Summary
Vision is widely accepted as the dominant sense in larger primates including humans, whereas olfaction is often considered a vestigial sense yielding only obscure object representations [1]. It is well documented that vision drives olfactory perception [2-3], but the converse is hardly known. Here we introduce smells to a well-established visual phenomenon termed binocular rivalry, perceptual alternations that occur when distinctively different images are separately presented to the two eyes [4]. We show that an odorant congruent to one of the competing images prolongs the time that image is visible and shortens its suppression time in a manner that is automatic, essentially independent of cognitive control, and partly subconscious. Our findings provide the first direct evidence that an olfactory cue biases the dynamic process of binocular rivalry, thereby demonstrating olfactory modulation of visual perception - an effect that has been hitherto unsuspected.
Results and Discussion
Whereas our perceptual world is interwoven with sensory inputs from various modalities, vision is commonly believed to dominate human perception ― as the saying goes “seeing is believing”. In comparison, human olfaction seems to be vague, fuzzy, and unreliable [2, 5]. It is thus not surprising that visual inputs strongly modulate olfactory perception. When visual and olfactory cues conflict with each other, olfaction is overridden by vision [2]. On the other hand, when visual cues and olfactory cues are congruent, visual cues facilitate olfactory detection, and such facilitation has been associated with enhanced neural activity in anterior hippocampus and rostromedial orbitofrontal cortex [3]. There has been little indication that the reverse could happen, that olfaction could modulate visual perception. Chemosensory emotional cues have been suggested to influence emotional perception subconsciously, but only when visual emotional cues are rendered extremely ambiguous [6]. To probe whether there is an active role of the sense of smell in the perceptual integrations of olfactory and visual cues (i.e., modulating visual perception rather than being modulated by vision), we introduced smells to a unique visual paradigm: binocular rivalry, which refers to the perceptual alternations that occur when distinctively different images are separately presented to the two eyes [4].
In Experiment 1, two odorants with the smell of rose (phenyl ethyl alcohol, PEA, 0.5% v/v in propylene glycol) and marker pen (butanol, 0.25% v/v in propylene glycol), respectively, were introduced to address whether the dynamics of binocular rivalry could be influenced by olfactory cues. In each run that lasted for 60s, the subjects viewed a composite rose/marker image through red/green anaglyph eye-glasses so that the rose and the marker images were dichoptically presented to the two eyes and engaged in rivalry. During this time subjects indicated what they saw by pressing buttons every time perception switched while being exposed continuously to PEA or butanol (Fig.1a, see Supplemental Methods for details). As compared with butanol, PEA was rated as much more like the smell of roses (p = 0.008), much less like the smell of marker pens (p < 0.0001), more pleasant (p = 0.026), and marginally less intense (p = 0.06). With the dominance time (the averaged duration between button presses) as dependent variable, repeated measures ANOVA revealed a significant interaction between olfactory condition (PEA vs. butanol) and visual image (rose vs. marker) [F(1, 11) = 8.21, p = 0.015, Fig.1b]. The dominance time of the rose image was significantly longer when the subjects smelled PEA as compared with butanol [t (11) = 2.26, p = 0.045]. Likewise, when the subjects smelled butanol as compared with PEA, the dominance time of the marker image was significantly longer [t (11) = 3.19, p = 0.009]. Although the two smells differed in pleasantness and marginally in intensity, these perceptual factors did not bias the subjects towards seeing one image versus the other (p = 0.38 and 0.35 for pleasantness and intensity, respectively, using mixed linear model analysis with olfactory condition as the factor and pleasantness and intensity ratings as the covariates). It could be argued that dominance time potentially includes instances of superimposed and piecemeal perceptions of the rivalry images, making the subjects prone to response biases. To address this possibility, we repeated the main results of Experiment 1 in a supplemental experiment (Fig. S1), in which subjects' responses were based on exclusive visibility (meaning seeing only one of the rivalry images and not any part of the other).
Figure 1.
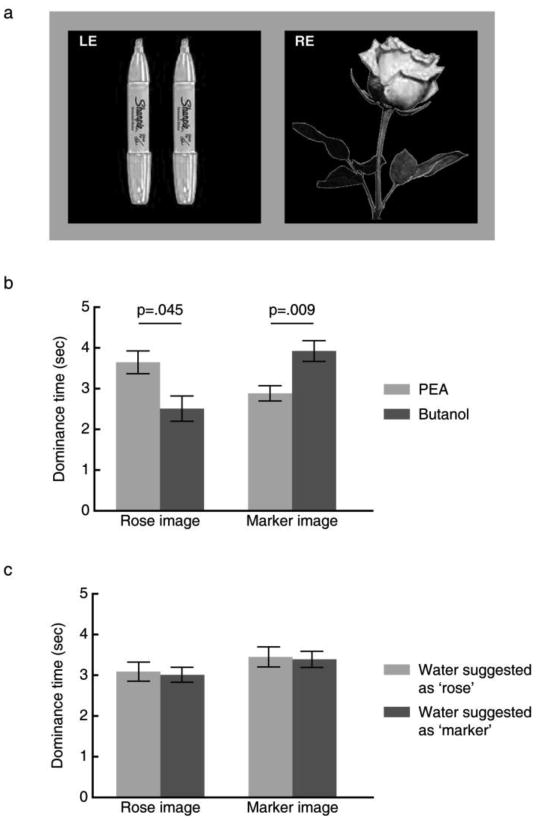
(a) Visual stimuli used in Experiment 1 and 2. When viewed through red-green anaglyph glasses, the rose image was projected to the subjects' one eye whereas the marker image was projected to the other eye. Subjects indicated when their perception switched from seeing predominantly the rose /marker image to predominantly the marker /rose image by pressing one of two buttons. (b) Olfactory cues influenced visual processing. Compared with butanol, the dominance time of the rose image was longer and the dominance time of the marker image was shorter when the subjects smelled PEA, and vice versa. (c) Suggestion did not affect binocular rivalry. The dominance time of both the rose image and the marker image remained the same under the two conditions in which purified water was suggested as containing a rose or marker smell. Error bars represent standard errors of the mean, adjusted for individual differences.
Still, the above findings could be due to a semantic bias (i.e., conceptual link between rose smell and rose image) or even to the possibility that subjects might have guessed the purpose of the experiment, rather than to the influence of olfactory cues. To investigate these alternative interpretations, we recruited an independent group of subjects in Experiment 2, who performed the same task while being exposed to two bottles of purified water. The subjects were however instructed that one of the bottles contained a low concentration of rose smell and the other contained a low concentration of marker smell, and were told which smell they were going to receive each time. Subjects rated the purified water as more pleasant (p = 0.05, one tailed), more like the smell of rose (p = 0.05, one tailed), but as similarly intense (p = 0.25), when the water was suggested as containing a rose smell as compared to a marker smell. However, despite being susceptible to suggestions when making olfactory judgments, subjects were not influenced by the suggested smell contents in perceiving one image versus the other in the binocular rivalry task. No interaction was found between olfactory condition (water suggested as containing a rose smell vs. water suggested as containing a marker smell) and visual image (rose vs. marker) [F(1, 11) = 0.004, p = 0.95]. In other words, there was no difference in the dominance time of either the rose image [t (11) = 0.27, p = 0.79] or of the marker image [t (11) = 0.18, p = 0.86] between the two olfactory conditions (water suggested as containing a rose smell vs. water suggested as containing a marker smell) (Fig.1c).
We thus conclude that the change of the temporal dynamics of binocular rivalry, as observed in Experiment 1, is not due to the intensity or pleasantness of the smells, to the semantically mediated conceptual bias, or to the cognitive control of the subjects who have guessed the purpose of the experiment. Instead, it results from the sensory congruency/incongruency between olfactory cues and visual inputs.
The olfactory cues could have exerted their modulation effect when they were congruent with the current dominant visual image, as is the case with the reported tactile modulation of binocular rivalry [7], or when they were congruent with the currently suppressed visual image. The latter would imply that olfactory modulation occurs unconsciously. To test this, in Experiment 3 we measured the time needed for the two images (rose vs. marker), respectively, to break from interocular continuous flash suppression [8-9] under the two olfactory conditions (PEA vs. butanol), a technique that targets the information processing while the stimuli remain invisible [10-11] (Fig. 2a, see Supplemental Methods for details). Again, a significant interaction was observed between olfactory condition (PEA vs. butanol) and visual image (rose vs. marker) [F (1, 13) = 52.50, p < 0.001]. When the subjects were exposed to PEA as compared with butanol, the suppression time of the rose image tended to be shorter [t (13) = −1.83, p = 0.09] and the suppression time of the marker image was longer [t (13) = 2.65, p = 0.02] (Fig. 2b) whereas accuracy was high (96.95% correct on average; see Supplemental Methods) and equal [F (1, 13) = 0.275, p = 0.61]. As the subjects did not know whether they were presented with the rose image or the marker image before they responded (by the nature of interocular suppression), this result suggests that olfactory modulation of visual processing occurs in the absence of visual awareness.
Figure 2.
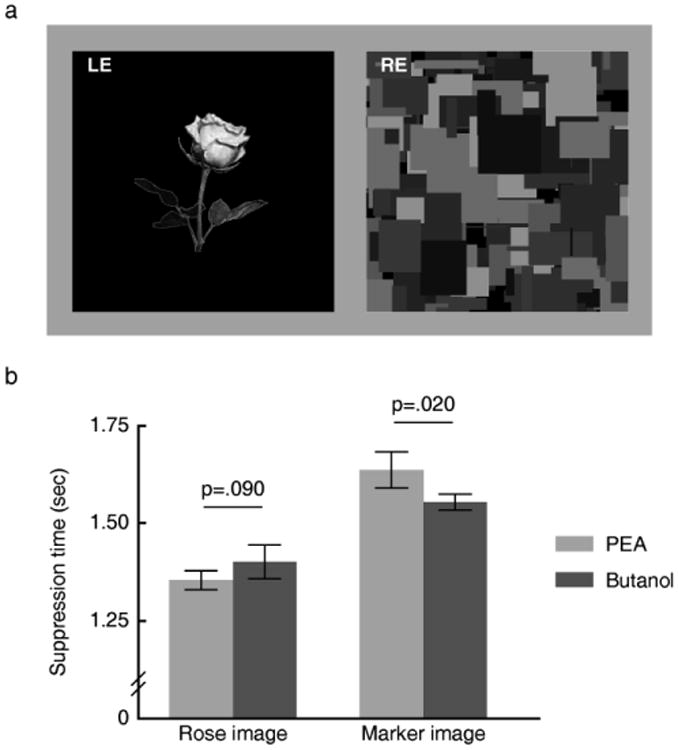
(a) Visual stimuli in Experiment 3 viewed through red-green anaglyph glasses. At the beginning of each trial, a standard dynamic noise pattern was presented to the subjects' dominant eye at full contrast, and the test figure (the rose image or the marker image) was presented to the non-dominant eye at a random location along the midline within the region corresponding to the location of the noise pattern. The contrast of the test figure was ramped up gradually from 0 to full contrast within 1 s starting from the beginning of the trial and then remained constant until the subjects made a button press to indicate whether they saw the rose image or the marker image, whereas the contrast of the dynamic noise was ramped down gradually from full contrast to 0 within 2s starting from 1s after the test figure reached its full contrast. (b) Olfactory cues modulated visual processing in the absence of visual awareness. Compared with butanol, when the subjects smelled PEA the suppression time of the rose image tended to be shorter and the suppression time of the marker image was longer. Error bars represent standard errors of the mean, adjusted for individual differences.
The dynamic process of binocular rivalry is known to be influenced by visual factors like contrast [12], brightness [13], contour density [14], visual context [15], and to a certain extent visual attention [16-17]. More recently it has been demonstrated to be modulated by auditory [18] and tactile [7] cues. Here we provide the first empirical evidence that it can also be affected by olfactory inputs.
Animals range and forage using a combination of olfactory and visual cues [19]. Extensive neuroanatomical convergence has been identified between retinal and olfactory projections [20] and higher visual and olfactory regions [21], which likely contributes to the integration of olfactory and visual inputs, and hence to the sensory modulation of vision by olfaction observed here. In binocular rivalry, the competition between the information from the two eyes potentially occurs at multiple stages of visual processing [4, 22] and has been suggested to be functionally accounted for in terms of predictive coding in a Bayesian framework [23]. As the observed effects rely on the association between a visual object and its smell, olfactory information may influence visual processing at visual object representation stages: strengthening the representation of one object and/or weakening the other, in a manner that is automatic, essentially independent of cognitive control, and partly subconscious.
In summary, by introducing olfactory cues to the binocular rivalry paradigm, we showed, for the first time, that the dynamic process of binocular rivalry can be influenced by olfactory cues. Our discovery adds to the sensory integration literature [24-26] and unambiguously demonstrates that olfaction can modulate visual processing. In other words, the eyes are inclined to see what the nose smells.
Supplementary Material
Highlights.
Olfactory information modulates the dominance and suppression of visual percepts in binocular rivalry.
Such modulation is essentially independent of cognitive control and can even occur in the absence of visual awareness.
Acknowledgments
We thank Jennifer Chen, Tracey Isidro, Shumaila Sarfani, and Li Wang for assistance. This work was supported by NIH R03DC4956 and the Knowledge Innovation Program of the Chinese Academy of Sciences (KSCX2-YW-R-250, KSCX2-YW-R-248, and 09CX192019).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ackerman D. A natural history of the senses. London: Vintage; 1991. [Google Scholar]
- 2.Morrot G, Brochet F, Dubourdieu D. The color of odors. Brain Lang. 2001;79:309–320. doi: 10.1006/brln.2001.2493. [DOI] [PubMed] [Google Scholar]
- 3.Gottfried JA, Dolan RJ. The nose smells what the eye sees: crossmodal visual facilitation of human olfactory perception. Neuron. 2003;39:375–386. doi: 10.1016/s0896-6273(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 4.Blake R, Logothetis NK. Visual competition. Nat Rev Neurosci. 2002;3:13–21. doi: 10.1038/nrn701. [DOI] [PubMed] [Google Scholar]
- 5.Cain WS. To know with the nose: keys to odor identification. Science. 1979;203:467–470. doi: 10.1126/science.760202. [DOI] [PubMed] [Google Scholar]
- 6.Zhou W, Chen D. Fear-related chemosignals modulate recognition of fear in ambiguous facial expressions. Psychol Sci. 2009;20:177–183. doi: 10.1111/j.1467-9280.2009.02263.x. [DOI] [PubMed] [Google Scholar]
- 7.Lunghi C, Binda P, Morrone MC. Touch disambiguates rivalrous perception at early stages of visual analysis. Curr Biol. 2010;20:R143–144. doi: 10.1016/j.cub.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 8.Tsuchiya N, Koch C. Continuous flash suppression reduces negative afterimages. Nat Neurosci. 2005;8:1096–1101. doi: 10.1038/nn1500. [DOI] [PubMed] [Google Scholar]
- 9.Fang F, He S. Cortical responses to invisible objects in the human dorsal and ventral pathways. Nat Neurosci. 2005;8:1380–1385. doi: 10.1038/nn1537. [DOI] [PubMed] [Google Scholar]
- 10.Jiang Y, Costello P, He S. Processing of invisible stimuli: advantage of upright faces and recognizable words in overcoming interocular suppression. Psychol Sci. 2007;18:349–355. doi: 10.1111/j.1467-9280.2007.01902.x. [DOI] [PubMed] [Google Scholar]
- 11.Yang E, Zald DH, Blake R. Fearful expressions gain preferential access to awareness during continuous flash suppression. Emotion. 2007;7:882–886. doi: 10.1037/1528-3542.7.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mueller TJ, Blake R. A fresh look at the temporal dynamics of binocular rivalry. Biol Cybern. 1989;61:223–232. doi: 10.1007/BF00198769. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan IT, Metlay W. Light Intensity and Binocular Rivalry. J Exp Psychol. 1964;67:22–26. doi: 10.1037/h0041379. [DOI] [PubMed] [Google Scholar]
- 14.Fahle M. Binocular rivalry: suppression depends on orientation and spatial frequency. Vision Res. 1982;22:787–800. doi: 10.1016/0042-6989(82)90010-4. [DOI] [PubMed] [Google Scholar]
- 15.Alais D, Blake R. Grouping visual features during binocular rivalry. Vision Res. 1999;39:4341–4353. doi: 10.1016/s0042-6989(99)00146-7. [DOI] [PubMed] [Google Scholar]
- 16.Meng M, Tong F. Can attention selectively bias bistable perception? Differences between binocular rivalry and ambiguous figures. J Vis. 2004;4:539–551. doi: 10.1167/4.7.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chong SC, Tadin D, Blake R. Endogenous attention prolongs dominance durations in binocular rivalry. J Vis. 2005;5:1004–1012. doi: 10.1167/5.11.6. [DOI] [PubMed] [Google Scholar]
- 18.van Ee R, van Boxtel JJ, Parker AL, Alais D. Multisensory congruency as a mechanism for attentional control over perceptual selection. J Neurosci. 2009;29:11641–11649. doi: 10.1523/JNEUROSCI.0873-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wyatt TD. Pheromones and animal behavior: Communication by smell and taste. London: Cambridge University Press; 2003. [Google Scholar]
- 20.Cooper HM, Parvopassu F, Herbin M, Magnin M. Neuroanatomical pathways linking vision and olfaction in mammals. Psychoneuroendocrinology. 1994;19:623–639. doi: 10.1016/0306-4530(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 21.Gottfried JA. Smell: central nervous processing. Adv Otorhinolaryngol. 2006;63:44–69. doi: 10.1159/000093750. [DOI] [PubMed] [Google Scholar]
- 22.Tong F, Meng M, Blake R. Neural bases of binocular rivalry. Trends Cogn Sci. 2006;10:502–511. doi: 10.1016/j.tics.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Hohwy J, Roepstorff A, Friston K. Predictive coding explains binocular rivalry: an epistemological review. Cognition. 2008;108:687–701. doi: 10.1016/j.cognition.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 24.McGurk H, MacDonald J. Hearing lips and seeing voices. Nature. 1976;264:746–748. doi: 10.1038/264746a0. [DOI] [PubMed] [Google Scholar]
- 25.Blake R, Sobel KV, James TW. Neural synergy between kinetic vision and touch. Psychol Sci. 2004;15:397–402. doi: 10.1111/j.0956-7976.2004.00691.x. [DOI] [PubMed] [Google Scholar]
- 26.Beauchamp MS. See me, hear me, touch me: multisensory integration in lateral occipital-temporal cortex. Curr Opin Neurobiol. 2005;15:145–153. doi: 10.1016/j.conb.2005.03.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


