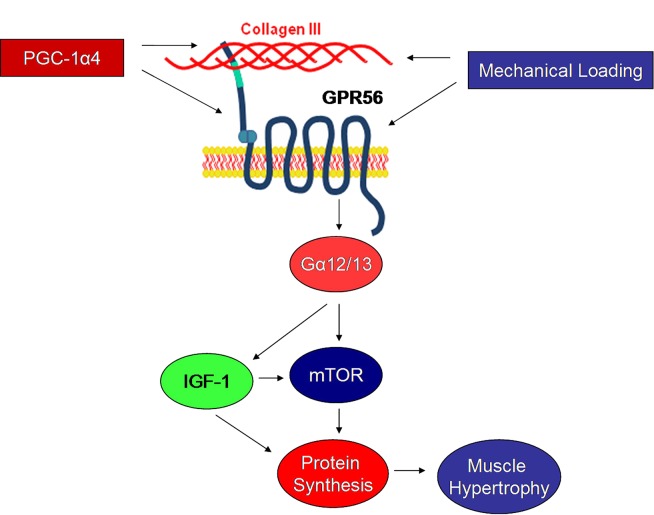
Fig. 7.
Working model of GPR56 signaling in skeletal muscle. Driven by PGC-1α4 or independently by mechanical loading, GPR56 signals through the Gα12/13 subunit to induce IGF1 expression and downstream mTOR activation. The activation in mTOR signaling is associated with increased muscle protein synthesis and subsequent hypertrophy.
We show here that GPR56 mRNA expression is induced in both human and mouse models of muscle hypertrophy; these observations are in contrast to our observations with endurance-based exercise, in which minimal or no changes in GPR56 expression were observed. These results are consistent with GPR56 elevations being selective for conditions of muscle hypertrophy, including mTOR activation. It is well established that resistance exercise yields higher anabolic potential than endurance exercise (5, 12). In addition, GPR56 expression appears to be very important in mediating at least some of the hypertrophic response of mechanical overload in mice. The translational impact of these findings implies that variations in GPR56 expression caused by disease or aging could affect the ability of muscle to adapt to mechanical loading. The ability of skeletal muscle to adapt to mechanical stimuli is essential for health and well-being, especially during conditions of sarcopenia or cachexia in which muscle mass is compromised. Signaling through GPR56 thus identifies a signaling pathway for further scientific exploration and a possible avenue for pharmaceutical interventions against muscle atrophy.
Methods
All animal experiments were performed according to procedures approved by the Institutional Animal Care and Use Committee of Dana–Farber Cancer Institute and the Beth Israel Deaconess Medical Center. All primers used in this article are listed with their sequences in Table S1. Primary satellite cells (myoblasts) were isolated, maintained, and differentiated as described previously (5). The generation of adenoviral expression of GPR56, GPR56 shRNA, and the DN Gα12/13 and experimental conditions are described in SI Methods. mRNA was processed and protein expression was quantified as previously described (23). Bioinformatic identification of PGC1α1/4-dependent signal-peptide proteins and mass spectrometry were performed as previously described (24). The right hindlimb plantaris muscle was functionally overloaded by a modified unilateral surgical ablation method as previously described (25). In vivo intramuscular electroporation of GPR56 shRNA or of a plasmid overexpressing GPR56 was used to determine acute changes in myofiber morphology during mechanical overload. The human exercise training protocol and subject characteristics have been described previously (5). The wheel-running protocol has been described previously (23). An acute stretching protocol of skeletal muscle was performed as previously described (26). Muscle wet weights and cross-sections were measured as previously described (5). All results are expressed as means + SE. The Student t test was used to determine P values. Significance was set at P < 0.05.
Supplementary Material
Acknowledgments
We thank the Harvard Histology Core Facility for assistance with embedding and processing muscle tissue. J.P.W. was supported by Postdoctoral Fellowship PF-13-385-01-TBE from the American Cancer Society. C.D.W. was supported by Fellowship WR 157/3-1 from the German Research Foundation. R.R.R. was supported by the Irvington Institute Fellowship Program of the Cancer Research Institute. T.A.H. was supported by National Institutes of Health (NIH) Grant AR057347. This project was supported by NIH Grant DK061562 and a grant from Novartis (to B.M.S.).
Footnotes
Conflict of interest statement: D.J.G. and Z.W. are employees of, and stockholders in, Novartis.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1417898111/-/DCSupplemental.
References
- 1.Hornberger TA. Mechanotransduction and the regulation of mTORC1 signaling in skeletal muscle. Int J Biochem Cell Biol. 2011;43(9):1267–1276. doi: 10.1016/j.biocel.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tesch PA. Skeletal muscle adaptations consequent to long-term heavy resistance exercise. Med Sci Sports Exerc. 1988;20(5) Suppl:S132–S134. doi: 10.1249/00005768-198810001-00008. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg AL. Protein synthesis during work-induced growth of skeletal muscle. J Cell Biol. 1968;36(3):653–658. doi: 10.1083/jcb.36.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunter GR, McCarthy JP, Bamman MM. Effects of resistance training on older adults. Sports Med. 2004;34(5):329–348. doi: 10.2165/00007256-200434050-00005. [DOI] [PubMed] [Google Scholar]
- 5.Ruas JL, et al. A PGC-1α isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell. 2012;151(6):1319–1331. doi: 10.1016/j.cell.2012.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Araç D, et al. A novel evolutionarily conserved domain of cell-adhesion GPCRs mediates autoproteolysis. EMBO J. 2012;31(6):1364–1378. doi: 10.1038/emboj.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langenhan T, Aust G, Hamann J. Sticky signaling—adhesion class G protein-coupled receptors take the stage. Sci Signal. 2013;6(276):re3. doi: 10.1126/scisignal.2003825. [DOI] [PubMed] [Google Scholar]
- 8.Luo R, et al. G protein-coupled receptor 56 and collagen III, a receptor-ligand pair, regulates cortical development and lamination. Proc Natl Acad Sci USA. 2011;108(31):12925–12930. doi: 10.1073/pnas.1104821108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iguchi T, et al. Orphan G protein-coupled receptor GPR56 regulates neural progenitor cell migration via a G alpha 12/13 and Rho pathway. J Biol Chem. 2008;283(21):14469–14478. doi: 10.1074/jbc.M708919200. [DOI] [PubMed] [Google Scholar]
- 10.Takefuji M, et al. G(13)-mediated signaling pathway is required for pressure overload-induced cardiac remodeling and heart failure. Circulation. 2012;126(16):1972–1982. doi: 10.1161/CIRCULATIONAHA.112.109256. [DOI] [PubMed] [Google Scholar]
- 11.Goodman CA, et al. The role of skeletal muscle mTOR in the regulation of mechanical load-induced growth. J Physiol. 2011;589(Pt 22):5485–5501. doi: 10.1113/jphysiol.2011.218255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drummond MJ, et al. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol. 2009;587(Pt 7):1535–1546. doi: 10.1113/jphysiol.2008.163816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spangenburg EE, Le Roith D, Ward CW, Bodine SC. A functional insulin-like growth factor receptor is not necessary for load-induced skeletal muscle hypertrophy. J Physiol. 2008;586(1):283–291. doi: 10.1113/jphysiol.2007.141507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minetti GC, et al. Gαi2 signaling is required for skeletal muscle growth, regeneration, and satellite cell proliferation and differentiation. Mol Cell Biol. 2014;34(4):619–630. doi: 10.1128/MCB.00957-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minetti GC, et al. Gαi2 signaling promotes skeletal muscle hypertrophy, myoblast differentiation, and muscle regeneration. Sci Signal. 2011;4(201):ra80. doi: 10.1126/scisignal.2002038. [DOI] [PubMed] [Google Scholar]
- 16.Li S, et al. GPR56 regulates pial basement membrane integrity and cortical lamination. J Neurosci. 2008;28(22):5817–5826. doi: 10.1523/JNEUROSCI.0853-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glass DJ. PI3 kinase regulation of skeletal muscle hypertrophy and atrophy. Curr Top Microbiol Immunol. 2010;346:267–78. doi: 10.1007/82_2010_78. [DOI] [PubMed] [Google Scholar]
- 18.Goodman CA, et al. Novel insights into the regulation of skeletal muscle protein synthesis as revealed by a new nonradioactive in vivo technique. FASEB J. 2011;25(3):1028–1039. doi: 10.1096/fj.10-168799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pérez-Schindler J, Summermatter S, Santos G, Zorzato F, Handschin C. The transcriptional coactivator PGC-1α is dispensable for chronic overload-induced skeletal muscle hypertrophy and metabolic remodeling. Proc Natl Acad Sci USA. 2013;110(50):20314–20319. doi: 10.1073/pnas.1312039110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu MP, et al. G-protein coupled receptor 56 promotes myoblast fusion through serum response factor- and nuclear factor of activated T-cell-mediated signalling but is not essential for muscle development in vivo. FEBS J. 2013;280(23):6097–6113. doi: 10.1111/febs.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McClung JM, Lee WJ, Thompson RW, Lowe LL, Carson JA. RhoA induction by functional overload and nandrolone decanoate administration in rat skeletal muscle. Pflugers Archiv. 2003;447(3):345–355. doi: 10.1007/s00424-003-1151-7. [DOI] [PubMed] [Google Scholar]
- 22.Kumar RN, Ha JH, Radhakrishnan R, Dhanasekaran DN. Transactivation of platelet-derived growth factor receptor alpha by the GTPase-deficient activated mutant of Galpha12. Mol Cell Biol. 2006;26(1):50–62. doi: 10.1128/MCB.26.1.50-62.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wrann CD, et al. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 2013;18(5):649–659. doi: 10.1016/j.cmet.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rao RR, et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014;157(6):1279–1291. doi: 10.1016/j.cell.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White JP, et al. Overload-induced skeletal muscle extracellular matrix remodelling and myofibre growth in mice lacking IL-6. Acta Physiol (Oxf) 2009;197(4):321–332. doi: 10.1111/j.1748-1716.2009.02029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.You JS, et al. The role of diacylglycerol kinase ζ and phosphatidic acid in the mechanical activation of mammalian target of rapamycin (mTOR) signaling and skeletal muscle hypertrophy. J Biol Chem. 2014;289(3):1551–1563. doi: 10.1074/jbc.M113.531392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.