Abstract
Purpose of review
A key factor driving AIDS-associated immunopathogenesis is chronic immune activation. SIV infection of African natural host species leads to high viremia, but low immune activation and absence of disease. Considerable progress in our understanding of pathological immune activation have come from comparative studies of SIV infection in pathogenic Asian macaque species and natural hosts. The focus of this review is to highlight recent work on the natural host model using high throughput genomics.
Recent findings
Several groups have independently conducted microarray gene expression profiling comparing in vivo SIV infection in natural and non-natural hosts. A consistent finding between these studies is that both pathogenic SIV infection of macaques and nonpathogenic infections of natural hosts have strong induction of interferon-stimulated genes (ISGs) early on, but a key difference was that natural hosts downmodulated the interferon response rapidly after acute infection. The development of new genome-based resources for further study of the natural host model is discussed.
Summary
Initial efforts using high throughput biology to study SIV infection of natural hosts have effectively identified the ability of natural hosts to resolve interferon responses and immune activation. Further application of ‘omic’-based technologies coupled with integrative systems-based analysis should continue to yield progress.
Keywords: Simian Immunodeficiency Virus, Microarray, Interferon, ISG, interferon stimulated genes, Genome, Sooty Mangabey, Cercocebus Atys, African green monkey, vervet, Chlorocebus pygerythrus, grivet, Chlorocebus aethiops, sabaeus, Chlorocebus sabaeus, tantalus, Chlorocebus tantalus, Natural host, Rhesus, Macaca mulatta, Systems biology
Introduction
HIV-induced immunodeficiency is characterized by progressive functional impairment, loss of CD4+ T cells, and excessive, generalized immune activation. Chronic immune activation is theorized to drive CD4+ T cell depletion and progression towards AIDS[1], and understanding the underlying pathophysiology remains one of the most important questions in HIV research. African nonhuman primates (NHPs) represent the only reservoir of simian immunodeficiency viruses (SIV) in the wild. These lentiviruses are the ancestors of the human immunodeficiency viruses (HIV-1 and HIV-2)[2-4]. For three African NHPs, sooty mangabeys (SMs), African green monkeys (AGMs) and mandrills, it has been demonstrated that SIV infection is nonpathogenic[5,6]. In contrast, Asian and New World NHPs are not infected with SIV in their natural habitat. Macaque species, endemic to Asia, can be experimentally infected with virus strains derived from SM, collectively termed SIVmac, and progress to AIDS with clinical hallmarks similar to human disease. An important distinction between nonpathogenic infection of natural reservoir species of SIV compared to pathogenic SIV infection of Asian macaques species is that natural hosts do not exhibit chronic immune activation despite virus replication levels in blood and gut as high as in HIV-1 infection in humans and SIVmac infection in macaques[5]. Comparative study of SIV infection in African natural host species and nonnatural, pathogenic macaques is an important tool for understanding the pathophysiology of AIDS[5]. Several hypotheses, not entirely mutually exclusive, have been proposed in the literature to explain the lack of chronic immune activation in natural hosts[5,6]
Amongst viral factors, a role for the differential activity of the NEF protein between SIVmac and SIVsm strains has been suggested[7]. NEF derived from SIVmac strains cannot downregulate CD3 on T cells as efficiently as viruses from natural hosts, which may protect infected cells from TCR-mediated activation[8]. Considering host factors, several important differences have been observed for CD4+ T cells between natural and nonnatural hosts. In SIV-infected natural hosts, CD4+ T cells do not show increased susceptibility to apoptosis and bystander cell death[9-13]. Infection of central memory CD4+ T cells (TCM) is limited in natural hosts compared to which might confer protection[14-17]. In the gut, TCM are dramatically reduced during acute infection in natural hosts, with partial reconstitution. Gut-resident Th17 cells, and the balance between Th17 and CD25+ Treg cells, are preserved in natural hosts[18,19], which may contribute to the lack of microbial translocation and chronic immune activation[14,20]. A comprehensive review comparing the immune system of natural and nonnatural hosts during SIV infection has recently been reported[21]. The remainder of this review will examine the impact of genomic technology on the study of natural host biology and highlight developments that will accelerate future research.
Lessons learned about SIV/natural host biology using high-throughput genomics: resolution of immune activation
In 2009 four separate studies were published using microarray technology to characterize genome-wide expression during in vivo SIV infection of SMs[22] and AGMs[19,23,24]. These studies were conducted in a comparative fashion, performing parallel SIV infection of natural hosts with non-natural host macaque species (Table 1). Despite using distinct platforms, the results from all four studies were in remarkable concordance. SIV infection induced expression of an expansive array of antiviral genes in all species during the acute infection phase (1 to ~30-60 days post-infection). This antiviral response was comprised of transcripts encoding restriction factors, chemokines, genes regulating adaptive immune responses, and interferon stimulated genes (ISGs). Collectively, these works demonstrated conclusively that natural host species exhibit widespread induction of innate immunity, including the interferon (IFN) system, in response to SIV. An important difference in the immune response between natural and pathogenic hosts was the duration: after the initial wave of ISG induction in SMs and AGMs, expression returned to baseline levels in the post-acute phase (>30 days). In contrast, rhesus macaques (RMs) maintained elevated expression of ISGs chronically (>180 days).
Table 1.
Large scale microarray analyses characterizing in vivo SIV infection of natural host species.
| Reference | Natural Host Species & Virus Strain |
Pathogenic Species & Virus Strain |
Tissue | Array Platform |
# chips |
Unique Feature |
|---|---|---|---|---|---|---|
| Lederer et al. PLoS Pathog 2009 [23] Favre et al. PLoS Pathog 2009 [19] |
AGM C. sabeus SIVagm.92018 |
PTM M. nemestrina SIVagm.92018 |
Blood LN Colon |
Agilent/ Katze 22k Rhesus Macaque |
90 | Same strain of virus for both species; includes colon; monitors Th17 cells in gut |
| Jacquelin et al. [24] J Clin Invest 2009 |
AGM C. sabeus SIVagm.92018 |
RM M. mulatta SIVmac251 |
PB CD4 LN CD4 |
Applied Biosystems Human |
201 | Transcriptomes from isolated CD4+ T cells; extensive longitudinal sampling (>1 yr) |
| Bosinger et al. J Clin Invest 2009 [22] |
SM C. atys SIVsmm |
RM M. mulatta SIVsmm & SIVmac239 |
Blood LN |
Affymetrix Rhesus Macaque |
120 | Used sooty mangabey; included infected RM cohorts of high and low pathogenicity; Same strain of virus for natural and pathogenic species |
Previously, most work on natural hosts had used cross-sectional analyses of chronically infected animals, or limited sampling during acute infection and did not observe the initial burst of antiviral responses[12,25,26]. As a result, initial models proposed that innate pathways in the anti-SIV response of natural hosts were silenced or largely reduced. Subsequent studies took advantage of more frequent sampling during very early intervals of infection (3-30 days) and reported three key findings: (i) during acute infection, elevated levels of activation (DR) or proliferation (Ki67) markers on CD4+ T cells in SMs[27] and CD8+ T cells in AGMs[28] and SMs[29]; (ii) transient recruitment of pDCs to lymph nodes during acute SIVagm infection[30]; and (iii) recruitment of cells expressing the activation/exhaustion marker PD1 in lymph nodes after SIVsm infection[31], Despite these observations, and the assertion by the authors that timely homeostatic mechanisms were at play, the concepts that natural hosts (i) displayed an acute-phase immune response to SIV, and (ii) subsequently resolved early innate responses, were not fully appreciated. In this regard, high-throughput genomics were key, as they showed that early immune activation after SIV infection in natural hosts was not limited to isolated pathways involving the CD8+ T cell response, but was indicative of system-wide activation.
The most striking finding in the microarray screens was the observation that SMs and AGMs exhibited widespread upregulation of ISGs during acute SIV infection, and that, for the majority of ISGs, expression returned to baseline during chronic infection[19,22-24]. In contrast, ISG expression remained elevated in chronic infection in RMs and PTMs (pig-tailed macaques). Furthermore, expression of IFNα protein in the lymph nodes of SIV-infected SMs and AGMs was readily apparent during acute infection[32]. Collectively, these studies indicate that SIV infection of natural hosts induces an intact IFN response during the acute phase of infection that resolves during the transition to chronic infection, while non-natural hosts maintain ISG expression indefinitely[33]. The role of Type I IFN in HIV infection has long been considered to be a double-edged sword; it promotes an antiviral state in target cells that act to limit viral replication and spread, however it also induces nonspecific activation in lymphocytes, inhibits hematopoietic potential, skews Th differentiation and contributes to T cell apoptosis and activation[34,35]. Similar to SIV-infected macaques, persistent ISG expression has also been demonstrated in CD4+ and CD8+ T cells from patients chronically infected with HIV[36,37], thus, the ability of natural host species to limit the type I Interferon response may be a critical mechanism to avoid long term immune activation.
Recent unpublished work from our group tested the hypothesis that IFNα is capable of driving immune activation in vivo by administering recombinant RM IFNα2 (rmIFNα) to SIV+ SMs weekly for a period of 4 months. Microarray analysis showed induction of ISG expression in blood for the first 21 days, albeit at lower fold-changes compared to those seen in acute SIV infection; by day 42 ISG expression had largely disappeared, coinciding with the appearance of rmIFNα-specific antibodies in sera. However, the period of ISG upregulation/rmIFNα bioactivity yielded several interesting observations: (i) plasma viral loads decreased by approximately one-log (ii) peripheral blood CD8+ T lymphocytes significantly decreased (iii) peripheral CD4+ T cells declined (iv) Ki67+ expression in CD4+ cells and CD4+ memory subsets were elevated, although modestly. While short-lived, the observed transient increase in Ki67+ lymphocytes, even in the face of reduced viremia, suggests that IFNα may be able to contribute to immune activation in natural hosts if left unchecked.
On the horizon - new technologies, new cohorts, new genomes
Microarrays, as applied to the study of natural hosts, are hampered by a few technical limitations: (i) static content (ii) probes specific for RM and/or human sequences (iii) limited dynamic range[38]. RNA-seq technology employs next-generation sequencing platforms for transcriptomic profiling[39]. The study of SIV infection in natural hosts using RNA-seq offers insight beyond microarrays, particularly in identifying sequence variation between orthologous transcripts and splicing differences and will be particularly useful for investigating the role of noncoding RNAs, such as snRNAs, lncRNAs, and regulatory miRNAs. RNA-seq in NHPs has been limited, in part, due to a lack of reference genomes to efficiently identify sequencing reads. To improve the accuracy and efficiency of RNA-seq in primates, a co-operative effort led by the Katze laboratory at the University of Washington, between Illumina Inc. and the primate genetics community, has assembled pooled tissue RNA libraries from 14 NHP species, obtained high density sequencing data and is currently annotating the transcriptomes for reference usage (http://physiology.med.cornell.edu/faculty/mason/lab/styled/). The advent of next generation sequencing technology has substantially reduced the cost of de novo genome sequencing projects such that they can be borne by individual centers. Sequencing of the SM and AGM genomes is currently underway, with details in Table 2. The complete genome sequences of two natural host species will be an invaluable tool for comparative genetics aimed at identifying genes potentially under selective pressure by SIV.
Table 2.
Genome sequencing projects of SIV natural host species.
| Natural Host |
Species | Centers | Sequencing Platforms |
Details | Website |
|---|---|---|---|---|---|
| AGM | Caribbean C. sabeus, C. aethiops, C. tantalus, C.pygerythr us, C. aethiops cynosures, West African C. sabeus |
McGill, UCLA, Wake Forest Primate Center |
Illumina | 10x average coverage of Caribbean C. Sabeus planned; paired end libraries of other species |
http://genomequebec.mcgill.ca/compgen/submit_db/vervet_web/index2
www.vervetgenome.org |
| SM | C. Atys | Baylor Human Genome Sequencing Center, Yerkes National Primate Research Center |
Pacific Biosciences, Illumina |
80x average coverage; Reference transcriptomes of multiple tissues |
n/a |
A conceptual challenge in the study of natural hosts is that of translation: comparative differences between SIV-infected natural hosts and macaques do not necessarily represent HIV infection. Recently, however, a rare group of HIV-infected patients, termed Viremic Non-progressors (VNPs), have been identified that exhibit clinical features similar to those observed in natural hosts: stable CD4+ T cell count, low levels of lymphocyte immune activation despite high plasma viremia[40]. Extensive genetic analysis of five VNPs was recently reported[41]. Interestingly, SMs shared overlapping features: genes upregulated by SIV in SMs (but not RMs) were enriched in VNPs compared to rapid progressors (RPs); further, ISGs identified as being downregulated by SMs were expressed at a higher level in RPs compared to VNPs. This latter finding is of special interest considering that plasma viral loads of VNPs were remarkably higher than RPs (median 5.4 vs 4.7 log10 copies/ml). Thus, a common response between SIV-infected SMs and VNPs is a lowered interferon response. The observation that VNPs and SMs have shared gene expression responses indicates that natural hosts are able to, at least in part, recapitulate immunologic features of lentivirus infection relevant to humans.
Harnessing the potential of systems biology to understand the natural host phenotype
The field of systems biology has been defined in different ways, but is consistently comprised of three activities: (i) acquisition of high-throughput data at multiple biological levels over time or between phenotypic classes; (ii) a computational component to integrate diverse data and make predictive models; (iii) an iterative cycle of local perturbations of the system that validate predictions and refine the model[42-45]. Microarray-based studies of transcriptional changes induced in SMs and AGMs by SIV infection have yielded considerable insight. However, a complete understanding of the molecular underpinnings that regulate the natural host phenotype will require a fully integrated analysis that incorporates the wealth of available clinical, immunological, genetic, and transcriptomic data. In the remaining section, we describe how systems-based approaches can be used to: (i) explore the restriction of SIV infection to ‘expendable’ target cells in natural hosts; (ii) investigate the resolution of immune activation, (iii) identify gene signatures predictive of disease progression.
Viral replication in SMs and AGMs occurs primarily in short-lived cells, i.e. CD4+ T cells, with estimates similar to HIV infection[46-48]. However, evidence indicates that natural hosts have evolved mechanisms to limit SIV entry into the CD4+ T cell pool: reduced levels of CCR5 on target cells[16]; loss-of-function mutations in CCR5 in SMs[49]; CD4null memory subsets capable of TH activity in AGMs[15] and reduced expression of CCR5 on CD4+ TCM in SMs[50]. Because the depletion of the CD4+ TCM pool predicts disease progression in SIV-infected macaques[51] reduction of entry receptors may be a mechanism by which natural hosts shift infection from the TCM pool into other ‘expendable’ targets. However, entry blockade is not the only weapon in the host arsenal to prevent lentiviral infection. Mammalian genomes encode genes for restriction factors, which function to make target cells nonpermissive to productive retroviral infection[52]. During SIV infection, natural and non-natural hosts express an overlapping, but different, array of restriction factors[22]. The prospect that restriction factors may be partially responsible for the observed differences in the pattern of infected cells between natural and nonnatural hosts is intriguing, however testing this hypothesis with conventional experimentation is challenging due to the vast number of potential restriction factors and interspecies diversity. Recently, a high-throughput method for screening the activity of restriction factors was described[53]. Restriction factor screening, when integrated with comparative genetics and transcriptomic data from discrete lymphocyte pools, should accelerate discovery of intrinsic antiviral effector molecules contributing to the natural host phenotype.
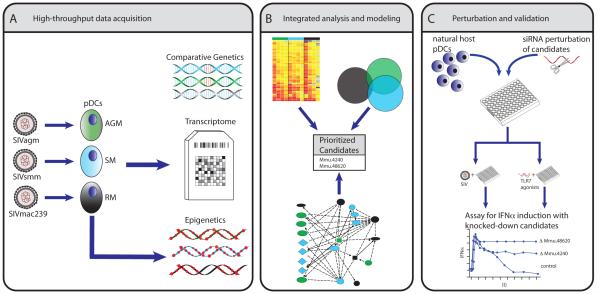
As described above, an important, feature of the natural host phenotype is resolution of the interferon response[33], predominately regulated by pDCs(reviewed in[53]). A number of seminal studies have elegantly used systems-level analyses to model TLR and antiviral responses, such as negative-feedback mechanisms in LPS/TLR4 signaling[54], reviewed by[45]; signaling cascades downstream of TLRs in dendritic cells[55], and influenza infection[56], reviewed in [57]. An example of how similar approaches can be applied to comparatively study SIV interactions with natural hosts aimed identifying differential regulatory pathways of IFN production is depicted in Figure 1.
Figure 1. Systems biology strategy to identify regulatory mechanisms controlling the resolution of interferon responses in natural host species.
pDCs have been implicated as a the predominant source of interferon production in lymph nodes of acutely infected SMs and AGMs [32]. (A) Transcriptional profiles of pDCs from natural and non-natural hosts activated over time with endogenous strains of SIV can be used to identify in a longitudinal analysis waves of genes expression and early activating ‘affector’ genes (i.e. signaling molecules; transcription factors) from ‘late’ effector genes. The ability of pDCs to suppress interferon expression despite chronic viremia suggest an epigenetic mechanism, and deep sequencing can be used to profile epigenetic modifications in pDCs before and after SIV exposure. The genome sequence of AGMs and SMs can be leveraged against epigenetic and transcriptome data to probe for species specific variation in the regulatory regions of genes with differential epigenetic/transcriptional profiles. (B) Importantly, potential candidates for negative feedback of interferon signaling in natural host species may not be readily identified as differential on highthroughput screens. Detailed reconstruction of gene networks, boolean and enrichment analysis of genes with sequence variation/differential behavior between natural and pathogenic hosts, kinetic modeling of transcription and correlation with IFNα/β production can be integrated to predict potential candidates and map biologically meaningful divergence in innate signaling. (C) Prioritized candidates can be perturbed in vitro using gene-knockdown techniques and assayed for their impact on prolonged interferon production. Candidates can also be examined using comparative in vivo study of SIV infected NHPs.
The oncology field established proof-of-principle for using transcriptional profiles to distinguish biological classes (e.g. clinically distinct subtypes of leukemias) over a decade ago[58,59]. Recently, similar molecular classification strategies have been adopted in the nascent field of ‘systems vaccinology’[60-62] to identify gene signatures predictive of the immunogenicity of well-established vaccines[63-65]. An advantage of this approach is that predictive models are built from direct associations between transcript measurements and phenotypes of interest; disadvantages are: (i) robust prediction algorithms require large numbers of biological replicates separated into independent ‘training’ and ‘validation’ groups[66]; (ii) identified classifier genes, although predictive, may not necessarily regulate the biological process under study. For biological discovery in the natural host model, the quantity of animals required may reduce its feasiblity compared to conventional gene expression profiling experiments. Instead, molecular discrimination techniques may have greater utility in identifying early marker gene signatures capable of predicting clinical progression. To this aim, the VNP phenotype will likely be an instructive model, and further establishment of this cohort is warranted.
Conclusion
High throughput genomics have allowed unprecedented insight into the immunological interplay between SIV and natural host species in vivo. Gene expression profiling studies from multiple groups have lead to the clear proof that African natural hosts mount an intact Type I interferon response during acute infection. A key difference with pathogenic HIV/SIV infections is that they only show a transient response, whereas interferon expression persists in infected Asian macaques and humans indefinitely. Ongoing developments in the natural host field include the genome sequencing of SMs and AGMs, development of NHP reference genome resources for RNA-seq, and the establishment of a novel cohort of HIV infected patients, VNPs, in which the natural host phenotypes is recapitulated. Chronic immune activation remains an important cause of disease in HIV infection, even in patients with good virologic responses to antiretroviral therapy. The use of system-based analyses to uncover pathways by which natural hosts avoid disease will hopefully identify novel targets for ameliorating HIV-associated immune activation.
Keypoints.
The immunological mechanisms of pathological immune activation in AIDS pathogenesis have recently been studied by using high-throughput gene expression profiling to compare pathogenic simian immunodeficiency virus (SIV) infection in RMs and nonpathogenic infection of sooty mangabeys and African green monkeys
Genome-wide expression profiling identified key difference in the innate immune response to SIV between pathogenic and natural hosts was strong induction of interferon stimulated genes (ISGs), which persisted indefinitely in pathogenic infection, but resolved to baseline rapidly in natural hosts
A novel cohort HIV-infected patients, termed Viremic Nonprogressors, have been described that share immunological features with natural host species, including preserved CD4+ T cell counts, low immune activation, and lowered ISG expression in CD4+ and CD8+ lymphocytes
Ongoing work to develop resources for the study of SIV infection in natural hosts include the sequencing of the genome of SMs and AGMs, and the development of reference transcriptomes for RNA-seq transcript analysis.
Acknowledgments
The authors would like to thank Dr. Donald Sodora and Dr. Zach Johnson for critical review of this manuscript. The authors apologize for the absence or abbreviated discussion of all relevant publications due to space limitations. This work was supported in part by the National Institutes of Health (R37-AI66998 to GS), Agence Nationale des Recherches sur le SIDA et les hépatites virales (ANRS, to MMT, AB), the Institut Pasteur (MMT), the Institut des Hautes Études Scientifiques, the Genopole Evry (AB) and the AREVA Foundation.
Abbreviations
- AGM
African Green monkey
- PTM
pig-tailed macaque
- SIV
simian immunodeficiency virus
- IFN
interferon
- ISG
interferon stimulated genes
- VNP
viremic non-progressor
- RM
rhesus macaque
- Tcm
central memory T cell
- Tem
effector memory T cell
- SM
sooty mangabey
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Douek DC, Roederer M, Koup RA. Emerging concepts in the immunopathogenesis of AIDS. Annual review of medicine. 2009;60:471–484. doi: 10.1146/annurev.med.60.041807.123549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirsch VM, Olmsted RA, Murphey-Corb M, Purcell RH, Johnson PR. An African primate lentivirus (SIVsm) closely related to HIV-2. Nature. 1989;339:389–392. doi: 10.1038/339389a0. [DOI] [PubMed] [Google Scholar]
- 3.Keele BF, Van Heuverswyn F, Li Y, Bailes E, Takehisa J, Santiago ML, Bibollet-Ruche F, Chen Y, Wain LV, Liegeois F, et al. Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science. 2006;313:523–526. doi: 10.1126/science.1126531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Heuverswyn F, Li Y, Neel C, Bailes E, Keele BF, Liu W, Loul S, Butel C, Liegeois F, Bienvenue Y, et al. Human immunodeficiency viruses: SIV infection in wild gorillas. Nature. 2006;444:164. doi: 10.1038/444164a. [DOI] [PubMed] [Google Scholar]
- 5.Liovat AS, Jacquelin B, Ploquin MJ, Barre-Sinoussi F, Muller-Trutwin MC. African non human primates infected by SIV - why don’t they get sick? Lessons from studies on the early phase of non-pathogenic SIV infection. Curr HIV Res. 2009;7:39–50. doi: 10.2174/157016209787048546. [DOI] [PubMed] [Google Scholar]
- 6.Sodora DL, Allan JS, Apetrei C, Brenchley JM, Douek DC, Else JG, Estes JD, Hahn BH, Hirsch VM, Kaur A, et al. Toward an AIDS vaccine: lessons from natural simian immunodeficiency virus infections of African nonhuman primate hosts. Nat Med. 2009;15:861–865. doi: 10.1038/nm.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirchhoff F, Silvestri G. Is Nef the elusive cause of HIV-associated hematopoietic dysfunction? The Journal of clinical investigation. 2008;118:1622–1625. doi: 10.1172/JCI35487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schindler M, Schmokel J, Specht A, Li H, Munch J, Khalid M, Sodora DL, Hahn BH, Silvestri G, Kirchhoff F. Inefficient Nef-mediated downmodulation of CD3 and MHC-I correlates with loss of CD4+T cells in natural SIV infection. PLoS Pathog. 2008;4:e1000107. doi: 10.1371/journal.ppat.1000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meythaler M, Martinot A, Wang Z, Pryputniewicz S, Kasheta M, Ling B, Marx PA, O’Neil S, Kaur A. Differential CD4+ T-lymphocyte apoptosis and bystander T-cell activation in rhesus macaques and sooty mangabeys during acute simian immunodeficiency virus infection. J Virol. 2009;83:572–583. doi: 10.1128/JVI.01715-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herbeuval JP, Shearer GM. HIV-1 immunopathogenesis: how good interferon turns bad. Clin Immunol. 2007;123:121–128. doi: 10.1016/j.clim.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Estaquier J, Idziorek T, de Bels F, Barre-Sinoussi F, Hurtrel B, Aubertin AM, Venet A, Mehtali M, Muchmore E, Michel P, et al. Programmed cell death and AIDS: significance of T-cell apoptosis in pathogenic and nonpathogenic primate lentiviral infections. Proc Natl Acad Sci U S A. 1994;91:9431–9435. doi: 10.1073/pnas.91.20.9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silvestri G, Sodora DL, Koup RA, Paiardini M, O’Neil SP, McClure HM, Staprans SI, Feinberg MB. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity. 2003;18:441–452. doi: 10.1016/s1074-7613(03)00060-8. [DOI] [PubMed] [Google Scholar]
- 13.Cumont MC, Diop O, Vaslin B, Elbim C, Viollet L, Monceaux V, Lay S, Silvestri G, Grand R Le, Muller-Trutwin M, et al. Early divergence in lymphoid tissue apoptosis between pathogenic and nonpathogenic simian immunodeficiency virus infections of nonhuman primates. J Virol. 2008;82:1175–1184. doi: 10.1128/JVI.00450-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brenchley JM, Silvestri G, Douek DC. Nonprogressive and progressive primate immunodeficiency lentivirus infections. Immunity. 2010;32:737–742. doi: 10.1016/j.immuni.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beaumier CM, Harris LD, Goldstein S, Klatt NR, Whitted S, McGinty J, Apetrei C, Pandrea I, Hirsch VM, Brenchley JM. CD4 downregulation by memory CD4+ T cells in vivo renders African green monkeys resistant to progressive SIVagm infection. Nat Med. 2009;15:879–885. doi: 10.1038/nm.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandrea I, Apetrei C, Gordon S, Barbercheck J, Dufour J, Bohm R, Sumpter B, Roques P, Marx PA, Hirsch VM, et al. Paucity of CD4+CCR5+ T cells is a typical feature of natural SIV hosts. Blood. 2007;109:1069–1076. doi: 10.1182/blood-2006-05-024364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silvestri G, Paiardini M, Pandrea I, Lederman MM, Sodora DL. Understanding the benign nature of SIV infection in natural hosts. J Clin Invest. 2007;117:3148–3154. doi: 10.1172/JCI33034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, Asher TE, Scheinberg P, Price DA, Hage CA, Kholi LM, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Favre D, Lederer S, Kanwar B, Ma ZM, Proll S, Kasakow Z, Mold J, Swainson L, Barbour JD, Baskin CR, et al. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 2009;5:e1000295. doi: 10.1371/journal.ppat.1000295. ** This study is one of two studies that were the first to characterize genome wide expression in natural hosts during in vivo SIV infection. The results are framed in the context of Th17 dynamics during acute SIV infection in RMs and AGMs.
- 20.Pandrea IV, Gautam R, Ribeiro RM, Brenchley JM, Butler IF, Pattison M, Rasmussen T, Marx PA, Silvestri G, Lackner AA, et al. Acute loss of intestinal CD4+ T cells is not predictive of simian immunodeficiency virus virulence. J Immunol. 2007;179:3035–3046. doi: 10.4049/jimmunol.179.5.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brenchley JM, Paiardini M. Immunodeficiency lentiviral infections in natural and non-natural hosts. Blood. 2011;118:847–854. doi: 10.1182/blood-2010-12-325936. * This review is a timely update of some of the changing concepts in natural host biology.
- 22.Bosinger SE, Li Q, Gordon SN, Klatt NR, Duan L, Xu L, Francella N, Sidahmed A, Smith AJ, Cramer EM, et al. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest. 2009;119:3556–3572. doi: 10.1172/JCI40115. ** This study was the first to profile in vivo SIV infection in sooty mangabeys using microarrays and identified that this species suppress their interferon expression. Immune activation markers correlated with gene expression of lymphocyte exhaustion markers.
- 23.Lederer S, Favre D, Walters KA, Proll S, Kanwar B, Kasakow Z, Baskin CR, Palermo R, McCune JM, Katze MG. **Transcriptional profiling in pathogenic and non-pathogenic SIV infections revealed significant distinctions in kinetics and tissue compartmentalization. PLoS Pathog. 2009;5:e1000296. doi: 10.1371/journal.ppat.1000296. ** This study one of two articles (Favre 2009) that the first to characterize genome wide expression during in vivo SIV infection of natural hosts. It demonstrated that ISGs are downregulated as early as 45 days post-infection in AGMs.
- 24.Jacquelin B, Mayau V, Targat B, Liovat AS, Kunkel D, Petitjean G, Dillies MA, Roques P, Butor C, Silvestri G, et al. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest. 2009;119:3544–3555. doi: 10.1172/JCI40093. ** This article conducted an exhaustive characterization of transcriptomic changes in purified CD4+ cells during SIV infection of African green monkeys. The data provide the proof of an innate response during acute SIVagm infection.
- 25.Mandl JN, Barry AP, Vanderford TH, Kozyr N, Chavan R, Klucking S, Barrat FJ, Coffman RL, Staprans SI, Feinberg MB. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat Med. 2008;14:1077–1087. doi: 10.1038/nm.1871. [DOI] [PubMed] [Google Scholar]
- 26.Silvestri G, Fedanov A, Germon S, Kozyr N, Kaiser WJ, Garber DA, McClure H, Feinberg MB, Staprans SI. Divergent host responses during primary simian immunodeficiency virus SIVsm infection of natural sooty mangabey and nonnatural rhesus macaque hosts. J Virol. 2005;79:4043–4054. doi: 10.1128/JVI.79.7.4043-4054.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muthukumar A, Zhou D, Paiardini M, Barry AP, Cole KS, McClure HM, Staprans SI, Silvestri G, Sodora DL. Timely triggering of homeostatic mechanisms involved in the regulation of T-cell levels in SIVsm-infected sooty mangabeys. Blood. 2005;106:3839–3845. doi: 10.1182/blood-2005-01-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kornfeld C, Ploquin MJ, Pandrea I, Faye A, Onanga R, Apetrei C, Poaty-Mavoungou V, Rouquet P, Estaquier J, Mortara L, et al. Antiinflammatory profiles during primary SIV infection in African green monkeys are associated with protection against AIDS. J Clin Invest. 2005;115:1082–1091. doi: 10.1172/JCI23006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gordon SN, Klatt NR, Bosinger SE, Brenchley JM, Milush JM, Engram JC, Dunham RM, Paiardini M, Klucking S, Danesh A, et al. Severe depletion of mucosal CD4+ T cells in AIDS-free simian immunodeficiency virus-infected sooty mangabeys. J Immunol. 2007;179:3026–3034. doi: 10.4049/jimmunol.179.5.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diop OM, Ploquin MJ, Mortara L, Faye A, Jacquelin B, Kunkel D, Lebon P, Butor C, Hosmalin A, Barre-Sinoussi F, et al. Plasmacytoid dendritic cell dynamics and alpha interferon production during Simian immunodeficiency virus infection with a nonpathogenic outcome. J Virol. 2008;82:5145–5152. doi: 10.1128/JVI.02433-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Estes JD, Gordon SN, Zeng M, Chahroudi AM, Dunham RM, Staprans SI, Reilly CS, Silvestri G, Haase AT. Early resolution of acute immune activation and induction of PD-1 in SIV-infected sooty mangabeys distinguishes nonpathogenic from pathogenic infection in rhesus macaques. J Immunol. 2008;180:6798–6807. doi: 10.4049/jimmunol.180.10.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris LD, Tabb B, Sodora DL, Paiardini M, Klatt NR, Douek DC, Silvestri G, Muller-Trutwin M, Vasile-Pandrea I, Apetrei C, et al. Downregulation of robust acute type I interferon responses distinguishes nonpathogenic simian immunodeficiency virus (SIV) infection of natural hosts from pathogenic SIV infection of rhesus macaques. J Virol. 2010;84:7886–7891. doi: 10.1128/JVI.02612-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mir KD, Gasper MA, Sundaravaradan V, Sodora DL. SIV infection in natural hosts: resolution of immune activation during the acute-to-chronic transition phase. Microbes Infect. 2011;13:14–24. doi: 10.1016/j.micinf.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Altfeld M, Fadda L, Frleta D, Bhardwaj N. DCs and NK cells: critical effectors in the immune response to HIV-1. Nat Rev Immunol. 2011;11:176–186. doi: 10.1038/nri2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–336. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 36.Hyrcza MD, Kovacs C, Loutfy M, Halpenny R, Heisler L, Yang S, Wilkins O, Ostrowski M, Der SD. Distinct transcriptional profiles in ex vivo CD4+ and CD8+ T cells are established early in human immunodeficiency virus type 1 infection and are characterized by a chronic interferon response as well as extensive transcriptional changes in CD8+ T cells. J Virol. 2007;81:3477–3486. doi: 10.1128/JVI.01552-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sedaghat AR, German J, Teslovich TM, Cofrancesco J, Jr., Jie CC, Talbot CC, Jr., Siliciano RF. Chronic CD4+ T-cell activation and depletion in human immunodeficiency virus type 1 infection: type I interferon-mediated disruption of T-cell dynamics. J Virol. 2008;82:1870–1883. doi: 10.1128/JVI.02228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shendure J. The beginning of the end for microarrays? Nat Methods. 2008;5:585–587. doi: 10.1038/nmeth0708-585. [DOI] [PubMed] [Google Scholar]
- 39.Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10:57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choudhary SK, Vrisekoop N, Jansen CA, Otto SA, Schuitemaker H, Miedema F, Camerini D. Low immune activation despite high levels of pathogenic human immunodeficiency virus type 1 results in long-term asymptomatic disease. J Virol. 2007;81:8838–8842. doi: 10.1128/JVI.02663-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rotger M, Dalmau J, Rauch A, McLaren P, Bosinger SE, Martinez R, Sandler NG, Roque A, Liebner J, Battegay M, et al. Comparative transcriptomics of extreme phenotypes of human HIV-1 infection and SIV infection in sooty mangabey and rhesus macaque. J Clin Invest. 2011;121:2391–2400. doi: 10.1172/JCI45235. ** This study was the first in depth genetic analysis of viremic non-progressor patients. Gene Set Enrichment analysis demonstrated overlap with SIVinfected sooty mangabey transcriptome profiles.
- 42.Tisoncik JR, Katze MG. What is systems biology? Future Microbiol. 2010;5:139–141. doi: 10.2217/fmb.09.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rappuoli R, Aderem A. A 2020 vision for vaccines against HIV, tuberculosis and malaria. Nature. 2011;473:463–469. doi: 10.1038/nature10124. [DOI] [PubMed] [Google Scholar]
- 44.Aderem A, Adkins JN, Ansong C, Galagan J, Kaiser S, Korth MJ, Law GL, McDermott JG, Proll SC, Rosenberger C, et al. A systems biology approach to infectious disease research: innovating the pathogen-host research paradigm. MBio. 2011;2:e00325–00310. doi: 10.1128/mBio.00325-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zak DE, Aderem A. Systems biology of innate immunity. Immunol Rev. 2009;227:264–282. doi: 10.1111/j.1600-065X.2008.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gordon SN, Dunham RM, Engram JC, Estes J, Wang Z, Klatt NR, Paiardini M, Pandrea IV, Apetrei C, Sodora DL, et al. Short-lived infected cells support virus replication in sooty mangabeys naturally infected with simian immunodeficiency virus: implications for AIDS pathogenesis. J Virol. 2008;82:3725–3735. doi: 10.1128/JVI.02408-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pandrea I, Ribeiro RM, Gautam R, Gaufin T, Pattison M, Barnes M, Monjure C, Stoulig C, Dufour J, Cyprian W, et al. Simian immunodeficiency virus SIVagm dynamics in African green monkeys. J Virol. 2008;82:3713–3724. doi: 10.1128/JVI.02402-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perelson AS, Essunger P, Cao Y, Vesanen M, Hurley A, Saksela K, Markowitz M, Ho DD. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387:188–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 49.Riddick NE, Hermann EA, Loftin LM, Elliott ST, Wey WC, Cervasi B, Taaffe J, Engram JC, Li B, Else JG, et al. A novel CCR5 mutation common in sooty mangabeys reveals SIVsmm infection of CCR5-null natural hosts and efficient alternative coreceptor use in vivo. PLoS Pathog. 2010;6:e1001064. doi: 10.1371/journal.ppat.1001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paiardini M, Cervasi B, Reyes-Aviles E, Micci L, Ortiz AM, Chahroudi A, Vinton C, Gordon SN, Bosinger SE, Francella N, et al. Low levels of SIV infection in sooty mangabey central memory CD T cells are associated with limited CCR5 expression. Nat Med. 2011;17:830–836. doi: 10.1038/nm.2395. ** This study demonstrated that during activation and transition to central memory cells, CD4+ lymphocytes in sooty mangabeys have lower expression of CCR5, in contrast to rhesus macaques, and that the ratio of SIV infected Tcm/Tem CD4+ T cells in vivo was lower in sooty mangabeys than in rhesus macaques. Thus natural hosts may maintain a CD4+ T cell pool by restricting virus entry in Tcm cells.
- 51.Okoye A, Meier-Schellersheim M, Brenchley JM, Hagen SI, Walker JM, Rohankhedkar M, Lum R, Edgar JB, Planer SL, Legasse A, et al. Progressive CD4+ central memory T cell decline results in CD4+ effector memory insufficiency and overt disease in chronic SIV infection. J Exp Med. 2007;204:2171–2185. doi: 10.1084/jem.20070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neil S, Bieniasz P. Human immunodeficiency virus, restriction factors, and interferon. J Interferon Cytokine Res. 2009;29:569–580. doi: 10.1089/jir.2009.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bosinger SE, Sodora DL, Silvestri G. Generalized immune activation and innate immune responses in simian immunodeficiency virus infection. Curr Opin HIV AIDS. 2011;6:411–418. doi: 10.1097/COH.0b013e3283499cf6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gilchrist M, Thorsson V, Li B, Rust AG, Korb M, Roach JC, Kennedy K, Hai T, Bolouri H, Aderem A. Systems biology approaches identify ATF3 as a negative regulator of Toll-like receptor 4. Nature. 2006;441:173–178. doi: 10.1038/nature04768. [DOI] [PubMed] [Google Scholar]
- 55.Amit I, Garber M, Chevrier N, Leite AP, Donner Y, Eisenhaure T, Guttman M, Grenier JK, Li W, Zuk O, et al. Unbiased reconstruction of a mammalian transcriptional network mediating pathogen responses. Science. 2009;326:257–263. doi: 10.1126/science.1179050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shapira SD, Gat-Viks I, Shum BO, Dricot A, de Grace MM, Wu L, Gupta PB, Hao T, Silver SJ, Root DE, et al. A physical and regulatory map of host-influenza interactions reveals pathways in H1N1 infection. Cell. 2009;139:1255–1267. doi: 10.1016/j.cell.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shapira SD, Hacohen N. Systems biology approaches to dissect mammalian innate immunity. Current opinion in immunology. 2011;23:71–77. doi: 10.1016/j.coi.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Golub TR, Slonim DK, Tamayo P, Huard C, Gaasenbeek M, Mesirov JP, Coller H, Loh ML, Downing JR, Caligiuri MA, et al. Molecular classification of cancer: class discovery and class prediction by gene expression monitoring. Science. 1999;286:531–537. doi: 10.1126/science.286.5439.531. [DOI] [PubMed] [Google Scholar]
- 59.Yeoh EJ, Ross ME, Shurtleff SA, Williams WK, Patel D, Mahfouz R, Behm FG, Raimondi SC, Relling MV, Patel A, et al. Classification, subtype discovery, and prediction of outcome in pediatric acute lymphoblastic leukemia by gene expression profiling. Cancer Cell. 2002;1:133–143. doi: 10.1016/s1535-6108(02)00032-6. [DOI] [PubMed] [Google Scholar]
- 60.Pulendran B. Learning immunology from the yellow fever vaccine: innate immunity to systems vaccinology. Nat Rev Immunol. 2009;9:741–747. doi: 10.1038/nri2629. [DOI] [PubMed] [Google Scholar]
- 61.Pulendran B, Li S, Nakaya HI. Systems vaccinology. Immunity. 2010;33:516–529. doi: 10.1016/j.immuni.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trautmann L, Sekaly RP. Solving vaccine mysteries: a systems biology perspective. Nat Immunol. 2011;12:729–731. doi: 10.1038/ni.2078. [DOI] [PubMed] [Google Scholar]
- 63.Querec TD, Akondy RS, Lee EK, Cao W, Nakaya HI, Teuwen D, Pirani A, Gernert K, Deng J, Marzolf B, et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol. 2009;10:116–125. doi: 10.1038/ni.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gaucher D, Therrien R, Kettaf N, Angermann BR, Boucher G, Filali-Mouhim A, Moser JM, Mehta RS, Drake DR, 3rd, Castro E, et al. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J Exp Med. 2008;205:3119–3131. doi: 10.1084/jem.20082292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakaya HI, Wrammert J, Lee EK, Racioppi L, Marie-Kunze S, Haining WN, Means AR, Kasturi SP, Khan N, Li GM, et al. Systems biology of vaccination for seasonal influenza in humans. Nat Immunol. 2011;12:786–795. doi: 10.1038/ni.2067. ** This work used systems biology techniques to identify genes correlated with immune responses to seasonal influenza vaccines. It built off previous work using a similar approach to identify genes capable of predicting immunogenicity of yellow fever vaccination, demonstrating that the methods have broad utility.
- 66.Ramaswamy S, Golub TR. DNA microarrays in clinical oncology. J Clin Oncol. 2002;20:1932–1941. doi: 10.1200/JCO.2002.20.7.1932. [DOI] [PubMed] [Google Scholar]