Abstract
Aim
To test the efficacy and safety of osmotic release oral system (OROS) methylphenidate (MPH) in doses up to 180 mg/day to treat attention deficit hyperactivity disorder (ADHD) and prevent any drug relapse in individuals with a co-diagnosis of ADHD and amphetamine dependence.
Design
Randomized placebo-controlled 24-week double-blind trial with parallel groups design.
Setting
Participants were recruited from medium security prisons in Sweden. The medication started within 2 weeks before release from prison and continued in out-patient care with twice-weekly visits, including once-weekly cognitive behavioural therapy.
Participants
Fifty-four men with a mean age of 42 years, currently incarcerated, meeting DSM-IV criteria for ADHD and amphetamine dependence.
Measurements
Change in self-reported ADHD symptoms, relapse to any drug use (amphetamine and other drugs) measured by urine toxicology, retention to treatment, craving and time to relapse.
Findings
The MPH-treated group reduced their ADHD symptoms during the trial (P = 0.011) and had a significantly higher proportion of drug-negative urines compared with the placebo group (P = 0.047), including more amphetamine-negative urines (P = 0.019) and better retention to treatment (P = 0.032).
Conclusions
Methylphenidate treatment reduces attention deficit hyperactivity disorder symptoms and the risk for relapse to substance use in criminal offenders with attention deficit hyperactivity disorder and substance dependence.
Keywords: ADHD, methylphenidate, pharmacotherapy, substance use
Introduction
More than a decade of research has established that adults with substance use disorders (SUD) show a high prevalence of comorbid attention deficit hyperactivity disorder (ADHD) 1. The disproportionately high prevalence rate of ADHD and SUD in prison populations 2–5 is noteworthy, and represents a major public health problem and challenge to psychiatric and prison services globally.
Methylphenidate (MPH) appears to be an effective and safe treatment in adults with ADHD in doses up to 1.3 mg/kg 6–8, but so far evidence is lacking on the efficacy of stimulant pharmacotherapy to treat comorbid ADHD and SUD. Several randomized clinical trials (RCT) of pharmacotherapy for ADHD in SUD, primarily cocaine dependence, have evaluated the utility of MPH in current users 9–11 and in recently abstinent users 12. Globally, however, the number of amphetamine-type stimulant users (estimated at 34 million) far exceeds the number of opiate and cocaine users combined 13. Recently, a high rate of ADHD has been reported in amphetamine users 14–16. Therefore, management of ADHD in amphetamine-dependent patients is of major importance.
A common denominator in clinical trials of MPH in adults with ADHD and SUD conducted to date is the dose (60–90 mg/day) tested. While effective in patients without a history of SUD 17, this dose level could be insufficient for the SUD population and might explain the poor treatment effects seen in earlier clinical trials 9–12. Evidence from brain imaging studies suggests that long-term drug use may down-regulate brain dopamine systems in chronic drug-dependent individuals 18, leading to increased tolerance to stimulants. A patient with a long history of daily illicit use of up to several grams of amphetamine is likely to need a higher dose of MPH to reduce ADHD symptoms than previously stimulant-naive patients. Clinical experience indicates a significant individual variation in therapeutic doses 17, with some individuals requiring a higher dose of stimulants to achieve a clinical response 17,19. Taken together, this suggests that a wider range of doses is needed to evaluate the efficacy and safety of MPH in substance-dependent patients with ADHD.
The study recruited participants from prison, as the majority of drug users in Swedish prisons report amphetamine as their primary substance of abuse 20. Many of these individuals are repeated offenders serving recurrent short sentences for drug-related crimes and having difficulties in accessing psychiatric services outside the prison. The study participants were included at the end of their prison term and received out-patient treatment following release from prison.
The present randomized placebo-controlled 24-week trial thus aimed to evaluate the safety and efficacy of the osmotic release oral system (OROS) MPH, in doses up to180 mg/day, to treat ADHD and prevent relapse in individuals with a co-diagnosis of ADHD and amphetamine dependence. The OROS delivery system allows an extended release of MPH over 12 hours.
Materials and methods
Participants
The study included men aged between 18 and 65 years, recruited from three medium-security prisons in Stockholm County, Sweden. Participation was voluntary and in no way affected the prison sentence. The participants received no financial compensation.
The study included participants who met the diagnostic criteria for ADHD according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) 21 and the DSM-IV diagnostic criteria for amphetamine dependence during the last 12 months prior to the current incarceration, and had used amphetamines on a minimum of 12 occasions during the last 12 weeks preceding the incarceration. Potential participants met with the study physician and the study psychologist and underwent an extensive clinical assessment, including the Adult ADHD Self-Rating Scale 22, the Wender Utah Rating Scale 23, the Structured Clinical Interview for DSM-IV I and II (SCID I and II) 24, the Addiction Severity Index (ASI) 25, Conners’ continuous performance test 26 and a short form of the Wechsler Adult Intelligence Scale–III 27. Collateral information from significant others was collected by telephone interviews. The study exclusion criteria were: (i) DSM-IV diagnosis of any other substance dependence except nicotine, currently or during the 12 months prior to incarceration, (ii) a major psychiatric disorder (e.g. schizophrenia, severe depression), (iii) current antipsychotic medication, (iv) current use of benzodiazepine, (v) traces of any of the following substances in urine: amphetamine, benzodiazepine, cannabis, cocaine, dextropropoxyphene and opiates, (vi) serious somatic disease (e.g. moderate to severe hypertension >150/95 mm Hg, hyperthyroidism) and (vii) known hypersensitivity to methylphenidate. Prior to inclusion participants underwent a physical examination, including laboratory tests for haematology and liver function, short neurological status and a basic cardiovascular examination. At any indication of heart problems the participant was referred to a specialized heart clinic for a cardiac examination, including electrocardiogram.
After receiving detailed information about the study, the research subjects signed a written consent. The trial was approved by the Regional Ethical Review Board in Stockholm and the Swedish Medical Products Agency, and conducted in accordance with Good Clinical Practice 28 and the Declaration of Helsinki 1975 29. The trial was registered in the International Standard Randomized Controlled Trial Number Register (ISRCTN) at http://www.controlled-trials.com/ISRCTN77940178.
Study design
The study profile is shown in Fig. 1. This study was a double-blind, placebo-controlled, randomized trial. The randomization list was generated by an independent pharmacist using the computer-based program design by Trombult Programing. Between March 2007 and February 2011, 54 subject numbers were randomized into two parallel groups (MPH or identical placebo) with the block size 2. Block randomization was used because of the length of the trial and the nature of the medication effect, and was unknown to the principle investigator and the study staff. The randomization code was retained by the Karolinska Pharmacy and disclosed after the end of the trial. No interim analysis was performed.
Figure 1.
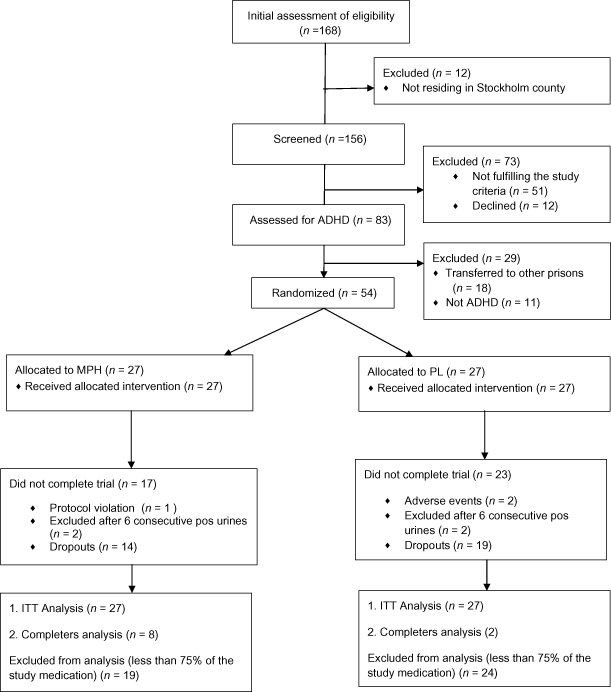
Study profile. Methylphenidate for attention deficit hyperactivity disorder (ADHD) and drug relapse in criminal offenders with substance dependence: a 24-week randomized placebo-controlled trial
Patients were required to abstain from any illicit substances during the 2 weeks preceding the inclusion, verified by patient self-reports and supervised urine toxicology. The medication started 14 days before release from prison (two participants started 3 days and one 5 days before release) and continued for 24 weeks. Like the majority of prisoners in Sweden, all participants were released on supervised probation involving mandatory meetings with a probation officer. The start dose was 18 mg MPH/placebo titrated over a period of 19 days (with 36 mg increments every 3 days), to a maximum dose of 180 mg/day. For participants who did not require or tolerate a dose increase, the dosage was adjusted and continued at that level. To enhance compliance, the subjects were picked up by a prepaid taxi at the prison gate on the day of their release and taken to the out-patient clinic, where they received study medication for 2–4 days and were asked to provide a supervised urine specimen. During the 22-week out-patient treatment phase, the participants visited the clinic twice weekly to meet the research nurse who dispensed the study medication and supervised the urine sampling. A trial completer was defined as a participant who received at least 75% of the study medication. For the MPH group, compliance was verified by analysing MPH in the urines at the end of the trial.
Once weekly, for the first 12 weeks, the participants attended individual manual-based cognitive–behavioural therapy sessions targeting relapse 30. In the case of relapse lasting longer than 3 weeks (defined as six consecutive positive or missing urines), the participant was excluded from the trial. This arbitrary time limit was selected in agreement with the Swedish Medical Products Agency as a clinically relevant requirement to balance efficacy and safety. No data were analysed after exclusions from the trial. Study participants, including dropouts, were offered medication after the 24-week trial period when this was medically justified. The hypothesis of the study was that treatment of ADHD would lead to a reduction in relapse to drug use. The primary efficacy variable was therefore defined as the proportion of urine samples negative for drugs of abuse.
Measures
ADHD symptoms were assessed with Conners’ adult ADHD self-rating scale (CAARS:SV). A total score was calculated of the 18 CAARS items measuring symptoms of inattention, impulsivity and hyperactivity, scored from 0 (not at all, never) to 3 (very much, very frequently) 26. Missing values were imputed using the individual’s mean rating for that subscale. The participants rated their ADHD symptoms once weekly for the first 6 weeks, and once every 4 weeks thereafter. Clinicians rated ADHD symptom severity and improvement on a seven-point Clinical Global Impression Scale (CGI). Outcome Questionnaire 45 (OQ45) 31 for psychiatric symptoms was completed at baseline and weeks 12 and 24.
Drug use was analysed as the proportion of urine samples negative for any of the following: amphetamines, cocaine (bensoylecgonine), cannabis [tetrahydrocannabinol carboxylic acid (THC-COOH)], opiates [morphine, codeine, 6-acetylmorphine (6-AM)], buprenorphine (buprenorphine and norbuprenorphine), benzodiazepines (oxazepam, temazepan, diazepam, 7-aminoflunitrazepam, 7-aminonitrazepam, 7-aminoclonazepam, α-hydroxyalprazolam) and dextropropoxyphene. As per protocol, the first sample each week was analysed for all drugs and the second sample for amphetamines only. The participants were unaware of the order of the analysis. Samples were screened by cloned enzyme donor immunoassay (CEDIA) 32,33. Liquid chromatography–tandem mass spectrometry was used for verification analysis of the above-mentioned drugs and for analysis of MPH and ritalinic acid in urine 32,33.
Weekly assessments included self-reports of craving for amphetamine using a seven-point Craving for Amphetamine Scale, adapted from the Desire for Alcohol scale 34, and safety assessments, including adverse events (AE) monitoring, using a standardized form. Blood pressure, pulse and weight were monitored weekly. Laboratory tests to monitor haematology and liver function were completed at weeks 4, 8, 12, 16 and 22.
Statistical analysis
The data were analysed for the intention-to-treat (ITT) population as the primary analysis. As per protocol, nine of 10 missing samples were regarded as positive and weighted with 0.9. When the participant refused to provide a sample it was always regarded as positive. The primary outcome and other drug use data were analysed using the Mann–Whitney U-test for data with non-Gaussian distributions.
For repeated measures, missing data were completed using the last observation carried forward (LOCF) method. Fisher’s exact test was calculated for the categorical variables. Retention in the trial, calculated up through the last visit at the clinic (or if the patient did not visit the clinic after the release, the day of release), was analysed using the Kaplan–Meier survival analysis. All statistical analyses were performed using IBM spss version 20.
Results
The participants were chronic intravenous amphetamine users, about 40 years of age and with 9 years of education (Table 1). The two treatment groups were comparable in terms of demographics and baseline characteristics.
Table 1.
Demographics and baseline characteristics for the methylphenidate (MPH) and placebo groups (PL): number, percentage or mean and standard deviation (SD)
| MPH (n = 27) | PL (n = 27) | P-value | |
|---|---|---|---|
| Age, mean (SD) yearsa | 41 (7.5) | 42 (11.7) | 0.874 |
| Married /cohabitant | 8 (31%) | 8 (31%) | 1.0 |
| Homelessb | 11 (41%) | 10 (37%) | 1.0 |
| Born in Swedenb | 24 (93%) | 23 (93%) | 1.0 |
| Hepatitisb | 20 (77%) | 20 (77%) | 1.0 |
| Education, mean (SD) yearsa | 9.6 (2.2) | 9.6 (1.9) | 0.866 |
| IQ estimate, mean (SD)a | 90 (9.9) | 94 (12.0) | 0.185 |
| Substance use measures | |||
| Age of onset in substance use, mean (SD) yearsa | 13.0 (1.8) | 12.2 (2.2) | 0.172 |
| Age of onset amphetamine use, mean (SD) yearsa | 18.2 (4.5) | 19.3 (7.2) | 0.535 |
| Amphetamine use by injectionb | 24 (89%) | 25 (93%) | 1.0 |
| Age of onset use by injection, mean (SD) years | 20.5 (6.2) | 20.8 (5.4) | 1.0 |
| Amphetamine use (years) life-time, mean (SD) yearsa | 20.6 (10.2) | 18.3 (12.7) | 0.495 |
| Psychiatric measures: SCID | |||
| Additional DSM-IV diagnosis | |||
| Axis Ib (n = 43) | 21 (96%) | 16 (76%) | 0.095 |
| Axis I diagnoses, mean (SD)a | 1.4 (0.7) | 1.5 (0.8) | 1.0 |
| Axis IIb | 19 (70%) | 15 (56%) | 0.264 |
| Axis II diagnoses mean (SD)a | 1.4 (1.8) | 1.6 (2.2) | 0.398 |
| Antisocial personality disorderb | 17 (63%) | 11 (41%) | 0.173 |
| Attempted suicide in life-timeb | 4 (15%) | 9 (35%) | 0.199 |
| OQ45 score mean (SD) | 111.5 (3.7) | 114.8 (3.6) | 0.522 |
| ADHD measures | |||
| Inattentive subtypeb | 4 (15%) | 3 (11%) | 0.827 |
| Hyperactive subtypeb | 3 (11%) | 5 (19%) | 0.827 |
| Combined subtypeb | 20 (74%) | 19 (70%) | 0.827 |
| Criminality measures | |||
| Age at first prison sentence, mean (SD) yearsa | 28.7 (8.7) | 27.4 (9.6) | 0.713 |
| Prison sentences, no (SD)a | 10.5 (7.3) | 12.3 (8.8) | 0.503 |
| Total length of prison sentences, mean (SD)a | 67.7 (79.4) | 62.0 (55.5) | 0.761 |
| Length of current prison sentence, mean (SD)a | 5.30 (3.76) | 6.89 (6.07) | 0.253 |
ADHD = attention deficit hyperactivity disorder; SCID = Structured Clinical Interview for DSM disorders; SD = standard deviation; at-test for independent samples; bFisher’s exact test.
Compared to the placebo group, the MPH group showed significantly greater improvement in CAARS:SV; all ADHD symptoms [95% confidence interval (CI) = −14.18 to −3.28, df = 50, P = 0.002) (Fig. 2); inattention (95% CI = −7.0 to −1.59, df = 50, P = 0.026) and hyperactivity (95% CI = −6.95 to −1.59, df = 50, P = 0.002). In the MPH group, 17 patients (65%, n = 26) decreased symptoms of inattention or hyperactivity by at least 30%, considered as a clinically relevant reduction, compared to seven patients (27%, n = 26) in the placebo group (P = 0.012).
Figure 2.
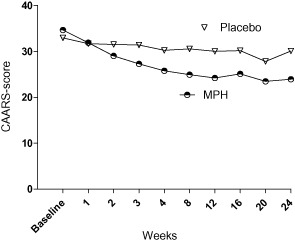
Change in self-rated attention deficit hyperactivity disorder (ADHD) symptoms (95% confidence interval = −13.78 to −1.91, P = 0.011)
In addition, in the ITT analysis, clinician-rated CGI-S decreased significantly (P = 0.039) from baseline to LOCF in the MPH group but not in the placebo group (P = 0.688); however, there was no significant difference between the treatment groups in clinician-rated improvement in CGI-I. There were no significant changes in other psychiatric symptoms.
Figure 3 shows the proportion of drug-negative urines for the two treatment groups. The ITT analysis of the primary efficacy variable (Fig. 3a) showed a significant difference in the proportion of drug-negative urines in the MPH group (Md = 23%, n = 27) compared to the placebo group (Md = 16%, n = 27) (U = 250, Z = −1.985, P = 0.047, r = 0.27). Similarly, the secondary analysis (Fig. 3b) showed significantly more amphetamine-negative urine samples in the MPH group (Md = 23%, n = 27) compared to the placebo group (Md = 14%) (U = 230, Z = −2.340, P = 0.019, r = 0.32). The same pattern was obtained when analysing for other drugs only (MPH group: Md = 44%, n = 27; placebo group: Md = 29%, n = 27) (U = 242, Z = −2.136, P = 0.032, r = 0.29) (Fig. 3c). The proportion of the respective drug (other than amphetamines) among the total number of analysed urine samples was: THC 3.7%, benzodiazepines 2.3%, buprenorphine 2.3%, cocaine 0.002% and opioids 0.002%. One sample could include traces of several drugs. Completers’ analysis was omitted due to lack of statistical power.
Figure 3.
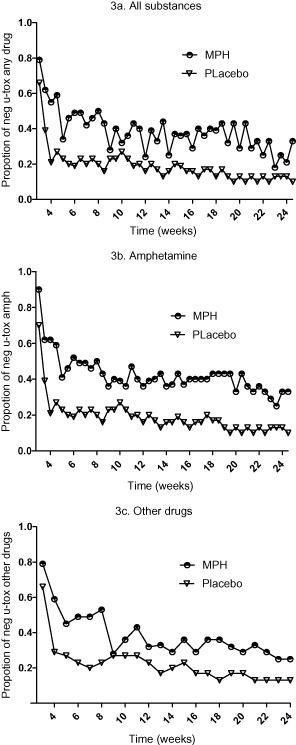
Proportion of negative urine-toxicology after release from prison (weeks 3–24) for the two treatment groups; methylphenidate (MPH) and placebo over 24 weeks of treatment: (a) any drugs amphetamine + other drugs, mean difference 95% confidence interval (CI) = 0.05–0.32; (b) amphetamines only, mean difference 95% CI = 0.07–0.36; and (c) other drugs, mean difference 95% CI = 0.02–0.25
To test the robustness of the results, we conducted several tests of sensitivity. First, urine samples that were missing in prison (due to administrative aberrations, e.g. shortage of staff, staff not complying with study protocol etc.) were omitted and only the urines in the out-patient phase were analysed. Secondly, the missing samples were imputed by 0.6–0.8. For all drugs, 0.8 gave a significance level of P = 0.037 and for amphetamines 0.6 resulted in P = 0.052.
Median retention to treatment (Fig. 4) for the MPH group was 51 days compared to 18 days for the placebo group (U = 176, Z = −3.269, P = 0.001, r = 0.44). Four patients (two in each arm) were excluded after six consecutive positive or missing urines and one additional early dropout from the MPH group was not allowed to rejoin the study at week 20. Time (days) to first positive urine for any drug was significantly shorter for the placebo group (Md = 15 days, n = 27) compared to the MPH group (Md = 29 days, n = 27) (U = 199, Z = −2.879, P = 0.004, r = 0.39), as was time to first amphetamine-positive urine (MPH: Md = 25; placebo: Md = 16) (U = 188, Zz = −3.068, P = 0.002, r = 0.42).
Figure 4.
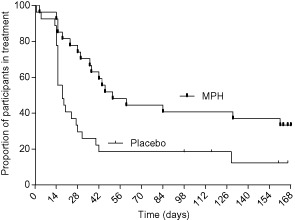
Kaplan–Meier curve for retention in treatment through to last visit at the clinic [methylphenidate (MPH): Md = 51, placebo: Md = 18; hazard ratio 0.38, 95% confidence interval = 0.174–0.647]
The two treatment groups did not differ with regard to craving at weeks 12 or 24 using LOCF. During the medication titration (weeks 1–4), craving decreased more in the MPH group compared to the placebo group. This difference reached significance at week 3 (P = 0.047).
Twenty-one patients (79%) in the MPH group and 16 patients (59%) in the placebo group completed the titration period. In the MPH group, 17 patients (63%) were stabilized on 180 mg, three (11%) on 144 mg and two (7%) on 96 mg/day. In the placebo group the median dose was 180 mg for 16 patients (59%), 144 mg for two (7%), 96 mg for eight (30%) and 18 mg for one person (4%).
Adverse events were generally mild to moderate. Table 2 presents the frequencies of AE for the two treatment groups.
Table 2.
Blood pressure, pulse and weight for the methylphenidate group from baseline to last observation carried forward (LOCF)
| Methylphenidate group | |||
|---|---|---|---|
| Baseline | LOCF | P | |
| Systolic mmHg | 129.6 ± 11.2 | 134.4 ± 16.2 | 0.901 |
| Diastolic mmHg | 83.7 ± 8.1 | 83.7 ± 12.4 | 0.163 |
| Pulse b.p.m. | 71.9 ± 4.5 | 91.7 ± 12.5 | 0.090 |
| Weight kg | 86.1 ± 11.6 | 84.1 ± 12.8 | 0.383 |
b.p.m. = beats per minute.
No unexpected adverse effects were reported. In the placebo group, one participant reported suicidal ideation at week 5 in treatment, at which point study medication was discontinued; two others required dose reduction because of high blood pressure and one because of palpitations. In the MPH group, two participants required dose reduction because of high blood pressure, one because of muscular cramps and one because of feeling edgy.
There was no significant increase in blood pressure, pulse or weight from baseline to LOCF in the MPH or the placebo group (Table 3) and no significant difference in change in blood pressure or pulse between the groups. However, in the MPH group, heart rate from baseline showed a trend to increase.
Table 3.
Frequency of adverse events in respective treatment group
| Adverse events | ||
|---|---|---|
| MPH | Placebo | |
| n = 27 | n = 27 | |
| Headache | 6 | 2 |
| Abdominal discomfort | 6 | 1 |
| Sleep problems | 6 | 2 |
| Loss of appetite | 7 | 0 |
| Depressed mood | 3 | 4 |
| Increased blood pressure | 2 | 4 |
| Sweating | 5 | 1 |
| Fatigue | 3 | 3 |
| Anxiety | 1 | 4 |
| Dry mouth | 2 | 1 |
| Craving | 1 | 2 |
| Chest pain | 1 | 2 |
| Muscular pain | 1 | 2 |
| Restlessness | 1 | 1 |
| Procrastination | 2 | 0 |
| Dizziness | 1 | 1 |
| Skin problems | 2 | 0 |
| Hears voices | 0 | 1 |
| Palpitations | 0 | 1 |
| Tics | 0 | 1 |
| Agitation | 0 | 1 |
| Lower self-esteem | 0 | 1 |
| Suicidal ideation | 0 | 1 |
MPH = methylphenidate.
The adherence to the study medication was 0.83 [standard deviation (SD) 0.25], calculated as the mean proportion of MPH-positive urines of all the urine samples provided in the MPH group (missing urines excluded). At week 24, 29% of the participants in the MPH group visited the clinic compared to 7.4% in the placebo group.
Within 6 months after the trial period, 14 participants (52%) in the MPH group and nine participants (33%) in the placebo group continued or initiated pharmacotherapy for ADHD at the trial clinic.
Discussion
To our knowledge, this is the first 24-week placebo-controlled trial evaluating the effects of high-dose MPH for the treatment of ADHD in adults with substance dependence. It was hypothesized that successful treatment of ADHD symptoms would lead to improvement in drug use outcomes.
Treatment with MPH compared to placebo significantly improved self-reported ADHD symptoms and clinician-rated severity of symptoms, reduced relapse to drug use (including amphetamine, the primary drug of abuse), and resulted in higher retention to treatment. Collectively, the outcome supports the feasibility of using stimulant medication in offenders with ADHD and substance dependence, and suggests that such treatment may act as an important reinforcer to treatment retention in this population, which is notoriously difficult to treat.
Although the primary drug of abuse in this population was amphetamine the effect of treatment with MPH was also investigated for other illicit substances, because drug-dependent individuals often use more than one type of drug. By screening for other commonly used drugs (in addition to amphetamine), the aim was to detect any potential risk of patients diverging in their drug use pattern to other illicit drugs while on treatment with MPH. The data provide evidence that a reduction in amphetamine use is mediated by the medication treatment, rather than a consequence of an increase in use of other drugs.
In the present study, MPH improved symptoms of inattention and hyperactivity/impulsivity. A minimum of 2 weeks’ abstinence was a prerequisite to be included in the trial, as it was hypothesized that treatment of ADHD would be more clinically meaningful in abstinent individuals, both in order to measure the risk for a relapse and the change in ADHD symptoms, which may otherwise be confounded with drug-induced symptoms. In the present study MPH improved symptoms of inattention and of hyperactivity/impulsivity. The lack of effect of ADHD treatment in non-abstinent individuals in earlier studies suggests that abstinence should also be a primary clinical end-point when evaluating the effect of stimulant treatment for comorbid ADHD and SUD. It may also be considered clinically less meaningful to assess the level of functional impairment with regard to ADHD in individuals who are currently using drugs.
Recruitment of confined and potentially vulnerable individuals raises important ethical questions. To minimize any harm to the potential participants, they and the prison staff were carefully informed about the voluntary nature of the study and that participation should in no way affect the circumstances in the prison. Those who were not enrolled into the study were referred to an appropriate clinic if they so desired. Studies in opiate-dependent patients 35,36 support the feasibility and effectiveness of starting medication for SUD prior to release from prison. Pharmacotherapy during a prison sentence, as found effective in long-term prisoners with ADHD and SUD 37, might even enhance retention to treatment post-incarceration, as it enables the inmates to utilize the treatment programmes more effectively while in prison.
The clinical concern of potentially inducing craving and thereby triggering relapse during titration of stimulant medication in abstinent substance-dependent individuals is not supported by this study. Instead, craving decreased significantly in the MPH group compared to the placebo group during titration. This is also corroborated by a laboratory study of MPH in cocaine-dependent patients 38 where there was no increase in cocaine craving, suggesting that the context of use may influence the subjective effects and abuse potential. Another important factor is the formulation of the medication. The reinforcing effects of stimulants are associated with rapid changes in serum concentration 39, and extended-release preparations of MPH are associated with less stimulant-like drug effects in healthy subjects 40. Of relevance to this specific psychiatric population is the fact that the OROS MPH is more difficult to use via a non-oral route (e.g. injection or intranasal use), thereby lowering the risk of abuse and diversion. However, abuse and diversion of medication should always be a concern in SUD treatment, and measures should be taken to provide a treatment setting that enhances medication compliance.
The present results are highly relevant in light of a recently published population-based study that investigated the association between the use of ADHD medication and criminality 41. In that study, ADHD medication was found to reduce all types of criminality with a 32% reduction of crime rate in men and a 41% reduction in women.
The adverse events observed in the present study were mild to moderate, and no unexpected severe adverse events were reported. Long-term safety data of MPH or other stimulant medication for ADHD in adults is largely lacking, but there is a concern from earlier studies regarding an increased risk of cardiovascular events 42. In the present study, there was no significant increase in blood pressure and pulse rate. A recent study by Stevens et al. 43 found no association between high doses of (OROS) MPH with unusually elevated plasma concentration of MPH or clinical toxicity. However, some individuals are sensitive to the cardiovascular effects of MPH, and blood pressure and pulse should always be monitored carefully.
The present clinical trial has important limitations to be considered. The sample size was relatively small, and the findings need to be replicated. The attrition rate, although significantly lower in the MPH group, was high overall. Exclusion following six consecutive positive urine samples should have only a limited effect on the results, as the excluded were few and distributed evenly (2 + 2) between the two treatment arms. Furthermore, the investigators had no means of checking whether or not the dropouts, although chronic amphetamine users, in fact relapsed to substance use for the remaining trial period. In SUD treatment trials, missing data are generally assumed to be positive, i.e. to represent relapse to drug use. By weighting the missing data by 0.9 we aimed to decrease a potential positive bias in favour of active medication.
Although efforts were made to maintain blinding, 48% of the participants receiving MPH and 48% of the placebo group identified their medication correctly during the titration phase or after reaching the maximum dose. However, many of the participants in both treatment groups remained uncertain (41% MPH, 26% placebo) or were wrong (11% MPH, 26% placebo) about which treatment arm they were allocated to.
Strengths of the study include trial length, dose range and an objectively verifiable primary outcome measure.
Taken together, the results in the present study suggest that diagnosing and treating ADHD in abstinent patients with comorbid ADHD and SUD is both feasible and effective, as it enhances the retention to treatment for SUD following release from prison, reduces the likelihood of a relapse and improves ADHD symptoms.
Acknowledgments
The authors would like to thank the Swedish National Board of Health and Welfare, the Swedish Research Council and Stockholm County Council for financial support, the Swedish Prison and Probation Service for generous practical support, the staff at Håga, Storboda and Täby prisons for excellent collaboration, the study staff for their commitment and the participants for their willingness to engage in the study.
Declaration of interests
The authors declare no conflicts of interest.
References
- van Emmerik-van Oortmerssena K, van de Glind G, van den Brink W, Smit F, Crunelle CL, Swets M, et al. Prevalence of attention-deficit hyperactivity disorder in substance use disorder patients: a meta-analysis and meta-regression analysis. Drug Alcohol Depend. 2012;122:11–19. doi: 10.1016/j.drugalcdep.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Ginsberg Y, Hirvikoski T, Lindefors N. Attention deficit hyperactivity disorder (ADHD) among longer-term prison inmates is a prevalent, persistent and disabling disorder. BMC Psychiatry. 2010;10:112. doi: 10.1186/1471-244X-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstenius M, Larsson H, Lundholm L, Philips B, van de Glind G, Jayaram-Lindström N, et al. An epidemiological study of ADHD, substance use, and comorbid problems in incarcerated women in Sweden. J Atten Disord. 2012 doi: 10.1177/1087054712451126. doi: 10.1177/1087054712451126 [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- Rosler M, Retz W, Retz-Junginger P, Hengesch G, Schneider M, Supprian T, et al. Prevalence of attention deficit-/hyperactivity disorder (ADHD) and comorbid disorders in young male prison inmates. Eur Arch Psychiatry Clin Neurosci. 2004;254:365–371. doi: 10.1007/s00406-004-0516-z. [DOI] [PubMed] [Google Scholar]
- Young SJ, Adamou M, Bolea B, Gudjonsson G, Muller U, Pitts M, et al. The identification and management of ADHD offenders within the criminal justice system: a consensus statement from the UK Adult ADHD Network and criminal justice agencies. BMC Psychiatry. 2011;11:32. doi: 10.1186/1471-244X-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler LA, Zimmerman B, Starr HL, Silber S, Palumbo J, Orman C, et al. Efficacy and safety of OROS methylphenidate in adults with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, double-blind, parallel group, dose-escalation study. J Clin Psychopharmacol. 2009;29:239–247. doi: 10.1097/JCP.0b013e3181a390ce. [DOI] [PubMed] [Google Scholar]
- Biederman J, Mick E, Surman C, Doyle R, Hammerness P, Harpold T, et al. A randomized, placebo-controlled trial of OROS methylphenidate in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2006;59:829–835. doi: 10.1016/j.biopsych.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Spencer T, Biederman J, Wilens T, Doyle R, Surman C, Prince J, et al. A large, double-blind, randomized clinical trial of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:456–463. doi: 10.1016/j.biopsych.2004.11.043. [DOI] [PubMed] [Google Scholar]
- Levin FR, Evans SM, Brooks DJ, Garawi F. Treatment of cocaine dependent treatment seekers with adult ADHD: double-blind comparison of methylphenidate and placebo. Drug Alcohol Depend. 2007;87:20–29. doi: 10.1016/j.drugalcdep.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Levin FR, Evans SM, Brooks DJ, Kalbag AS, Garawi F, Nunes EV, et al. Treatment of methadone-maintained patients with adult ADHD: double-blind comparison of methylphenidate, bupropion and placebo. Drug Alcohol Depend. 2006;81:137–148. doi: 10.1016/j.drugalcdep.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Schubiner H, Saules KK, Arfken CL, Johanson CE, Schuster CR, Lockhart N, et al. Double-blind placebo-controlled trial of methylphenidate in the treatment of adult ADHD patients with comorbid cocaine dependence. Exp Clin Psychopharmacol. 2002;10:286–294. doi: 10.1037//1064-1297.10.3.286. [DOI] [PubMed] [Google Scholar]
- Konstenius M, Jayaram-Lindstrom N, Beck O, Franck J. Sustained release methylphenidate for the treatment of ADHD in amphetamine abusers: a pilot study. Drug Alcohol Depend. 2010;108:130–133. doi: 10.1016/j.drugalcdep.2009.11.006. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime (UNODC) World Drug Report. (United Nations publication, Sales No. E.12.XI.1) [Google Scholar]
- Jayaram-Lindstrom N, Hammarberg A, Beck O, Franck J. Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. Am J Psychiatry. 2008;165:1442–1448. doi: 10.1176/appi.ajp.2008.08020304. [DOI] [PubMed] [Google Scholar]
- Kaye S, Darke S, Torok M. Attention deficit hyperactivity disorder (ADHD) among illicit psychostimulant users: a hidden disorder? Addiction. 2013;108:923–931. doi: 10.1111/add.12086. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Kamijo A, Yamaguchi A, Iseki E, Hirayasu Y. Childhood histories of attention-deficit hyperactivity disorders in Japanese methamphetamine and inhalant abusers: preliminary report. Psychiatry Clin Neurosci. 2005;59:102–105. doi: 10.1111/j.1440-1819.2005.01340.x. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Morrison NR, Prince J. An update on the pharmacotherapy of attention-deficit/hyperactivity disorder in adults. Expert Rev Neurother. 2011;11:1443–1465. doi: 10.1586/ern.11.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry. 2004;9:557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- Liebrenz M, Hof D, Buadze A, Stohler R, Eich D. High dose methylphenidate treatment in adult attention deficit hyperactivity disorder: a case report. J Med Case Rep. 2012;6:125–129. doi: 10.1186/1752-1947-6-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakansson A, Schlyter F, Berglund M. Characteristics of primary amphetamine users in Sweden: a criminal justice population examined with the Addiction Severity Index. Eur Addict Res. 2009;15:10–18. doi: 10.1159/000173004. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Press, Inc; 1994. [Google Scholar]
- Kessler RC, Adler L, Ames M, Johanson CE, Schuster CR, Lockhart N, et al. The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychol Med. 2005;35:245–256. doi: 10.1017/s0033291704002892. [DOI] [PubMed] [Google Scholar]
- Ward MF, Wender PH, Reimherr FW. The Wender Utah Rating Scale: an aid in the retrospective diagnosis of childhood attention deficit hyperactivity disorder. Am J Psychiatry. 1993;150:885–890. doi: 10.1176/ajp.150.6.885. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) Washington, DC: American Psychiatric Press, Inc; 1997. [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The fifth edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Conners C, Erhardt D, Sparrow E. Conners’ Adult ADHD Rating Scales (CAARS): Technical Manual. North Tonawanda, NY: Multi-Health Systems Inc; 1999. [Google Scholar]
- Wechsler D. WAIS-III Administration and Scoring Manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- International Conference on Harmonization (ICH) 2002. Guideline for Good Clinical Practice.
- World Medical Organization. Available at: http://www.wma.net/en/30publications/10policies/b3/
- Caroll K. A Cognitive-Behavioral Approach: Treating Cocaine Addiction. Rockville, MD: National Institute on Drug Abuse; 1998. NIH Publication no. 98-4308. [Google Scholar]
- Lambert MJ, Burlingame GM, Umphress V, Hansen NB, Vermeersch DA, Clouse GC. The reliability and validity of the Outcome Questionnaire. Clin Psychol Psychother. 1996;3:249–258. [Google Scholar]
- Gustavsson E, Andersson M, Stephanson N, Beck O. Validation of direct injection electrospray LC-MS/MS for confirmation of opiates in urine drug testing. J Mass Spectrom. 2007;42:881–889. doi: 10.1002/jms.1219. [DOI] [PubMed] [Google Scholar]
- Karlsson A, Gustavsson H, Mansson S, McAuley KB, Back SA. Dose integration characteristics in normoxic polymer gel dosimetry investigated using sequential beam irradiation. Phys Med Biol. 2007;52:4697–4706. doi: 10.1088/0031-9155/52/15/021. [DOI] [PubMed] [Google Scholar]
- Love A, James D, Willner P. A comparison of two alcohol craving questionnaires. Addiction. 1998;93:1091–1102. doi: 10.1046/j.1360-0443.1998.937109113.x. [DOI] [PubMed] [Google Scholar]
- Gordon MS, Kinlock TW, Schwartz RP, O’Grady KE. A randomized clinical trial of methadone maintenance for prisoners: findings at 6 months post-release. Addiction. 2008;103:1333–1342. doi: 10.1111/j.1360-0443.2008.002238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinlock TW, Battjes RJ, Schwartz RP. A novel opioid maintenance program for prisoners: report of post-release outcomes. Am J Drug Alcohol Abuse. 2005;31:433–454. doi: 10.1081/ada-200056804. [DOI] [PubMed] [Google Scholar]
- Ginsberg Y, Lindefors N. Methylphenidate treatment of adult male prison inmates with attention-deficit hyperactivity disorder: randomised double-blind placebo-controlled trial with open-label extension. Br J Psychiatry. 2012;200:68–73. doi: 10.1192/bjp.bp.111.092940. [DOI] [PubMed] [Google Scholar]
- Roache JD, Grabowski J, Schmitz JM, Creson DL, Rhoades HM. Laboratory measures of methylphenidate effects in cocaine-dependent patients receiving treatment. J Clin Psychopharmacol. 2000;20:61–68. doi: 10.1097/00004714-200002000-00011. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Newcorn J, Telang F, Solanto MV, Fowler JS, et al. Depressed dopamine activity in caudate and preliminary evidence of limbic involvement in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2007;64:932–940. doi: 10.1001/archpsyc.64.8.932. [DOI] [PubMed] [Google Scholar]
- Parasrampuria DA, Schoedel KA, Schuller R, Silber SA, Ciccone PE, Gu J, et al. Do formulation differences alter abuse liability of methylphenidate? A placebo-controlled, randomized, double-blind, crossover study in recreational drug users. J Clin Psychopharmacol. 2007;27:459–467. doi: 10.1097/jcp.0b013e3181515205. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Halldner L, Zetterqvist J, Sjolander A, Serlachius E, Fazel S, et al. Medication for attention deficit-hyperactivity disorder and criminality. N Engl J Med. 2012;367:2006–2014. doi: 10.1056/NEJMoa1203241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerness PG, Wilens TE, Berul CI, Elkort MS. Supraventricular tachycardia in an adolescent with attention-deficit/hyperactivity disorder (ADHD) J Am Acad Child Adolesc Psychiatry. 2008;47:219–220. doi: 10.1097/chi.0b013e31815d7485. [DOI] [PubMed] [Google Scholar]
- Stevens JR, George RA, Fusillo S, Stern TA, Wilens TE. Plasma methylphenidate concentrations in youths treated with high-dose osmotic release oral system formulation. J Child Adolesc Psychopharmacol. 2010;20:49–54. doi: 10.1089/cap.2008.0128. [DOI] [PubMed] [Google Scholar]


