Abstract
Background
Statin use is frequently associated with muscle-related symptoms. Coenzyme Q10 supplementation has yielded conflicting results in decreasing statin myopathy. Herein, we tested whether coenzyme Q10 supplementation could decrease statin-associated muscular pain in a specific group of patients with mild-to-moderate muscle symptoms.
Material/Methods
Fifty patients treated with statins and reporting muscle pain were recruited. The Q10 group (n=25) received coenzyme Q10 supplementation over a period of 30 days (50 mg twice daily), and the control group (n=25) received placebo. The Brief Pain Inventory (BPI) questionnaire was used and blood testing was performed at inclusion in the study and after 30 days of supplementation.
Results
The intensity of muscle pain, measured as the Pain Severity Score (PSS), in the Q10 group was reduced from 3.9±0.4 to 2.9±0.4 (P<0.001). The Pain Interference Score (PIS) after Q10 supplementation was reduced from 4.0±0.4 to 2.6±0.4 (P<0.001). In the placebo group, PSS and PIS did not change. Coenzyme Q10 supplementation decreased statin-related muscle symptoms in 75% of patients. The relative values of PSS and PIS significantly decreased (−33.1% and −40.3%, respectively) in the Q10 group compared to placebo group (both P<0.05). From baseline, no differences in liver and muscle enzymes or cholesterol values were found.
Conclusions
The present results show that coenzyme Q10 supplementation (50 mg twice daily) effectively reduced statin-related mild-to-moderate muscular symptoms, causing lower interference of statin-related muscular symptoms with daily activities.
MeSH Keywords: Hydroxymethylglutaryl-CoA Reductases, NAD-Dependent, Myalgia, Ubiquinone
Background
Treatment with statins is highly effective in primary and secondary cardiovascular disease prevention [1,2], but their use can be associated with a variety of adverse effects, mainly affecting the muscles and liver. The most frequent symptom is myalgia, which occurs in 10–15% of patients treated with statins [3]. In primary prevention, up to 25.4% of patients stop taking statins by 6 months after the beginning of treatment [4]. For patients who cannot tolerate statin therapy, alternatives include rechallenge with a different statin, dose reduction, or statin withdrawal [5,6].
Statins inhibit the rate-limiting step in cholesterol synthesis through acting on the key enzyme 3-hydroxy-3-methylglutaryl coenzyme A (HMGCoA) reductase. Besides inhibiting the synthesis of cholesterol, biosynthesis of coenzyme Q10 is also reduced, since coenzyme Q10 is an intermediate in the mevalonate pathway [7,8]. Statins were shown to reduce serum/plasma levels of coenzyme Q10 by 16–54% and coenzyme Q10 supplementation was reported to increase the levels of coenzyme Q10 in plasma [9]. Coenzyme Q10, also known as ubiquinone, is localized in cellular membranes and participates in electron transport, and during oxidative phosphorylation in mitochondria, protects against oxidative stress and regenerates active forms of the antioxidant vitamin E [9,10]. Deficiency of coenzyme Q10 affects oxidative phosphorylation and mitochondrial adenosine triphosphate (ATP) production, and consequently impairs muscle energy metabolism. Therefore, a deficiency of coenzyme Q10 is the most probable mechanism of statin-related myopathy [7–9].
If the proposed mechanism of statin-related muscle pain is at least partially correct, coenzyme Q10 supplementation seems a logical step in its reduction. Several previous studies have reported conflicting results regarding decrease of statin-related muscle pain by coenzyme Q10 supplementation in different study populations [6,11–13]. Therefore, the aim of the present study was to test whether coenzyme Q10 supplementation could decrease mild-to-moderate statin-associated muscle pain. The specifics of the chosen group represent one of the most frequent clinical scenarios in statin-related adverse effects.
Material and Methods
Patients
Fifty patients of both sexes, aged between 40 and 65 years, with statin-related muscle pain were recruited in this study. Important inclusion criteria were the use of a statin for more than 6 months and the presence of statin-associated muscular symptoms for at least 6 months. In all enrolled patients, before the inclusion to the study, all possible efforts to decrease symptoms and to identify possible association with statin use were made as follows: gradual decreases of statin dose, de-challenge and re-challenge of statin treatment, and switch to another statin. Symptoms repeated after re-challenge or switching. Thus, the association between symptoms and statin treatment was clearly shown. Where other possible identifiable causes of myopathy were suspected, patients were not eligible for enrollment. Also, patients with serious medical conditions or any other specific disease (e.g., hepatic, vascular, renal, or endocrine) or coagulopathy were excluded. A neurologist and an orthopedist also excluded possible neurological and orthopedic reasons for muscle pain before the inclusion to the study. Exclusion criterion also included coenzyme Q10 supplementation or anticoagulant therapy.
This was a double-blind, placebo-controlled study with balanced randomization (1:1). An independent pharmacist, who was not clinically involved in the study, packed containers with either placebo or Q10 (MGC Company, Japan) and numbered them according to a simple randomization procedure (computerized random numbers). The substances, placebo or Q10, were in the form of tablets identical in appearance, packed in the same white, opaque containers. The key to the content of each numbered container was stored in the safe deposit box of the pharmacist. Each participant was assigned an order number and received the corresponding prepacked container according to the simple randomization procedure. The allocation sequence obtained was concealed in a sealed envelope from the researchers enrolling and assessing participants. The envelope was kept in possession of an independent physician. The same independent physician carried out the implementation procedure.
The control group (n=25) received placebo, and the Q10 group (n=25) received coenzyme Q10 supplementation over a period of 30 days. The patients were recruited at the Department of Vascular Diseases, University of Ljubljana Medical Center. They reported myopathic symptoms consisting of muscle pain per se or accompanied by other symptoms, such as muscle weakness, muscle fatigue, or both. A definition of mild-to-moderate muscle symptoms was used, representing the Pain Severity Score (PSS) or Pain Interference Score (PIS) of 2–6.
Study protocol
The study protocol was approved by the National Medical Ethics Committee of Slovenia and informed consent was obtained from all the patients. Enrolled patients were assessed in 2 separate visits: at the beginning and at the end of the study (i.e., Day 0 and Day 30 of the study). On the first visit, a clinical examination of each subject was performed and each subject completed a Brief Pain Inventory (BPI) questionnaire for evaluation of myopathic symptoms before intervention. The questionnaire consisted of 2 parts: a Pain Severity Score (PSS), calculated by averaging scores of the 4 pain intensity categories and a Pain Interference Score (PIS), computed by averaging values of the 7 interference items from the questionnaire. A blood sample was also obtained for the following laboratory analyses: fasting plasma lipid profile (total cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides), creatine kinase (CK) concentration, and liver function enzymes (transaminases and gamma-glutamyl transpeptidase).
Patients were then randomly assigned to receive either coenzyme Q10 supplementation (a daily supplement consisting of 50 mg of water-soluble coenzyme Q10 twice daily) or placebo twice daily for 30 days. Their assigned statin therapy was continued during the course of the study. On the second visit after completion of supplementation therapy, myopathic symptoms were re-evaluated with the BPI questionnaire and another blood sample was obtained for the above-listed laboratory analyses.
Statistical analysis
All values are expressed as means ±SEM. Intragroup differences in Q10 and placebo groups from the beginning to the end of the supplementation period were assessed by the paired t test. Intergroup comparisons of relative changes (before/after study) between Q10 and placebo group were assessed by the t test. A P-value of less than 0.05 was considered significant. All statistical analyses were performed using Graph Pad Prism 5.0 and SPSS 15.0 software.
Power analysis for the difference between the placebo and the Q10 groups for the Pain Severity Score (PSS) and Pain Interference Score (PIS) was conducted. Parameters needed for the power analysis – the difference between groups and standard deviations – were estimated from the sample. A power of 99.1% and 98.5% was achieved when PIS and PSS, respectively, were used as a response.
Results
Patient characteristics
Fifty patients were enrolled in the study, of whom 25 received coenzyme Q10 and 25 received placebo. Nineteen patients received statin therapy in secondary prevention and 31 in primary prevention. In patients who received statin in secondary prevention, intensive dose reduction was not performed and statin discontinuation lasted for a short time (not more than for 2 weeks). Patient characteristics at inclusion in the study are summarized in Table 1 and did not differ between groups. The distribution of types of statin among the patients was as follows: 22 patients received rosuvastatin 10–20 mg daily, 20 patients received atorvastatin (10 patients 40 mg daily, and 10 patients 10–20 mg daily), 4 patients received simvastatin 10–20 mg daily, 3 patients received fluvastatin 80 mg daily, and 1 patient received lovastatin 20 mg daily.
Table 1.
Characteristics of Q10 and placebo groups of patients.
| Placebo group (n=25) | Q10 group (n=25) | |
|---|---|---|
| Sex (women/men) | 13/12 | 14/11 |
| Age (years) | 65.6±2.1 | 64.5±1.9 |
| BMI (kg/m2) | 24.6±1.5 | 25.3±1.2 |
| Duration of therapy (years) | 3.2±0.9 | 4.8±1.2 |
| Duration of symptoms (years) | 2.4±0.7 | 1.9±0.4 |
All data expressed as means ±SEM. BMI – body mass index.
Intensity of myopathic pain
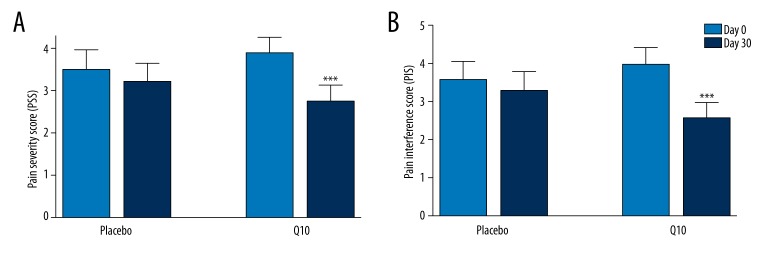
At inclusion in the study, the intensity of myopathic pain, measured as the Pain Severity Score (PSS), was similar in both groups (placebo and Q10 group). Intragroup comparison showed that after 30 days of intervention, the intensity of myopathic pain in the Q10 group was significantly reduced, from 3.9±0.4 to 2.9±0.4 (P<0.001), but there was no significant change in myopathic pain intensity in the placebo group (Figure 1A). When comparing the percentage of change between the groups (intergroup comparison), we found that the intensity of myopathic pain was significantly reduced after 30 days of intervention in the Q10 group compared to the placebo group (−33.1±5.7% and −0.4±6.3%, respectively; both P<0.05). Coenzyme Q10 supplementation decreased statin-related muscle symptoms in 75% of patients.
Figure 1.
(A) Pain Severity Score (PSS) and (B) Pain Interference Score (PIS) in placebo and Q10 groups before (Day 0, light blue columns) and after Q10 or placebo supplementation (Day 30, navy columns). All data expressed as means ±SEM. *** represents P<0.001 Day 0 vs. Day 30 in the Q10 group.
Interference of pain with daily activities
Interference of pain with daily activities was measured as the Pain Interference Score (PIS). At inclusion in the study, no significant differences in PIS between the Q10 and placebo groups were observed. Intragroup comparison showed that after Q10 intervention, PIS was significantly reduced, from 4.0±0.4 to 2.6±0.4 (P<0.001). In the placebo group, the PIS did not change significantly (Figure 1B). When comparing the percentage of change between the groups (intergroup comparison), we found that the intensity of myopathic pain was significantly reduced after 30 days of intervention in the Q10 group compared to the placebo group (−40.3±7.0% and −11.5±10.2%, respectively; both P<0.05).
Laboratory serum analyses
The results of the biochemical analyses made at the beginning and at the end of the study are presented in Table 2. There were no differences in liver and muscle enzymes or cholesterol levels in the Q10 or placebo groups during the course of the study (intragroup comparison) (Table 2).
Table 2.
Laboratory serum analysis in Q10 and placebo groups.
| Q10 group | Placebo group | |||||
|---|---|---|---|---|---|---|
| Day 0 | Day 30 | Day 0 | Day 30 | |||
| Blood lipids | ||||||
| Cholesterol (mmol/l) | 5.69±0.27 | 5.52±0.24 | NS | 5.02±0.27 | 4.88±0.24 | NS |
| LDL cholesterol (mmol/l) | 3.21±0.22 | 3.11±0.17 | NS | 2.83±0.24 | 2.71±0.20 | NS |
| Triglycerides (mmol/l) | 1.57±0.13 | 1.78±0.20 | NS | 1.65±0.22 | 1.78±0.27 | NS |
| Liver function enzymes | ||||||
| AST (μkat/L) | 0.56±0.04 | 0.56±0.04 | NS | 0.52±0.03 | 0.56±0.04 | NS |
| ALT (μkat/L) | 0.54±0.05 | 0.53±0.04 | NS | 0.55±0.06 | 0.57±0.05 | NS |
| γ-GT (μkat/L) | 0.78±0.22 | 0.81±0.21 | NS | 0.65±0.11 | 0.60±0.07 | NS |
| Muscle enzymes | ||||||
| CK (μkat/L) | 2.69±0.40 | 3.05±0.50 | NS | 2.58±0.44 | 2.86±0.64 | NS |
All data expressed as means ±SEM. LDL – low-density lipoprotein; AST – aspartate aminotransferase; ALT – alanine aminotransferase; γ-GT – γ-glutamyl transpeptidaze; CK – creatine kinase. NS – no significance
Discussion
In the present study, the possible beneficial effects of coenzyme Q10 supplementation on mild-to-moderate statin-related muscle symptoms were studied, since this is one of the most frequent reasons for statin therapy withdrawal. Previously, conflicting results have been published. We found that supplementation with coenzyme Q10 (50 mg twice daily for 30 days) reduced muscle pain and produced less interference by pain with daily life activities. Overall, coenzyme Q10 supplementation decreased statin-related muscle symptoms in 75% of patients. We observed no significant effects of coenzyme Q10 supplementation on liver and muscle enzymes, which were within normal ranges during the course of the study in both treatment groups. Also, cholesterol levels did not change significantly from the beginning to the end of the study. The observed beneficial effects of coenzyme Q10 supplementation could lead to better statin treatment compliance.
The present study was designed as a double-blind, randomized, placebo-controlled trial to evaluate the possible beneficial effect of coenzyme Q10 supplementation (50 mg twice daily for 30 days) on statin-associated muscle pain. A 30-day period was chosen because data in the literature indicate that the effect should reveal itself within that period. In its absence, extended supplementation is not recommended. Using the standardized and simple Brief Pain Inventory (BPI) questionnaire, consisting of a Pain Severity Score (PSS) and a Pain Interference Score (PIS), we found that after 30 days with coenzyme Q10 supplementation, muscle pain and the interference of pain with daily life activities in the mild-to-moderate symptom group were significantly reduced from the beginning to the end of the study (from 3.9±0.4 to 2.9±0.4 and from 4.0±0.4 to 2.6±0.4, respectively, both P<0.001). The relative values of PSS and PIS significantly decreased in the Q10 group compared to placebo group (−33.1 and −40.3%, respectively; both P<0.05), showing the effectiveness of Q10. Coenzyme Q10 supplementation did not affect liver and muscle enzymes, which were in the normal ranges in both groups during the course of the study. According to the guidelines [7,14], all patients included in our study had myalgia – muscle pain per se that could also be accompanied with other muscular symptoms such as muscle weakness, muscle fatigue, or both. Muscle enzyme creatine kinase (CK) was at normal levels. The levels of all cholesterols did not differ significantly before and after intervention in separate groups.
Conflicting results of coenzyme Q10 supplementation on statin-induced muscle pain were observed in previous studies. The beneficial effects of coenzyme Q10 were confirmed in 2 studies [11,13], but not in others [6,12]. Interestingly, Young et al. reported that coenzyme Q10 supplementation at a 200-mg daily dose (twice that used in our study) for 12 weeks did not improve myalgia in patients receiving simvastatin (in a dosage of 10–40 mg daily) [6]. In that study, the myalgia score was assessed by a visual analogue scale, which is known to be less accurate than the more complex Brief Pain Inventory (BPI) questionnaire used in our study, and this could be the reason for the negative result observed. Similar results were observed in a study by Bookstaver et al., in which coenzyme Q10 was supplemented in a dosage of 120 mg daily for 3 months [12]. It is also interesting that the intervals of observation were longer in both of these 2 studies compared to the present study. On the other hand, the most similar regimen to ours was in the study by Caso et al., in which the same dosage of coenzyme Q10 was prescribed (100 mg daily) and the BPI questionnaire was used to assess pain severity and pain interference with daily activities. Also, the duration of supplementation was the same. Like our results, a decrease in both parameters was observed after 30 days of coenzyme Q10 supplementation, but its major limitation was not being placebo-controlled [11]. From that point of view, the results obtained in the present study have greater value and confirm the beneficial effect of coenzyme Q10 supplementation. A recent study by Fedacko et al. showed that coenzyme Q10 supplementation decreased symptoms of statin-associated myopathy, intensity of muscle pain, muscle weakness, muscle cramps, and muscle tiredness [13]. Considering all data from the above-mentioned studies together, we believe that the conflicting results show a complex relation between statin-related muscle symptoms and coenzyme Q10 supplementation, probably due to the many confounding factors that influence the efficacy of coenzyme Q10 supplementation.
The limitation of the present study was the relatively small number of patients involved, which consequently limits extensive generalization of the results obtained. The main reason for the relatively small population group lay in the strict inclusion criteria, which ensured recruiting only patients with “real” statin-related muscular symptoms, who were highly motivated and informed, and consequently an accurate estimate of the intervention efficacy could be obtained. Specifically, over-subjective estimation of statin-associated muscular symptoms is a well-known problem, which could importantly affect the results and conclusions. Undoubtedly, further studies enrolling larger numbers of participants are warranted to address this problem. Importantly, patients with mild-to-moderate muscular symptoms were included, which is probably the most frequent clinical scenario in patients with statin-related symptoms. Furthermore, it is important to note that it was placebo-controlled and used widely accessible, relatively objective standardized questionnaires, allowing for great comparability. On the other hand, we do not know whether the proposed therapy is effective in all patients with statin-associated symptoms, but according to our data, it should be effective in the mild-to-moderate statin-associated symptoms group. The present study was patient-oriented and designed to focus on clinical effects of coenzyme Q10 supplementation in statin-induced muscle symptoms, and not its mechanistic background. Therefore, the concentration of coenzyme Q10 in plasma or muscles and possible mechanistic background (e.g., anti-inflammatory and anti-oxidative) were not addressed in this study.
Conclusions
The present study revealed that by simple coenzyme Q10 supplementation of the regular statin treatment, associated muscle pain in the mild-to-moderate symptoms group could be significantly reduced. Consequently, it may lead to lower interference with daily activities and higher compliance with statin treatment. These observations mean a better quality of life, besides adequate cardiovascular protection, which is the primary goal of statin therapy. The results of the present study are very promising, but require further testing in larger clinical trials, mainly to allow them to be generalized.
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
Source of support: Departmental sources
References
- 1.Naci H, Brugts JJ, Fleurence R, et al. Comparative benefits of statins in the primary and secondary prevention of major coronary events and all-cause mortality: a network meta-analysis of placebo-controlled and active-comparator trials. Eur J Prev Cardiol. 2013;20(4):641–57. doi: 10.1177/2047487313480435. [DOI] [PubMed] [Google Scholar]
- 2.Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;1:CD00481. doi: 10.1002/14651858.CD004816.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients – the PRIMO study. Cardiovasc Drugs Ther. 2005;19(6):403–14. doi: 10.1007/s10557-005-5686-z. [DOI] [PubMed] [Google Scholar]
- 4.Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA. 2002;288:462–67. doi: 10.1001/jama.288.4.462. [DOI] [PubMed] [Google Scholar]
- 5.Pasternak RC, Smith SC, Jr, Bairey-Merz CN, et al. ACC/AHA/NHLBI Clinical Advisory on the Use and Safety of Statins. Stroke. 2002;33:2337–41. doi: 10.1161/01.str.0000034125.94759.41. [DOI] [PubMed] [Google Scholar]
- 6.Young JM, Florkowski CM, Molyneux SL, et al. Effect of coenzyme Q(10) supplementation on simvastatin-induced myalgia. Am J Cardiol. 2007;100:1400–3. doi: 10.1016/j.amjcard.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 7.Harper CR, Jacobson TA. The broad spectrum of statin myopathy: from myalgia to rhabdomyolysis. Curr Opin Lipidol. 2007;18:401–8. doi: 10.1097/MOL.0b013e32825a6773. [DOI] [PubMed] [Google Scholar]
- 8.Wyman M, Leonard M, Morledge T. Coenzyme Q10: a therapy for hypertension and statin-induced myalgia? Cleve Clin J Med. 2010;77:435–42. doi: 10.3949/ccjm.77a.09078. [DOI] [PubMed] [Google Scholar]
- 9.Marcoff L, Thompson PD. The role of coenzyme Q10 in statin-associated myopathy: a systematic review. J Am Coll Cardiol. 2007;49:2231–37. doi: 10.1016/j.jacc.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 10.Potgieter M, Pretorius E, Pepper MS. Primary and secondary coenzyme Q10 deficiency: the role of therapeutic supplementation. Nutr Rev. 2013;71:180–88. doi: 10.1111/nure.12011. [DOI] [PubMed] [Google Scholar]
- 11.Caso G, Kelly P, McNurlan MA, Lawson WE. Effect of coenzyme q10 on myopathic symptoms in patients treated with statins. Am J Cardiol. 2007;99:1409–12. doi: 10.1016/j.amjcard.2006.12.063. [DOI] [PubMed] [Google Scholar]
- 12.Bookstaver DA, Burkhalter NA, Hatzigeorgiou C. Effect of coenzyme Q10 supplementation on statin-induced myalgias. Am J Cardiol. 2012;110:526–29. doi: 10.1016/j.amjcard.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 13.Fedacko J, Pella D, Fedackova P, et al. Coenzyme Q(10) and selenium in statin-associated myopathy treatment. Can J Physiol Pharmacol. 2013;91:165–70. doi: 10.1139/cjpp-2012-0118. [DOI] [PubMed] [Google Scholar]
- 14.Mancini GB, Tashakkor AY, Baker S, et al. Diagnosis, prevention, and management of statin adverse effects and intolerance: Canadian Working Group Consensus update. Can J Cardiol. 2013;29:1553–68. doi: 10.1016/j.cjca.2013.09.023. [DOI] [PubMed] [Google Scholar]