Alternative oxidase reduces feedback on the photosynthetic apparatus that can otherwise limit photosynthetic capacity during moderate drought.
Abstract
The mitochondrial electron transport chain includes an alternative oxidase (AOX) that is hypothesized to aid photosynthetic metabolism, perhaps by acting as an additional electron sink for photogenerated reductant or by dampening the generation of reactive oxygen species. Gas exchange, chlorophyll fluorescence, photosystem I (PSI) absorbance, and biochemical and protein analyses were used to compare respiration and photosynthesis of Nicotiana tabacum ‘Petit Havana SR1’ wild-type plants with that of transgenic AOX knockdown (RNA interference) and overexpression lines, under both well-watered and moderate drought-stressed conditions. During drought, AOX knockdown lines displayed a lower rate of respiration in the light than the wild type, as confirmed by two independent methods. Furthermore, CO2 and light response curves indicated a nonstomatal limitation of photosynthesis in the knockdowns during drought, relative to the wild type. Also relative to the wild type, the knockdowns under drought maintained PSI and PSII in a more reduced redox state, showed greater regulated nonphotochemical energy quenching by PSII, and displayed a higher relative rate of cyclic electron transport around PSI. The origin of these differences may lie in the chloroplast ATP synthase amount, which declined dramatically in the knockdowns in response to drought. None of these effects were seen in plants overexpressing AOX. The results show that AOX is necessary to maintain mitochondrial respiration during moderate drought. In its absence, respiration rate slows and the lack of this electron sink feeds back on the photosynthetic apparatus, resulting in a loss of chloroplast ATP synthase that then limits photosynthetic capacity.
The plant mitochondrial electron transport chain (ETC) is bifurcated such that electrons in the ubiquinone pool partition between the cytochrome (cyt) pathway (consisting of Complex III, cyt c, and Complex IV) and alternative oxidase (AOX; Finnegan et al., 2004; Millar et al., 2011; Vanlerberghe, 2013). AOX directly couples ubiquinol oxidation with O2 reduction to water. This reduces the energy yield of respiration because, unlike Complexes III and IV, AOX is not proton pumping. Hence, AOX is an electron sink, the capacity of which is little encumbered by rates of ATP turnover. In this way, AOX might be well suited to prevent cellular over-reduction. Supporting this, transgenic Nicotiana tabacum leaves with suppressed amounts of AOX have increased concentrations of mitochondrial-localized superoxide radical (O2−) and nitric oxide, the products that can arise when an over-reduced ETC results in electron leakage to O2 or nitrite (Cvetkovska and Vanlerberghe, 2012, 2013).
In angiosperms, AOX is encoded by a small gene family (Considine et al., 2002). In Arabidopsis (Arabidopsis thaliana), mutation or knockdown of the stress-responsive AOX1a gene family member dramatically reduces AOX protein and the capacity of the AOX respiration pathway to consume O2. Several studies have shown that this loss of AOX capacity in Arabidopsis aox1a plants affected processes such as growth, carbon and energy metabolism, and/or the cellular network of reactive oxygen species (ROS) scavengers (Fiorani et al., 2005; Umbach et al., 2005; Watanabe et al., 2008; Giraud et al., 2008; Skirycz et al., 2010). However, in studies in which respiration was measured, it was consistently reported that the lack of AOX capacity had no significant impact on the respiration rate in the dark (RD; Umbach et al., 2005; Giraud et al., 2008; Strodtkötter et al., 2009; Florez-Sarasa et al., 2011; Yoshida et al., 2011b; Gandin et al., 2012). The exceptions are two reports that RD was actually higher in aox1a than in the wild type under some conditions (Watanabe et al., 2008; Vishwakarma et al., 2014). To our knowledge, how the lack of AOX affects respiration rate in the light (RL) is not reported in Arabidopsis or other species.
Numerous studies have established the importance of mitochondrial metabolism in the light to optimize photosynthesis (Hoefnagel et al., 1998; Raghavendra and Padmasree, 2003). In recent years, the potential importance of specifically AOX respiration during photosynthesis has been examined using the Arabidopsis aox1a plants (Giraud et al., 2008; Strodtkötter et al., 2009; Zhang et al., 2010; Florez-Sarasa et al., 2011; Yoshida et al., 2011a, 2011b). In general, these studies reported small perturbations of photosynthesis in standard-grown aox1a plants, including slightly lower rates of CO2 uptake or O2 release (Gandin et al., 2012; Vishwakarma et al., 2014), slightly higher rates of cyclic electron transport (CET; Yoshida et al., 2011b), and slightly increased susceptibility to photoinhibition after a high light treatment (Florez-Sarasa et al., 2011). Generally, these studies concluded that aox1a plants exhibit a biochemical limitation of photosynthesis, in line with the hypothesis that AOX serves as a sink for excess photogenerated reducing power, with the reductant likely reaching the mitochondrion via the malate valve (Noguchi and Yoshida, 2008; Taniguchi and Miyake, 2012). Similar to these Arabidopsis studies, we recently reported that well-watered N. tabacum AOX knockdowns grown at moderate irradiance display a slight reduced rate of photosynthesis (approximately 10%–15%) when measured at high irradiance. However, we established that the lower photosynthetic rate was the result of a stomatal rather than biochemical limitation of photosynthesis, and provided evidence that this stomatal limitation resulted from disrupted nitric oxide homeostasis within the guard cells of AOX knockdown plants (Cvetkovska et al., 2014).
Drought is a common abiotic stress that can substantially curtail photosynthesis because stomatal closure, meant to conserve water, also restricts CO2 availability to the Calvin cycle. Besides this well established stomatal limitation of photosynthesis, there may also be water deficit-sensitive biochemical components that contribute to the reduction of photosynthesis during drought. However, the nature of this biochemical limitation and the degree to which it contributes to the curtailment of photosynthesis during drought remain areas of active debate (Flexas et al., 2004; Lawlor and Tezara, 2009; Pinheiro and Chaves, 2011). Additional factors, such as patchy stomatal closure (Sharkey and Seemann, 1989; Gunasekera and Berkowitz, 1992) or changes in the conductance to CO2 of mesophyll cells (Perez-Martin et al., 2009), can further complicate analyses of photosynthesis during drought.
Metabolism can experience energy imbalances, when there is a mismatch between rates of synthesis and rates of utilization of ATP and/or NADPH, and the importance of mechanisms to minimize such imbalances has been emphasized (Cruz et al., 2005; Kramer and Evans, 2011; Vanlerberghe, 2013). For example, such imbalances may occur in the chloroplast when the use of ATP and NADPH by the Calvin cycle does not keep pace with the harvesting of light energy (Hüner et al., 2012). This can result in excess excitation energy that can damage photosynthetic components, perhaps through the generation of ROS (Asada, 2006; Noctor et al., 2014). Such a scenario has been hypothesized to underlie the development of the biochemical limitations of photosynthesis reported during drought (Lawlor and Tezara, 2009).
In this study, we find that N. tabacum AOX knockdowns show a compromised rate of mitochondrial respiration in the light during moderate drought. This corresponds with a strong nonstomatal limitation of photosynthesis in these plants relative to the wild type, and we describe a biochemical basis for this photosynthetic limitation. The results indicate that AOX is a necessary electron sink to support photosynthesis during drought, a condition when the major photosynthetic electron sink, the Calvin cycle, is becoming limited by CO2 availability.
RESULTS
In well-watered N. tabacum, leaf relative water content (RWC) was approximately 88% and did not differ significantly between wild-type plants, two knockdown transgenic lines with suppressed amounts of AOX (RI9 and RI29), and a transgenic line with constitutive overexpression of AOX (B7; Table I). After moderate drought stress (withholding water for 4 d), leaf RWC declined to approximately 77% and again did not differ between plant lines (Table I).
Table I. Leaf and photosynthetic characteristics of wild-type N. tabacum, two knockdown lines (RI9 and RI29) with reduced AOX, and an AOX-overexpressing line (B7).
All analyses used the fourth or fifth fully developed leaves of well-watered or moderate drought-stressed plants. The ΦPSII, gs, gm, Ci, and Cc values were measured at saturating irradiance (1,600 PPFD) and Ca of 400 µmol mol−1. Results are the mean ± se of at least three independent experiments. Within each treatment, plant lines not sharing a common superscript letter are significantly different from one another (P < 0.05).
| Parameter | Well-Watered Plants |
Moderate Drought-Stressed Plants |
||||||
|---|---|---|---|---|---|---|---|---|
| B7 | Wild Type | RI9 | RI29 | B7 | Wild Type | RI9 | RI29 | |
| Leaf RWC (%) | 87 ± 2a | 89 ± 2a | 87 ± 2a | 88 ± 3a | 76 ± 1a | 77 ± 3a | 78 ± 2a | 77 ± 1a |
| Fv/Fm | 0.81 ± 0.009a | 0.80 ± 0.007a | 0.82 ± 0.009a | 0.81 ± 0.003a | 0.79 ± 0.012a | 0.80 ± 0.007a | 0.81 ± 0.006a | 0.80 ± 0.012a |
| ΦPSII | 0.211 ± 0.014a | 0.221 ± 0.013a | 0.220 ± 0.017a | 0.208 ± 0.018a | 0.156 ± 0.010a | 0.141 ± 0.060a | 0.100 ± 0.011b | 0.089 ± 0.011b |
| gs (mol m−2 s−1) | 0.150 ± 0.015a | 0.160 ± 0.012a | 0.143 ± 0.014a,b | 0.126 ± 0.015b | 0.100 ± 0.006a | 0.103 ± 0.009a | 0.090 ± 0.005a | 0.087 ± 0.007a |
| gm (mol m−2 s−1) | 0.115 ± 0.003a | 0.121 ± 0.004a | 0.113 ± 0.005a | 0.121 ± 0.013a | 0.113 ± 0.004a | 0.111 ± 0.005a | 0.110 ± 0.008a | 0.119 ± 0.008a |
| Ci (µmol mol−1) | 309 ± 14a | 320 ± 10a | 318 ± 13a | 263 ± 8b | 212 ± 11a | 201 ± 16a | 189 ± 5a | 196 ± 9a |
| Cc (µmol mol−1) | 227 ± 8a,b | 240 ± 9a | 237 ± 8a | 194 ± 9b | 160 ± 10a | 154 ± 9a | 156 ± 18a | 150 ± 8a |
| CE (CO2 m−2 s−1/mol−1 CO2) | 0.071 ± 0.001a | 0.066 ± 0.003a | 0.073 ± 0.005a | 0.061 ± 0.007a | 0.046 ± 0.003a | 0.051 ± 0.008a | 0.039 ± 0.006a | 0.030 ± 0.003b |
| Total Chl (mg m−2 leaf area) | 334 ± 35a | 320 ± 36a | 337 ± 26a | 331 ± 20a | 300 ± 33a | 288 ± 31a,b | 259 ± 29a,b | 248 ± 16b |
| Chl a (mg m−2) | 223 ± 19a | 231 ± 23a | 235 ± 12a | 234 ± 14a | 202 ± 16a | 196 ± 15a,b | 165 ± 13b,c | 154 ± 10c |
| Chl b (mg m−2) | 111 ± 16a | 89 ± 13a | 102 ± 14a | 97 ± 8a | 97 ± 17a | 93 ± 17a | 94 ± 16a | 94 ± 14a |
| Chl a/b (ratio) | 2.18 ± 0.14b | 2.63 ± 0.15a | 2.35 ± 0.21a,b | 2.44 ± 0.16a,b | 2.19 ± 0.27a | 2.20 ± 0.25a | 1.81 ± 0.18b | 1.73 ± 0.33b |
| Protein (g m−2 leaf area) | 2.82 ± 0.12a | 2.94 ± 0.10a | 2.97 ± 0.06a | 2.85 ± 0.09a | 2.63 ± 0.15a | 2.55 ± 0.06a,b | 2.29 ± 0.13b | 2.40 ± 0.19a,b |
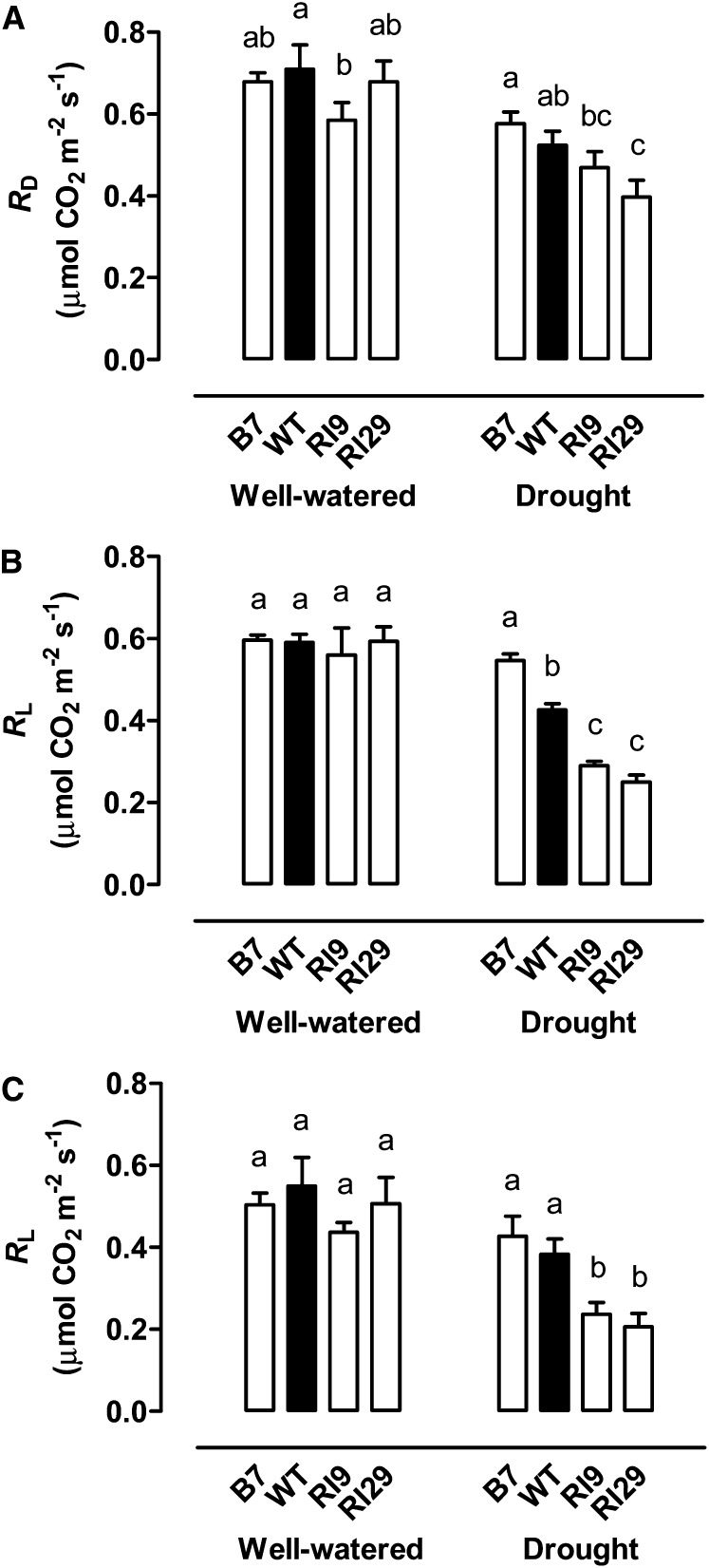
In well-watered plants, both mitochondrial RD and mitochondrial RL (with RL estimated by either the Laisk or Kok method; see “Materials and Methods”) were similar between the wild-type, knockdown, and overexpression lines (Fig. 1). During moderate drought stress, respiration rate differed significantly between the plant lines. RD was lower in the AOX knockdowns, being reduced to 89% (RI9) and 76% (RI29) of the wild-type rate, although only the reduction in RI29 was statistically significant (Fig. 1A). Conversely, RD was slightly higher (1.1-fold) in the AOX overexpressor (B7) than in the wild type, although this difference was not statistically significant. The differences in respiration rate between plant lines under drought were magnified further in the light. In RI9, RL was reduced to 68% (Laisk method) or 62% (Kok method) of the wild type (Fig. 1, B and C). In RI29, RL was reduced to 59% (Laisk method) or 54% (Kok method) of the wild type. Furthermore, RL was higher in B7 than in the wild type although this difference was only significant when estimated using the Laisk method, in which case the rate was 1.3-fold higher than the wild type (Fig. 1B).
Figure 1.
RD (A), RL as estimated by the Laisk method (B), and RL as estimated by the Kok method (C) of wild-type N. tabacum and three transgenic lines with altered AOX amount, in both well-watered and moderate drought-stressed plants. Data are the mean ± se of six (A) or three (B and C) independent experiments. Within each treatment, plant lines not sharing a common letter above the data bar are significantly different from one another (P < 0.05). WT, Wild type.
To examine whether the lower respiration rate of the knockdowns under moderate drought might be attributable to a substrate limitation, we examined leaf amounts of Suc, starch, and monosaccharides (Glu, Fru, and Fru-6-P) in well-watered and moderate drought-stressed plants. All lines showed a strong decrease in starch and a severalfold increase in Glu, Fru, and Fru-6-P in response to drought, whereas Suc amounts were similar under the two growth conditions (Supplemental Fig. S1). However, the amounts of starch, Suc, and monosaccharides did not differ across plant lines for either well-watered or drought-stressed plants (Supplemental Fig. S1). In general, these metabolite changes in response to drought are similar to those previously seen in barley (Hordeum vulgare; Wingler et al., 1999).
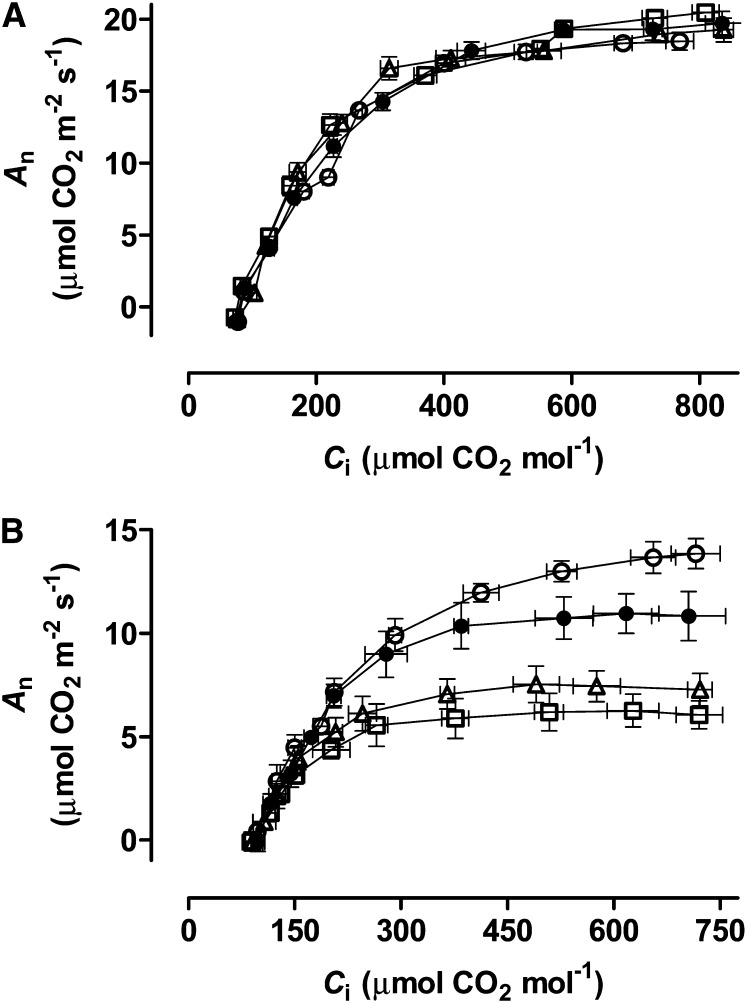
Combined gas exchange and chlorophyll (Chl) a fluorescence were used to characterize photosynthesis of the wild-type and transgenic lines (see “Materials and Methods” for brief descriptions of the parameters examined). For well-watered plants, CO2 response curves (net CO2 assimilation rate [An] as a function of leaf intercellular CO2 concentration [Ci]) generated at a saturating photosynthetic photon flux density (PPFD) were similar across all of the plant lines (Fig. 2A). For moderate drought-stressed plants, however, the curves differed between lines. At moderate to high measurement Ci, An was substantially lower in RI9 and RI29 than in the wild type, whereas it was slightly higher than the wild type in B7 (Fig. 2B). Hence, the maximal An rate in the AOX knockdowns was only about 68% (RI9) and 56% (RI29) of the wild type, whereas it was about 1.2-fold higher than the wild type in B7. In the wild type and knockdowns, the CO2 response curve at moderate to high Ci was much flatter for drought-stressed than well-watered plants but, interestingly, this was not the case for B7 (Fig. 2). Compared with the wild type, the carboxylation efficiency (CE) of Rubisco under drought was lower in knockdowns, especially RI29 (Table I). On the other hand, no differences were seen between the wild type and knockdown lines during drought in total Chl, stomatal conductance (gs), mesophyll conductance (gm), Ci, or the concentration of CO2 at the Rubisco carboxylation site (Cc; Table I). However, gs, Ci, and Cc were slightly lower in RI29 than in the wild type in well-watered plants, which is consistent with a previously reported stomatal limitation (Cvetkovska et al., 2014).
Figure 2.
CO2 response curves (An as a function of Ci) of wild-type N. tabacum (black circles) and three transgenic lines (B7, white circle; RI9, white triangle; and RI29, white square) with altered AOX amount, in both well-watered plants (A) and moderate drought-stressed plants (B). Data are the mean ± se of three independent experiments.
Light response curves (An as a function of PPFD) generated similar results to those above. For well-watered plants, the curves were similar across lines, except for an approximately 10% to 15% lower maximal An in RI29 (Supplemental Fig. S2A). For drought-stressed plants at moderate to high measurement PPFD, An was much lower in RI9 and RI29 than in the wild type, whereas it was higher than the wild type in B7 (Supplemental Fig. S2E).
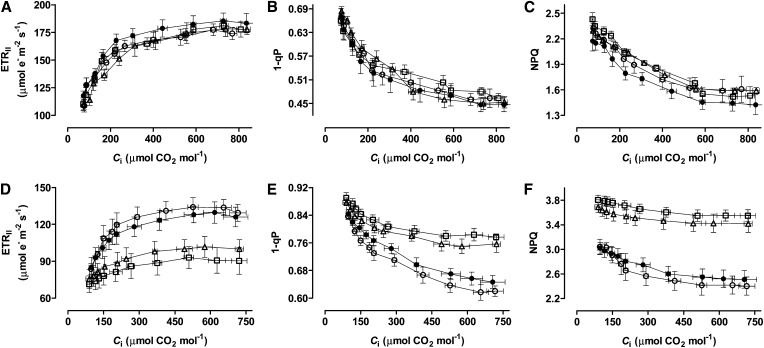
Chl fluorescence analyses done in conjunction with the CO2 response curves were used to estimate the rate of electron transport through PSII (ETRII), the proportion of closed (reduced) PSII reaction centers (excitation pressure), and nonphotochemical energy quenching (NPQ). As expected, ETRII increased with increasing Ci, whereas excitation pressure and NPQ declined (Fig. 3). In well-watered plants, all of these parameters acted similarly across the four plant lines (Fig. 3, A–C). In moderate drought-stressed plants, however, clear differences were seen, particularly between the wild type and two knockdown lines. The knockdowns displayed a much lower ETRII and a much higher excitation pressure and NPQ than the wild type or B7 (Fig. 3, D–F). Similar results were obtained when Chl fluorescence analyses were done in conjunction with the light response curves (Supplemental Fig. S2, B–D and F–H). Furthermore, the operating efficiency of PSII (ΦPSII) at saturating PPFD was much lower during drought in the AOX knockdowns than the wild type or B7, whereas no differences across plant lines were seen in the well-watered condition (Table I). On the other hand, no differences were seen across lines in the maximum photochemical efficiency of PSII in the dark-adapted state (Fv/Fm) in either well-watered or drought-stressed plants (Table I).
Figure 3.
ETRII (A and D), PSII excitation pressure (B and E), and NPQ (C and F) of wild-type N. tabacum (black circles) and three transgenic lines (B7, white circle; RI9, white triangle; and RI29, white square) with altered AOX amount, in both well-watered plants (A–C) and moderate drought-stressed plants (D–F). Data are the mean ± se of three independent experiments.
Comparing just wild-type well-watered to drought-stressed plants, both the CO2 response curves (Supplemental Fig. S3) and the light response curves (Supplemental Fig. S4) showed clear differences in An, ETRII, excitation pressure, and NPQ between the two watering regimes.
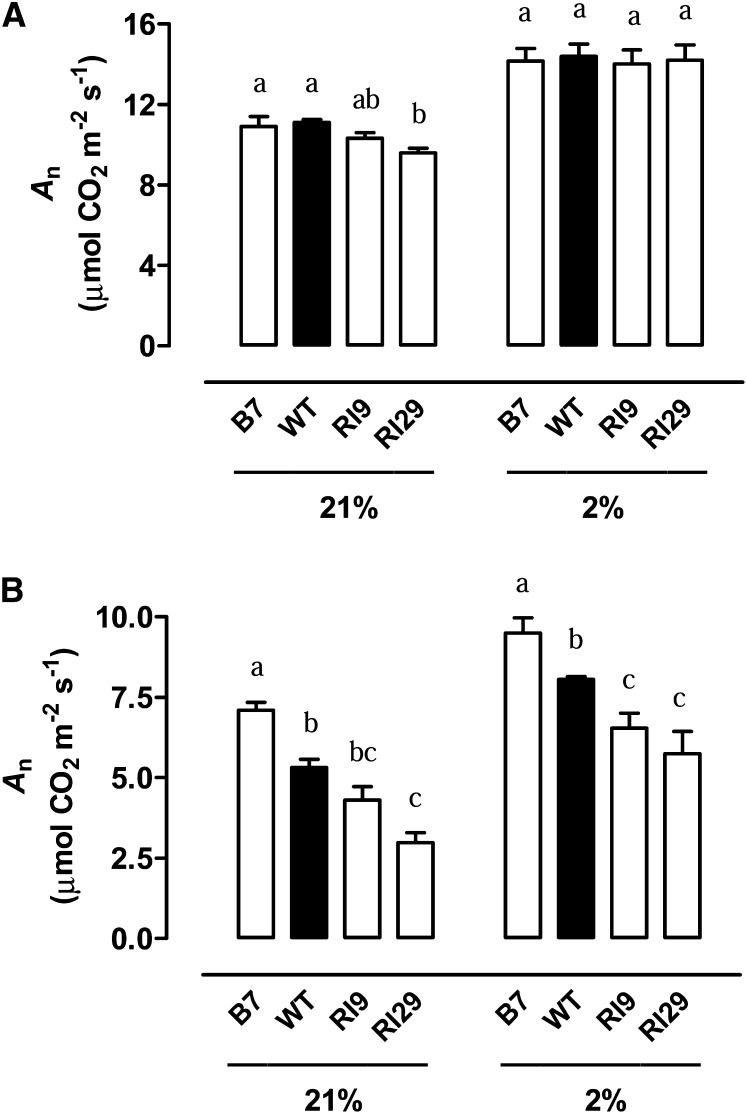
To examine whether the differences in An rates across the plant lines would persist under nonphotorespiratory conditions, we measured photosynthesis in an atmosphere containing either 21% or 2% O2. In 21% O2, we again saw the slight lower An in RI29 than in the wild type when examining well-watered plants (Fig. 4A). The An rate of well-watered plants increased when measured in 2% O2 compared with 21% O2, and now no differences were seen across the plant lines (Fig. 4A). Similarly in moderate drought-stressed plants, An in all plant lines was higher at 2% compared with 21% O2 (Fig. 4B). In this case, however, the large differences in An rate between plant lines at 21% O2 also persisted at 2% O2.
Figure 4.
An rate of wild-type N. tabacum and three transgenic lines with altered AOX amount, in both well-watered plants (A) and moderate drought-stressed plants (B). An rate was measured at saturating irradiance (1,600 PPFD) in an atmosphere containing 400 µmol CO2 mol−1 and either 21% or 2% O2. Data are the mean ± se of three independent experiments. Within each treatment, plant lines not sharing a common letter above the data bar are significantly different from one another (P < 0.05). WT, Wild type.
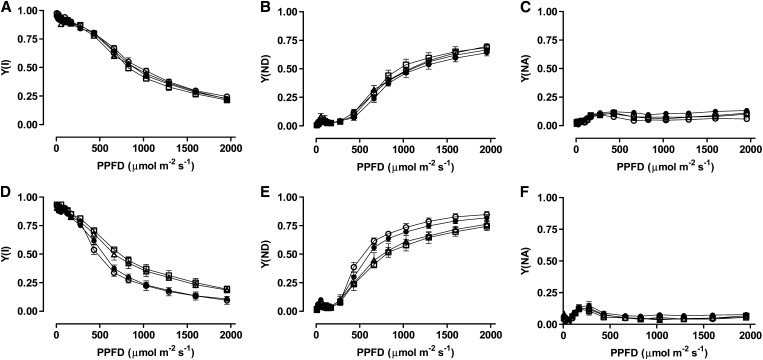
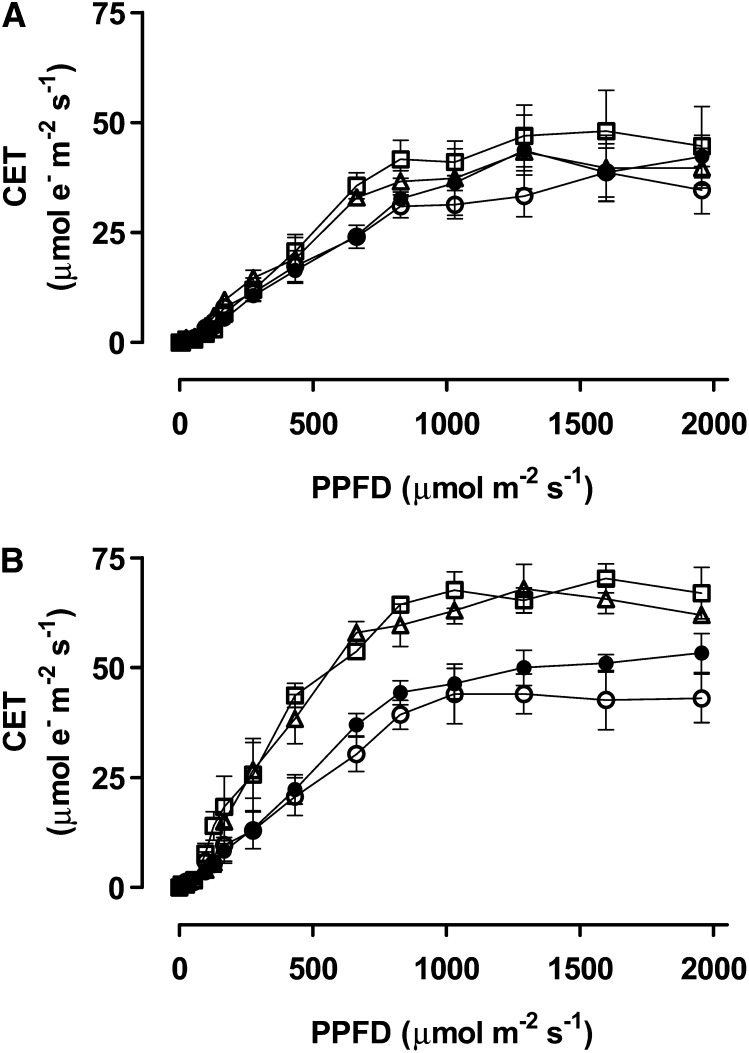
In well-watered plants, there were no differences across the plant lines in the photochemical quantum yield of PSI [Y(I)] (Fig. 5A) or in the relative rate of CET around PSI (Fig. 6A). After moderate drought, Y(I) was slightly higher in the two knockdown lines than in the wild type or B7 at moderate to high PPFD, indicating a more reduced PSI reaction center (P700) in the knockdowns (Fig. 5D). This was accompanied by higher rates of CET in the two knockdowns, relative to the wild type and B7 (Fig. 6B). Alternatively, a comparison of just the well-watered to drought-stressed wild-type plants shows that drought reduced Y(I), whereas CET was unchanged or perhaps slightly increased (Supplemental Fig. S5).
Figure 5.
Y(I) (A and D), Y(ND) (B and E), and Y(NA) (C and F) in wild-type N. tabacum (black circles) and three transgenic lines (B7, white circle; RI9, white triangle; and RI29, white square) with altered AOX amount, in both well-watered plants (A–C) and moderate drought-stressed plants (D–F). Data are the mean ± se of three independent experiments.
Figure 6.
Estimated rate of CET around PSI in wild-type N. tabacum (black circles) and three transgenic lines (B7, white circle; RI9, white triangle; and RI29, white square) with altered AOX amount, in both well-watered plants (A) and moderate drought-stressed plants (B). Data are the mean ± se of three independent experiments.
The higher Y(I) in knockdowns under drought was the result of a slightly lower nonphotochemical quantum yield resulting from donor side limitation [Y(ND)], relative to the wild type or B7 (Fig. 5E). On the other hand, the nonphotochemical quantum yield resulting from acceptor side limitation [Y(NA)] remained low (approximately 0.1) and similar across lines in both well-watered and drought-stressed plants (Fig. 5, C and F).
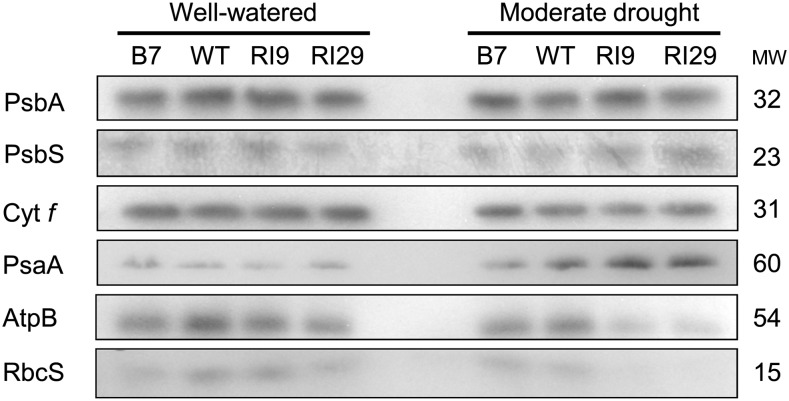
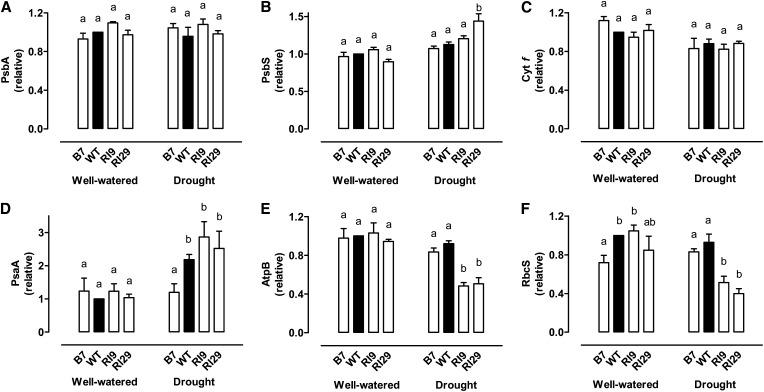
Immunoblots were used to compare the amounts of key photosynthetic components (as described in “Materials and Methods”) across the plant lines. Figure 7 shows representative immunoblots of the proteins examined while Figure 8 shows quantitative results from multiple experiments. In well-watered plants, the amount of each of these proteins was similar across all plant lines (Fig. 8), the only exception being a 28% lower amount of the small subunit of Rubisco (RbcS) in B7 than in the wild type (Fig. 8F). This particular difference, however, did not persist under drought.
Figure 7.
Representative immunoblots for several photosynthesis-related proteins (PsbA, PsbS, Cyt f, PsaA, AtpB, and RbcS) in wild-type N. tabacum and three transgenic lines with altered AOX amount, in both well-watered and moderate drought-stressed plants. Note that Figure 8 shows the results of three independent experiments in which such immunoblots were generated and quantified. MW, Protein molecular weight in kilodaltons; WT, wild type.
Figure 8.
The amount of several photosynthesis-related proteins (PsbA, PsbS, Cyt f, PsaA, AtpB, and RbcS) in wild-type N. tabacum and three transgenic lines with altered AOX amount, in both well-watered and moderate drought-stressed plants. Data are the mean ± se of three independent experiments. Protein amounts are relative to that of the well-watered wild-type amount, which was set to 1. Within each treatment, plant lines not sharing a common letter above the data bar are significantly different from one another (P < 0.05). Note that Figure 7 shows a representative immunoblot result from these experiments. WT, Wild type.
Under moderate drought, the amount of PsbA (the D1 reaction center protein of PSII) and Cyt f (a subunit of the Cyt b6f complex) did not differ across plant lines (Fig. 8, A and C), but each of the other proteins did exhibit significant differences between lines. In particular, the two AOX knockdown lines each had approximately 50% lower amounts of AtpB (a subunit of the catalytic subcomplex of ATP synthase) than the wild type or B7 (Fig. 8E). Because the AtpB antibody recognizes both chloroplast and mitochondrial ATP synthase β-subunits (Agrisera), we did separate organelle isolations and confirmed that the observed decline of whole-leaf AtpB in knockdowns was indeed due primarily to loss of chloroplast AtpB. Although some decline in mitochondrial AtpB was also seen in response to drought, it accounted for only 11% of the total (chloroplast + mitochondrial) decline of AtpB (Supplemental Fig. S6). The knockdowns also had an approximately 50% reduced RbcS amount under drought compared with the wild type (Fig. 8F), consistent with their lower CE (Table I). In addition, PsbS (a sensor of lumen pH necessary for NPQ induction) was significantly higher (by 1.3-fold) in RI29 than the wild type under drought, with RI9 showing an intermediate amount (Fig. 8B). Overall, the trend in PsbS amount across plant lines under drought mirrored the trend seen in NPQ. Finally, although PsaA (a reaction center protein of PSI) amount increased greater than 2-fold in the wild type and knockdown plants in response to drought, this increase was not seen in B7, such that the PsaA amount in B7 was now significantly lower than in the other lines (Fig. 8D).
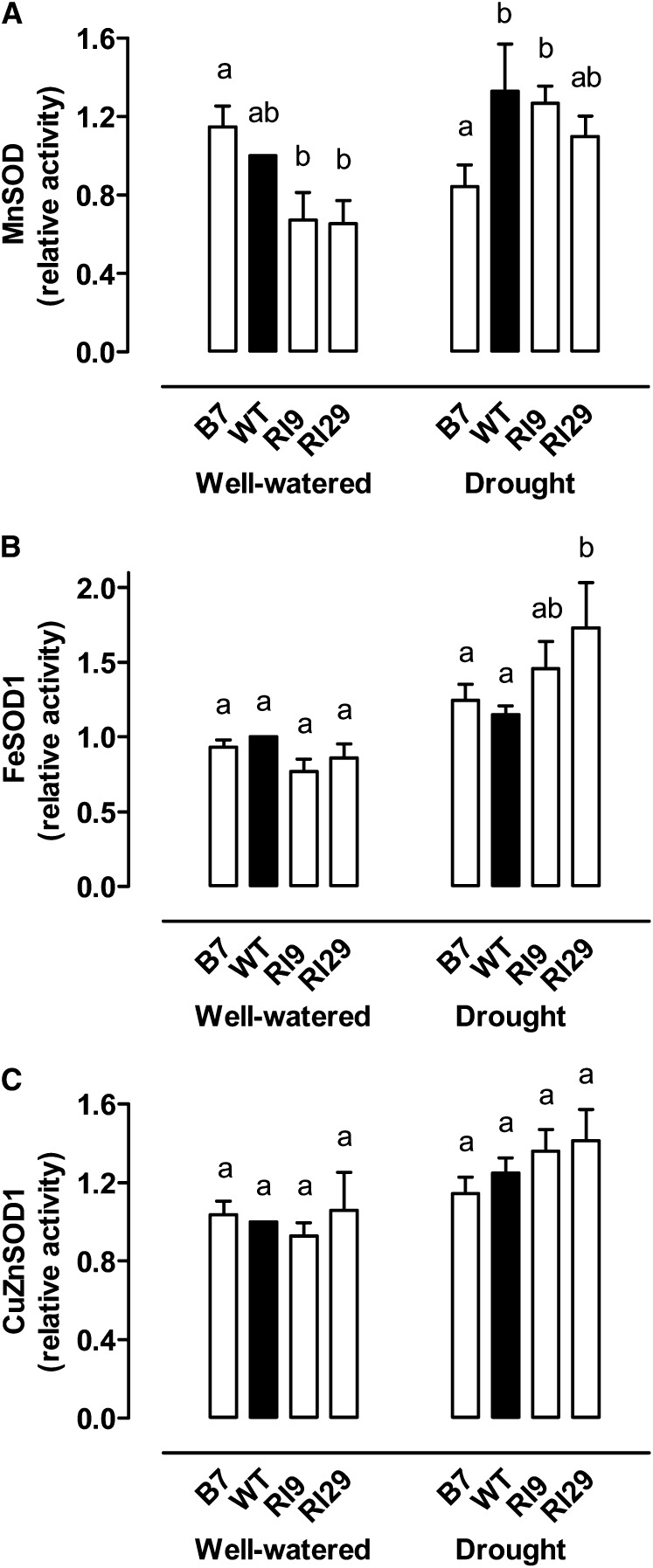
An in-gel assay was used to measure the maximum activity of the different leaf cellular superoxide dismutase (SOD) isozymes, responsible for O2− scavenging. Five SOD activities could be visualized, which, based upon their inhibitor sensitivities (see “Materials and Methods”; Cvetkovska and Vanlerberghe, 2012), included one manganese SOD (MnSOD), two iron SODs (FeSODs), and two copper zinc SODs (CuZnSODs). For each of the FeSODs and CuZnSODs, there was one major band of activity (which we termed FeSOD1 and CuZnSOD1) and one minor band of activity (termed FeSOD2 and CuZnSOD2). In well-watered plants, the activity of the nonmitochondrial SOD isozymes (FeSOD1 and CuZnSOD1 [Fig. 9, B and C] and FeSOD2 and CuZnSOD2 [Supplemental Fig. S7]) did not differ between any of the plant lines. However, the mitochondrial MnSOD activity was one-third lower in the AOX knockdowns than in the wild type, and was slightly higher (although not significantly) in B7 than in the wild type (Fig. 9A). After moderate drought, MnSOD activity declined 27% in B7, whereas it increased 1.3-fold in the wild type and 1.7- to 1.9-fold in the AOX knockdowns. As a result, MnSOD activity was now similar between the wild type and knockdowns, and was significantly lower in B7 (Fig. 9A). FeSOD1 activity increased slightly (1.2- to 1.3-fold) in the wild type and B7 in response to drought, but increased strongly (1.9- to 2-fold) in the two knockdowns (Fig. 9B). CuZnSOD1 activity increased slightly in all lines in response to drought (1.1-, 1.2-, 1.3-, and 1.5-fold in B7, the wild type, RI9, and RI29, respectively; Fig. 9C). FeSOD2 and CuZnSOD2 activities increased only marginally in response to drought and, similar to the well-watered condition, no differences were seen between plant lines (Supplemental Fig. S7).
Figure 9.
Maximal activities of MnSOD (A), FeSOD1 (B), and CuZnSOD1 (C) in wild-type N. tabacum and three transgenic lines with altered AOX amount, in both well-watered and moderate drought-stressed plants. Data are the mean ± se of six independent experiments. Activities are relative to that of the well-watered wild-type activity, which was set to 1. Within each treatment, plant lines not sharing a common letter above the data bar are significantly different from one another (P < 0.05). WT, Wild type.
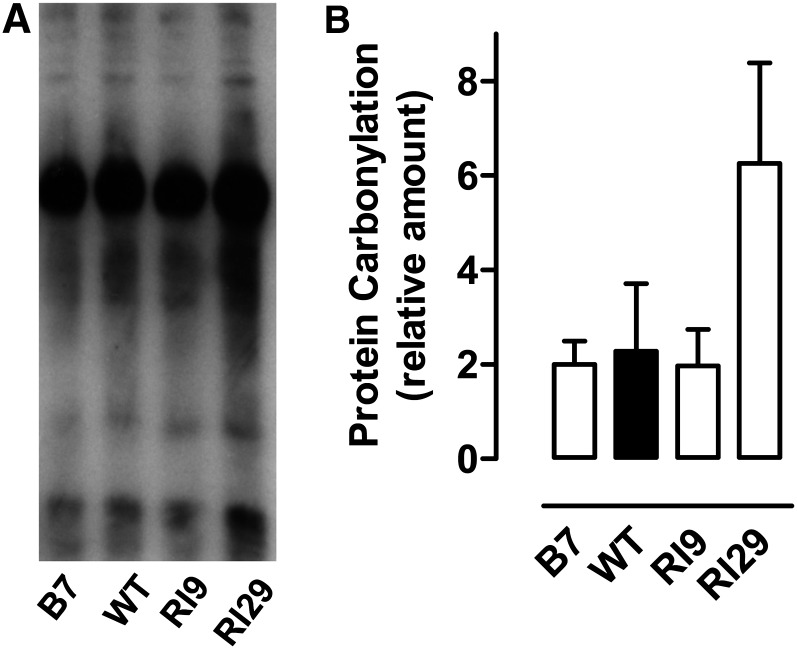
Immunoblots were used to compare the amounts of leaf protein oxidative modification (carbonylation) across the plant lines. Under well-watered conditions, carbonylation amounts were variable, with no reproducible trends across plant lines from one experiment to another (data not shown). Conversely, under drought conditions, protein carbonylation was always highest in RI29, although the amount in RI9 was similar to the wild type and B7 (Fig. 10).
Figure 10.
Protein carbonylation amounts after moderate drought in wild-type N. tabacum and three transgenic lines with altered AOX amount. A, A representative immunoblot. B, Quantitative results (mean ± se) from three independent experiments. Image analysis for quantification did not include the one prominent (always overexposed) band at approximately one-third down the gel but otherwise included the entire gel lane. WT, Wild type.
DISCUSSION
Knockdown of AOX Restricts Respiration Rate during Moderate Drought, Particularly in the Light
In wild-type N. tabacum, RL was about 21% lower than RD in both well-watered and moderate drought-stressed plants (Fig. 1), consistent with the general consensus in the literature that light moderately reduces respiration rate (Nunes-Nesi et al., 2007). There is less consensus in the literature of how drought impacts respiration rate (Atkin and Macherel, 2009). In some cases, drought was reported to have little or no effect on respiration rate (Ribas-Carbo et al., 2005; Giraud et al., 2008; Gimeno et al., 2010), whereas other studies reported decreases (Haupt-Herting et al., 2001; Haupt-Herting and Fock, 2002; Taylor et al., 2005; Vassileva et al., 2009; Galle et al., 2010; Ayub et al., 2011) or even increases (Bartoli et al., 2005; Feng et al., 2008; Hummel et al., 2010; Begcy et al., 2011). We found that both RD and RL tended to be lower (by about 27%) in moderate drought-stressed than well-watered wild-type N. tabacum (Fig. 1).
Our analyses showed that AOX knockdowns have a reduced rate of respiration during drought relative to the wild type, particularly in the light (Fig. 1, B and C). This implies that AOX respiration is an important component of RL in wild-type N. tabacum during drought. It also indicates that during drought, the cyt pathway of the knockdowns did not increase in activity to compensate for the lack of AOX. Three possible explanations are (1) that cyt pathway activity is limited by substrate availability, (2) that cyt pathway activity is limited by the turnover rate of ATP (adenylate control), or (3) that cyt pathway activity is already operating at the maximum capacity of one or more of its component parts (e.g. Complex III, cyt c, or Complex IV) during drought. All plant lines maintained similar amounts of Suc and monosaccharides during drought (Supplemental Fig. S1). In addition, the monosaccharide pools were severalfold higher during drought and the Suc amount was similar in well-watered and drought-stressed plants. Hence it seems unlikely that substrate availability accounts for the cyt pathway not compensating for the lack of AOX in knockdowns. It also seems unlikely that the cyt pathway is limited by ATP turnover, given the strong declines of chloroplast ATP synthase in knockdowns (see below), which should restrict overall ATP synthesis in the light relative to the wild type. Rather, it is possible that the cyt pathway is already operating at its maximum capacity during drought and is hence unable to compensate for the lack of AOX. In both soybean (Glycine max; Ribas-Carbo et al., 2005) and Nicotiana sylvestris (Galle et al., 2010), experiments using an oxygen isotope discrimination technique to analyze RD showed that cyt pathway activity declined during drought while AOX activity increased (soybean) or remained unchanged (N. sylvestris). Although these studies did not decipher the underlying mechanism(s) responsible for the cyt pathway decline (i.e. substrate availability, adenylate control, or overall capacity), the results are consistent with there being an inherent susceptibility of the cyt pathway to drought, relative to AOX respiration.
It is clear that RL is a smaller direct sink for electrons in AOX knockdowns than the wild type during moderate drought. Lower RL will likely also reduce the rate of reassimilation of respiratory CO2 by photosynthesis (Busch et al., 2013), hence indirectly reducing this electron sink as well. This provided a unique system to examine the importance of mitochondrial respiration as an electron sink to support photosynthesis during drought.
AOX Respiration Provides a Critical Electron Sink Supporting Photosynthetic Metabolism during Moderate Drought
In well-watered plants, all lines had similar RD and RL and, based upon several disparate types of analyses, all had similar photosynthetic characteristics. RI29 (and RI9 to a lesser extent) did show a slight (10%–15%) reduction in the light-saturated rate of photosynthesis estimated from light response curves. As previously described, this reduction is attributable to a stomatal limitation relating to disruption of guard cell nitric oxide homeostasis in well-watered knockdowns (Cvetkovska et al., 2014). Consistent with it resulting from a stomatal limitation, this reduction was not evident when An between lines was compared at equivalent Ci (Fig. 2A) or measured at low O2 (Fig. 4A).
During moderate drought stress, wild-type N. tabacum showed a reduced An rate over a wide range of Ci or irradiance. This was accompanied by a reduced ETRII, higher excitation pressure at PSII, lower ΦPSII, and increased NPQ relative to well-watered wild-type plants (Table I; Supplemental Figs. S3 and S4). These are all documented responses of photosynthesis to drought, when reduced stomatal aperture limits the availability of CO2 (Flexas et al., 2004; Lawlor and Tezara, 2009; Pinheiro and Chaves, 2011). Under such conditions, high stromal NADPH and/or a high difference in pH across the thylakoid membrane, due to decreased Calvin cycle demand for NADPH and ATP, down-regulates intersystem electron transport, likely at the level of the cyt b6f complex (Golding and Johnson, 2003; Hald et al., 2008; Kohzuma et al., 2009; Tikhonov, 2014). This prevents over-reduction at PSI, which could otherwise result in O2− generation (Asada, 2006; Joliot and Johnson, 2011). Meanwhile, the low lumenal pH induces NPQ, protecting PSII against overenergization that could otherwise generate singlet oxygen and damage the photosystem (Golding and Johnson, 2003; Tezara et al., 2008; Lawlor and Tezara, 2009; Vass, 2012). Consistent with many previous studies (Wingler et al., 1999; Golding and Johnson, 2003; Tezara et al., 2008), we saw that moderate drought had no impact on Fv/Fm, indicating that PSII functionality was not compromised (Table I). Drought-stressed wild-type plants also showed a marginal increase in CET, despite a decline in PSI reduction state [Y(I)] relative to the well-watered condition (Supplemental Fig. S5).
RL during drought was most disrupted in RI29, with RI9 showing an intermediate response between RI29 and the wild type (Fig. 1). This correlates well with our previous studies showing that RI29 is the stronger of the two knockdowns (Wang et al., 2011; Wang and Vanlerberghe, 2013; Cvetkovska et al., 2014). In RI29, the effects of drought on An, ETRII, excitation pressure, ΦPSII, and NPQ were all strongly magnified relative to the wild type, whereas RI9 (the slightly weaker AOX knockdown) always showed an intermediate response between RI29 and the wild type (Figs. 2 and 3; Table I; Supplemental Fig. S2). In other words, under drought conditions, lack of AOX correlates well with both a reduced RL and a reduced photosynthetic capacity relative to wild-type plants. The additional photosynthetic limitation in the knockdowns could not be explained by differences in PSII functionality or CO2 availability at Rubisco because Fv/Fm, gs, gm, Ci, and Cc were all similar between plant lines (Table I). It is known that restrictions in photorespiration can feedback-limit photosynthesis (Timm et al., 2012). However, the lower An of AOX knockdowns than the wild type during drought persisted even under nonphotorespiratory (2% O2) conditions (Fig. 4B). This suggests that the low An rate in knockdowns is not due to short-term feedback effects resulting from impeded photorespiration, although it should be realized that interpretation of such low O2 experiments can be complex (Sharkey, 1988). In summary, lack of AOX compromises photosynthetic capacity during moderate drought, presumably because the smaller respiratory electron sink further aggravates the drought-induced increases in stromal NADPH that arise due to limiting CO2 availability.
AOX Respiration Maintains Photosynthetic Capacity during Drought by Preventing Loss of Chloroplast ATP Synthase
Because our results suggested that plants lacking AOX had reduced photosynthetic capacity under drought and that these limitations were not due to any obvious differences in PSII functionality (Fv/Fm) or CO2 availability relative to the wild type, we sought to identify a biochemical basis for this additional limitation. Over the past decade, chloroplast ATP synthase has emerged as a central control point for photosynthesis, particularly as it relates to the lumenal pH-induced regulation of NPQ. Lumenal pH is a function of the rate of electron transport generating the H+ gradient and the rate of ATP synthase dissipating the H+ gradient. Regulation of ATP synthase amount and conductance can therefore influence the pH of the lumen relative to the rate of ATP synthesis, which in turn can feedback and modulate NPQ (Herbert, 2002; Kanazawa and Kramer, 2002; Cruz et al., 2005; Rott et al., 2011; Yamori et al., 2011b; Kohzuma et al., 2013).
The AOX knockdowns showed a large (approximately 50%) decline in the AtpB subunit of chloroplast ATP synthase, whereas wild-type plants saw only a small decline (Fig. 8E). We hypothesize that this difference relates to a higher stromal NADPH in the knockdown lines relative to the wild type under drought. Higher stromal NADPH will enhance the down-regulation of intersystem electron transport, as observed in the knockdowns. The question is how, with lower intersystem electron transport rates, is the knockdown able to maintain the low lumenal pH necessary to support its enhanced photoprotective NPQ relative to the wild type? The answer, at least in part, may be the strong reduction of the ATP synthase amount, which could act to preserve the H+ gradient being established by limited rates of electron transport.
The reduction state of the stromal NAD(P)H pool is likely a key factor regulating CET around PSI (Joët et al., 2002; Okegawa et al., 2008; Livingston et al., 2010; Joliot and Johnson, 2011; Johnson, 2011; Takahashi et al., 2013). We observed higher relative rates of CET in knockdown than in wild-type plants under drought (Fig. 6B), associated with a higher PSI reduction state and yield [i.e. higher Y(I) and lower Y(ND); Fig. 5]. This observation further supports the idea that lack of AOX results in a more highly reduced stromal NAD(P)H pool. By enhancing H+ gradient formation, CET is an important mechanism to boost NPQ, particularly under conditions when intersystem electron transport is limiting (Miyake et al., 2005; Johnson, 2011; Joliot and Johnson, 2011; Shikanai, 2014). Hence, its heightened activity in the knockdowns under drought likely contributes to their greater NPQ. Finally, the higher amount of PsbS in knockdowns (particularly RI29) than the wild type under drought (Fig. 8B) is consistent with their higher NPQ (Niyogi et al., 2005).
It was previously shown in sunflower (Helianthus annuus; Tezara et al., 1999) and watermelon (Citrullis vulgaris; Kohzuma et al., 2009) that chloroplast ATP synthase amount can progressively decline in response to increasing drought severity and contributes to a drought-induced biochemical limitation of photosynthesis. In another study, a drought-induced biochemical limitation of photosynthesis in sunflower correlated with reduced Rubisco (Tezara et al., 2002). Interestingly, we found that the AOX knockdowns had both strongly reduced ATP synthase and Rubisco amounts (Fig. 8, E and F). Such parallels between these previous studies and ours indicate that the AOX knockdowns may simply be more predisposed to a series of photosynthetic adjustments that will also manifest themselves in wild-type plants with increasing drought severity. We did observe a hint of decline (<10%) of both ATP synthase and Rubisco in wild-type N. tabacum with our moderate drought treatment. We suggest that with increasing drought severity, mitochondrial respiration becomes increasingly important as an electron sink necessary to maintain photosynthetic capacity. Hence, when this electron sink is lessened by removal of AOX, photosynthetic capacity becomes compromised at milder levels of drought stress.
It was hypothesized that drought-induced loss of ATP synthase results from chloroplast oxidative stress (Lawlor and Tezara, 2009). Two approaches were taken to address this possibility. First, we measured activity of the different compartment-specific SOD isozymes (MnSOD, CuZnSOD, and FeSOD), our rationale being that changes in maximal activity of a particular SOD isozyme could be indicative of the propensity for O2− generation within that compartment. In both N. tabacum and Arabidopsis, FeSOD is essential to managing chloroplast O2− amounts (Myouga et al., 2008; Zhang et al., 2011). Second, we examined protein carbonyl content as an estimate of protein oxidative modifications (Møller et al., 2007).
In well-watered plants, only MnSOD differed across lines, being paradoxically lower in the AOX knockdowns relative to the wild type and B7 (Fig. 9A). After drought, MnSOD activity increased 1.3-fold in the wild type, consistent with drought increasing the propensity for mitochondrial O2− generation and hence prompting a response by the O2− scavenger. It was previously shown that MnSOD expression is drought inducible in wheat (Triticum durum; Wu et al., 1999) and that wheat mitochondria suffer relatively more drought-induced oxidative damage, estimated by protein carbonyl content, than do chloroplasts or peroxisomes (Bartoli et al., 2004). Interestingly, the relative increase in MnSOD with drought was greater in the knockdowns (1.7- to 1.9-fold), whereas activity declined 27% with drought in B7. These results suggest a role for AOX in controlling O2− generation in the mitochondrion during drought, although absolute MnSOD activity still did not differ between the wild type and knockdowns under drought (Fig. 9A).
The only SOD isozyme whose absolute activity differed between lines during drought was the major chloroplast isozyme (FeSOD1), whose activity was higher in both knockdowns than in the wild type, although only significantly higher in RI29 (Fig. 9B). Consistent with this result, RI29 also consistently showed higher amounts of protein oxidative modification (carbonylation) than the other plant lines under drought (Fig. 10).
Overall, our data are consistent with there being higher rates of ROS generation in knockdowns than in the wild type, and specifically during drought. This corresponds with the higher reduction state of PSI in the knockdowns than the wild type, which should promote O2− generation in the chloroplast (Asada, 2006). However, whether enhanced protein oxidative damage can account for the enhanced loss of ATP synthase in knockdowns is questionable because this loss was clearly evident in both knockdown lines, whereas increased protein carbonylation was only evident in the stronger knockdown. Hence, we favor the hypothesis that loss of ATP synthase represents a photosynthetic regulatory response to drought and that in plants lacking AOX as an electron sink, this response is engaged at milder stress levels than in the wild type (see above). Consistent with this view, we have found that more severe drought stress manifests a similar loss of ATP synthase in wild-type N. tabacum (K. Dahal and G.C. Vanlerberghe, unpublished data).
Does AOX Have a General Role in Optimizing Photosynthetic Performance during Stress?
Studies in wheat (Bartoli et al., 2005) and Arabidopsis (Giraud et al., 2008) provided some support that AOX may aid photosynthesis during drought. In wheat, chemical inhibition of AOX by salicylhydroxamic acid was shown to decrease ΦPSII and increase PSII excitation pressure and NPQ specifically in drought-stressed leaves, although effects on An were not reported. However, experiments utilizing salicylhydroxamic acid must be interpreted cautiously given its ability to inhibit components of the cellular ROS network and plastid terminal oxidase. In Arabidopsis, an aox1a mutant similarly showed decreased ΦPSII and increased NPQ in drought-stressed leaves (again An was not reported). However, these results are difficult to interpret because drought reduced the leaf RWC of aox1a by about 10%, whereas no decline occurred in the wild type. This may be attributable to a root growth defect in aox1a, reported in the same study (Giraud et al., 2008). Given the different leaf water status of the Arabidopsis wild type and aox1a, it seems possible that their differences in Chl fluorescence were due to differences in stomatal aperture and hence CO2 availability.
Most studies of the relationship between AOX and photosynthesis have investigated the importance of AOX in short-term or long-term acclimation to changes in irradiance. Such studies comparing the Arabidopsis wild type and aox1a plants have, in general, demonstrated modest perturbations of An and/or Chl fluorescence parameters (Zhang et al., 2010; Florez-Sarasa et al., 2011; Yoshida et al., 2011b; Gandin et al., 2012; Vishwakarma et al., 2014). The results are consistent with there being little if any perturbation of respiration rate in the Arabidopsis aox1a plants (see the introduction), albeit only RD rates (not RL) have been reported thus far. This study emphasizes a much greater perturbation of photosynthesis, as well as a compromising of RD but especially RL in N. tabacum AOX knockdowns. This might result from species differences or may indicate a relatively greater importance of AOX to optimize photosynthesis during drought than in response to irradiance changes. This could be the case, particularly if the cyt pathway is indeed drought susceptible (Ribas-Carbo et al., 2005). Our results emphasize the need to now examine the interplay of AOX and photosynthesis in a greater range of species and in response to additional abiotic factors such as temperature, salinity, nutrient availability, and high CO2. Further investigation of the relationship between AOX and the chloroplast ATP synthase is of particular interest, given their common ability to manage changes in supply and demand of ATP and NAD(P)H.
CONCLUSION
Our results show that, during drought in N. tabacum, AOX is a necessary ETC component to maintain mitochondrial respiration during photosynthesis. In the absence of this electron sink, respiration is slowed, and this is accompanied by changes in the composition of the photosynthetic apparatus that then compromise photosynthetic capacity. These data contribute to our understanding of the metabolic role of AOX respiration during photosynthesis under drought (Ribas-Carbo et al., 2005). These data may also have some bearing on the more general (and controversial) question of how photosynthesis in wild-type plants during drought may be impacted, not just by the primary diffusive limitations (i.e. CO2 supply), but also potentially by biochemical factors (Flexas et al., 2004; Lawlor and Tezara, 2009; Pinheiro and Chaves, 2011). Our data suggest that if those mechanisms available to prevent chloroplast over-reduction are overwhelmed, changes in composition of the photosynthetic apparatus can result that then compromise photosynthetic capacity. In this regard, chloroplast ATP synthase amount may be a key biochemical factor, given its central regulatory role in photosynthesis (Kanazawa and Kramer, 2002). An intriguing question is whether these changes in composition of the photosynthetic apparatus are the result of damage (e.g. oxidative damage) or whether they represent inherent regulatory phenomenon, perhaps meant to preserve the photosynthetic apparatus from just such damage.
MATERIALS AND METHODS
Plants and Growth Conditions
Nicotiana tabacum ‘Petit Havana SR1’ was used for all experiments. Transgenic plant lines with suppressed levels of AOX protein (RI9 and RI29) attributable to the presence of an AOX1a RNA interference construct, or elevated levels of AOX protein (B7) due to the presence of an AOX1a transgene driven by a constitutive promoter were previously described (Wang et al., 2011; Wang and Vanlerberghe, 2013; Cvetkovska et al., 2014). In particular, it has been shown that AOX protein levels are strongly suppressed in the knockdowns, even under the strongly AOX-inducing conditions of low temperature (Wang et al., 2011) or severe drought (Wang and Vanlerberghe, 2013). Seeds were germinated in vermiculite for 16 d and then transferred individually to 4-inch plastic pots containing a general purpose growing medium with 4 parts soil (Promix BX; Premier Horticulture) and 1 part vermiculite. These were transferred to controlled-environment growth chambers (models PGR-15 and PGC-20; Conviron) with a 16-h photoperiod, temperature of 28°C/22°C (light/dark), relative humidity of 60%, and PPFD of 150 µmol m−2 s−1 (150 PPFD). Plants were irrigated daily with one-tenth-strength Hoagland solution. After 3 weeks in the growth chambers, a drought treatment was applied to some plants by withholding water. All analyses were subsequently performed on the fully developed fourth or fifth leaves of control well-watered plants (analyzed at 1 d after their last irrigation) or moderate drought-stressed plants (analyzed at 4 d after their last irrigation).
Analyses of Respiration and Photosynthesis
All respiration and photosynthesis measurements were performed on attached leaves at 4 to 5 h into the light period using a portable system (GFS-3000; Heinz Walz) able to simultaneously measure CO2 exchange and Chl a fluorescence from PSII. Light was provided through red and blue light-emitting diodes (model 3055-FL; Heinz Walz). The gas flow rate was set to 750 µmol s−1, and impeller speed was set to step 7. In some cases, the GFS-3000 was combined with a DUAL-PAM-100 measuring system (Heinz Walz) so that Chl fluorescence analyses could be done simultaneously with dual absorbance measurements (875 and 830 nm) reflective of the redox state of the PSI reaction center Chl (P700).
RD was estimated after a 30-min preincubation in the dark. RL was estimated by both the Laisk (1977) method (as described in Brooks and Farquhar, 1985; Galmés et al., 2006) and the Kok (1948) method, each of which generated similar quantitative and qualitative results. For the Laisk method, An was measured at 100, 300, and 500 PPFD over a leaf Ci range of approximately 50 to 175 µmol mol−1. RL was then estimated by identifying the intersection of the linear relationships between An and Ci measured at each PPFD. For the Kok method, An was measured at 0, 5, 10, 15, 20, 30, 40, 60, 80, 100, and 120 PPFD, which consistently resulted in a Kok break point at 20 PPFD. RL was then estimated by extrapolating to 0 PPFD the linear relationship between An and PPFD over the range of 20 to 120 PPFD. Supplemental Figure S8 shows typical examples of raw gas exchange results obtained using these Laisk and Kok protocols.
For photosynthetic light response curves, An was measured at intervals over the range of 0 to 2,000 PPFD (from low to high PPFD) with CO2 concentration of 400 µmol mol−1. An was determined after 6 min at a particular PPFD. For photosynthetic CO2 response curves, An was measured at saturating irradiance (1,600 PPFD), with CO2 supplied at a range of concentrations (with 2 min at each concentration) in the following sequence: 400, 50, 100, 200, 300, 400, 400, 600, 800, 1,000, and 1,200 µmol mol−1. The initial measure at 400 was discarded, and the following two repeated measures at 400 were averaged. The slope of the initial linear portion of the curve was used to estimate the CE of Rubisco (Sage, 1994).
Chl a fluorescence analyses always followed a dark-adaptation period of at least 30 min. Initial (minimum) PSII fluorescence in the dark-adapted state (F0), maximum PSII fluorescence in the dark-adapted state (Fm), maximum PSII fluorescence in the light-adapted state (Fm′), steady-state fluorescence (Fs), and initial (minimum) PSII fluorescence in the light-adapted state (F0′) were determined as previously described (Maxwell and Johnson, 2000). The maximal quantum yield of PSII was calculated as Fv/Fm = (Fm − F0)/Fm whereas the effective quantum yield (operating efficiency) of PSII was calculated as ΦPSII = (Fm′ − Fs)/Fm′. ETRII was calculated as: ETRII = (ΦPSII)(PPFD)(0.84)(0.5), where 0.84 and 0.5 represent the reasonable first approximations that leaves absorb 84% of incident photons and that 50% of these are absorbed by PSII (Baker et al., 2007). Photochemical energy quenching was calculated as: qP = (Fm′ − Fs)/(Fm′ − F0′) and used to determine the proportion of closed (reduced) PSII reaction centers, also known as excitation pressure, and calculated as 1 − qP. NPQ, a measure of heat dissipation of absorbed light energy, was calculated as: NPQ = (Fm − Fm′)/Fm′ (Maxwell and Johnson, 2000).
Dual-PAM-100 absorbance measurements were used to estimate PSI parameters including Y(I), Y(ND), Y(NA), and the rate of PSI electron transport (ETRI), as previously described (Klughammer and Schreiber, 2008). Y(I) is the fraction of overall P700 that is reduced and not acceptor side limited. As for ETRII, the ETRI estimate assumed that leaves absorb 84% of incident photons and that 50% of these are absorbed by PSI. The steady-state relative rate of CET around PSI was then estimated by subtracting ETRII from ETRI, with each electron transport rate having been estimated concurrently by simultaneous Chl fluorescence and PSI absorbance measurements (Johnson, 2011; Yamori et al., 2011a).
Stomatal conductance (gs) was calculated with the CO2 concentration in air (Ca) and Ci as follows: gs = 1.6 (An)/(Ca − Ci) (Farquhar and Sharkey, 1982). Mesophyll conductance (gm) was calculated from gas exchange and fluorescence data using the variable J method (Harley et al., 1992), where An and Ci were calculated from CO2 gas exchange measurements at saturating irradiance (1,600 PPFD), and RL was measured using the Laisk method, as described above. Cc was calculated as: Cc = Ci − (An/gm) (Harley et al., 1992).
Protein Analyses
After respiration and photosynthesis measurements, leaves were snap-frozen in liquid N2 and stored at −80°C. The frozen samples were then ground to a fine powder using liquid N2 and a mortar and pestle, and the leaf protein was extracted as previously described (Busch et al., 2007). Protein (15 µg) was then added to sample buffer (2% [w/v] SDS, 2% [v/v] 2-mercaptoethanol, 10% [v/v] glycerol, and 42 mm Tris, pH 6.8), heated to 75°C for 5 min, followed by addition of 0.01% bromophenol blue and loading on a gel for separation by SDS-PAGE. The resolved proteins were then transferred to nitrocellulose membrane using a TE 50X electrotransfer unit (Hoefer Pharmacia Biotech) and transfer buffer (25 mm Tris, pH 8.3, 192 mm Gly, and 20% [v/v] methanol). The membranes were then washed briefly in Tris-buffered saline (TBS)-Tween (20 mm Tris, pH 7.2, 125 mm NaCl, and 0.05% [v/v] Tween 20), allowed to dry, and then incubated at room temperature for 1 h with gentle rotation in SuperBlock blocking buffer (Thermo Scientific). The membranes were then incubated (1 h at room temperature, with gentle rotation) in TBS-Tween containing primary antibodies (Agrisera) diluted from 1,000- to 5,000-fold, and raised against the following proteins: PsbA (the plastid-encoded D1 reaction center protein of PSII), PsbS (the nuclear-encoded and PSII-associated sensor of lumen pH that is necessary for NPQ induction), PsaA (a plastid-encoded reaction center protein of PSI), Cyt f (the plastid-encoded c-type cyt subunit of the Cyt b6f complex), AtpB (the plastid-encoded β-subunit of the F1 catalytic subcomplex of ATP synthase), and RbcS (the nuclear-encoded small subunit of Rubisco; Allen et al., 2011). After incubation, the blots were briefly rinsed in TBS-Tween and then washed in TBS-Tween (4 × 15 min at room temperature, with gentle rotation). The membranes were then incubated (1 h at room temperature, with gentle rotation) in TBS-Tween with secondary antibody (goat anti-rabbit or donkey anti-rabbit IgG horseradish peroxidase-conjugated, diluted 20,000-fold; Agrisera). The membranes were then washed as described above and the antibodies detected using a chemiluminescent substrate (SuperSignal West Pico; Thermo Scientific) and x-ray film. The signals were quantified using an image analysis system (Chemidoc XRS+ with Image Lab Software v.3.0; BioRad).
Other Methods
Hexoses, Suc, and starch were quantified by enzyme-coupled assays, as previously described (Wang et al., 2011). In-gel SOD activity assays were done as previously described (Cvetkovska and Vanlerberghe, 2012). Protein carbonyl content was estimated by immunoblotting using the OxyBlot protein oxidation detection kit, following the manufacturer’s instructions (EMD Millipore). Leaf water status was determined by measuring the RWC of leaves, as previously described (Wang and Vanlerberghe, 2013). Separate chloroplast and mitochondrial fractions for immunoblotting were isolated simultaneously from 40 g fresh weight of leaf tissue using the protocol developed by Rödiger et al. (2010). Chl a and b were determined according to Arnon (1949) using leaf samples that had been snap-frozen in liquid N2. Protein concentration was determined by a modified Lowry method (Larson et al., 1986). Statistical analyses (two-way ANOVA, followed by a Bonferroni post-test comparing plant lines within a treatment) were done using Prism 5.0 (GraphPad Software).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Amounts of hexoses, Suc, and starch in wild-type N. tabacum and three transgenic lines with altered AOX amount, in both well-watered and moderate drought-stressed plants.
Supplemental Figure S2. Light response curves (for An, ETRII, PSII excitation pressure, and NPQ) of wild-type N. tabacum and three transgenic lines with altered AOX amount, in both well-watered and moderate drought-stressed plants.
Supplemental Figure S3. CO2 response curves (for An, ETRII, 1 − qP, and NPQ) of wild-type N. tabacum in both well-watered and moderate drought-stressed plants.
Supplemental Figure S4. Light response curves (for An, ETRII, 1 − qP, and NPQ) of wild-type N. tabacum in both well-watered and moderate drought-stressed plants.
Supplemental Figure S5. Y(I) and CET around PSI of wild-type N. tabacum in both well-watered and moderate drought-stressed plants.
Supplemental Figure S6. Amount of AtpB in chloroplast and mitochondrial fractions from both well-watered and moderate drought-stressed AOX knockdown plants.
Supplemental Figure S7. Maximal activities of the minor SOD isoforms (FeSOD2 and CuZnSOD2) in wild-type N. tabacum and three transgenic lines with altered AOX amount, in both well-watered and moderate drought-stressed plants.
Supplemental Figure S8. Examples of gas exchange data used to estimate RL using either the Laisk or Kok method.
Supplementary Material
Glossary
- AOX
alternative oxidase
- ETC
electron transport chain
- cyt
cytochrome
- O2−
superoxide radical
- ROS
reactive oxygen species
- RD
respiration rate in the dark
- RL
respiration rate in the light
- CET
cyclic electron transport
- RWC
relative water content
- Chl
chlorophyll
- An
net CO2 assimilation rate
- Ci
leaf intercellular CO2 concentration
- PPFD
photosynthetic photon flux density
- CE
carboxylation efficiency
- gs
stomatal conductance
- gm
mesophyll conductance
- Cc
concentration of CO2 at the Rubisco carboxylation site
- ETRII
rate of electron transport through PSII
- NPQ
nonphotochemical energy quenching
- ΦPSII
operating efficiency of PSII
- Fv/Fm
maximum photochemical efficiency of PSII in the dark-adapted state
- Y(I)
photochemical quantum yield of PSI
- Y(ND)
nonphotochemical quantum yield resulting from donor side limitation
- Y(NA)
nonphotochemical quantum yield resulting from acceptor side limitation
- RbcS
small subunit of Rubisco
- SOD
superoxide dismutase
- F0
initial (minimum) PSII fluorescence in the dark-adapted state
- Fm
maximum PSII fluorescence in the dark-adapted state
- Fm′
maximum PSII fluorescence in the light-adapted state
- Fs
steady-state fluorescence
- F0′
initial (minimum) PSII fluorescence in the light-adapted state
- qP
photochemical energy quenching
- ETRI
rate of PSI electron transport
- Ca
air intercellular CO2 concentration
- TBS
Tris-buffered saline
Footnotes
This work was supported by the Natural Sciences and Engineering Research Council of Canada (grant no. RGPIN-2014-06553 to G.C.V.).
The online version of this article contains Web-only data.
References
- Allen JF, de Paula WBM, Puthiyaveetil S, Nield J. (2011) A structural phylogenetic map for chloroplast photosynthesis. Trends Plant Sci 16: 645–655 [DOI] [PubMed] [Google Scholar]
- Arnon DI. (1949) Copper enzymes in isolated chloroplasts: polyphenoloxidases in Beta vulgaris. Plant Physiol 24: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada K. (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141: 391–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkin OK, Macherel D. (2009) The crucial role of plant mitochondria in orchestrating drought tolerance. Ann Bot (Lond) 103: 581–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayub G, Smith RA, Tissue DT, Atkin OK. (2011) Impacts of drought on leaf respiration in darkness and light in Eucalyptus saligna exposed to industrial-age atmospheric CO₂ and growth temperature. New Phytol 190: 1003–1018 [DOI] [PubMed] [Google Scholar]
- Baker NR, Harbinson J, Kramer DM. (2007) Determining the limitations and regulation of photosynthetic energy transduction in leaves. Plant Cell Environ 30: 1107–1125 [DOI] [PubMed] [Google Scholar]
- Bartoli CG, Gomez F, Gergoff G, Guiamét JJ, Puntarulo S. (2005) Up-regulation of the mitochondrial alternative oxidase pathway enhances photosynthetic electron transport under drought conditions. J Exp Bot 56: 1269–1276 [DOI] [PubMed] [Google Scholar]
- Bartoli CG, Gómez F, Martínez DE, Guiamet JJ. (2004) Mitochondria are the main target for oxidative damage in leaves of wheat (Triticum aestivum L.). J Exp Bot 55: 1663–1669 [DOI] [PubMed] [Google Scholar]
- Begcy K, Mariano ED, Mattiello L, Nunes AV, Mazzafera P, Maia IG, Menossi M. (2011) An Arabidopsis mitochondrial uncoupling protein confers tolerance to drought and salt stress in transgenic tobacco plants. PLoS ONE 6: e23776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks A, Farquhar GD. (1985) Effect of temperature on the CO2/O2 specificity of ribulose-1,5-bisphosphate carboxylase/oxygenase and the rate of respiration in the light: estimates from gas-exchange measurements on spinach. Planta 165: 397–406 [DOI] [PubMed] [Google Scholar]
- Busch F, Hüner NPA, Ensminger I. (2007) Increased air temperature during simulated autumn conditions does not increase photosynthetic carbon gain but affects the dissipation of excess energy in seedlings of the evergreen conifer Jack pine. Plant Physiol 143: 1242–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch FA, Sage TL, Cousins AB, Sage RF. (2013) C3 plants enhance rates of photosynthesis by reassimilating photorespired and respired CO2. Plant Cell Environ 36: 200–212 [DOI] [PubMed] [Google Scholar]
- Considine MJ, Holtzapffel RC, Day DA, Whelan J, Millar AH. (2002) Molecular distinction between alternative oxidase from monocots and dicots. Plant Physiol 129: 949–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz JA, Avenson TJ, Kanazawa A, Takizawa K, Edwards GE, Kramer DM. (2005) Plasticity in light reactions of photosynthesis for energy production and photoprotection. J Exp Bot 56: 395–406 [DOI] [PubMed] [Google Scholar]
- Cvetkovska M, Dahal K, Alber NA, Jin C, Cheung M, Vanlerberghe GC. (2014) Knockdown of mitochondrial alternative oxidase induces the ‘stress state’ of signaling molecule pools in Nicotiana tabacum, with implications for stomatal function. New Phytol 203: 449–461 [DOI] [PubMed] [Google Scholar]
- Cvetkovska M, Vanlerberghe GC. (2012) Alternative oxidase modulates leaf mitochondrial concentrations of superoxide and nitric oxide. New Phytol 195: 32–39 [DOI] [PubMed] [Google Scholar]
- Cvetkovska M, Vanlerberghe GC. (2013) Alternative oxidase impacts the plant response to biotic stress by influencing the mitochondrial generation of reactive oxygen species. Plant Cell Environ 36: 721–732 [DOI] [PubMed] [Google Scholar]
- Farquhar GD, Sharkey TD. (1982) Stomatal conductance and photosynthesis. Annu Rev Plant Physiol 33: 317–345 [Google Scholar]
- Feng H, Duan J, Li H, Liang H, Li X, Han N. (2008) Alternative respiratory pathway under drought is partially mediated by hydrogen peroxide and contributes to antioxidant protection in wheat leaves. Plant Prod Sci 11: 59–66 [Google Scholar]
- Finnegan PM, Soole KL, Umbach AL. (2004) Alternative electron transport proteins in plants. In Day DA, Millar AH, Whelan J, eds. Plant Mitochondria: From Genome to Function, Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 163–230 [Google Scholar]
- Fiorani F, Umbach AL, Siedow JN. (2005) The alternative oxidase of plant mitochondria is involved in the acclimation of shoot growth at low temperature; A study of Arabidopsis AOX1a transgenic plants. Plant Physiol 139: 1795–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flexas J, Bota J, Loreto F, Cornic G, Sharkey TD. (2004) Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol (Stuttg) 6: 269–279 [DOI] [PubMed] [Google Scholar]
- Florez-Sarasa I, Flexas J, Rasmusson AG, Umbach AL, Siedow JN, Ribas-Carbo M. (2011) In vivo cytochrome and alternative pathway respiration in leaves of Arabidopsis thaliana plants with altered alternative oxidase under different light conditions. Plant Cell Environ 34: 1373–1383 [DOI] [PubMed] [Google Scholar]
- Galle A, Florez-Sarasa I, Thameur A, de Paepe R, Flexas J, Ribas-Carbo M. (2010) Effects of drought stress and subsequent rewatering on photosynthetic and respiratory pathways in Nicotiana sylvestris wild type and the mitochondrial complex I-deficient CMSII mutant. J Exp Bot 61: 765–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galmés J, Medrano H, Flexas J. (2006) Acclimation of Rubisco specificity factor to drought in tobacco: discrepancies between in vitro and in vivo estimations. J Exp Bot 57: 3659–3667 [DOI] [PubMed] [Google Scholar]
- Gandin A, Duffes C, Day DA, Cousins AB. (2012) The absence of alternative oxidase AOX1A results in altered response of photosynthetic carbon assimilation to increasing CO2 in Arabidopsis thaliana. Plant Cell Physiol 53: 1627–1637 [DOI] [PubMed] [Google Scholar]
- Gimeno TE, Sommerville KE, Valladares F, Atkin OK. (2010) Homeostasis of respiration under drought and its important consequences for foliar carbon balance in a drier climate: insights from two contrasting Acacia species. Funct Plant Biol 37: 323–333 [Google Scholar]
- Giraud E, Ho LHM, Clifton R, Carroll A, Estavillo G, Tan YF, Howell KA, Ivanova A, Pogson BJ, Millar AH, et al. (2008) The absence of ALTERNATIVE OXIDASE1a in Arabidopsis results in acute sensitivity to combined light and drought stress. Plant Physiol 147: 595–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding AJ, Johnson GN. (2003) Down-regulation of linear and activation of cyclic electron transport during drought. Planta 218: 107–114 [DOI] [PubMed] [Google Scholar]
- Gunasekera D, Berkowitz GA. (1992) Heterogenous stomatal closure in response to leaf water deficits is not a universal phenomenon. Plant Physiol 98: 660–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hald S, Nandha B, Gallois P, Johnson GN. (2008) Feedback regulation of photosynthetic electron transport by NADP(H) redox poise. Biochim Biophys Acta 1777: 433–440 [DOI] [PubMed] [Google Scholar]
- Harley PC, Loreto F, Di Marco G, Sharkey TD. (1992) Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. Plant Physiol 98: 1429–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haupt-Herting S, Fock HP. (2002) Oxygen exchange in relation to carbon assimilation in water-stressed leaves during photosynthesis. Ann Bot (Lond) 89: 851–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haupt-Herting S, Klug K, Fock HP. (2001) A new approach to measure gross CO2 fluxes in leaves; gross CO2 assimilation, photorespiration, and mitochondrial respiration in the light in tomato under drought stress. Plant Physiol 126: 388–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert SK. (2002) A new regulatory role for the chloroplast ATP synthase. Proc Natl Acad Sci USA 99: 12518–12519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefnagel MHN, Atkin OK, Wiskich JT. (1998) Interdependence between chloroplasts and mitochondria in the light and the dark. Biochim Biophys Acta 1366: 235–255 [Google Scholar]
- Hummel I, Pantin F, Sulpice R, Piques M, Rolland G, Dauzat M, Christophe A, Pervent M, Bouteillé M, Stitt M, et al. (2010) Arabidopsis plants acclimate to water deficit at low cost through changes of carbon usage: an integrated perspective using growth, metabolite, enzyme, and gene expression analysis. Plant Physiol 154: 357–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüner NPA, Bode R, Dahal K, Hollis L, Rosso D, Krol M, Ivanov AG. (2012) Chloroplast redox imbalance governs phenotypic plasticity: the “grand design of photosynthesis” revisited. Front Plant Sci 3: 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joët T, Cournac L, Peltier G, Havaux M. (2002) Cyclic electron flow around photosystem I in C3 plants. In vivo control by the redox state of chloroplasts and involvement of the NADH-dehydrogenase complex. Plant Physiol 128: 760–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GN. (2011) Physiology of PSI cyclic electron transport in higher plants. Biochim Biophys Acta 1807: 384–389 [DOI] [PubMed] [Google Scholar]
- Joliot P, Johnson GN. (2011) Regulation of cyclic and linear electron flow in higher plants. Proc Natl Acad Sci USA 108: 13317–13322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa A, Kramer DM. (2002) In vivo modulation of nonphotochemical exciton quenching (NPQ) by regulation of the chloroplast ATP synthase. Proc Natl Acad Sci USA 99: 12789–12794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klughammer C, Schreiber U. (2008) Saturation pulse method for assessment of energy conversion in PSI. PAM Application Notes 1: 11–14 [Google Scholar]
- Kohzuma K, Cruz JA, Akashi K, Hoshiyasu S, Munekage YN, Yokota A, Kramer DM. (2009) The long-term responses of the photosynthetic proton circuit to drought. Plant Cell Environ 32: 209–219 [DOI] [PubMed] [Google Scholar]
- Kohzuma K, Dal Bosco C, Meurer J, Kramer DM. (2013) Light- and metabolism-related regulation of the chloroplast ATP synthase has distinct mechanisms and functions. J Biol Chem 288: 13156–13163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok B. (1948) A critical consideration of the quantum yield of Chlorella-photosynthesis. Enzymologia 13: 1–56 [Google Scholar]
- Kramer DM, Evans JR. (2011) The importance of energy balance in improving photosynthetic productivity. Plant Physiol 155: 70–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laisk AK. (1977) Kinetics of Photosynthesis and Photorespiration in C3-Plants. Nauka, Moscow [Google Scholar]
- Larson E, Howlett B, Jagendorf A. (1986) Artificial reductant enhancement of the Lowry method for protein determination. Anal Biochem 155: 243–248 [DOI] [PubMed] [Google Scholar]
- Lawlor DW, Tezara W. (2009) Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: a critical evaluation of mechanisms and integration of processes. Ann Bot (Lond) 103: 561–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston AK, Kanazawa A, Cruz JA, Kramer DM. (2010) Regulation of cyclic electron flow in C₃ plants: differential effects of limiting photosynthesis at ribulose-1,5-bisphosphate carboxylase/oxygenase and glyceraldehyde-3-phosphate dehydrogenase. Plant Cell Environ 33: 1779–1788 [DOI] [PubMed] [Google Scholar]
- Maxwell K, Johnson GN. (2000) Chlorophyll fluorescence: a practical guide. J Exp Bot 51: 659–668 [DOI] [PubMed] [Google Scholar]
- Millar AH, Whelan J, Soole KL, Day DA. (2011) Organization and regulation of mitochondrial respiration in plants. Annu Rev Plant Biol 62: 79–104 [DOI] [PubMed] [Google Scholar]
- Miyake C, Miyata M, Shinzaki Y, Tomizawa K. (2005) CO2 response of cyclic electron flow around PSI (CEF-PSI) in tobacco leaves: relative electron fluxes through PSI and PSII determine the magnitude of non-photochemical quenching (NPQ) of Chl fluorescence. Plant Cell Physiol 46: 629–637 [DOI] [PubMed] [Google Scholar]
- Møller IM, Jensen PE, Hansson A. (2007) Oxidative modifications to cellular components in plants. Annu Rev Plant Biol 58: 459–481 [DOI] [PubMed] [Google Scholar]
- Myouga F, Hosoda C, Umezawa T, Iizumi H, Kuromori T, Motohashi R, Shono Y, Nagata N, Ikeuchi M, Shinozaki K. (2008) A heterocomplex of iron superoxide dismutases defends chloroplast nucleoids against oxidative stress and is essential for chloroplast development in Arabidopsis. Plant Cell 20: 3148–3162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi KK, Li XP, Rosenberg V, Jung HS. (2005) Is PsbS the site of non-photochemical quenching in photosynthesis? J Exp Bot 56: 375–382 [DOI] [PubMed] [Google Scholar]
- Noctor G, Mhamdi A, Foyer CH. (2014) The roles of reactive oxygen metabolism in drought: not so cut and dried. Plant Physiol 164: 1636–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi K, Yoshida K. (2008) Interaction between photosynthesis and respiration in illuminated leaves. Mitochondrion 8: 87–99 [DOI] [PubMed] [Google Scholar]
- Nunes-Nesi A, Sweetlove LJ, Fernie AR. (2007) Operation and function of the tricarboxylic acid cycle in the illuminated leaf. Physiol Plant 129: 45–56 [Google Scholar]
- Okegawa Y, Kagawa Y, Kobayashi Y, Shikanai T. (2008) Characterization of factors affecting the activity of photosystem I cyclic electron transport in chloroplasts. Plant Cell Physiol 49: 825–834 [DOI] [PubMed] [Google Scholar]
- Perez-Martin A, Flexas J, Ribas-Carbó M, Bota J, Tomás M, Infante JM, Diaz-Espejo A. (2009) Interactive effects of soil water deficit and air vapour pressure deficit on mesophyll conductance to CO2 in Vitis vinifera and Olea europaea. J Exp Bot 60: 2391–2405 [DOI] [PubMed] [Google Scholar]
- Pinheiro C, Chaves MM. (2011) Photosynthesis and drought: can we make metabolic connections from available data? J Exp Bot 62: 869–882 [DOI] [PubMed] [Google Scholar]
- Raghavendra AS, Padmasree K. (2003) Beneficial interactions of mitochondrial metabolism with photosynthetic carbon assimilation. Trends Plant Sci 8: 546–553 [DOI] [PubMed] [Google Scholar]
- Ribas-Carbo M, Taylor NL, Giles L, Busquets S, Finnegan PM, Day DA, Lambers H, Medrano H, Berry JA, Flexas J. (2005) Effects of water stress on respiration in soybean leaves. Plant Physiol 139: 466–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rödiger A, Baudisch B, Klösgen RB. (2010) Simultaneous isolation of intact mitochondria and chloroplasts from a single pulping of plant tissue. J Plant Physiol 167: 620–624 [DOI] [PubMed] [Google Scholar]
- Rott M, Martins NF, Thiele W, Lein W, Bock R, Kramer DM, Schöttler MA. (2011) ATP synthase repression in tobacco restricts photosynthetic electron transport, CO2 assimilation, and plant growth by overacidification of the thylakoid lumen. Plant Cell 23: 304–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF. (1994) Acclimation of photosynthesis to increasing atmospheric CO2: the gas exchange perspective. Photosynth Res 39: 351–368 [DOI] [PubMed] [Google Scholar]
- Sharkey TD. (1988) Estimating the rate of photorespiration in leaves. Physiol Plant 73: 147–152 [Google Scholar]
- Sharkey TD, Seemann JR. (1989) Mild water stress effects on carbon-reduction-cycle intermediates, ribulose bisphosphate carboxylase activity, and spatial homogeneity of photosynthesis in intact leaves. Plant Physiol 89: 1060–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikanai T. (2014) Central role of cyclic electron transport around photosystem I in the regulation of photosynthesis. Curr Opin Biotechnol 26: 25–30 [DOI] [PubMed] [Google Scholar]
- Skirycz A, De Bodt S, Obata T, De Clercq I, Claeys H, De Rycke R, Andriankaja M, Van Aken O, Van Breusegem F, Fernie AR, et al. (2010) Developmental stage specificity and the role of mitochondrial metabolism in the response of Arabidopsis leaves to prolonged mild osmotic stress. Plant Physiol 152: 226–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strodtkötter I, Padmasree K, Dinakar C, Speth B, Niazi PS, Wojtera J, Voss I, Do PT, Nunes-Nesi A, Fernie AR, et al. (2009) Induction of the AOX1D isoform of alternative oxidase in A. thaliana T-DNA insertion lines lacking isoform AOX1A is insufficient to optimize photosynthesis when treated with antimycin A. Mol Plant 2: 284–297 [DOI] [PubMed] [Google Scholar]
- Takahashi H, Clowez S, Wollman FA, Vallon O, Rappaport F. (2013) Cyclic electron flow is redox-controlled but independent of state transition. Nat Commun 4: 1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi M, Miyake H. (2012) Redox-shuttling between chloroplast and cytosol: integration of intra-chloroplast and extra-chloroplast metabolism. Curr Opin Plant Biol 15: 252–260 [DOI] [PubMed] [Google Scholar]
- Taylor NL, Heazlewood JL, Day DA, Millar AH. (2005) Differential impact of environmental stresses on the pea mitochondrial proteome. Mol Cell Proteomics 4: 1122–1133 [DOI] [PubMed] [Google Scholar]
- Tezara W, Driscoll S, Lawlor DW. (2008) Partitioning of photosynthetic electron flow between CO2 assimilation and O2 reduction in sunflower plants under water deficit. Photosynthetica 46: 127–134 [Google Scholar]
- Tezara W, Mitchell V, Driscoll SP, Lawlor DW. (2002) Effects of water deficit and its interaction with CO2 supply on the biochemistry and physiology of photosynthesis in sunflower. J Exp Bot 53: 1781–1791 [DOI] [PubMed] [Google Scholar]
- Tezara W, Mitchell VJ, Driscoll SP, Lawlor DW. (1999) Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature 401: 914–917 [Google Scholar]
- Tikhonov AN. (2014) The cytochrome b6f complex at the crossroad of photosynthetic electron transport pathways. Plant Physiol Biochem 81: 163–183 [DOI] [PubMed] [Google Scholar]
- Timm S, Florian A, Arrivault S, Stitt M, Fernie AR, Bauwe H. (2012) Glycine decarboxylase controls photosynthesis and plant growth. FEBS Lett 586: 3692–3697 [DOI] [PubMed] [Google Scholar]
- Umbach AL, Fiorani F, Siedow JN. (2005) Characterization of transformed Arabidopsis with altered alternative oxidase levels and analysis of effects on reactive oxygen species in tissue. Plant Physiol 139: 1806–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC. (2013) Alternative oxidase: a mitochondrial respiratory pathway to maintain metabolic and signaling homeostasis during abiotic and biotic stress in plants. Int J Mol Sci 14: 6805–6847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vass I. (2012) Molecular mechanisms of photodamage in the Photosystem II complex. Biochim Biophys Acta 1817: 209–217 [DOI] [PubMed] [Google Scholar]
- Vassileva V, Simova-Stoilova L, Demirevska K, Feller U. (2009) Variety-specific response of wheat (Triticum aestivum L.) leaf mitochondria to drought stress. J Plant Res 122: 445–454 [DOI] [PubMed] [Google Scholar]
- Vishwakarma A, Bashyam L, Senthilkumaran B, Scheibe R, Padmasree K. (2014) Physiological role of AOX1a in photosynthesis and maintenance of cellular redox homeostasis under high light in Arabidopsis thaliana. Plant Physiol Biochem 81: 44–53 [DOI] [PubMed] [Google Scholar]
- Wang J, Rajakulendran N, Amirsadeghi S, Vanlerberghe GC. (2011) Impact of mitochondrial alternative oxidase expression on the response of Nicotiana tabacum to cold temperature. Physiol Plant 142: 339–351 [DOI] [PubMed] [Google Scholar]
- Wang J, Vanlerberghe GC. (2013) A lack of mitochondrial alternative oxidase compromises capacity to recover from severe drought stress. Physiol Plant 149: 461–473 [DOI] [PubMed] [Google Scholar]
- Watanabe CK, Hachiya T, Terashima I, Noguchi K. (2008) The lack of alternative oxidase at low temperature leads to a disruption of the balance in carbon and nitrogen metabolism, and to an up-regulation of antioxidant defence systems in Arabidopsis thaliana leaves. Plant Cell Environ 31: 1190–1202 [DOI] [PubMed] [Google Scholar]
- Wingler A, Quick WP, Bungard RA, Bailey KJ, Lea PJ, Leegood RC. (1999) The role of photorespiration during drought stress: an analysis utilizing barley mutants with reduced activities of photorespiratory enzymes. Plant Cell Environ 22: 361–373 [Google Scholar]
- Wu G, Wilen RW, Robertson AJ, Gusta LV. (1999) Isolation, chromosomal localization, and differential expression of mitochondrial manganese superoxide dismutase and chloroplastic copper/zinc superoxide dismutase genes in wheat. Plant Physiol 120: 513–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamori W, Sakata N, Suzuki Y, Shikanai T, Makino A. (2011a) Cyclic electron flow around photosystem I via chloroplast NAD(P)H dehydrogenase (NDH) complex performs a significant physiological role during photosynthesis and plant growth at low temperature in rice. Plant J 68: 966–976 [DOI] [PubMed] [Google Scholar]
- Yamori W, Takahashi S, Makino A, Price GD, Badger MR, von Caemmerer S. (2011b) The roles of ATP synthase and the cytochrome b6/f complexes in limiting chloroplast electron transport and determining photosynthetic capacity. Plant Physiol 155: 956–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Watanabe CK, Hachiya T, Tholen D, Shibata M, Terashima I, Noguchi K. (2011a) Distinct responses of the mitochondrial respiratory chain to long- and short-term high-light environments in Arabidopsis thaliana. Plant Cell Environ 34: 618–628 [DOI] [PubMed] [Google Scholar]
- Yoshida K, Watanabe CK, Terashima I, Noguchi K. (2011b) Physiological impact of mitochondrial alternative oxidase on photosynthesis and growth in Arabidopsis thaliana. Plant Cell Environ 34: 1890–1899 [DOI] [PubMed] [Google Scholar]
- Zhang DW, Xu F, Zhang ZW, Chen YE, Du JB, Jia SD, Yuan S, Lin HH. (2010) Effects of light on cyanide-resistant respiration and alternative oxidase function in Arabidopsis seedlings. Plant Cell Environ 33: 2121–2131 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ding S, Lu Q, Yang Z, Wen X, Zhang L, Lu C. (2011) Characterization of photosystem II in transgenic tobacco plants with decreased iron superoxide dismutase. Biochim Biophys Acta 1807: 391–403 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.