Significance
This study unmasks a previously unidentified functional role of adiponectin (a hormone secreted by adipocytes) in modulating hippocampal neurogenesis and alleviating depression-like behaviors. To our knowledge, this is the first report showing that adiponectin may be an essential factor that mediates the antidepressant effects of physical exercise on the brain by adiponectin receptor 1-mediated activation of AMP-activated protein kinase. Our results reveal a possible mechanism by which exercise increases hippocampal neurogenesis and also suggest a promising therapeutic treatment for depression.
Keywords: adiponectin, adiponectin receptor, physical exercise, hippocampal neurogenesis, depression-like behavior
Abstract
Adiponectin (ADN) is an adipocyte-secreted protein with insulin-sensitizing, antidiabetic, antiinflammatory, and antiatherogenic properties. Evidence is also accumulating that ADN has neuroprotective activities, yet the underlying mechanism remains elusive. Here we show that ADN could pass through the blood–brain barrier, and elevating its levels in the brain increased cell proliferation and decreased depression-like behaviors. ADN deficiency did not reduce the basal hippocampal neurogenesis or neuronal differentiation but diminished the effectiveness of exercise in increasing hippocampal neurogenesis. Furthermore, exercise-induced reduction in depression-like behaviors was abrogated in ADN-deficient mice, and this impairment in ADN-deficient mice was accompanied by defective running-induced phosphorylation of AMP-activated protein kinase (AMPK) in the hippocampal tissue. In vitro analyses indicated that ADN itself could increase cell proliferation of both hippocampal progenitor cells and Neuro2a neuroblastoma cells. The neurogenic effects of ADN were mediated by the ADN receptor 1 (ADNR1), because siRNA targeting ADNR1, but not ADNR2, inhibited the capacity of ADN to enhance cell proliferation. These data suggest that adiponectin may play a significant role in mediating the effects of exercise on hippocampal neurogenesis and depression, possibly by activation of the ADNR1/AMPK signaling pathways, and also raise the possibility that adiponectin and its agonists may represent a promising therapeutic treatment for depression.
The therapeutic effects of physical exercise in treating depressive disorders are increasingly recognized (1). Previous studies have reported that physical exercise decreases depressive behaviors, improves hippocampal-dependent learning, enhances hippocampal neurogenesis, and increases dendritic plasticity (2–5). Nevertheless, understanding the mechanisms involved in the functional and structural benefits of exercise has proven to be somewhat of a challenge. It has recently been found that adiponectin, a hormone secreted predominantly from adipocytes, can mimic many of the metabolic effects of physical exercise. Adiponectin exists in the circulation primarily as three oligomeric complexes, including trimers, hexamers, or high molecular weight (HMW) oligomers (6, 7). Both the trimeric and hexameric forms are present in the human cerebrospinal fluid (CSF), implying their roles in the CNS (8, 9). In addition, only the trimeric and hexameric forms are detected in the CSF after i.v. injection of full-length recombinant adiponectin in mice (10), suggesting that these low molecular weight (LMW) forms of adiponectin may have the ability to pass through the blood–brain barrier.
There are two subtypes of adiponectin receptors (ADNRs), including ADNR1, which is highly expressed in skeletal muscle, and ADNR2, which is abundantly expressed in liver. Additionally, both receptors have been found in several brain regions, including cortex, hypothalamus, pituitary gland, and hippocampus (11). One of the unique features of adiponectin is that, like exercise, it promotes glucose uptake in skeletal muscle and suppresses glucose production in liver (12). Furthermore, both adiponectin and exercise exhibit antidiabetic, antiinflammatory, antiatherogenic and cardio-protective properties (13). Recent evidence also suggests that adiponectin has neuroprotective effects in the CNS (14, 15) in addition to its peripheral effects (13, 16).
Clinical studies have reported lower levels of plasma adiponectin in patients with depression (17), which could be increased after antidepressant treatments (18). A recent study has also shown that adiponectin deficiency increases depressive symptoms in mice, whereas reintroduction of adiponectin exerts antidepressant effects (19). However, the mechanism(s) underlying the antidepressive effects of adiponectin have remained unexplored. Although the globular or full-length adiponectin has been found to promote cell proliferation in the rat neural stem cell cultures (20), it is still unclear whether the antidepressant effects of adiponectin are mediated by altered hippocampal neurogenesis. Furthermore, the involvement of ADNR1 and ADNR2 in promoting hippocampal neurogenesis has not yet been examined.
In the present study we investigated the potential role of adiponectin in mediating the beneficial effects of running on promoting hippocampal neurogenesis and decreasing depression-like behaviors, and also elucidated the mechanism by which adiponectin promotes neurogenesis.
Materials and Methods
Animals and Experimental Design.
All experimental procedures were approved and followed the guidelines of the Committee on the Use of Live Animals in Teaching and Research, the University of Hong Kong. Eight- to nine-week-old male WT C57BL/6J mice or adiponectin knockout (adipo−/−) mice with the same genetic background (21) were randomly assigned into different treatment groups and fed with standard chow and water ad libitum in a room with a 12-h:12-h light/dark cycle. We used group housing together with shared wheels to avoid social isolation-triggered stress (22) and excessive exercise-induced anxiety (23).
Following a 2-d adaptation to the cages, running wheels were placed into the cages for 14 d. For studying hippocampal neurogenesis, proliferating cells were labeled by i.p. injection of BrdU (50 μg/g body weight, dissolved in 0.9% saline at a concentration of 10 mg/mL; Sigma-Aldrich) once daily during the last 5 days of running. Individual cohorts of animals were subjected to 14-d nonrunning or running and killed at the time points specified.
Intracerebroventricular and Tail Vein Injections.
WT mice were i.c.v. (intracerebroventricularly) injected with 2 µL of adenovirus expressing adiponectin (Ad-Adn, 108 pfu) or control luciferase (Ad-Luc, 108 pfu). To test the permeability to the blood–brain barrier, recombinant trimeric adiponectin proteins (20 µg per mouse) or the control PBS were injected into adipo−/− mice through the tail vein (SI Materials and Methods).
Behavioral Tests.
The forced swim test (FST) and tail suspension test (TST) were conducted as previously described (24). The immobility time was applied as the indicator for behavioral despair. The sucrose preference test (SPT) was performed as previously reported (25). Data were presented as the percentage of sucrose consumption. Loss of preference to sucrose suggests anhedonia, a core symptom of depressive patients. Experimental details of behavioral tests are given in SI Materials and Methods.
Tissue Preparation and Immunostaining.
The immunostaining was performed as previously described (5, 26, 27). Immunofluorescent colabeling of BrdU and doublecortin (DCX, immature neuronal marker) was performed as previously reported (5, 28). Further details are provided in SI Materials and Methods.
Immunostaining Analysis.
Coronal sections stained with antibodies against BrdU, Ki67 as the proliferating marker, and DCX were counted in the 1-in-6 series (from bregma −1.34 mm to −3.80 mm) (29) using the optical fractionators system (grid size: 55 μm × 55 μm; counting frame: 35 μm × 35 μm) of StereoInvestigator (MicroBrightfield Inc.). Data were presented as cells/mm2 of the dentate gyrus. Further details are provided in SI Materials and Methods.
Immunoassays and Western Blot Analysis.
The tissue homogenates were analyzed with immunoassays specific to adiponectin, insulin-like growth factor 1 (IGF-1), and BDNF. Protein expression or phosphorylation was analyzed with Western blot. Further details are provided in SI Materials and Methods.
Details regarding cell culture, proliferation assays, and gene knockdown are provided in SI Materials and Methods. Information about PCR primers and siRNA is listed in Tables S1–S3.
Statistical Analysis.
Data are shown as means ± SEM. The Student t test was applied for comparisons between two groups, whereas one-way or two-way ANOVA with running and genotype between-subject factors was used to compare three or more sets of data, followed by Tukey or Fisher post hoc tests, as appropriate, with SPSS 13.0 software. Correlation was determined by Pearson’s correlation analysis. A probability (P) value of <0.05 was considered statistically significant.
Results
Increase of Adiponectin in the CNS Reduced Depression-Like Behavior.
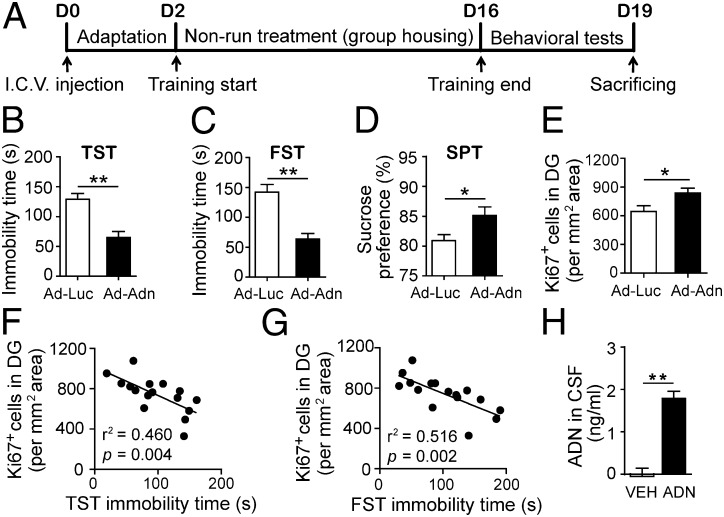
We injected recombinant Ad-Adn or control Ad-Luc to determine whether overexpressing adiponectin would alter depression-like behaviors in C57BL/J mice (Fig. 1A). Two weeks after i.c.v. injection of Ad-Adn, there were significant decreases of depression-like behaviors in all behavioral tests, including TST (Fig. 1B), FST (Fig. 1C), and SPT (Fig. 1D; P < 0.05 vs. Ad-Luc controls). Notably, mice exhibiting decreased depression-like behaviors also showed a marked increase in hippocampal cell proliferation (Fig. 1E; P < 0.05). Significant negative correlations between the number of proliferating cells and the immobility time were also observed (Fig. 1 F and G), suggesting an association between an increase in the number of proliferating cells and a reduction in depression-like behaviors.
Fig. 1.
Elevation of adiponectin levels in the brain reduced depressive phenotype in mice. (A) Experimental timeline for administration of recombinant adenovirus and behavioral tests. C57BL/6J mice with i.c.v. injection of recombinant Ad-Adn or control Ad-Luc received the nonrunning treatment for 2 wk, followed by behavioral tests. (B) The tail TST and (C) the FST assessed the duration of immobility that parallels depressive severity. (D) The SPT examined the loss of preference to sucrose (anhedonia), a core symptom in depression. (E) Cell proliferation in the hippocampal dentate gyrus was determined by measuring the density of Ki67+ cells. *P < 0.05, **P < 0.005; n = 8 mice per group. (F and G) The immobility time of TST and FST was negatively correlated with the density of hippocampal Ki67+ cells. (H) Trimeric adiponectin (ADN, 20 μg) administered through the tail vein became detectable in CSF in adipo−/− mice at 3 h after injection. VEH, PBS as vehicle. **P < 0.005; n = 4 mice per group.
The Trimeric Form of Adiponectin Was Permeable to the Blood–Brain Barrier.
To prove that adiponectin could pass through the blood–brain barrier, trimeric adiponectin (20 µg per mouse) was administered to adipo−/− mice through tail vein injection. Three hours after injection, there was a significant increase of adiponectin levels in the CSF, whereas adiponectin remained undetectable in the control adipo−/− mice injected with PBS vehicle (Fig. 1H; P < 0.005). This result further confirmed that LMW adiponectin could enter the CNS from the circulation (30).
Adiponectin Deficiency Diminished the Beneficial Effects of Running on Depression-Like Behaviors in Mice.
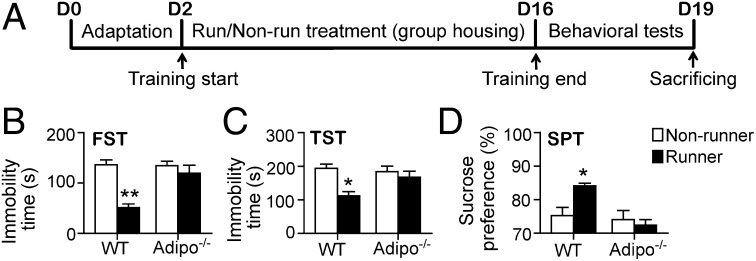
Adipo−/− mice displayed similar running activity compared with WT littermates under either the single or paired housing condition (Fig. S1 A and B). Furthermore, adipo−/− mice did not show any appreciable difference in locomotor activity compared with WT mice (Fig. S1 C and D). Next, we examined the potential involvement of adiponectin in running-induced decrease in depression-like behaviors. The results from FST showed that there were significant main effects of running and genotype (Fig. 2B; effect of running: F3,38 = 21.85, P < 0.001; effect of genotype: F3,38 = 9.847, P = 0.003; interaction: F3,38 = 10.89, P = 0.002). The immobility time was essentially identical in adipo−/− and WT nonrunners. In contrast, running significantly decreased immobility time in WT (P < 0.005 vs. WT nonrunners) but not adipo−/− mice (Fig. 2B). Similar results were also observed in the TST (Fig. 2C; effect of running: F3,38 = 2.403, P = 0.137; effect of genotype: F3,38 = 10.79, P = 0.002; interaction: F3,38 = 4.725, P = 0.037). Running-induced reduction of immobility time observed in WT mice (P < 0.05 for WT nonrunners vs. runners) was remarkably diminished by adiponectin knockout (Fig. 2C).
Fig. 2.
Depressive phenotype of WT and adipo−/− mice after the 2-wk running. (A) Experimental timeline for behavioral tests and sample collections. WT or adipo−/− mice received the 2-wk nonrunning or running treatment, followed by behavioral tests, including FST (B), TST (C), and SPT (D). Depression-like behaviors were significantly reduced by running in WT, but not adipo−/− mice. *P < 0.05 and **P < 0.005 vs. WT nonrunners; n = 8–10 mice per group.
WT runners showed an increase of sucrose consumption in SPT compared with WT nonrunners (Fig. 2D; P = 0.033). In addition, adipo−/− runners also showed a significant decrease of sucrose consumption compared with WT runners (P < 0.005), indicating that adiponectin knockout diminished running-elicited mitigation of anhedonia (Fig. 2D; effect of running: F3,35 = 3.016, P = 0.092; effect of genotype: F3,35 = 9.726, P = 0.003; interaction: F3,35 = 6.427, P = 0.017).
Adiponectin Knockout Diminished Running-Induced Hippocampal Neurogenesis.
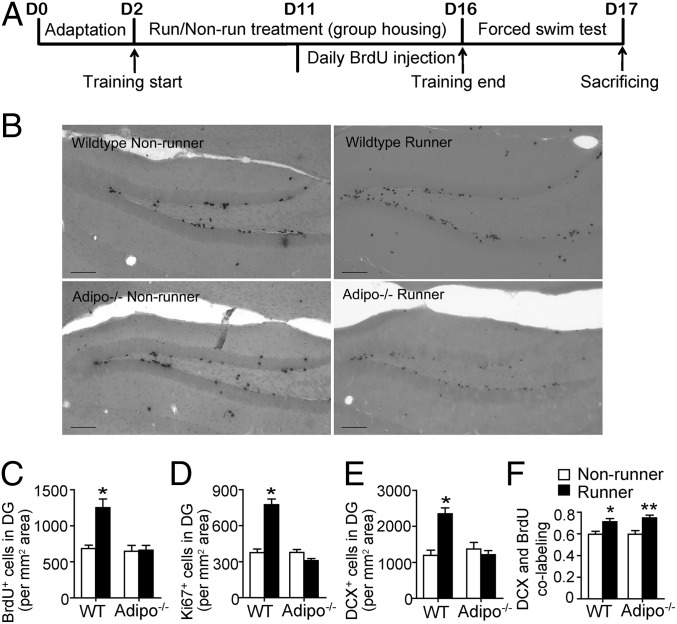
We subsequently investigated whether the behavioral alterations were correlated to the corresponding changes in hippocampal neurogenesis using BrdU, Ki67, and DCX stainings (Fig. 3B and Fig. S2 A and B). Running significantly increased the number of BrdU+ cells, which was diminished in adipo−/− mice (Fig. 3C; effect of running: F3,24 = 14.74, P = 0.001; effect of genotype: F3,24 = 17.21, P < 0.005; interaction: F3,24 = 13.303, P = 0.002). Adiponectin deficiency did not affect the basal cell proliferation (P > 0.05 vs. WT nonrunners) but abolished running-promoted hippocampal cell proliferation. Changes of hippocampal cell proliferation were reconfirmed by quantifying Ki67+ cells (Fig. S2A). ANOVA analysis supported the main effects of running (Fig. 3D; F3,22 = 47.70, P < 0.005) and genotype (F3,22 =93.956, P < 0.001) and an interaction (F3,22 = 95.114, P < 0.001). Running considerably enhanced hippocampal cell proliferation in WT (P < 0.005 vs. WT nonrunners) but not in adipo−/− mice (P > 0.05 vs. adipo−/− nonrunners).
Fig. 3.
Cellular changes in the hippocampus of WT and adipo−/− mice after exercise. (A) Experimental timeline for the running or nonrunning treatment, FST, and immunohistochemical analyses. WT and adipo−/− mice receiving the nonrunning or running treatment were daily injected with BrdU to label newborn cells during the last 5 consecutive days of the 2-wk training period. (B) Representative images of newborn (BrdU+) cells in the hippocampal dentate gyrus. (Scale bars, 100 µm.) (C and D) Running-enhanced hippocampal cell proliferation, reflected by the density of BrdU+ or Ki67+ population, was observed in WT but not adipo−/− mice. (E) Adiponectin knockout also diminished the effect of running on increasing the number of immature neurons (DCX+), without affecting the baseline. *P < 0.005 vs. WT nonrunners. (F) Neuronal differentiation estimated by the colabeling ratio of BrdU and DCX was comparable between WT and adipo−/− mice receiving the same treatments. *P < 0.05 vs. WT nonrunners, **P < 0.01 vs. adipo−/− nonrunners; n = 5–6 mice per group.
Analysis on DCX+ cells revealed the main effects of running (Fig. 3E; F3,20 = 12.162, P = 0.003) and genotype (F3,20 = 11.216, P = 0.004) and an interaction between running and genotype (F3,20 = 21.01, P < 0.005). WT runners showed a higher number of DCX+ cells compared with WT nonrunners (P < 0.005). Running did not increase DCX+ cells in adipo−/− mice, suggesting that adiponectin is required for running-induced generation of newborn neurons.
We further explored whether such a running-induced increase of immature neurons resulted from the enhanced neuronal differentiation by estimating the ratio of BrdU/DCX colabeling (Fig. S2C). Adiponectin deficiency did not affect running-enhanced neuronal differentiation (Fig. 3F; P < 0.01 for adipo−/− runners vs. adipo−/− nonrunners) or basal neuronal differentiation (P > 0.05 for WT nonrunners vs. adipo−/− nonrunners).
Levels of Hippocampal Neurotrophins and Adiponectin After Running.
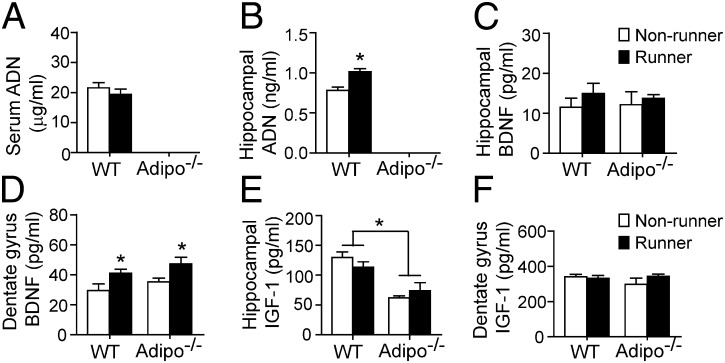
As expected, adiponectin was undetectable in adipo−/− mice (Fig. 4 A and B). Although running did not raise serum adiponectin levels in WT mice (Fig. 4A; P > 0.05 vs. WT nonrunners), hippocampal adiponectin levels were significantly elevated (Fig. 4B; P < 0.05 for WT runners vs. WT nonrunners). On the other hand, the expression of hippocampal ADNR1 and ADNR2 and their downstream adaptor APPL1 was unchanged in WT mice after running (Fig. S3A).
Fig. 4.
Effects of running on neurotrophic factors. The brain samples collected from adipo−/− mice or WT littermates receiving the 2-wk running or nonrunning treatment as described in Fig. 2A were homogenized and subjected to immunoassays. (A and B) Running significantly increased the hippocampal but not circulating adiponectin levels in WT mice. *P < 0.05 vs. WT nonrunners. Note that adiponectin was undetectable in either the serum or hippocampal lysate of Adipo−/− mice. (C and D) The levels of BDNF in the whole hippocampus were unaffected by either exercise or adiponectin knockout. Running raised the BDNF levels specifically in the dentate gyrus of WT and adipo−/− mice essentially to the same extent. (E and F) adipo−/− mice showed lower protein levels of IGF-1 in the whole hippocampus compared with WT animals (*main effect of genotype: P < 0.001), whereas IGF-1 levels in the dentate region remained comparable in all four groups. n = 4–6 mice per group.
Neurotrophic factors, such as BDNF and IGF-1, have been suggested to modulate exercise-induced hippocampal neurogenesis (31). We next examined whether adiponectin knockout affected exercise-induced elevation of BDNF and IGF-1 in vivo. The results of quantitative immunoassays showed that BDNF protein levels in the whole hippocampal homogenates were comparable among all groups (Fig. 4C; effect of running: F3,16 = 1.074, P = 0.320; effect of genotype: F3,16 = 0.013, P = 0.913). Interestingly, running specifically increased BDNF levels in the dentate gyrus subregion of both WT and adipo−/− mice (Fig. 4D; effect of running: F3,22 = 16.274, P < 0.001). In adipo−/− mice there was no reduction of BDNF levels in the dentate region of either nonrunners or runners (effect of genotype: F3,22 = 0.749, P = 0.398), suggesting that BDNF is not regulated by adiponectin.
Two weeks of running failed to increase the protein levels of IGF-1 in either the whole hippocampus (Fig. 4E; F3,16 = 0.058, P = 0.813) or the dentate gyrus (Fig. 4F; F3,22 = 0.806, P = 0.381) in WT mice. Of note, protein levels of IGF-1 were significantly reduced in adipo−/− mice in the whole hippocampal lysates (Fig. 4E; effect of genotype: F3,16 = 31.231; P < 0.001). In these animals there was also no significant effect of running (F3,16 = 0.058, P = 0.813) or interaction (F3,16 = 2.151, P = 0.168). Furthermore, IGF-1 levels in the dentate gyrus homogenates were comparable among all groups (Fig. 4F; effect of running: F3,22 = 0.194, P = 0.665; effect of genotype: F3,22 = 0.092; P = 0.765: interaction: F3,22 = 0.915, P = 0.351), indicating that altered IGF-1 levels are not involved in adiponectin-mediated antidepressant effects of running.
Running-Induced Activation of AMPK in the Hippocampus Was Compromised in adipo−/− Mice.
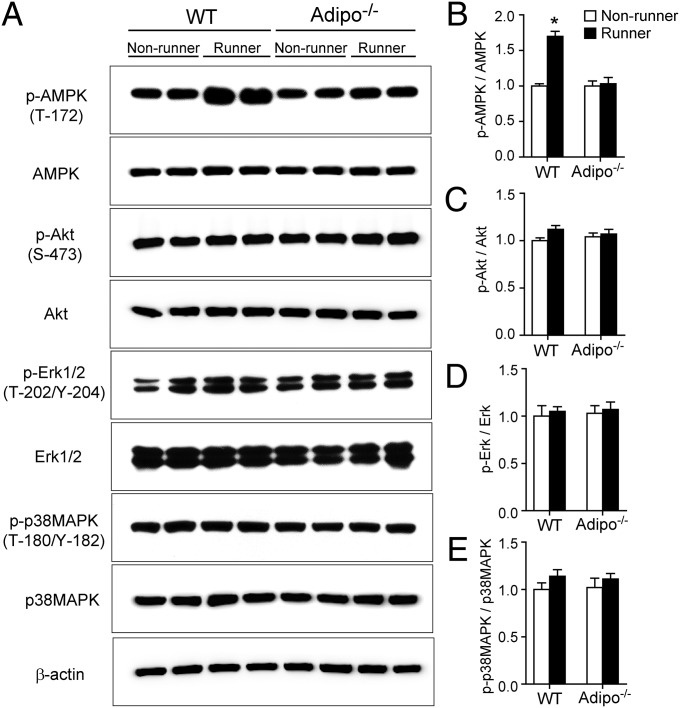
We next examined the possible downstream molecules involved in adiponectin-induced neurogenesis after running, by Western blot analysis. AMP-activated protein kinase (AMPK), protein kinase B (Akt), and extracellular signal-regulated kinases (Erk), as well as p38 mitogen-activated protein kinase (p38MAPK), have been considered the major pathways involved in adiponectin receptor-mediated signaling transduction (11, 13). Our results showed that running significantly increased phosphorylation of AMPKT172 in WT mice but not in adipo−/− mice (Fig. 5 A and B; effect of running: F3,36 = 23.46, P < 0.001; effect of genotype: F3,36 = 27.18, P < 0.001; interaction: F3,36 = 23.62, P < 0.001), suggesting the involvement of this kinase in running-induced neurogenesis. In contrast, the amount of phospho-AktS473 (Fig. 5 A and C), phospho-Erk1/2T202/204 (Fig. 5 A and D), or p38MAPKT180/Y182 (Fig. 5 A and E) was unaltered by running or adiponectin deficiency (effect of running: P > 0.05; effect of genotype: P > 0.05; interaction: P > 0.05).
Fig. 5.
Effects of running and adiponectin on several signaling pathways in the hippocampus. The homogenates of hippocampal tissues collected from adipo−/− mice or WT littermates receiving the 2-wk running or nonrunning treatment as described in Fig. 2A were subjected to Western blot analysis. (A) Representative immunoblotting images for phospho-AMPK (T172), total AMPK, phospho-Akt (S473), total Akt, phospho-Erk1/2 (T202/Y204), total Erk1/2, phospho-p38MAPK (T180/Y182), total p38MAPK, and the loading control β-actin. (B–E) Semiquantitative analysis for phosphor-AMPK (T172), phospho-Akt (S473), phospho-Erk1/2 (T202/Y204), and phospho-p38MAPK (T180/Y182). The data were expressed as fold changes over WT nonrunners. *P < 0.005 vs. WT nonrunners. n = 9 mice per group.
Adiponectin-Stimulated Hippocampal Cell Proliferation Was Mediated by Its Receptor 1 (ADNR1).
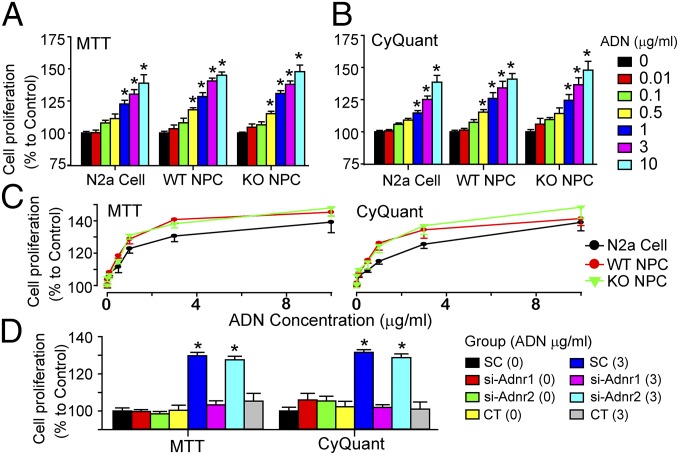
As demonstrated above, the trimeric adiponectin can be detected in the CSF of adipo−/− mice at 3 h after tail vein injection. Increasing adiponectin levels in the brain were in concurrence with enhanced hippocampal neurogenesis. Thus, we speculated that the running-enhanced neurogenesis observed in mice was, at least, partially attributed to the increase of adiponectin levels. Our in vitro data showed that incubation with trimeric adiponectin for 48 h promoted proliferation of neural progenitor cells (NPCs) cultured from WT and adipo−/− mice, as well as Neuro2a (N2a) cells in a concentration-dependent manner, as determined by the thiazolyl blue tetrazolium bromide (MTT) (Fig. 6 A and C, Left) and the CyQuant assays (Fig. 6 B and D, Right). In addition, we did not observe any growth defect for NPCs derived from adipo−/− mice (Fig. S4). This is in agreement with our in vivo results showing that adipo−/− nonrunners had the similar number of hippocampal newborn cells compared with their WT counterparts.
Fig. 6.
Adiponectin-induced enhancement of cell proliferation was mediated by ADNR1. The N2a cell line as well as the primary NPCs isolated from WT (WT NPC) and adipo−/− mice (KO NPC) were incubated with different concentrations of trimeric adiponectin and measured for proliferation using MTT (A and C, Left) and CyQuant assays (B and C, Right). *P < 0.05 vs. control cells without adiponectin treatment. (C) The concentration-dependent curves replotted using the data from A and B show the comparable responses to adiponectin in these three cell preparations. (D) Down-regulating ADNR1 but not ADNR2 in N2a cells with siRNA abolished adiponectin-enhanced proliferation. *P < 0.05 vs. Scramble siRNA-transfected cells (SC) without adiponectin treatment. n = 4–5 independent experiments for each assay. Numbers enclosed in the bracelets indicate the concentrations of the trimeric adiponectin applied. CT, combined transfection of si-Adnr1 and si-Adnr2.
We subsequently explored whether ADNRs were required for the adiponectin-elicited neurotrophic effect, as well as the specific subtype of ADNRs potentially involved in this process. Using RT-PCR and subsequent DNA sequencing, we identified ADNR1 and ADNR2, as well as their adaptor protein APPL1, in WT NPCs and N2a cells (Fig. S5A). Notably, NPCs cultured from adipo−/− mice had an expression profile of these three genes comparable to that from WT mice and N2a cells (Figs. S5 and S6) and responded similarly to adiponectin stimulation (Fig. 6C).
Given the intrinsic resistant nature of NPCs to transfection, we used the N2a cell line as an alternative to study the involvement of ADNRs in mediating adiponectin-induced cell proliferation. Knocking down ADNR1 and ADNR2 with siRNA significantly decreased the mRNA and protein expressions of these two receptors to a similar extent (Fig. S7). Down-regulation of ADNR1 abolished adiponectin-triggered increase in cell proliferation [Fig. 6D; P > 0.05 vs. SC (scramble siRNA-transfected cells) control treated with 3 μg/mL adiponectin], whereas knockdown of ADNR2 had no such an effect (P > 0.05 vs. SC control treated with 3 μg/mL adiponectin), indicating that adiponectin-enhanced proliferation may be mostly mediated by ADNR1 but not ADNR2.
Discussion
This study revealed a previously unidentified role of adiponectin and ADNR1 in running-stimulated increase of neurogenesis. We found that adiponectin deficiency alone does not affect the basal neurogenesis but causes a remarkable attenuation of running-induced hippocampal progenitor cell proliferation in mice. Furthermore, running-exerted antidepressant effects were also diminished in adipo−/− mice, suggesting that running-elicited neurogenesis and antidepressant effects, which are closely correlated, are dependent on adiponectin.
Previous studies have suggested the indispensable role of hippocampal neurogenesis in mediating antidepressive effects of physical exercise and antidepressants (5, 32). Consistently, we observed a significant negative correlation between the number of newborn cells and depressive severity in mice treated with adiponectin, suggesting that the antidepressant effects of adiponectin may be mediated by enhanced hippocampal neurogenesis. Our data reinforced the antidepressive effects of adiponectin observed in a mouse model of depression induced by chronic social defeat (19).
The origin of adiponectin found in the CNS has been debated. However, the trimeric and hexameric forms of adiponectin have been confirmed to enter the brain by passing through the blood–brain barrier (11). Hence, the peripheral adipose tissues are likely the major source of adiponectin in the CNS. Interestingly, we observed an increase in hippocampal adiponectin without a concomitant increase in the serum of WT runners. This could reflect that the 2-wk training is insufficient to produce a substantial elevation of adiponectin in the circulation (12) or that exercise potentially facilitates the transport of adiponectin from the circulation into the brain. It is also possible that this increase was produced by adipose or nervous tissues inside the brain (33).
The present results indicate that adiponectin promotes neurogenesis by increasing cell proliferation, and adiponectin deficiency does not influence neuronal differentiation in runners. This accords with a recent study that adiponectin does not affect neuronal or glial differentiation in vitro (20). Taken together, these data suggest that adiponectin may not be required for normal neuronal differentiation but is indispensable for running-induced increases in adult hippocampal cell proliferation and neurogenesis.
The molecular mechanisms mediating adiponectin-elicited beneficial effects on the CNS are not yet fully clear. Our cell proliferation assays showed that trimeric adiponectin increased proliferation of both N2a cell line and NPCs. These results support the observations of Zhang et al. (20) that adding globular and full-length adiponectin to cultured rat NPCs could accelerate cell growth. However, the involvement of ADNRs was not examined in their study. Different oligomeric forms of adiponectin have distinct affinities to different adiponectin receptors: the LMW adiponectin tends to bind ADNR1, and the HMW adiponectin displays preferential binding to ADNR2. Given that the major forms of adiponectin in the CNS are trimeric and hexameric, and that the distribution of ADNR1 is mostly detected at the mood-regulatory regions (e.g., medial prefrontal cortex, hippocampus and amygdala) (20), ADNR1 seemed to be the possible candidate for mediating the antidepressant effects of physical exercise.
We explored this possibility by siRNA-mediated knockdown of adiponectin receptor subtypes in N2a cells, which have been proven to have similar concentration responses to adiponectin compared with primary NPCs. The results confirmed that down-regulating ADNR1, but not ADNR2, abolished adiponectin-enhanced proliferation. Additionally, the concurrent knockdown of ADNR1 and ADNR2 made no additive inhibitory effect, further supporting the notion that activation of ADNR1, but not ADNR2, is critical for adiponectin-triggered neurogenesis. Given that expression levels of ADNRs and their binding adaptor APPL1 were comparable in mice with or without running (Fig. S3), it is likely that physical exercise-elicited increase of adiponectin in the hippocampus could be the enabling step in the antidepressive process.
Although knockdown of receptors in vitro may not adequately reflect the response of hippocampal neurons in vivo, our cell culture work using N2a cells is in agreement with the in vivo finding that ADNR1 has high affinity to LMW adiponectin (11) and is highly expressed in the hippocampus (20). Future work on primary progenitor cell cultures with virus-mediated delivery of shRNA against ADNR1 or mice with hippocampus-specific knockout of ADNR1 is needed to reinforce the important role of ADNR1 in mediating adiponectin-elicited proliferating effect.
BDNF and IGF-1 are known to be the proneurogenic factors mediating the effect of running on neurogenesis (31). In the present study, BDNF levels in the dentate region were elevated in WT mice after running, and a comparable increase was also observed in adipo−/− runners whose neurogenesis was unaltered. IGF-1 levels in the whole hippocampus of adipo−/− nonrunners were lower than in WT counterparts, regardless of the similar hippocampal neurogenesis and depressive state. Reducing IGF-1 levels could diminish running-enhanced neurogenesis without affecting the basal level of neurogenesis (34). Whether the reduction reflects the potential interaction between IGF-1 and adiponectin warrants further investigation.
The antiinflammatory and cytoprotective effects of adiponectin are known to be partly mediated through APPL1-dependent AMPK activation of the PI3K-Akt signaling pathway in endothelial cells (35). Of note, inhibition of PI3K-Akt signaling blocks exercise-enhanced adult neurogenesis in the dentate gyrus (36). We detected a significant increase of phosphorylated AMPK at T172 in WT runners, which was attenuated in adipo−/− runners. Our previous study has shown that adiponectin protects against kainic acid-induced excitotoxicity in an AMPK-dependent way in primary hippocampal neurons that express ADNRs (37). Meanwhile, moderate AMPK activation has also been documented to enhance hippocampal neurogenesis and cognitive functions (38). Zhang et al. (20) have suggested that globular adiponectin triggered proliferation of cultured rat NPCs through a p38MAPK/GSK-3β/β-catenin cascade, as such an enhancement was abolished by the p38MAPK inhibitor SB203580. However, we did not observed any significant activation of other candidate pathways, including Akt, Erk1/2, and p38MAPK, suggesting that these pathways are unlikely to play a role in transducing adiponectin/ADNR1 signal on cell proliferation after running.
Adiponectin is a well-known insulin sensitizer with multiple metabolic activities, including promotion of glucose uptake and induction of fatty acid oxidation in liver and skeletal muscle, and inhibition of hepatic glucose production (39, 40) via activation of AMPK (41). In obese individuals, both circulating levels of adiponectin and its mRNA expression in adipose tissue are markedly decreased compared with healthy subjects (42, 43), possibly because hypoxia and inflammatory cytokines suppress adiponectin gene expression in enlarged adipocytes (44). Physical exercise has been shown to enhance adiponectin production by reducing fat mass and inflammation in obese/diabetic patients (12). Given the important role of AMPK in mitochondrial biogenesis (45), which is required for running-accelerated newborn neuron maturation (46), increase in hippocampal adiponectin after running may modulate mitochondria biogenesis via activation of AMPK in the newborn cells, thereby enhancing neurogenesis.
In summary, the present study demonstrates that the antidepressant effects of physical exercise are mediated partly by inducing production of adiponectin, which in turn promotes hippocampal neurogenesis (Fig. S8). Notably, adiponectin is a well-known downstream mediator of the peroxisome proliferation-activated receptor-γ (PPARγ) agonist thiazolidinediones. The insulin-sensitizing effect of the PPARγ agonist is abrogated in adiponectin-knockout mice (47). PPARy agonist has also been shown to exert a potent antidepressant effect (48, 49). It is possible that such an antidepressant effect of the PPARγ agonist is mediated in part by adiponectin (50). Therefore, pharmacological interventions to elevate endogenous adiponectin, for example the medicinal herb Radix Astragali (51) and the PPARγ agonists, may represent an effective strategy for treatment or/and prevention of depression.
Supplementary Material
Acknowledgments
We thank Dr. Li Lu for technical assistance and Dr. Milton Wong for comments on the manuscript. The work is supported by Hong Kong Health and Medical Research Fund and by funds of Leading Talents of Guangdong (2013), Programme of Introducing Talents of Discipline to Universities (B14036), and Project of International, as well as Hong Kong, Macao & Taiwan Science and Technology Cooperation Innovation Platform in Universities in Guangdong Province (2013gjhz0002). Support was also provided through grants to Jinan University Guangdong-Hong Kong-Macau Cooperation and Innovation Center for Tissue Regeneration and Repair, and to State Key Laboratory of Pharmaceutical Biotechnology, Hong Kong SAR.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1415219111/-/DCSupplemental.
References
- 1.Rimer J, et al. Exercise for depression. Cochrane Database Syst Rev. 2012;7:CD004366. doi: 10.1002/14651858.CD004366.pub5. [DOI] [PubMed] [Google Scholar]
- 2.Déry N, et al. Adult hippocampal neurogenesis reduces memory interference in humans: Opposing effects of aerobic exercise and depression. Front Neurosci. 2013;7:66. doi: 10.3389/fnins.2013.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999;96(23):13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eadie BD, Redila VA, Christie BR. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J Comp Neurol. 2005;486(1):39–47. doi: 10.1002/cne.20493. [DOI] [PubMed] [Google Scholar]
- 5.Yau SY, et al. Hippocampal neurogenesis and dendritic plasticity support running-improved spatial learning and depression-like behaviour in stressed rats. PLoS One. 2011;6(9):e24263. doi: 10.1371/journal.pone.0024263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Lam KS, Yau MH, Xu A. Post-translational modifications of adiponectin: Mechanisms and functional implications. Biochem J. 2008;409(3):623–633. doi: 10.1042/BJ20071492. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, et al. Adiponectin inhibits cell proliferation by interacting with several growth factors in an oligomerization-dependent manner. J Biol Chem. 2005;280(18):18341–18347. doi: 10.1074/jbc.M501149200. [DOI] [PubMed] [Google Scholar]
- 8.Glintborg D, et al. Total and high molecular weight (HMW) adiponectin levels and measures of glucose and lipid metabolism following pioglitazone treatment in a randomized placebo-controlled study in polycystic ovary syndrome. Clin Endocrinol (Oxf) 2008;68(2):165–174. doi: 10.1111/j.1365-2265.2007.03015.x. [DOI] [PubMed] [Google Scholar]
- 9.Aroda V, et al. Circulating and cellular adiponectin in polycystic ovary syndrome: Relationship to glucose tolerance and insulin action. Fertil Steril. 2008;89(5):1200–1208. doi: 10.1016/j.fertnstert.2007.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kubota N, et al. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 2007;6(1):55–68. doi: 10.1016/j.cmet.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Thundyil J, Pavlovski D, Sobey CG, Arumugam TV. Adiponectin receptor signalling in the brain. Br J Pharmacol. 2012;165(2):313–327. doi: 10.1111/j.1476-5381.2011.01560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vu V, Riddell MC, Sweeney G. Circulating adiponectin and adiponectin receptor expression in skeletal muscle: Effects of exercise. Diabetes Metab Res Rev. 2007;23(8):600–611. doi: 10.1002/dmrr.778. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Zhou M, Lam KS, Xu A. Protective roles of adiponectin in obesity-related fatty liver diseases: Mechanisms and therapeutic implications. Arq Bras Endocrinol Metabol. 2009;53(2):201–212. doi: 10.1590/s0004-27302009000200012. [DOI] [PubMed] [Google Scholar]
- 14.Jeon BT, et al. Adiponectin protects hippocampal neurons against kainic acid-induced excitotoxicity. Brain Res Brain Res Rev. 2009;61(2):81–88. doi: 10.1016/j.brainresrev.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Nishimura M, et al. Adiponectin prevents cerebral ischemic injury through endothelial nitric oxide synthase dependent mechanisms. Circulation. 2008;117(2):216–223. doi: 10.1161/CIRCULATIONAHA.107.725044. [DOI] [PubMed] [Google Scholar]
- 16.Hui X, Lam KS, Vanhoutte PM, Xu A. Adiponectin and cardiovascular health: An update. Br J Pharmacol. 2012;165(3):574–590. doi: 10.1111/j.1476-5381.2011.01395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leo R, et al. Decreased plasma adiponectin concentration in major depression. Neurosci Lett. 2006;407(3):211–213. doi: 10.1016/j.neulet.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 18.Narita K, et al. Plasma levels of adiponectin and tumor necrosis factor-alpha in patients with remitted major depression receiving long-term maintenance antidepressant therapy. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(6):1159–1162. doi: 10.1016/j.pnpbp.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, et al. Adiponectin is critical in determining susceptibility to depressive behaviors and has antidepressant-like activity. Proc Natl Acad Sci USA. 2012;109(30):12248–12253. doi: 10.1073/pnas.1202835109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang D, Guo M, Zhang W, Lu XY. Adiponectin stimulates proliferation of adult hippocampal neural stem/progenitor cells through activation of p38 mitogen-activated protein kinase (p38MAPK)/glycogen synthase kinase 3β (GSK-3β)/β-catenin signaling cascade. J Biol Chem. 2011;286(52):44913–44920. doi: 10.1074/jbc.M111.310052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma K, et al. Increased beta-oxidation but no insulin resistance or glucose intolerance in mice lacking adiponectin. J Biol Chem. 2002;277(38):34658–34661. doi: 10.1074/jbc.C200362200. [DOI] [PubMed] [Google Scholar]
- 22.Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nat Neurosci. 2006;9(4):526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuss J, et al. Voluntary exercise induces anxiety-like behavior in adult C57BL/6J mice correlating with hippocampal neurogenesis. Hippocampus. 2010;20(3):364–376. doi: 10.1002/hipo.20634. [DOI] [PubMed] [Google Scholar]
- 24.Gould TD, et al. Involvement of AMPA receptors in the antidepressant-like effects of lithium in the mouse tail suspension test and forced swim test. Neuropharmacology. 2008;54(3):577–587. doi: 10.1016/j.neuropharm.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren C, et al. Direct retino-raphe projection alters serotonergic tone and affective behavior. Neuropsychopharmacology. 2013;38(7):1163–1175. doi: 10.1038/npp.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lau BW, et al. Effect of corticosterone and paroxetine on masculine mating behavior: Possible involvement of neurogenesis. J Sex Med. 2011;8(5):1390–1403. doi: 10.1111/j.1743-6109.2010.02081.x. [DOI] [PubMed] [Google Scholar]
- 27.Yau SY, et al. Low dose of corticosterone treatment with exercise increases hippocampal cell proliferation, and improves cognition. Neural Regeneration Research. 2011;6(34):2645–2655. [Google Scholar]
- 28.Yau SY, et al. Effects of voluntary running on plasma levels of neurotrophins, hippocampal cell proliferation and learning and memory in stressed rats. Neuroscience. 2012;222:289–301. doi: 10.1016/j.neuroscience.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 29.Paxinos G, Franklin KBJ. 2001. The Mouse Brain in Stereotaxic Coordinates (Academic Press, San Diego)
- 30.Neumeier M, et al. Detection of adiponectin in cerebrospinal fluid in humans. Am J Physiol Endocrinol Metab. 2007;293(4):E965–E969. doi: 10.1152/ajpendo.00119.2007. [DOI] [PubMed] [Google Scholar]
- 31.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 32.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 33.Wilkinson M, Brown R, Imran SA, Ur E. Adipokine gene expression in brain and pituitary gland. Neuroendocrinology. 2007;86(3):191–209. doi: 10.1159/000108635. [DOI] [PubMed] [Google Scholar]
- 34.Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21(5):1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chandrasekar B, et al. Adiponectin blocks interleukin-18-mediated endothelial cell death via APPL1-dependent AMP-activated protein kinase (AMPK) activation and IKK/NF-kappaB/PTEN suppression. J Biol Chem. 2008;283(36):24889–24898. doi: 10.1074/jbc.M804236200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruel-Jungerman E, et al. Inhibition of PI3K-Akt signaling blocks exercise-mediated enhancement of adult neurogenesis and synaptic plasticity in the dentate gyrus. PLoS One. 2009;4(11):e7901. doi: 10.1371/journal.pone.0007901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qiu G, et al. Adiponectin protects rat hippocampal neurons against excitotoxicity. Age (Dordr) 2011;33(2):155–165. doi: 10.1007/s11357-010-9173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dagon Y, et al. Nutritional status, cognition, and survival: A new role for leptin and AMP kinase. J Biol Chem. 2005;280(51):42142–42148. doi: 10.1074/jbc.M507607200. [DOI] [PubMed] [Google Scholar]
- 39.Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: An adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13(2):84–89. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- 40.Tsao TS, Lodish HF, Fruebis J. ACRP30, a new hormone controlling fat and glucose metabolism. Eur J Pharmacol. 2002;440(2-3):213–221. doi: 10.1016/s0014-2999(02)01430-9. [DOI] [PubMed] [Google Scholar]
- 41.Yamauchi T, Kadowaki T. Physiological and pathophysiological roles of adiponectin and adiponectin receptors in the integrated regulation of metabolic and cardiovascular diseases. Int J Obes (Lond) 2008;32(Suppl 7):S13–S18. doi: 10.1038/ijo.2008.233. [DOI] [PubMed] [Google Scholar]
- 42.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271(18):10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 43.Arita Y, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 2012;425(3):560–564. doi: 10.1016/j.bbrc.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 44.Kita A, et al. Identification of the promoter region required for human adiponectin gene transcription: Association with CCAAT/enhancer binding protein-beta and tumor necrosis factor-alpha. Biochem Biophys Res Commun. 2005;331(2):484–490. doi: 10.1016/j.bbrc.2005.03.205. [DOI] [PubMed] [Google Scholar]
- 45.Reznick RM, Shulman GI. The role of AMP-activated protein kinase in mitochondrial biogenesis. J Physiol. 2006;574(Pt 1):33–39. doi: 10.1113/jphysiol.2006.109512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steib K, Schäffner I, Jagasia R, Ebert B, Lie DC. Mitochondria modify exercise-induced development of stem cell-derived neurons in the adult brain. J Neurosci. 2014;34(19):6624–6633. doi: 10.1523/JNEUROSCI.4972-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nawrocki AR, et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem. 2006;281(5):2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- 48.Eissa Ahmed AA, Al-Rasheed NM, Al-Rasheed NM. Antidepressant-like effects of rosiglitazone, a PPARγ agonist, in the rat forced swim and mouse tail suspension tests. Behav Pharmacol. 2009;20(7):635–642. doi: 10.1097/FBP.0b013e328331b9bf. [DOI] [PubMed] [Google Scholar]
- 49.Sepanjnia K, Modabbernia A, Ashrafi M, Modabbernia MJ, Akhondzadeh S. Pioglitazone adjunctive therapy for moderate-to-severe major depressive disorder: Randomized double-blind placebo-controlled trial. Neuropsychopharmacology. 2012;37(9):2093–2100. doi: 10.1038/npp.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu JG, et al. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes. 2002;51(10):2968–2974. doi: 10.2337/diabetes.51.10.2968. [DOI] [PubMed] [Google Scholar]
- 51.Xu A, et al. Selective elevation of adiponectin production by the natural compounds derived from a medicinal herb alleviates insulin resistance and glucose intolerance in obese mice. Endocrinology. 2009;150(2):625–633. doi: 10.1210/en.2008-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.