Abstract
IMPORTANCE
In clinical and research settings worldwide, low-density lipoprotein cholesterol (LDL-C) is typically estimated using the Friedewald equation. This equation assumes a fixed factor of 5 for the ratio of triglycerides to very low-density lipoprotein cholesterol (TG:VLDL-C); however, the actual TG:VLDL-C ratio varies significantly across the range of triglyceride and cholesterol levels.
OBJECTIVE
To derive and validate a more accurate method for LDL-C estimation from the standard lipid profile using an adjustable factor for the TG:VLDL-C ratio.
DESIGN, SETTING, AND PARTICIPANTS
We used a convenience sample of consecutive clinical lipid profiles obtained from 2009 through 2011 from 1 350 908 children, adolescents, and adults in the United States. Cholesterol concentrations were directly measured after vertical spin density-gradient ultracentrifugation, and triglycerides were directly measured. Lipid distributions closely matched the population-based National Health and Nutrition Examination Survey (NHANES). Samples were randomly assigned to derivation (n = 900 605) and validation (n = 450 303) data sets.
MAIN OUTCOMES AND MEASURES
Individual patient-level concordance in clinical practice guideline LDL-C risk classification using estimated vs directly measured LDL-C (LDL-CD).
RESULTS
In the derivation data set, the median TG:VLDL-C was 5.2 (IQR, 4.5–6.0). The triglyceride and non–high-density lipoprotein cholesterol (HDL-C) levels explained 65% of the variance in the TG:VLDL-C ratio. Based on strata of triglyceride and non–HDL-C values, a 180-cell table of median TG:VLDL-C values was derived and applied in the validation data set to estimate the novel LDL-C (LDL-CN). For patients with triglycerides lower than 400 mg/dL, overall concordance in guideline risk classification with LDL-CD was 91.7% (95% CI, 91.6%–91.8%) for LDL-CN vs 85.4% (95% CI, 85.3%–85.5%) for Friedewald LDL-C (LDL-CF) (P < .001). The greatest improvement in concordance occurred in classifying LDL-C lower than 70 mg/dL, especially in patients with high triglyceride levels. In patients with an estimated LDL-C lower than 70 mg/dL, LDL-CD was also lower than 70 mg/dL in 94.3% (95% CI, 93.9%–94.7%) for LDL-CN vs 79.9% (95% CI, 79.3%–80.4%) for LDL-CF in samples with triglyceride levels of 100 to 149 mg/dL; 92.4% (95% CI, 91.7%–93.1%) for LDL-CN vs 61.3% (95% CI, 60.3%–62.3%) for LDL-CF in samples with triglyceride levels of 150 to 199 mg/dL; and 84.0% (95% CI, 82.9%–85.1%) for LDL-CN vs 40.3% (95% CI, 39.4%–41.3%) for LDL-CF in samples with triglyceride levels of 200 to 399 mg/dL (P < .001 for each comparison).
CONCLUSIONS AND RELEVANCE
A novel method to estimate LDL-C using an adjustable factor for the TG:VLDL-C ratio provided more accurate guideline risk classification than the Friedewald equation. These findings require external validation, as well as assessment of their clinical importance. The implementation of these findings into clinical practice would be straightforward and at virtually no cost.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT01698489
Low-density lipoprotein cholesterol (LDL-C) is of longstanding clinical and research interest and the primary target in national and international clinical practice guidelines.1–10 Conventionally, LDL-C is estimated by the Friedewald equation, obviating need for an ultracentrifuge.1 This equation is based on an analysis of 448 patients from 1972 and estimates LDL-C as (total cholesterol) – (high-density lipoprotein cholesterol [HDL-C]) – (triglycerides/5) in mg/dL.1 The final term assumes a fixed ratio of triglyceride levels to very low-density lipoprotein cholesterol (TG:VLDL-C) of 5:1.
Applying a factor of 5 to every individual patient is problematic given variance in the TG:VLDL-C ratio across the range of triglyceride and non–HDL-C levels. Indeed, Friedewald and colleagues1 noted that simply dividing triglyceride values by 5 does not give an accurate estimate of VLDL-C. Providing further evidence of variance, the mean TG:VLDL-C ratio ranged from 5.2 to 8.9 across clinics in the Lipid Research Clinics Prevalence Study.2 DeLong and colleagues2 proposed a fixed factor of 6, effectively resetting the population mean, although not addressing interindividual variance in the TG:VLDL-C ratio.
In the eras in which the Friedewald equation1 and De-Long modification2 were proposed, an LDL-C lower than 70 mg/dL was not yet established as an ideal secondary prevention target for treatment of high-risk patients.5,7 In fact, an LDL-C level in this range was at the low end or outside of the distribution of the original training data set used in deriving the Friedewald equation.1 At higher LDL-C concentrations, error in VLDL-C estimation was relatively small with respect to non–HDL-C and actual LDL-C levels. Hence, it was believed that the VLDL-C estimation using a fixed factor was sufficiently accurate, and this approach simplified computation.
Leveraging improvements in computing and data availability, we aimed to derive and validate a novel method for estimation of LDL-C from the standard lipid profile using an adjustable factor for the TG:VLDL-C ratio.
Methods
Study Population and Lipid Testing
The Johns Hopkins institutional review board declared our study exempt. We examined a convenience sample of consecutive lipid profiles from the Very Large Database of Lipids (VLDL).11 Samples were obtained for clinical indications by Atherotech Diagnostics Laboratory (Birmingham, AL) from 2009 through 2011 from persons living in the United States. To enhance generalizability, we included children (aged <11 years), adolescents (aged 11 to <18 years), and adults (aged ≥18 years) in our primary analyses. We also included both sexes and did not exclude any patient based on his/her lipid profile. We subsequently explored the contribution of age, sex, and lipid profile characteristics to variance in TG:VLDL-C and also evaluated the performance of the novel method for LDL-C estimation within subgroups.
Deidentified data were transferred to the investigators. As previously reported,11,12 lipid distributions in the study sample closely matched those from a population-based survey, the National Health and Nutrition Examination Survey (NHANES) 2007–2008.13 Our study (VLDL-1B) is the second phase of the VLDL-1 study,11,12 based on a registered data set14 and peer-reviewed statistical analysis plan.15
Cholesterol concentrations were directly measured by the Vertical Auto Profile (VAP; Atherotech), an inverted rate zonal, single-vertical spin, density-gradient ultracentrifugation technique that separates lipoprotein subfractions and then measures the cholesterol content, including LDL-C, VLDL-C, and HDL-C.16 Triglycerides were directly measured using the ARCHITECT C-8000 system (Abbott).
Analytical performances of direct measures met guideline-established benchmarks.17 For the vertical spin density-gradient ultracentrifugation, accuracy was monitored by yearly random split-sample comparison with β quantification at Washington University’s Core Laboratory for Clinical Studies (St Louis, Missouri), and triglyceride measurements were compared with those obtained at the University of Alabama School of Medicine laboratory (Birmingham). From 2009 through 2011, the following correlation coefficients were typically obtained: total cholesterol, 0.99; HDL-C, 0.99; LDL-C, 0.98; VLDL-C, 0.98; triglycerides, 0.99. Between-day intra-assay coefficients of variation were lower than 3.0% for each of these lipid parameters.
Derivation
Two-thirds of patients were randomly assigned to a derivation data set. We first explored the distribution of TG: VLDL-C, anticipating, based on prior literature,1,2 interindividual variance. After confirming interindividual variance, we performed multiple linear regression analysis examining the extent to which TG:VLDL-C was explained by information in the standard lipid profile, age, and sex. Results from this analysis guided the choice of parameters for stratification to determine strata-specific median TG:VLDL-C ratios.
Validation
One-third of patients in the study sample were randomly assigned to a validation data set. Patients were grouped by those fulfilling the Friedewald equation criterion of triglycerides lower than 400 mg/dL (to convert to millimoles per liter, multiply by 0.0113) and those with triglycerides of 400 or higher.
Friedewald LDL-C (LDL-CF) was estimated as (non–HDL-C) – (triglycerides/5) in mg/dL.1 Novel LDL-C (LDL-CN) estimates were derived as (non–HDL-C) – (triglycerides/adjustable factor mg/dL), where the adjustable factor was determined as the strata-specific median TG:VLDL-C ratio. Numerical subscripts (eg, LDL-C180 for 180-cell stratification) were used to identify variants of LDL-CN. Alternative LDL-C estimates were also calculated based on previously proposed formulas.2,18–23 The reference standard direct LDL-C (LDL-CD) was subtracted from each LDL-C estimate to determine the absolute difference in their values in milligrams per deciliter.
Direct and estimated LDL-C values were classified according to clinical practice guidelines in the United States (<70, 70–99, 100–129, 130–159, 160–189, and ≥190 mg/dL; to convert to millimole per liter, multiply by 0.0259) and Europe (<70, 70–99, 100–154, 155–189, and ≥190 mg/dL).3–5,7,8 Concordance in classification between LDL-C estimates and LDL-CD was examined in the whole study population and subgroups. The initial classification was defined by the estimated parameter because this is the parameter routinely available in clinical practice. Odds ratios (ORs) for discordance in subgroups were calculated using logistic regression. Based on prior literature,24–30 definitions of Fredrickson-Levy phenotypes are provided in eTable 4 in the Supplement.
Statistical analyses were performed in Stata (StataCorp), version 11.0, and logarithmically scaled pseudocolor encoded data density plots were generated in R (http://r-project.org), version 2.14.1. We considered a 2-tailed P of less than .05 statistically significant.
Results
Study Samples
Of 1 350 908 samples, there were 2129 children, 8165 adolescents, and 1 340 614 adults. Table 1 shows age, sex, and lipid characteristics of the derivation (n = 900 605) and validation (n = 450 303) data sets. Triglycerides were lower than 400 mg/dL in 440 179 samples in the validation data set. Patients were generally middle-aged and evenly distributed by sex, with lipid distributions similar to that seen in NHANES.
Table 1.
Characteristics of Derivation and Validation Data Sets
| Median (IQR) | |||
|---|---|---|---|
| Derivation (n = 900 605) | Validation (n = 450 303) | NHANES (n = 3035) | |
| Age, No. (%), y | 59 (49–69) | 59 (49–69) | 45 (27–63) |
| <11 | 1430 (0.2) | 699 (0.2) | 0 (0) |
| 11–<18 | 5465 (0.6) | 2700 (0.6) | 356 (11.7) |
| ≥18 | 893 710 (99.2) | 446 904 (99.2) | 2679 (88.3) |
| Sex, No. (%) a | |||
| Men | 430 148 (47.8) | 215 120 (47.8) | 1521 (50.1) |
| Women | 465 276 (51.7) | 232 539 (51.6) | 1514 (49.9) |
| Cholesterol, mg/dL | |||
| Total | 188 (159–219) | 188 (159–219) | 185 (159–214) |
| HDL-C | 52 (42–63) | 52 (42–63) | 51 (43–62) |
| Friedewald LDL-Cb | 106 (82–134) | 106 (82–134) | 108 (85–132) |
| Direct LDL-C | 108 (84–135) | 108 (84–135) | NA |
| Non–HDL-C | 133 (106–162) | 133 (106–163) | 131 (105–160) |
| VLDL-C | 22 (17–29) | 22 (17–29) | NA |
| IDL-C | 12 (8–16) | 12 (8–16) | NA |
| Lp(a)-C | 6 (4–10) | 6 (4–10) | NA |
| Triglycerides, mg/dL | 115 (82–166) | 115 (82–167) | 105 (72–154) |
| TC:HDL-C | 3.5 (2.9–4.4) | 3.5 (2.9–4.4) | 3.5 (2.9–4.4) |
| TG:VLDL-C | 5.2 (4.5–6.0) | 5.2 (4.5–6.0) | NA |
| 5th to 95th percentile, range | 3.7–7.8 | 3.7–7.8 | |
| 1st to 99th percentile, range | 3.1–9.9 | 3.1–9.9 | |
Abbreviations: HDL-C, high-density lipoprotein cholesterol; IDL-C, intermediate-density lipoprotein cholesterol; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; Lp(a)-C, lipoprotein (a) cholesterol; NA, data not available; NHANES, National Health and Nutrition Examination Survey; TG:VLDL-C, ratio of triglycerides to very low-density lipoprotein cholesterol.
SI conversion factors: To convert HDL-C, LDL-C, and total cholesterol, multiply by 0.0259; triglycerides, multiply by 0.0113.
There were 5181 patients (0.6%) missing sex data in the derivation sample and 2644 patients (0.6%) in the validation sample.
After excluding samples with triglyceride levels of 400 mg/dL or higher, there were 880 403 samples in the derivation cohort and 440 179 samples in the validation cohort.
Estimation Method Derivation and Development
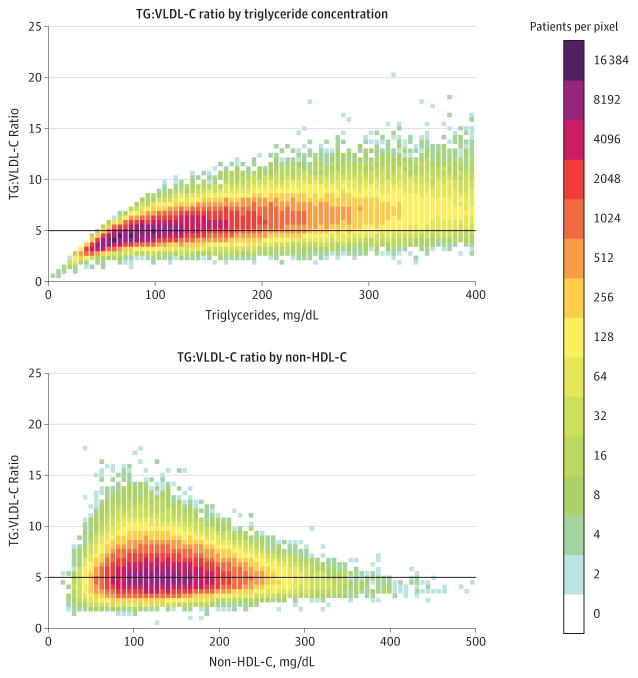
Figure 1 illustrates the distribution of TG:VLDL-C ratios in relation to triglyceride and non–HDL-C concentrations. The median ratio of TG:VLDL-C was 5.2 (interquartile range [IQR], 4.5–6.0). Approximately one-third of the samples had a TG: VLDL-C ratio of 4.5 to 5.5, and approximately two-thirds had a ratio from 4.0 to 6.0. The 5th to 95th percentile was 3.7 to 7.8; 1st to 99th percentile, 3.1 to 9.9; and the full range, 0.4 to 145.
Figure 1. Ratio of Triglycerides to Very Low-Density Lipoprotein Cholesterol by Concentrations of Triglycerides and Non–High-Density Lipoprotein Cholesterol.
Non–HDL-C indicates non–high-density lipoprotein cholesterol; TG:VLDL-C, the ratio of triglycerides to very low-density lipoprotein cholesterol. SI conversion factors: To convert non-HDL-C, multiply by 0.0259; triglycerides, multiply by 0.0113. Figure generated from derivation data set (n = 900 605). Dark horizontal lines represent a TG:VLDL-C ratio of 5, the constant factor used in the Friedewald equation. If the true TG:VLDL-C ratio is greater than 5 (pixels above line), then the Friedewald formula will tend to underestimate low-density lipoprotein cholesterol; and vice versa, if the true TG:VLDL-C is less than 5 (pixels below line). The shades of color represent increasing densities of patients per pixel, from light blue to purple.
The distribution of the TG:VLDL-C ratio was not normal (skewness, 7.1; kurtosis, 295.8). After log transformation, the TG:VLDL-C ratio was more normally distributed (skewness, 0.5; kurtosis, 5.6). In regression, the fraction of variance in the log-transformed TG:VLDL-C ratio explained by log-transformed triglycerides was 0.56 (P < .001), 0.65 (P < .001) after adding non–HDL-C to the model, and 0.66 (P < .001) if the total cholesterol and HDL-C were added as individual components. Adding age and sex to this model did not materially improve the fraction of variance explained (<0.01 improvement). There was also no material improvement by using ratio variables (total cholesterol to HDL-C, triglycerides to HDL-C, triglycerides to total cholesterol) or using higher degree fractional polynomial regressions rather than linear regression.
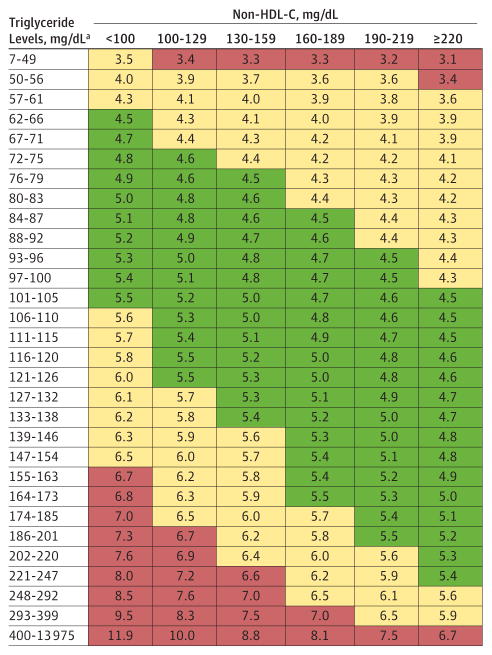
For stratification, we used triglycerides and non–HDL-C because of their performance in explaining variance in the TG: VLDL-C ratio compared with other combinations of parameters and because they capture information on the 3 core elements from the standard lipid profile. Varying the number of triglycerides and non–HDL-C strata based on quantiles or accepted cut points, we generated 2-dimensional tables of median TG:VLDL-C ratios using 10, 20, 30, 60, 90, 120, 150, 180, 200, 300, 360, 400, 720, 800, 1000, and 2000 cells. The 180-cell table is shown in Figure 2. We focused on the 180-cell results because there was a less than 0.1% overall increase in concordance estimates for guideline classification using larger tables. Cell counts and IQRs for the 180-cell table are provided in eTables 1A and 1B in the Supplement. The 10-cell, 360-cell, and 2000-cell tables of median TG:VLDL-C ratios are provided in eTables 2A, 2B, and 2C in the Supplement.
Figure 2. Median for the Ratio of Triglycerides to Very Low-Density Lipoprotein Cholesterol by Non–High-Density Lipoprotein Cholesterol and Triglyceride Strata (180-Cell).
HDL-C indicates high-density lipoprotein cholesterol. SI conversion factors: To convert HDL-C, multiply by 0.0259; triglycerides, multiply by 0.0113. Green, 4.5–5.5; yellow, 3.5–4.4, 5.6–6.5; red, <3.5, >6.5. Color banding is used to help visualize the pattern, but numerical results should be used for LDL-C estimation, rather than the boundaries or midpoints of the color ranges. Data are from the derivation data set (n = 900 605).
Validation and Concordance in Guideline Classification
Strata-specific median TG:VLDL-C ratios from the derivation data set were applied in the validation data set to generate LDL-CN estimates, including those using a 10-cell (LDL-C10), 180-cell (LDL-C180), or 360-cell (LDL-C360) table. Compared with LDL-CF, these LDL-CN estimates more closely approximated LDL-CD in patients with triglyceride levels lower than 400 mg/dL (P < .001 for each comparison). The median for (LDL-CF) – (LDL-CD) was 0.6mg/dL (5th–95th percentile, −15.4 to 5.0mg/dL), root-mean-squared error, 6.6. Examining LDL-CN – LDL-CD, the median was 0.0 mg/dL (5th–95th percentile, −6.0 to 6.6 mg/dL) for LDL-C10, root-mean- squared error, 4.4; 0.0mg/dL (5th–95th percentile, −5.0 to 6.4 mg/dL) for LDL-C180, root-mean-squared error, 4.1; and 0.0 mg/dL (5th–95th percentile, −5.0 to 6.3 mg/dL) for LDL-C360, root-mean-squared error, 4.1.
Overall concordance in guideline classification by LDL-C estimates and LDL-CD if triglyceride levels were lower than 400 mg/dL was 85.4% [95% CI, 85.3%–85.5%] for LDL-CF; 90.5% [95% CI, 90.4%–90.6%] for LDL-C10; 91.7% [95% CI, 91.6%–91.8%] for LDL-C180, and 91.7% [95% CI, 91.6%–91.8%] for LDL-C360. By individual guideline LDL-C classes, concordances are shown in Table 2. The greatest improvement in concordance with LDL-CN estimates compared with LDL-CF was observed in classifying LDL-C lower than 70 mg/dL.
Table 2.
Concordance in Guideline Classification by Friedewald vs Novel Estimates of Low-Density Lipoprotein Cholesterol (LDL-C) in Relation to Direct LDL-C if Triglycerides are Lower Than 400 mg/dL
| LDL-C, mg/dL | LDL-CF
|
LDL-C10
|
LDL-C180
|
LDL-C360
|
||||
|---|---|---|---|---|---|---|---|---|
| No. Concordant/ Total Group |
% (95% CI) | No. Concordant/ Total Group |
% (95% CI) | No. Concordant/ Total Group |
% (95% CI) | No. Concordant/ Total Group |
% (95% CI) | |
| United Statesa | ||||||||
|
| ||||||||
| ≥190 | 11 891/12 854 | 92.5 (92.0–93.0) | 12 683/14 346 | 88.4 (87.9–88.9) | 12 067/12 912 | 93.5 (93.0–93.9) | 12 084/12 942 | 93.4 (92.9–93.8) |
|
| ||||||||
| 160 to 189 | 27 322/31 232 | 87.5 (87.1–87.8) | 29 076/33 072 | 87.9 (87.6–88.3) | 29 449/33 371 | 88.3 (87.9–88.6) | 29 402/33 284 | 88.3 (88.0–88.7) |
|
| ||||||||
| 130 to 159 | 69 670/78 974 | 88.2 (88.0–88.4) | 73 471/80 368 | 91.4 (91.2–91.6) | 75 838/83 885 | 90.4 (90.2–90.6) | 76 082/84 306 | 90.3 (90.1–90.5) |
|
| ||||||||
| 100 to 129 | 109 881/126 094 | 87.1 (87.0–87.3) | 119 277/132 437 | 90.1 (89.9–90.2) | 120 515/131 804 | 91.4 (91.3–91.6) | 120 526/131 704 | 91.5 (91.4–91.7) |
|
| ||||||||
| 70 to 99 | 107 307/126 742 | 84.7 (84.5–84.9) | 114 826/125 533 | 91.5 (91.3–91.6) | 117 320/126 670 | 92.6 (92.5–92.8) | 117 258/126 491 | 92.7 (92.6–92.8) |
|
| ||||||||
| <70 | 49 641/64 283 | 77.2 (76.9–77.5) | 49 062/54 423 | 90.2 (89.9–90.4) | 48 512/51 618 | 94.0 (93.8–94.2) | 48 433/51 452 | 94.1 (93.9–94.3) |
|
| ||||||||
| Europea | ||||||||
|
| ||||||||
| ≥190 | 11 891/12 854 | 92.5 (92.0–93.0) | 12 683/14 346 | 88.4 (87.9–88.9) | 12 067/12 912 | 93.5 (93.0–93.9) | 12 084/12 942 | 93.4 (92.9–93.8) |
|
| ||||||||
| 155 to 189 | 36 218/40 625 | 89.2 (88.8–89.5) | 38 297/42 643 | 89.8 (89.5–90.1) | 38 830/43 244 | 89.8 (89.5–90.1) | 38 830/43 212 | 89.9 (89.6–90.1) |
|
| ||||||||
| 100 to 154 | 185 353/195 675 | 94.7 (94.6–94.8) | 194 068/203 234 | 95.5 (95.4–95.6) | 196 557/205 809 | 95.5 (95.4–95.6) | 196 735/206 082 | 95.5 (95.4–95.6) |
|
| ||||||||
| 70 to 99 | 107 307/126 742 | 84.7 (84.5–84.9) | 114 826/125 533 | 91.5 (91.3–91.6) | 117 320/126 670 | 92.6 (92.5–92.8) | 117 258/126 491 | 92.7 (92.6–92.8) |
|
| ||||||||
| <70 | 49 641/64 283 | 77.2 (76.9–77.5) | 49 062/54 423 | 90.2 (89.9–90.4) | 48 512/51 618 | 94.0 (93.8–92.4) | 48 433/51 452 | 94.1 (93.9–94.3) |
Abbreviations: LDL-C, low-density lipoprotein cholesterol; LDL-CF, Friedewald LDL-C; LDL-C10, 10-cell method LDL-C; LDL-C180, 180-cell method LDL-C; LDL-C360, 360-cell method LDL-C.
SI conversion factors: To convert LDL-C, multiply by 0.0259.
Data are from those with triglyceride levels lower than 400 mg/dL in the validation data set (n = 440 179). P value for difference in concordance rates between LDL-CF and each novel estimate is P < .001. Initial classification was defined by the estimated parameter; concordance was defined by agreement with direct LDL-C. United States indicates the National Cholesterol Education Program Adult Treatment Panel, American Heart Association and American College of Cardiology Foundation guidelines; Europe indicates the European Society of Cardiology and European Atherosclerosis Society guidelines.
This was particularly the case in samples with high triglyceride levels (Figure 3; P < .001 for those with triglyceride levels of 100 mg/dL or lower vs higher than 100 mg/dL). For example, of patients with estimated LDL-C levels lower than 70 mg/dL, LDL-CD was also lower than 70 mg/dL for 94.3% (95% CI, 93.9%–94.7%) of LDL-CN samples vs 79.9% (95% CI, 79.3%–80.4%) of LDL-CF samples with triglyceride levels of 100 to 149 mg/dL; 92.4% (95% CI, 91.7%–93.1%) of LDL-CN samples vs 61.3% (95% CI, 60.3%–62.3%) of LDL-CF samples with triglyceride levels of 150 to 199 mg/dL; and 84.0% (95% CI, 82.9%–85.1%) of LDL-CN samples vs 40.3% (95% CI, 39.4%–41.3%) of LDL-CF samples with triglyceride levels of 200 to 399 mg/dL.
Figure 3. Concordance of Direct Measurement With Friedewald and Novel Estimates in Classifying LDL-C Lower Than 70 mg/dL by Triglyceride Strata.
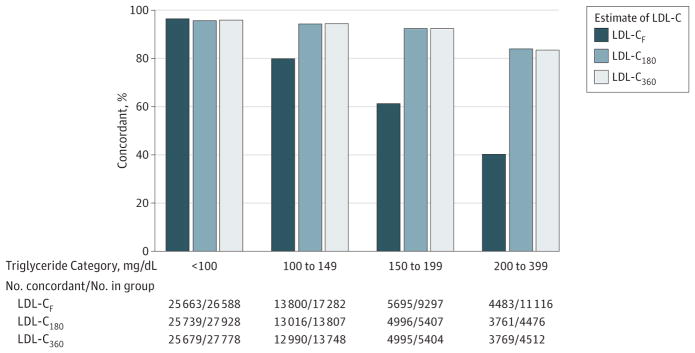
LDL-C indicates low-density lipoprotein cholesterol; F, Friedewald; 180, novel estimate by 180-cell method; and 360, novel estimate by 360-cell method. SI conversion factors: To convert LDL-C, multiply by 0.0259; triglycerides, multiply by 0.0113. Figure generated from validation data set.
In classifying LDL-C lower than 70 mg/dL, LDL-C180 and LDL-C360 were within 0.5% of each other in each triglyceride category (Figure 2), and overall concordance with LDL-CD in classifying LDL-C levels lower than 70 mg/dL was 94.0% (95% CI, 93.8%–94.2%) for LDL-C180 and 94.1% (95% CI, 93.9%–94.3%) for LDL-C360. Confidence intervals for concordance were also overlapping with LDL-C estimates using more than 360 cells, up to 2000 cells (94.3% [95% CI, 94.1%–94.5%]).
Adjusting for non–HDL-C and log-transformed triglyceride levels, there was an inverse association of age with discordance in guideline classification between LDL-C180 and LDL-CD with an OR close to 1 (OR per 10-year increase in age, 0.98 [95% CI, 0.97–0.99], P < .001), and there was no association of sex with discordance (men vs women, P = .91). In contrast, adjusting for non–HDL-C, those with triglyceride levels of 400 mg/dL or higher vs lower than 400 mg/dL had higher odds of discordance (OR, 4.73 [95% CI, 4.53–4.94]; P < .001).
Adjusting for non–HDL-C and log-transformed triglycerides, greater discordance was strongly associated with the type III phenotype, characterized by excess of remnants and higher cholesterol content of VLDL (OR, 49.9 [95% CI, 38.1–65.3], P < .001). More modest associations with greater discordance were present for the type IIa (OR, 1.05 [95% CI, 1.00–1.10], P = .049) and type IV phenotype (OR, 1.64 [95% CI, 1.58–1.71], P < .001). However, the type IIb phenotype was associated with less discordance (OR, 0.71 [95% CI, 0.66–0.77], P < .001).
eTable 3 in the Supplement shows concordance in patients with triglyceride levels of 400 mg/dL or higher; concordance with LDL-CD improved using LDL-C180 relative to LDL-CF at lower LDL-C levels, although concordance remained modest. eTable 4 in the Supplement shows concordance in the setting of Fredrickson-Levy phenotypes; compatible with the above results and the report of Friedewald et al,1 the largest discordance occurred for LDL-C180 and LDL-CF in those with type 3 phenotype.
Although multiple groups have previously proposed alternative methods for LDL-C estimation, these have not supplanted LDL-CF in routine practice.2, 18–23 To our knowledge, none have used a stratification approach as done in our study and none perform as well in classifying LDL-C based on clinical practice guidelines (see eTable 5 in the Supplement).
Discussion
We present the development and validation of a novel method for estimating LDL-C from the standard lipid profile. Rather than assuming a fixed factor of 5, it applies an adjustable factor for the TG:VLDL-C ratio based on triglyceride and non–HDL-C concentrations. The 180-cell approach could be coded into an online calculator, smartphone application, or automated laboratory reporting system. Compared with Friedewald estimation, classifications based on US and European clinical practice guidelines using LDL-C estimates by the novel method are more concordant with those by LDL-CD. The greatest advantage occurs in classification of LDL-C concentrations lower than 70 mg/dL, especially in patients with elevated triglyceride concentrations. In addition to the novel analytic approach, a major strength of this study is its size, 3015 times larger than the original Friedewald database.
The Friedewald Equation and Other Previous Methods for LDL-C Estimation
Considering interindividual variance in the TG:VLDL-C ratio and that the Friedewald equation was developed in only 448 patients with familial hyperlipoproteinemia or their relatives,1 it is remarkable how well the equation has withstood the test of time. Nevertheless, Friedewald et al1 recognized that inaccuracies in VLDL-C estimation could become more important at lower cholesterol concentrations and higher triglyceride concentrations, because VLDL-C constitutes a greater portion of the equation and errors in its estimate introduce larger relative errors in the resulting LDL-C estimate.15 It is under these circumstances that our proposed method produces the greatest improvement and in which an accurate LDL-C estimate is most crucial—in the range required for secondary prevention treatment of high-risk patients with hypertriglyceridemia.
Regarding previous methods, Delong et al2 proposed a fixed factor of 6 rather than 5; however, the overall median TG: VLDL-C ratio in our sample is closer to the original Friedewald factor of 5. Moreover, any fixed factor will not account for variance in the TG:VLDL-C ratio. This issue applies to the majority of other previous methods,18–22 including that of Chen and colleagues,20 which sets LDL-C equal to 90% of non–HDL-C plus 10% of triglycerides, and de Cordova and de Cordova,22 which takes 75% of non–HDL-C. In contrast, the equation of Rao and colleagues23 applies an adjustable factor that considers triglycerides but not cholesterol concentrations.
Implications for Patient Care
Measurement of LDL-C is of wide interest and deeply ingrained in practice. Guidelines around the globe focus on the LDL-C cut points, including guidelines from the National Heart, Lung, and Blood Institute,3–5 Canadian Cardiovascular Society,6 European Society of Cardiology and European Atherosclerosis Society,7 and the American Heart Association and American College of Cardiology.8 Some of these guidelines6,7 assign the highest level of evidence (class 1A) to LDL-C treatment goals. Low-density lipoprotein cholesterol has been a focus in the inclusion criteria of numerous clinical trials, serially quantified during trials, and used as a target for drug titration in some trials.9,10 The Cholesterol Treatment Trialists summarize the totality of evidence for statin therapy as the risk reduction indexed to a 39 mg/dL lowering of LDL-C.9,10
Without resorting to direct assays, estimation using an adjustable factor for estimation of VLDL-C seems to provide the most accurate quantification of LDL-C from patient to patient. Nevertheless, one-third of variance in the TG:VLDL-C ratio is not explained by the standard lipid profile and remains a point of caution. This unexplained variance in the TG: VLDL-C ratio represents an intrinsic error in VLDL-C estimation and is most problematic when the clinical question is if a high-risk patient with hypertriglyceridemia has attained a LDL-C level lower than 70 mg/dL. Our method is notably limited in the setting of severe hypertriglyceridemia and type III Fredrickson-Levy dyslipidemia. However, these conditions do not define the full extent of circumstances in which patients may deviate considerably from average. Although relatively uncommon in clinical practice, type III dyslipidemia serum lipid phenotype cannot be reliably identified using the standard lipid panel alone.
Another cholesterol-based parameter, non–HDL-C, is not dependent on VLDL-C estimation and has multiple other favorable characteristics, which have been reviewed elsewhere.31 Most notably, non–HDL-C includes cholesterol carried by all atherogenic apolipoprotein B–containing lipoproteins, not only that carried by LDL. Nevertheless, as one expert panel stated, “many years of public and professional education geared toward measurement of LDL cholesterol has resulted in its successful integration into the fabric of CVD [cardiovascular disease] prevention and treatment, and it would be a mistake to discontinue its use.”31
Ultimately, LDL-C and non–HDL-C may be best viewed in tandem. At low triglyceride levels, as VLDL-C approaches 0, LDL-CF approaches non–HDL-C. At triglyceride levels of 200 mg/dL or higher, guidelines recommend non–HDL-C as a treatment goal.3–5,8 Still, inherent limitations in LDL-C estimation are not a binary situation confined to patients with triglyceride levels either of 200 mg/dL or higher, supporting broader consideration of non–HDL-C in practice.
Study Limitations
The method requires validation in an independent population and using other laboratory techniques. Although lipid distributions in our sample closely matched a nationally representative population-based survey, patients who have their cholesterol concentrations measured by vertical spin, density-gradient ultracentrifugation may still be a special population. Factors such as race/ethnicity, obesity, diabetes, and insulin resistance, which may affect variance in the TG:VLDL-C ratio, were not available for analysis. It is unknown to what extent patients in our study sample were treated with lipid-modifying drug therapies, although LDL-CF is used ubiquitously in clinical practice for patients regardless of coincident drug therapy. Fasting status was not known in this study. Nonfasting lipid analysis is a common and accepted practice,32–34 and variance in TG:VLDL-C also exists in completely fasting samples.2
This study examines 1-time measurements. Therefore, this study does not address the problem of intraindividual variation in lipid levels, which is a major limitation in assessing the value of this study. It remains possible that intra-individual variation may exceed the improvement in classification provided by the new method so that there may not be a significant clinical improvement in classification. Due largely to biological variation, as well as analytical variation, there may be a 5% to 10% coefficient of variation when repeating lipid levels on the same individuals.35 Moreover, guidelines also support serial measurements to calculate a relative change with intervention (eg, goal of 50% lowering in LDL-C for high-risk patients).6,7 This study does not examine the effect of the new method on serial assessment of relative changes in LDL-C. Rather than relying on measurements at a single time point, a strategy that deserves further study is bringing the patient who returns in a few months for repeat risk factor measurements, including lipids, to further refine risk assessment and treatment decisions.36
Conclusion
In a very large sample of lipid profiles, we derived and validated a novel method to estimate LDL-C. If externally validated, when LDL-C must be known for clinical or research purposes and when direct measurement is not available or too costly, there may be an advantage to automated LDL-C estimation using a 180-cell array of TG:VLDL-C factors determined by triglyceride and non–HDL-C levels. This estimation method provided higher-fidelity estimates than the Friedewald equation or other methods, particularly when classifying LDL-C levels lower than 70 mg/dL in the presence of high triglyceride levels.
These findings require external validation, as well as assessment of their clinical importance. The novel method could be easily implemented in most laboratory reporting systems at virtually no cost.
Supplementary Material
Acknowledgments
Funding/Support: Atherotech participated in the collection of data by providing the investigators with deidentified data generated from commercial lipid analyses. Dr Martin is supported by the Pollin Cardiovascular Prevention Fellowship, as well as the Marie-Josée and Henry R. Kravis endowed fellowship. Dr Blumenthal is supported by the Kenneth Jay Pollin Professorship in Cardiology.
Role of the Sponsor: Atherotech had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Glossary
- HDL-C
high-density lipoprotein cholesterol
- LDL-C
low-density lipoprotein cholesterol
- TG
triglycerides
- VLDL-C
very low-density lipoprotein cholesterol
Footnotes
Author Contributions: Drs Martin and Jones had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Martin, Blaha, Elshazly, Kwiterovich, Jones.
Acquisition of data: Martin, Blaha, Jones.
Analysis and interpretation of data: Martin, Elshazly, Toth, Blumenthal, Jones.
Drafting of the manuscript: Martin, Blaha, Jones.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Martin, Elshazly, Jones.
Administrative, technical, or material support: Blaha, Toth, Kwiterovich, Jones.
Study supervision: Blaha, Jones.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Blaha reports pending grant funding from the National Institutes of Health and the US Food and Drug Administration and participating in a roundtable discussion with Regeneron. Dr Toth reports consulting for Amgen, AstraZeneca, Atherotech, Boehringer-Ingelheim, GlaxoSmithKline, Kowa, Liposcience, and Merck; serving on the speakers bureau for Amarin, Amgen, AstraZeneca, Genzyme, Kowa, Merck; and receiving travel accommodations from Atherotech. Dr Kwiterovich reports receiving compensation for consultancy from Merck and research grants from Pfizer, Amarin, and Merck. Dr Jones reports serving on the medical advisory board for, and receiving grant funding from, Atherotech. Drs Martin and Jones are listed on a planned provisional patent related to the novel method. No other disclosures were reported.
References
- 1.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 2.DeLong DM, DeLong ER, Wood PD, Lippel K, Rifkind BM. A comparison of methods for the estimation of plasma low- and very low-density lipoprotein cholesterol: the Lipid Research Clinics Prevalence Study. JAMA. 1986;256(17):2372–2377. [PubMed] [Google Scholar]
- 3.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 4.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). . Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 5.Grundy SM, Cleeman JI, Merz CN, et al. National Heart, Lung, and Blood Institute; American College of Cardiology Foundation; American Heart Association. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 6.Genest J, McPherson R, Frohlich J, et al. 2009 Canadian Cardiovascular Society/Canadian guidelines for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease in the adult—2009 recommendations. Can J Cardiol. 2009;25(10):567–579. doi: 10.1016/s0828-282x(09)70715-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reiner Z, Catapano AL, De Backer G, et al. European Association for Cardiovascular Prevention & Rehabilitation; ESC Committee for Practice Guidelines (CPG) 2008–2010 and 2010–2012 Committees. ESC/EAS Guidelines for the management of dyslipidaemias. Eur Heart J. 2011;32(14):1769–1818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- 8.Smith SC, Jr, Benjamin EJ, Bonow RO, et al. World Heart Federation and the Preventive Cardiovascular Nurses Association. AHA/ACCF Secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124(22):2458–2473. doi: 10.1161/CIR.0b013e318235eb4d. [DOI] [PubMed] [Google Scholar]
- 9.Baigent C, Keech A, Kearney PM, et al. Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 10.Baigent C, Blackwell L, Emberson J, et al. Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170 000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin SS, Blaha MJ, Toth PP, et al. Very large database of lipids: rationale and design [published online October 1, 2013] Clin Cardiol. 2013 doi: 10.1002/clc.22214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin SS, Blaha MJ, Elshazly MB, et al. Friedewald-estimated versus directly measured low-density lipoprotein cholesterol and treatment implications. J Am Coll Cardiol. 2013;62(8):732–739. doi: 10.1016/j.jacc.2013.01.079. [DOI] [PubMed] [Google Scholar]
- 13.Kuklina EV, Yoon PW, Keenan NL. Trends in high levels of low-density lipoprotein cholesterol in the United States, 1999–2006. JAMA. 2009;302(19):2104–2110. doi: 10.1001/jama.2009.1672. [DOI] [PubMed] [Google Scholar]
- 14.Ioannidis JP. The importance of potential studies that have not existed and registration of observational data sets. JAMA. 2012;308(6):575–576. doi: 10.1001/jama.2012.8144. [DOI] [PubMed] [Google Scholar]
- 15.Thomas L, Peterson ED. The value of statistical analysis plans in observational research: defining high-quality research from the start. JAMA. 2012;308(8):773–774. doi: 10.1001/jama.2012.9502. [DOI] [PubMed] [Google Scholar]
- 16.Kulkarni KR. Cholesterol profile measurement by vertical auto profile method. Clin Lab Med. 2006;26(4):787–802. doi: 10.1016/j.cll.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Bachorik PS, Ross JW The National Cholesterol Education Program Working Group on Lipoprotein Measurement. National Cholesterol Education Program recommendations for measurement of low-density lipoprotein cholesterol: executive summary. Clin Chem. 1995;41(10):1414–1420. [PubMed] [Google Scholar]
- 18.Hattori Y, Suzuki M, Tsushima M, et al. Development of approximate formula for LDL-chol, LDL-apo B and LDL-chol/LDL-apo B as indices of hyperapobetalipoproteinemia and small dense LDL. Atherosclerosis. 1998;138(2):289–299. doi: 10.1016/s0021-9150(98)00034-3. [DOI] [PubMed] [Google Scholar]
- 19.Anandaraja S, Narang R, Godeswar R, Laksmy R, Talwar KK. Low-density lipoprotein cholesterol estimation by a new formula in Indian population. Int J Cardiol. 2005;102(1):117–120. doi: 10.1016/j.ijcard.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Zhang X, Pan B, et al. A modified formula for calculating low-density lipoprotein cholesterol values. Lipids Health Dis. 2010;9:52. doi: 10.1186/1476-511X-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmadi SA, Boroumand MA, Gohari-Moghaddam K, Tajik P, Dibaj SM. The impact of low serum triglyceride on LDL-cholesterol estimation. Arch Iran Med. 2008;11(3):318–321. [PubMed] [Google Scholar]
- 22.de Cordova CM, de Cordova MM. A new accurate, simple formula for LDL-cholesterol estimation based on directly measured blood lipids from a large cohort. Ann Clin Biochem. 2013;50(pt 1):13–19. doi: 10.1258/acb.2012.011259. [DOI] [PubMed] [Google Scholar]
- 23.Rao A, Parker AH, el-Sheroni NA, Babelly MM. Calculation of low-density lipoprotein cholesterol with use of triglyceride/cholesterol ratios in lipoproteins compared with other calculation methods. Clin Chem. 1988;34(12):2532–2534. [PubMed] [Google Scholar]
- 24.Fredrickson DS, Levy RI, Lees RS. Fat transport in lipoproteins–an integrated approach to mechanisms and disorders. N Engl J Med. 1967;276(1):34–42. doi: 10.1056/NEJM196701052760107. [DOI] [PubMed] [Google Scholar]
- 25.Wang T, Nakajima K, Leary ET, et al. Ratio of remnant-like particle-cholesterol to serum total triglycerides is an effective alternative to ultracentrifugal and electrophoretic methods in the diagnosis of familial type III hyperlipoproteinemia. Clin Chem. 1999;45(11):1981–1987. [PubMed] [Google Scholar]
- 26.Hopkins PN, Toth PP, Ballantyne CM, Rader DJ. National Lipid Association Expert Panel on Familial Hypercholesterolemia. Familial hypercholesterolemias: prevalence, genetics, diagnosis and screening recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5(3 suppl):9–17. doi: 10.1016/j.jacl.2011.03.452. [DOI] [PubMed] [Google Scholar]
- 27.Fredrickson DS, Levy RI, Lees RS. Fat transport in lipoproteins–an integrated approach to mechanisms and disorders. N Engl J Med. 1967;276(2):94–103. doi: 10.1056/NEJM196701122760206. [DOI] [PubMed] [Google Scholar]
- 28.Fredrickson DS, Levy RI, Lees RS. Fat transport in lipoproteins–an integrated approach to mechanisms and disorders. N Engl J Med. 1967;276(3):148–56. doi: 10.1056/NEJM196701192760305. [DOI] [PubMed] [Google Scholar]
- 29.Fredrickson DS, Levy RI, Lees RS. Fat transport in lipoproteins–an integrated approach to mechanisms and disorders. N Engl J Med. 1967;276(4):215–25. doi: 10.1056/NEJM196701262760406. [DOI] [PubMed] [Google Scholar]
- 30.Fredrickson DS, Levy RI, Lees RS. Fat transport in lipoproteins–an integrated approach to mechanisms and disorders. N Engl J Med. 1967;276(5):273–81. doi: 10.1056/NEJM196702022760507. [DOI] [PubMed] [Google Scholar]
- 31.Brunzell JD, Davidson M, Furberg CD, et al. Lipoprotein management in patients with cardiometabolic risk: consensus conference report from the American Diabetes Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2008;51(15):1512–1524. doi: 10.1016/j.jacc.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 32.Mora S, Rifai N, Buring JE, Ridker PM. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation. 2008;118(10):993–1001. doi: 10.1161/CIRCULATIONAHA.108.777334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sidhu D, Naugler C. Fasting time and lipid levels in a community-based population: a cross-sectional study. Arch Intern Med. 2012;172(22):1707–1710. doi: 10.1001/archinternmed.2012.3708. [DOI] [PubMed] [Google Scholar]
- 34.Gaziano JM. Should we fast before we measure our lipids? Arch Intern Med. 2012;172(22):1705–1706. doi: 10.1001/jamainternmed.2013.1771. [DOI] [PubMed] [Google Scholar]
- 35.Hegsted DM, Nicolosi RJ. Individual variation in serum cholesterol levels. Proc Natl Acad Sci U S A. 1987;84(17):6259–6261. doi: 10.1073/pnas.84.17.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaziano JM, Wilson PW. Cardiovascular risk assessment in the 21st century. JAMA. 2012;308(8):816–817. doi: 10.1001/2012.jama.10456. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.