Divergent duplicated genes in the Brassica oleracea gain a novel function that could restore the fertility of a Brassica napus male-sterile mutant.
Abstract
Gene duplication followed by functional divergence in the event of polyploidization is a major contributor to evolutionary novelties. The Brassica genus evolved from a common ancestor after whole-genome triplication. Here, we studied the evolutionary and functional features of Brassica spp. homologs to Tic40 (for translocon at the inner membrane of chloroplasts with 40 kDa). Four Tic40 loci were identified in allotetraploid Brassica napus and two loci in each of three basic diploid Brassica spp. Although these Tic40 homologs share high sequence identities and similar expression patterns, they exhibit altered functional features. Complementation assays conducted on Arabidopsis thaliana tic40 and the B. napus male-sterile line 7365A suggested that all Brassica spp. Tic40 homologs retain an ancestral function similar to that of AtTic40, whereas BolC9.Tic40 in Brassica oleracea and its ortholog in B. napus, BnaC9.Tic40, in addition, evolved a novel function that can rescue the fertility of 7365A. A homologous chromosomal rearrangement placed bnac9.tic40 originating from the A genome (BraA10.Tic40) as an allele of BnaC9.Tic40 in the C genome, resulting in phenotypic variation for male sterility in the B. napus near-isogenic two-type line 7365AB. Assessment of the complementation activity of chimeric B. napus Tic40 domain-swapping constructs in 7365A suggested that amino acid replacements in the carboxyl terminus of BnaC9.Tic40 cause this functional divergence. The distribution of these amino acid replacements in 59 diverse Brassica spp. accessions demonstrated that the neofunctionalization of Tic40 is restricted to B. oleracea and its derivatives and thus occurred after the divergence of the Brassica spp. A, B, and C genomes.
Polyploidy or whole-genome duplication is thought to be a prominent evolutionary force in eukaryotes (Wolfe, 2001; Udall and Wendel, 2006), especially for flowering plants (Blanc and Wolfe, 2004; Van de Peer et al., 2009). Almost 95% of angiosperms show evidence of having undergone at least one round of whole-genome duplication in their evolutionary history, suggesting that most extant diploid flowering plants have evolved from ancient polyploids (Cui et al., 2006; Soltis et al., 2009). Gene duplications in the event of polyploidization provide sources for evolutionary novelties that could benefit plants (Lukens et al., 2004; Chen, 2007). Divergence after gene duplication could result in three primary evolutionary fates of duplicated genes: pseudogenization, neofunctionalization, and subfunctionalization (Force et al., 1999; Conant and Wolfe, 2008; Liu and Adams, 2010). Pseudogenization implies that duplicated genes with redundant functions lose their function by accumulating negative mutations; neofunctionalization denotes that the redundant gene evolves a new adaptive function; while subfunctionalization causes the duplicated genes to adopt a different part of the function of an ancestral gene (Rodríguez-Trelles et al., 2003; Flagel and Wendel, 2009; Liu and Adams, 2010).
The Brassica genus consists of three basic diploid species: Brassica rapa (AA; n = 10), Brassica nigra (BB; n = 8), and Brassica oleracea (CC; n = 9), and their derivative allotetraploid species: Brassica juncea (AABB; n = 18), Brassica napus (AACC; n = 19), and Brassica carinata (BBCC; n = 17; Beilstein et al., 2006). Comparative genetic mapping demonstrated that these Brassica spp., which diverged from Arabidopsis thaliana approximately 20 to 40 million years ago (Lagercrantz and Lydiate, 1996; Blanc et al., 2003; Town et al., 2006), descended from a common ancestor after whole-genome triplication (Parkin et al., 2002). Collinear comparison showed that for each of 24 ancestral genomic blocks defined in the ancestral karyotype in A. thaliana, three syntenic copies were identified in each of the diploid Brassica spp. genomes, with only one exception (Schranz et al., 2006; Wang et al., 2011; Cheng et al., 2013). Fractionation (gene loss from homologous genomic regions) and chromosomal rearrangements were prevalent in the diploidization process of the hexaploid Brassica spp. common ancestor (Lagercrantz, 1998; Town et al., 2006; Ziolkowski et al., 2006; Mun et al., 2009). Based on gene density differences caused by varying gene loss rates in the three collinear genomic block copies, these genomic blocks were classified into three subgenomes in the diploid Brassica spp. genomes: the least fractionated (LF), the medium fractionated (MF1), and the most fractionated (MF2) subgenomes (Wang et al., 2011; Tang and Lyons, 2012; Cheng et al., 2013). The different rates of gene loss of the three subgenomes support a two-step origin of the Brassiceae ancestral genome involving a tetraploidization process followed by substantial fractionation of the subgenomes MF1 and MF2 and more recently hybridization with the third subgenome LF to form a hexaploid (Wang et al., 2011; Tang et al., 2012). Although collinearity and changes in genomic structure, including duplications, deletions, and rearrangements of the Brassica spp. and A. thaliana are well studied, there is limited knowledge of the molecular and functional divergence of duplicated or homologous genes in the Brassica spp.
To utilize heterosis in B. napus breeding, hybrid production is based mainly on male sterility. Currently, the recessive epistatic genic male-sterile three-type line system 7365ABC is widely used for oilseed heterosis due to its advantages, producing 100% sterile offspring for realizing the triple-cross hybrid (Huang et al., 2007; Xia et al., 2012). The male sterility in this system is controlled by two genes, a recessive male sterile gene Bnms3 and a epistatic gene BnRf (Zhou et al., 2012). Bnms3 was recently reported to be a homolog to A. thaliana Tic40 (Dun et al., 2011). Tic40 was identified as a member of the TIC (for translocon at the inner envelope membrane of chloroplasts) complex that functions as a cochaperone to coordinate Tic110 and the stromal chaperone heat shock protein93 (Hsp93) (Chou et al., 2003, 2006). The A. thaliana tic40 mutant displayed a chlorotic phenotype throughout development (Chou et al., 2003) but a male-fertile phenotype with mature pollen grains (Dun et al., 2011). Interestingly, one allele of Bnms3, BnaC.Tic40, can rescue the fertility of the B. napus male-sterile line 7365A (Dun et al., 2011).
In this study, we identified and characterized Tic40 homologs in B. napus and three basic diploid Brassica spp. We suggested that neofunctionalization of BnaC9.Tic40 after the divergence of the Brassica spp. A, B, and C genomes was caused by amino acid replacements in the C terminus of BnaC9.Tic40. In addition, we validated that the allelic genes BnaC9.Tic40 (equivalent to BnaC.Tic40 as described by Dun et al. [2011]) and bnac9.tic40 originate from the C and A genomes, respectively, and became allelic due to a homologous chromosomal rearrangement in B. napus. These results provide further knowledge for the effective utilization of the restoring gene of 7365A and a better insight into the functional divergence of homologous duplicated genes in paleoploid Brassica spp.
RESULTS
Identification of Tic40 Homologs in B. napus and Three Basic Diploid Brassica spp.
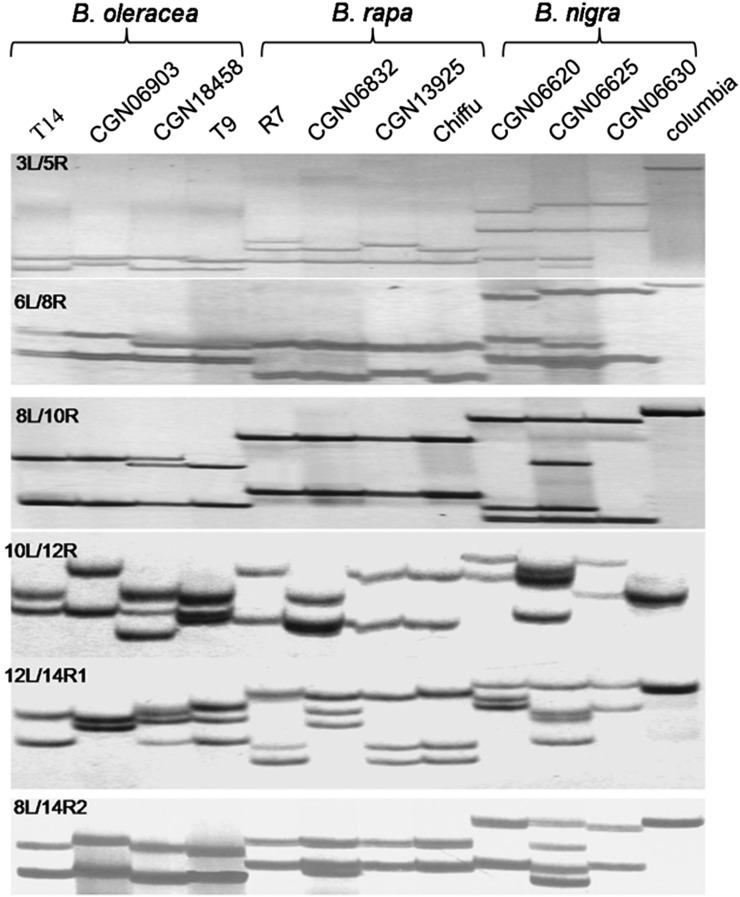
A. thaliana contains a single Tic40 locus (At5g16620) located on the short arm of chromosome 5. The complementary DNA (cDNA) sequence of AtTic40 was used to query the Brassica spp. EST database in GenBank (National Center for Biotechnology Information). Overall, 53 Brassica spp. sequences were identified that were aligned together with cDNAs of Tic40 homologs from several Brassicaceae spp. with available whole-genome sequence data: B. rapa (Wang et al., 2011), B. oleracea (Liu et al., 2014), Arabidopsis lyrata (Hu et al., 2011), Capsella rubella (Slotte et al., 2013), and Thellungiella halophila (Yang et al., 2013). Degenerate primers were designed using the conserved sequences identified from this alignment (for details of degenerate primers, see Supplemental Table S1; for expected annealing positions of degenerate primers, see Supplemental Fig. S1). From these primers, seven combinations were assayed in PCR to amplify Tic40 homologs in A. thaliana and three diploid Brassica spp., B. rapa, B. oleracea, and B. nigra. For each primer combination, one PCR fragment was obtained in A. thaliana, while two to four fragments were observed in the diploid Brassica spp. genotypes (Fig. 1), demonstrating multiple Tic40 copies in Brassica spp. genomes. For each amplified Brassica spp. genotype, 60 sequence reads obtained from PCR products of three primer combinations (6L/14R1, 8L/14R2, and 10L/12R) were assembled. Within genotypes, each unique sequence from one primer combination could be well overlapped with a unique sequence from another primer combination (excluding rare PCR recombination events), suggesting that all Tic40 copies in each genotype were amplified. This result supports the suitability of these primer combinations for amplifying Tic40 genes in Brassica spp. Based on sequence divergence, these assembled sequences were condensed into two groups in all amplified Brassica spp. genotypes, indicating the existence of two Tic40 loci in the diploid Brassica spp. (Table I). This successful PCR cloning approach was also used to isolate Tic40 homologs from homozygous B. napus genotypes, the wild-type 7365B and the male-sterile 7365A from the B. napus near-isogenic line 7365AB. A total of four Tic40 loci were identified in B. napus, and the fertility-restoring gene BnaC.Tic40 and its allele were identified at the Bnms3 locus in linkage group N19 of 7365B and 7365A, respectively, which has been reported by Dun et al. (2011).
Figure 1.
Amplification profiles of Tic40 genes from three diploid Brassica spp. Seven primer combinations, 3L/5R, 6L/8R, 8L/10R, 10L/12R, 12L/14R1, 8L/14R2, and 6L/14R1, were used to amplify DNA from genotypes of B. oleracea (T14, CGN06903, and CGN18458, T9), B. rapa (R7, CGN06832, CGN13925, and Chiffu), B. nigra (CGN06620, CGN06625, and CGN06630), and A. thaliana (Columbia). For 6L/14R1, the amplification profile is not shown because of the low resolution for large amplified fragments (about 1,500 bp).
Table I. Identification of Tic40 loci in three diploid Brassica spp.
| Genotype | Taxonomic Group | No. of Polymorphism Fragments | Sequence Identity between Polymorphism Fragmentsa | No. of Tic40 Locib |
|---|---|---|---|---|
| T14 | B. oleracea group Borecole | 2 | 74% | 2 |
| CGN06903 | Wild-type B. oleracea | 3 | 98%, 75%, 75% | 2 |
| CGN18458 | B. oleracea group Kohlrabi | 3 | 95%, 73%, 75% | 2 |
| T9 | Wild-type B. oleracea | 3 | 96%, 74%, 75% | 2 |
| R7 | B. rapa group Broccoletto | 2 | 74% | 2 |
| CGN06832 | Wild-type B. rapa group | 2 | 93%, 76%, 77% | 2 |
| CGN13925 | B. rapa group Pak Choi | 3 | 96%, 74%, 75% | 2 |
| Chiffu | B. rapa group Chinese Cabbage | 2 | 74% | 2 |
| CGN06620 | B. nigra var. Abyssinica | 3 | 96%, 70%, 70% | 2 |
| CGN06625 | B. nigra var. Abyssinica | 4 | 99%, 99%, 70%, 69%, 70%, 70% | 2 |
| CGN06630 | B. nigra var. Abyssinica | 2 | 70% | 2 |
Fragments from within a diploid Brassica spp. with sequence identity over 90% were considered alleles at a Tic40 locus. bDeduced number of loci based on number of polymorphic fragments and sequence identity.
Genome Synteny of the Tic40 Genomic Regions in Several Brassicaceae Genomes
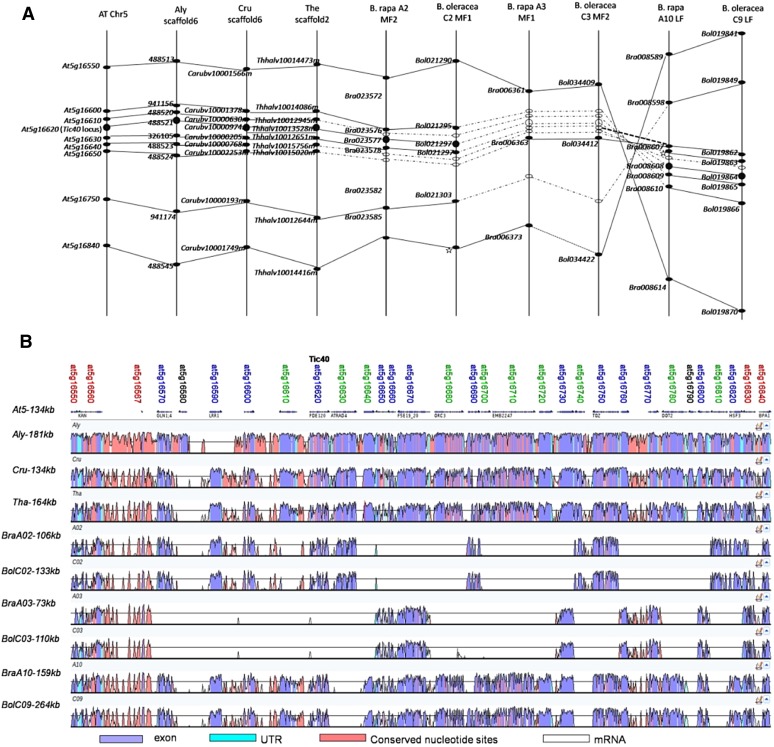
To study the evolutionary features of genomic regions around Tic40 in A. thaliana and Brassica spp., genes flanking the Tic40 locus in A. thaliana were used to identify its syntenic regions in the genomes of several Brassicaceae spp. As a result, one syntenic region was identified in the genomes of A. lyrata, C. rubella, and T. halophila, and three syntenic regions were identified in the genomes of B. rapa and B. oleracea (Fig. 2A). Notably, the genomes of B. rapa and B. oleracea each harbor two Tic40 loci, and no Tic40 homologs are present at the proposed syntenic regions of B. rapa A3 and B. oleracea C3 (Fig. 2A), which is consistent with the results presented above. Interestingly, gene deletions were prevalent in the three subgenomes of Brassica spp., as evident from the absence of several prospective homologous genes (Fig. 2A).
Figure 2.
Synteny of Tic40 genomic regions in Brassicaceae genomes. A, Alignment of homologous Tic40 regions from six Brassicaceae genomes. The loci depicted by black circles are Tic40 homologs, and ellipses represent homologs of Tic40 flanking genes. White circles and ellipses represent the absence of prospective homologous genes. The star indicates that the transcript of a corresponding homolog was absent in the data but the homologous genomic sequence was present. Solid lines connect homologs, and broken lines were used when at least one homolog was absent. B, Genomic multiple alignment of 10 Brassicaceae Tic40 genomic regions aligned using BLASTZ and visualized with a VISTA plot. The names of the 31 gene models in this A. thaliana region (from At5g16550 to At5g16840) are depicted with different colors to indicate the number of homologs detected in the two diploid Brassica spp. genomes: red, three homologs in each Brassica spp. genome; blue, two homologs; green, one homolog; and black, loss of homologs in these Brassica spp. genomes. Conserved regions in the similarity plots are colored to illustrate annotation of the region. At, A. thaliana; Aly, A. lyrata; Cru, C. rubella; The, T. halophila; Bra, B. rapa; Bol, B. oleracea; UTR, untranslated region.
Tic40 genomic regions orthologous to the A. thaliana Tic40 genomic region (from At5g16550 to At5g16840) in each genome were aligned using BLASTZ. The resulting VISTA plot (Fig. 2B) reveals a high degree of conservation among genomic regions, although a noncontinuous pattern of conserved subsets of genes are present in Brassica spp. Tic40 genomic regions due to a number of deletion events. The regions from A. lyrata, C. rubella, and T. halophila showed high similarity to that of A. thaliana. The apparent absence of homologs of At5g16580 and At5g16790 from all Brassica spp. as well as T. halophila might be the result of insertions in the A. thaliana lineage after the separation of the A. thaliana lineage and the one leading to the genera Brassica and Thellungiella. The degree of conservation between A. thaliana and the different Brassica spp. was considerably lower. Out of the other 29 gene models in the region, only five genes (17.24%) are represented in all six Brassica spp. paralogous regions; 14 genes (48.28%, including Tic40) have conserved gene models in four Brassica spp. paralogous regions, and 10 genes (34.48%) have one homolog in the diploid Brassica spp. genomes (Fig. 2B). These results suggest numerous deletion events in the diploidization process of the Brassica spp. ancestor. Furthermore, in agreement with a previous report (Cheng et al., 2013), deletion events in the Tic40 genomic regions from B. rapa A10 and B. oleracea C9 subgenome LF were much fewer than those from B. rapa A02 and B. oleracea C02 subgenome MF2 and the B. rapa A03 and B. oleracea C03 subgenome MF1 (Fig. 2B). In summary, these analyses demonstrate that duplication and deletion of Tic40 homologs in the Brassica spp. genomes are a result of genome triplication following diploidization in the Brassica spp. common ancestor.
According to their location in the Brassica spp. chromosomes, the identified Tic40 genes were named BraA2.Tic40 and BraA10.Tic40 in B. rapa, BolC2.Tic40 and BolC9.Tic40 in B. oleracea, BniB.Tic40a and BniB.Tic40b in B. nigra, and BnaA2.Tic40, BnaA10.Tic40, BnaC2.Tic40, and BnaC9.Tic40 (equivalent to the fertility-recovering gene BnaC.Tic40) in B. napus. The allele of BnaC9.Tic40 in the male-sterile line 7365A was named bnac9.tic40.
Evolutionary Analysis of Brassica spp. Tic40 Homologs
In B. napus, the A and C genomes were considered to be homologous subgenomes due to their evolutionary origins. Because of this complex genome background, Tic40 homologs in B. napus were classified into paralogs (duplicated genes in the same subgenome) and homologs (orthologous genes in different subgenomes).
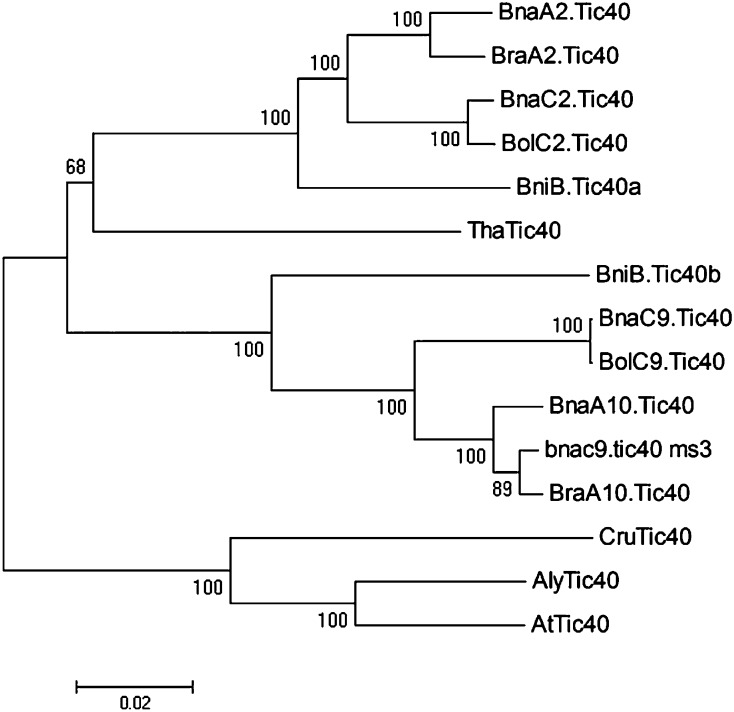
The full-length cDNAs and genomic sequences of the identified Brassica spp. Tic40 genes were isolated and compared with other Brassicaceae Tic40 genes. The phylogenetic tree constructed using the full-length Tic40 open reading frames (ORFs) revealed that these Brassicaceae Tic40 genes fell into three groups: AtTic40, AlyTic40, and CruTic40 in group 1; BnaC9.Tic40, BnaA10.Tic40, BolC9.Tic40, BraA10.Tic40, bnac9.tic40, and BniB.Tic40a in group 2; and ThaTic40, BnaA2.Tic40, BnaC2.Tic40, BolC2.Tic40, BraA2.Tic40, and BniB.Tic40b in group 3, which is consistent with the hypothesis stating a duplication within the Brassica spp. ancestor after the divergence from the lineage leading to A. thaliana (Fig. 3). With the exception of bnac9.tic40, each Tic40 gene existing in B. napus clustered with its corresponding diploid progenitor ortholog (Fig. 3). Sequence identities shared between Brassica spp. Tic40 orthologous gene pairs (98%–100%; e.g. BnaC9.Tic40 and BolC9.Tic40) were higher than those displayed between homologous gene pairs (92%–96%; e.g. BnaC9.Tic40 and BnaA10.Tic40; Supplemental Table S2). Interestingly, bnac9.tic40 showed only 94% sequence identity with BolC9.Tic40, less than that with BnaA10.Tic40 (98%) and BraA10.Tic40 (99%), while its allele BnaC9.Tic40 displayed 100% sequence identity with BolC9.Tic40 (Supplemental Table S2). Phylogenetic tree analysis also suggested that bnac9.tic40 was evolutionarily closer to BraA10.Tic40 than BolC9.Tic40 (Fig. 3). These data support the conclusion that BnaC9.Tic40 originated from BolC9.Tic40, but its allele bnac9.tic40 was derived from BraA10.Tic40, most likely due to homologous recombination between the A and C genomes (see below).
Figure 3.
A neighbor-joining (NJ) distance tree for Tic40 homologs in the Brassicaceae. The full-length ORF sequences of Tic40 homologs were used for phylogenetic analysis using MEGA4.0 (Tamura et al., 2007). Bna, B. napus; Bol, B. oleracea; Bra, B. rapa; Bni, B. nigra; At, A. thaliana; Aly, A. lyrata; Cru, C. rubella; Tha, T. halophila. The numbers on nodes are bootstrap values.
Homologous Chromosomal Rearrangement around BnaC9.Tic40 in B. napus
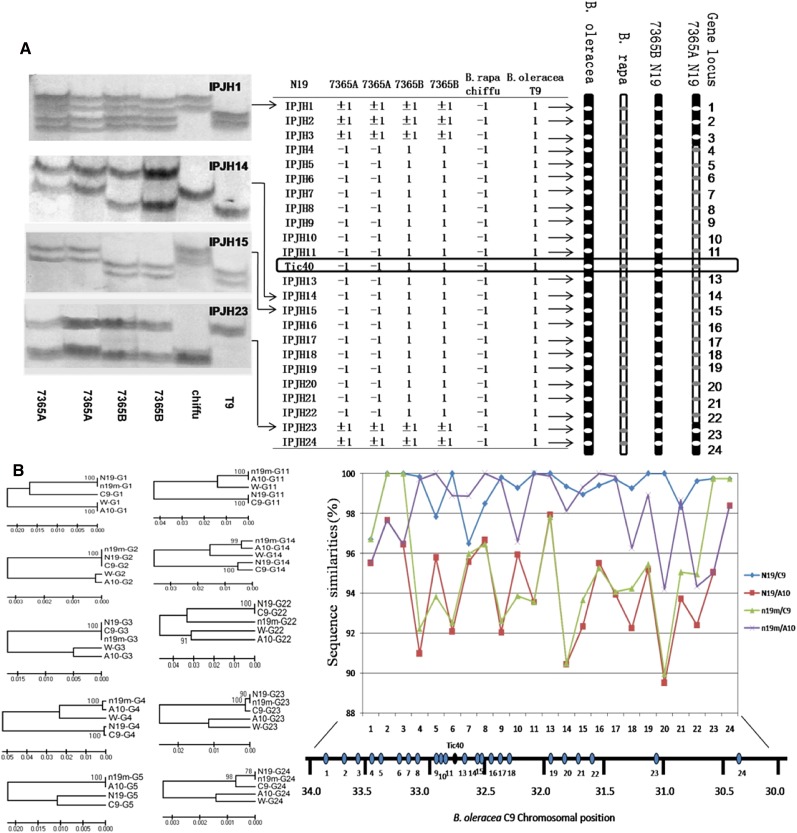
Due to high synteny between the A and C genomes in B. napus, chromosomal rearrangements caused by homologous recombination events between these two related genomes are not uncommon (Udall et al., 2005). We hypothesized that the genes BnaC9.Tic40 and bnac9.tic40, originating from the C and A genomes, respectively, became allelic on N19 as the result of an earlier chromosomal rearrangement between homologous Tic40 genomic regions of linkage groups N10 and N19 in B. napus. To confirm this deduction, we developed 23 intron polymorphism (IP) molecular markers using information of flanking genes spanning an approximately 3.5-Mb region around BolC9.Tic40. These 23 IP markers (from IPJH1 to IPJH24, without IPJH12 because the gene locus 12 is condescending to Tic40 locus) detected polymorphic fragments between the highly homozygous genotypes B. rapa Chiffu and B. oleracea T9 (Fig. 4A), and 18 of those (from IPJH4 to IPJH22) detected segregating loci for which the two homozygous B. napus genotypes 7365A and 7365B had different allelic fragments (Fig. 4A). In these cases, 7365A had the polymorphic fragment coincident to that detected by the same marker in B. rapa Chiffu and 7365B had the other polymorphic fragment corresponding to that in B. oleracea T9 (Fig. 4A). For other IP markers, IPJH1, IPJH2, IPJH3, IPJH23, and IPJH24, 7365A and 7365B had the fragments from both Chiffu and T9 (Fig. 4A). The most likely explanation for this pattern is that the genomic region from IPJH4 to IPJH22 on N19 in 7365A is derived from B. rapa (see outline of linkage groups in Fig. 4A).
Figure 4.
Analysis of the subgenome origin of genomic regions around BnaC9.Tic40 and bnac9.tic40 in B. napus. A, Segregation of IP molecular markers on linkage group N19 of B. napus 7365A and 7365B. Twenty-three IP molecular markers (IPJH1–IPJH24) corresponded to 23 loci flanking Tic40 in B. rapa A10 and B. oleracea C9 (Supplemental Table S3). For IPJH1, IPJH14, IPJH15, and IPJH23, amplification patterns are shown on the left. For each molecular marker, numbers were assigned to polymorphic fragments: −1 if they were identical to those from B. rapa Chiffu and +1 if they were identical to those from B. oleracea T9. The presence of these fragments in B. napus 7365A and 7365B is shown in the center table, and the result is visualized on the right, with white ovals representing C genome alleles and gray ovals representing A genome alleles. For gene loci IPJH1, IPJH2, IPJH3, IPJH23, and IPJH24, 7365A and 7365B displayed the expected pattern with fragments from both Chiffu and T9 (e.g. IPJH1 and IPJH23). However, for loci IPJH4 to IPJH22, B. rapa-specific fragments were present in 7365A; conversely, for IPJH13 and IPJH14, B. oleracea-specific fragments were present in 7365B. B, Sequence divergence of homologs of 23 flanking genes. The approximate genomic locations of the flanking genes at B. oleracea C9 are indicated by ovals (bottom right). In both 7365A and 7365B, partial sequences (300–800 bp) of 23 flanking genes were aligned with their orthologs in B. rapa A10 and B. oleracea C09. Phylogenetic trees of homologs of several flanking genes constructed by MEGA4.0 are shown on the left. Sequence divergences between these homologs are shown on the right. A10, Homologs from B. rapa; C9, homologs from B. oleracea; G1 to G24, gene loci 1 to 24 flanking Tic40; N19, homologs from 7365B; n19m, homologs from 7365A; W, homologs existing in both 7365B and 7365A, representing homologs from other syntenic regions. For genes G1, G2, G3, G23, and G24, sequences from N19 and n19m cluster together with the sequence from C9. For the other genes, only N19 sequences cluster with C9 sequences, while n19m sequences cluster with those from A10, supporting the conclusion that the region around Tic40 on N19 in 7365A is derived from N10 (corresponding to A10).
Furthermore, partial sequences (300–800 bp) of 23 flanking genes around BnaC9.Tic40 and bnac9.tic40 were sequenced, and the resulting sequences were aligned with their orthologs in B. rapa A10 and B. oleracea C09. Based on these alignments, we constructed phylogenetic trees of homologs of these flanking genes and compared the sequence divergence between these homologs. As shown in Figure 3B, both the phylogenetic trees and sequence divergence analysis suggested that flanking genes 1, 2, 3, 23, and 24 of 7365A and all analyzed flanking genes of 7365B were evolutionarily closer to their homologs from B. oleracea C09, whereas flanking gene models 4 to 22 of 7365A were more similar to their homologs from B. rapa A10 rather than B. oleracea C09. These data showed that an approximately 2-Mb fragment (from gene models 4 to 22) surrounding bnac9.tic40 is derived from B. rapa A10 (Fig. 4B). These results collectively support our hypothesis that the different origins of the allelic genes BnaC9.Tic40 and bnac9.tic40 were due to a homologous chromosomal rearrangement around the Tic40 locus in B. napus N19. Thus, BnaC9.Tic40 and bnac9.tic40 are evolutionary homologs, although they are allelic in B. napus.
BolC9.Tic40 and BnaC9.Tic40 Gained a Novel Function Related to Male Fertility
BnaC9.Tic40 has been reported as a fertility-restorer gene of the B. napus male-sterile line 7365A, the sterility of which is controlled by two genes, Bnms3 (bnac9.tic40) and BnRf, whereas loss of Tic40 in A. thaliana causes a chlorotic phenotype throughout development but does not affect male fertility (Chou et al., 2003; Dun et al., 2011). The A. thaliana tic40 mutant and the B. napus male-sterile 7365A mutant provide good systems for studying the functional features of Tic40 family members. To pursue a better understanding of the functional characteristics of Brassica spp. Tic40 genes, genomic fragments of BnaC9.Tic40, bnac9.tic40, BnaA10.Tic40, BnaC2.Tic40, BnaA2.Tic40, six diploid Brassica spp. Tic40 genes, and AtTic40, including approximately 2,000 bp of the putative upstream promoter region and 700 bp of the downstream region, were cloned into the pCAMBIA1305.1 binary vector. The 12 resulting constructs were transformed into both the A. thaliana attic40 mutant and the B. napus 7365A male-sterile line. Genetic complementation assays suggested that all Tic40 genes could restore the attic40 mutant to the wild type (Table II). We carefully investigated the phenotypes of more than 10 T0 transgenic B. napus 7365A plants for each Tic40 construct at the flowering stage. For BolC9.Tic40 and BnaC9.Tic40, eight out of 11 and 11 out of 13 transgenic lines displayed recovered fertility when grown to maturity, respectively (Table II). However, for all other Tic40 constructs, no 7365A transgenic plants exhibited a fertility-recovered phenotype (Table II). These results indicated that bnac9.tic40 is not a loss-of-function mutant but that the dominant gene BnaC9.Tic40 and its ortholog BolC9.Tic40 have gained a novel function relating to male fertility.
Table II. Results of genetic complementation assays for 12 Tic40 genes introduced into the 7365A mutant and the attic40 mutant.
+ indicates that the phenotype of the mutant was restored; − indicates that the phenotype of the mutant was not restored. Numbers in parentheses represent number of restored plants/number of plants assayed. na, Not applicable, as the genotype 7365A already harbors the Tic40 homologs introduced.
| Transformed Genotype | Introduced Tic40 Gene |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AtTic40 | BolC9.Tic40 | BolC2.Tic40 | BraA10.Tic40 | BraA2.Tic40 | BniB.Tic40a | BniB.Tic40b | BnaC9.tic40 | Bnac9.tic40 | BnaA10.tic40 | BnaC2.tic40 | BnaA2.tic40 | |
| 7365A | − (0/13) | + (8/11) | − (0/17) | − (0/10) | − (0/14) | − (0/11) | − (0/13) | + (11/13) | na | na | na | na |
| attic40 | + (7/7) | + (5/5) | + (8/9) | + (5/5) | + (5/5) | + (6/6) | + (7/7) | + (14/14) | + (11/11) | + (6/6) | + (8/8) | + (10/10) |
Expression Profiles of Tic40 Homologs in B. napus, B. rapa, and B. oleracea
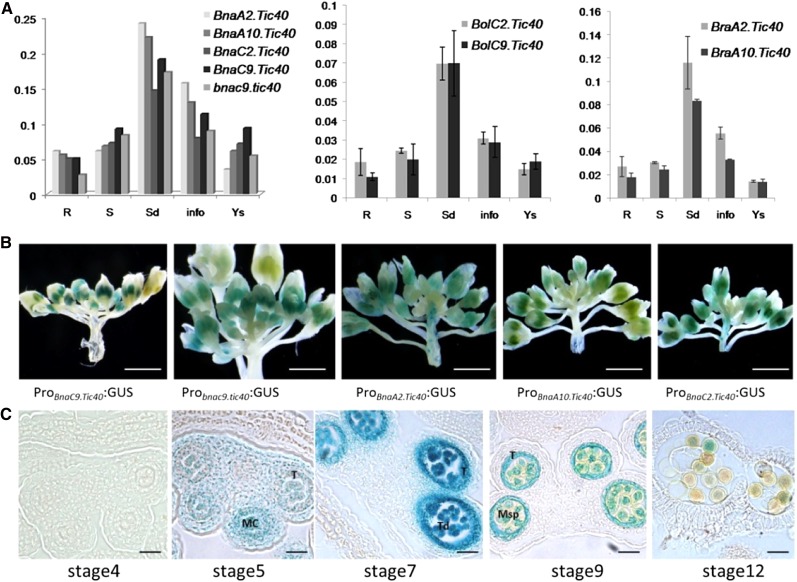
To determine whether the expression patterns of Brassica spp. Tic40 homologs have diverged, we compared the expression patterns of bnac9.tic40, BnaC9.Tic40, BnaC2.Tic40, BnaA2.Tic40, and BnaA10.Tic40 in B. napus, BraA2.Tic40 and BraA10.Tic40 in B. rapa, and BolC2.Tic40 and BolC9.Tic40 in B. oleracea by real-time PCR analysis. The data revealed that these Tic40 homologs are expressed in various organs, such as roots, seedlings, stems, young siliques, and inflorescences (Fig. 5A). Furthermore, expression patterns were similar for all Tic40 homologs throughout development (Fig. 5A).
Figure 5.
Expression patterns of Brassica spp. Tic40 genes. A, Spatial and temporal expression analyses of Brassica spp. Tic40 genes in selected tissues using real-time PCR. R, Root; S, stem; Sd, seedling; info, inflorescence; Ys, young silique. Expression patterns of Brassica spp. Tic40 genes are shown for B. napus (left), B. oleracea (middle), and B. rapa (right). B, Histochemical analysis of the GUS activities (blue staining) directed by individual B. napus Tic40 promoters in transgenic A. thaliana plants. GUS expression was driven by the five promoters of BnaC9.Tic40, bnac9.tic40, BnaA2.Tic40, BnaA10.Tic40, and BnaC2.Tic40. At least three independent transgenic plants for each construct displayed similar GUS activities in the anthers of young buds, one of which is shown in each image. C, GUS expression patterns in the anthers of the B. napus Tic40 promoter-GUS transgenic line. Transgenic lines of the five B. napus Tic40 promoters displayed similar GUS staining patterns; thus, only anther sections of one Probnac9.tic40-GUS transgenic line is shown. The developmental stages of anthers were described according to the previous report by Ma (2005). GUS staining signals were obviously detectable in the tapetum, tetrads, and microspores during anther development. MC, Microsporocyte; Msp, microspore; T, tapetum; Td, tetrad. Bars = 5 mm in B and 100 μm in C.
We also analyzed five B. napus Tic40 homolog promoter activities in anther tissues by constructing GUS reporter gene fusion vectors. Promoter fragments of about 2,000 bp from five B. napus Tic40 genes were inserted into the pBI201-GUS vector. These fusion constructs were introduced into wild-type A. thaliana by Agrobacterium tumefaciens-mediated transformation, and GUS histochemical assays were performed on inflorescences of A. thaliana transgenic plants. Analysis of transformed plants showed that transcriptional activities of these Tic40 genes were high in the anthers of young buds, and those spatial and temporal expression patterns of the five B. napus Tic40 homologs were similar during anther development (Fig. 5, B and C). These results suggested that the novel function related to male fertility probably arose through altered protein function rather than through changes in gene expression patterns.
Functional Divergence of Brassica spp. Tic40 Homologs Resulted from Amino Acid Sequence Differences in the C Terminus
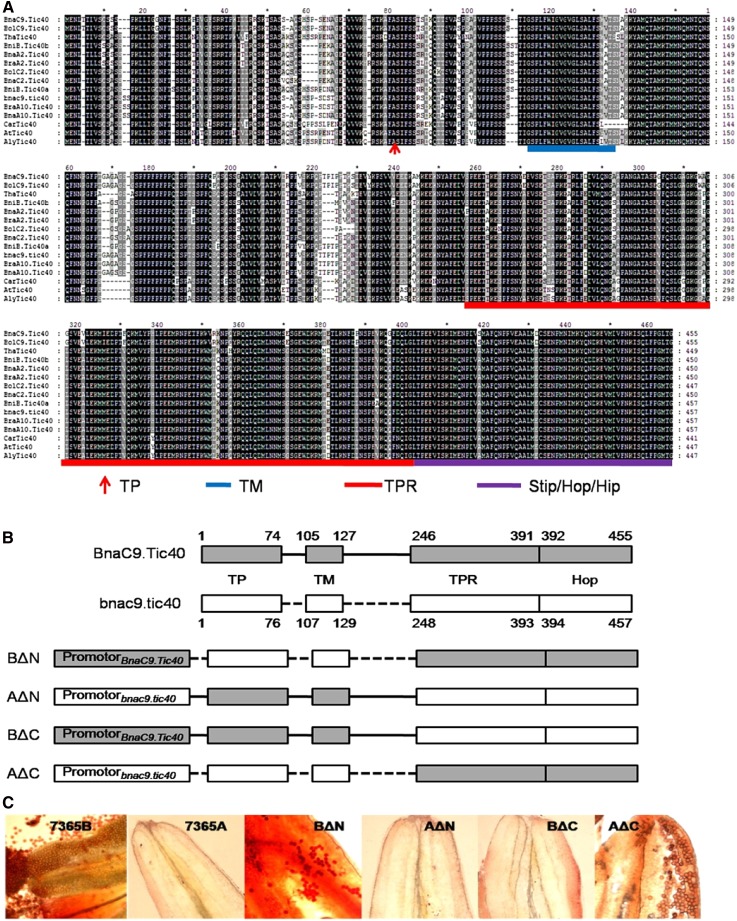
Analysis of the identified Brassicaceae Tic40 deduced amino acid sequences revealed the presence of several functional domains, a predicted chloroplast/plastid targeting transit peptide, and a transmembrane domain at their N-terminal region, mediating a protein-protein interaction domain Tetratricopeptide repeats (TPR) and a Sti1p/Hop/Hip (Hop, known as Hsp70 and Hsp90 organizing protein, and also known as Sti1p; Hip, Hsp70 interacting protein) domain known to bind to the ATPase domain of Hsp93 at the C-terminal region (Fig. 6A; Chou et al., 2003). The predicted Brassicaceae protein sequences share high sequence similarity (over 81%). Further analysis of the predicted protein sequences revealed 19 unique amino acid replacements in the gain-of-function Tic40 proteins as compared with other Brassicaceae Tic40 homologs. Four of these were found before the transmembrane domain, three were found between the transmembrane domain and the TPR domain, 10 were found inside the TPR domain, and the rest were found inside the Sti1p/Hop/Hip domain (Fig. 6A). This high degree of variation makes it difficult to confirm which mutation is responsible for the fertility-recovered phenotype. To get a better insight into the functional importance of different parts of BnaC9.Tic40, four binary constructs were made using the promoters and the full-length cDNAs of BnaC9.Tic40 and bnac9.tic40. BΔN was constructed using the promoter and the cDNA of BnaC9.Tic40, but with the N terminus before the TPR domain substituted by the corresponding fragment from bnac9.tic40. Conversely, AΔN included the promoter of bnac9.tic40, the N terminus of BnaC9.Tic40, and the C terminus (TPR and Hop domains) of bnac9.tic40. Likewise, in the BΔC and AΔC constructs, the C termini of BnaC9.Tic40 and bnac9.tic40 were replaced by each other.
Figure 6.
Functional analysis of B. napus Tic40 complementation constructs. A, ClustalW alignment and structural features of the deduced Tic40 proteins. The conserved domains were predicted by comparison with the structure of A. thaliana Tic40. TP, Transit peptide; TM, transmembrane domain; TPR, tetratricopeptide domain; Sti1p/Hop/Hip, Sti1p/Hop/Hip domain. B, Schematic drawings showing the positions of protein domains of BnaC9.Tic40 and bnac9.tic40 and the organization of the Tic40 N- and C-terminal domain-swap constructs. C, Typical anthers from four representative 7365A lines carrying the indicated B. napus Tic40 chimeric complementation constructs, alongside wild-type 7365B and untransformed 7365A controls.
These constructs were used to transform 7365A plants. More than 21 transformants were identified for each of the four constructs. The constructs BΔN and AΔC both complemented the 7365A plants, restoring a wild-type-like appearance with visible pollen grains in the anthers (Fig. 6D). By contrast, none of the AΔN and BΔC transformants resulted in a complemented phenotype (Fig. 6D). These results suggested that amino acid substitutions present in the C terminus of BolC9.Tic40 and BnaC9.Tic40, comprising the TPR and Sti1p/Hop/Hip domains, caused the observed functional divergence of Brassica spp. Tic40 homologs.
In addition to A. thaliana and several Brassicaceae spp., we identified additional Tic40 homologs by BLASTp searches in various plant species, including several monocots and eudicots. Despite their sequence divergence, comparisons show a similar structure of all studied Tic40 homologs with conserved functional domains, a transit peptide and a transmembrane domain at the N terminus, a TPR domain, and a Sti1p/Hop/Hip domain at the C terminus (Supplemental Fig. S1). Furthermore, direct sequence comparison of these Tic40 homologs revealed six amino acid substitutions unique to BnaC9.Tic40 and BolC9.Tic40 at their C-terminal regions (Supplemental Fig. S1). Five of these were found in the TPR domain at positions 307, 321, 343, 378, and 386. The sixth substitution occurred at position 408 in the Sti1p/Hop/Hip domain. These sites are highly conserved in other Tic40 homologs with amino acids identical to bnac9.tic40 (Supplemental Fig. S1). These data support that mutations in the TPR and/or Sti1p/Hop/Hip domains of BnaC9.Tic40 and BolC9.Tic40 are the causal mutations resulting in the restoration of fertility to 7365A.
Neofunctionalization of Tic40 in B. oleracea Lineages after Divergence of the Brassica spp. A, B, and C Genomes
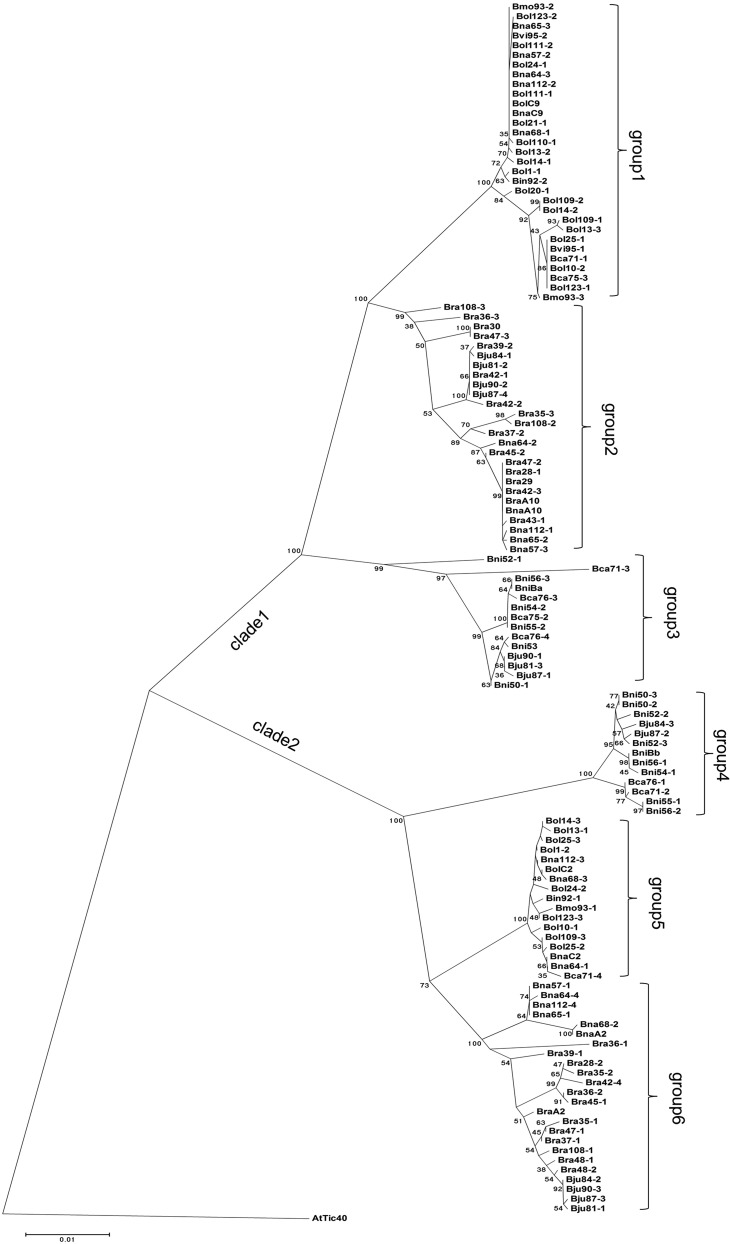
To investigate the diversity of Tic40 in Brassica spp., the primer combination 6L/14R1 as described above was used to isolate partial Tic40 sequences, encoding TPR and Hop domains, from a set of 59 diverse Brassica spp. accessions (Supplemental Table S4). To avoid the effects of modern breeding, these Brassica spp. accessions were chosen from collections in the wild, landraces and breeders varieties from regions around the world in order to represent a large part of the existing genetic diversity (Supplemental Table S4). Due to the genome triplication of the Brassica spp. ancestor and the heterozygosity in these accessions, more than one copy with high sequence similarity to AtTic40 was present in any specific accession. Thus, the different copies obtained from an accession were labeled with the suffix 1, 2, or 3 and so on. As a result, we obtained 115 Tic40 sequences from these accessions. These Tic40 homologs were combined with previously identified Brassica spp. Tic40 homologs for further analysis. An NJ distance tree was constructed using Tic40 from A. thaliana as an outgroup (Fig. 7). As expected, the Brassica spp. Tic40 genes were divided into two main clusters, clade 1 and clade 2, with an NJ bootstrap value of 100% (Fig. 7). This dichotomy strongly supports that two Tic40 loci existed in the common ancestor to extant Brassica spp. The Brassica spp. Tic40 genes were further divided into six major groups, in which these Tic40 genes were grouped together with a Brassica spp. Tic40 homolog of three basic Brassica diploid species, respectively (Fig. 7). For example, for group1, 30 sequences with an NJ bootstrap value of 100% were grouped together with the BolC9.Tic40 gene, and all of these sequences are from accessions belonging to B. oleracea and its derivatives, like Brassica montana, Brassica villosa, B. carinata, and B. napus (Fig. 7; Supplemental Table S4).
Figure 7.
An NJ distance tree for Tic40 homologs from Brassica spp. Tic40 from A. thaliana (AtTic40) was used as an outgroup. The numbers on nodes are bootstrap values. Bvi, B. villosa; Bmo, B. montana; Bin, Brassica incana; Bca, B. carinata; Bju, B. juncea; Bna, B. napus; Bol, B. oleracea; Bra, B. rapa; Bni, B. nigra. B. incana, B. villosa, and B. montana belong to the B. oleracea species complex.
Furthermore, we analyzed the prevalence of the 12 amino acid substitution sites, identified in the C-terminal domain of BnaC9.Tic40 and BolC9.Tic40, in the set of 115 Tic40 sequences. In our collection, all Tic40 homologs from group 2 to group 6 carried identical amino acids with bnac9.tic40 at these 12 unique substitution sites (Table III). In contrast, most Tic40 homologs from group 1 carried identical amino acids with BnaC9.Tic40 and BolC9.Tic40 at these sites, although some Tic40 homologs from group 1 carried amino acids identical to bnac9.tic40 at three sites, positions 294, 304, and 321 (Table III). These results indicate that the amino acid replacements in the C terminus of Tic40 that are related to functional divergence are confined to B. oleracea lineages and its derivatives. Overall, based on the evolutionary and functional analysis of Brassica Tic40 homologs, we proposed that BolC9.Tic40 and its ortholog BnaC9.Tic40 underwent neofunctionalization after the separation of the B. oleracea lineage from the other diploid Brassica spp. lineages, although the Tic40 duplication occurred much earlier.
Table III. Nucleotide and amino acid variations at 12 replacement sites of 115 sequences orthologous (group 1 in Fig. 7) and homologous (groups 2–6 in Fig. 7) to BolC9.Tic40.
| Group | Sequence Type | Replacement Amino Acid Sites in BolC9.Tic40 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 261 | 294 | 304 | 307 | 311 | 321 | 343 | 361 | 378 | 386 | 408 | 422 | ||
| Group 1 in Figure 7 | Nucleotide sequence | GAA | GTT/GGT | TGG/GGG | GGG/GGC | GTT | TTT/GTT | CGT | GAG | CCG | GGA | TCA | GAC |
| Amino acid | Gln | Phe/Ala | Trp/Gly | Gly | Val | Phe/Val | Leu | Gln | Leu | Gly | Ser | Asp | |
| Groups 2 to 6 in Figure 7 | Nucleotide sequence | GCA | GTT | GGG | TTA/CTG/TTG | GCT/GCG | GTT/GTC/GTG | CTT | GGG/GGA | CTG/CTT/CTA/CTC/TTG | CAA/CAG | GCA/GCG /GCT/GCC | GAA |
| Amino acid | Ala | Phe | Gly | Leu | Ala | Val | Arg | Gly | Pro | Gln | Ala | Gln | |
DISCUSSION
Two Ancient Tic40 Loci in Brassica spp.
In this study, we identified Tic40 homologs in several representative Brassicaceae spp. The A. thaliana genome that represents the ancestral ploidy state for the Brassicaceae family has one Tic40 locus, as do the closely related genomes of A. lyrata and C. rubella. The genomes of B. rapa, B. oleracea, and B. nigra are generally expected to contain three Tic40 loci due to the whole-genome triplication of the Brassica spp. ancestor after divergence from A. thaliana (Lysak et al., 2005; Parkin et al., 2005; Cheng et al., 2013). B. napus is expected to contain all of the loci present in B. rapa and B. oleracea, which merged to form B. napus (Cheung et al., 2009). However, only four Tic40 loci were identified in B. napus, and their genome origins from the A and C genomes were determined by phylogenetic tree and sequence identity analysis. The recent genome sequencing of B. rapa and B. oleracea also uncovered two Tic40 homologs in each genome (Wang et al., 2011), demonstrating that our results represent correct information of Tic40 homologs in B. napus and three Brassica diploid species.
The loss of one Tic40 locus took place on the MF1 subgenome as defined in B. rapa and B. oleracea. In addition, consistent with the estimated gene loss rate in three subgenomes of diploid Brassica spp. (Cheng et al., 2013), greater gene loss was observed in the Tic40 genomic regions in MF1 and MF2 subgenomes than in the LF subgenome. These data indicate that deletion of one Tic40 locus occurred in the common ancestor of Brassica spp. due to the diploidization process.
Functional Conservation and Divergence of Tic40 Genes
Initial analyses of the attic40 mutant demonstrated that Tic40 is required for the vegetative development of plants and functions as part of the protein translocon complex to assist protein translocation across the chloroplast inner membrane (Chou et al., 2003, 2006). The high conservation of Tic40 homologs in flowering plants, including monocots and eudicots, implies a general importance of Tic40 for plant development. The C-terminal domain of Tic40 shares sequence similarity with the C-terminal domains of the mammalian cochaperones Hip and Hop, acting as Hsp70-interacting protein and Hsp70/Hsp90-organizing protein, respectively (Bédard et al., 2007), and the Saccharomyces cerevisiae cochaperone Sti1p (Chou et al., 2003). Notably, the complementation efficiency of the Tic40:Hip fusion construct with the Sti1p/Hop/Hip domain of Tic40 exchanged with the human counterpart showed functional equivalence between the Sti1p/Hop/Hip domain of AtTic40 and that of human Hip (Bédard et al., 2007). Furthermore, among Tic40 sequences, this region is the most conserved in various plant species, suggesting that the function from Saccharomyces spp. (Sti1p) to human (Hop and Hip) to plant (Tic40) is evolutionarily conserved (Fig. 4, A and B; Bédard et al., 2007). Tic40 homologs in plants exhibit high conservation of additional functional domains, including the highly conserved predicted chloroplast/plastid-targeting transit peptide, the transmembrane domain at their N-terminal region, and the TPR. The highly conserved protein structure suggests that Tic40 homologs should maintain a similar biochemical and cellular function in protein translocation across the chloroplast/plastid inner membrane, and the four functional domains of Tic40 presented here should be important for plant development.
In this study, we have characterized the structure and function of a set of Tic40 genes in Brassica spp. These Brassica spp. Tic40 genes showed low levels of sequence divergence but did show functional diversity. BolC9.Tic40 and its ortholog BnaC9.Tic40, identified as gain-of-function Tic40 copies in the B. oleracea lineages, confer a fertility-restoring effect on the B. napus male-sterile mutant 7365A, while other representative Brassica Tic40 copies display an identical function with AtTic40. The analysis of deduced amino acid sequences and the assessment of complementation activity of different B. napus Tic40 constructs in the 7365A mutant suggested that amino acid replacements in the TPR and/or the Sti1p/Hip/Hop domains are responsible for this functional divergence. Furthermore, six amino acids specific to BnaC9.tic40 and BolC9.tic40 are highly conserved among other plant Tic40 homologs carrying amino acids identical to bnac9.tic40, suggesting that they are important for protein function. And five of them were found inside the TPR domain. As mentioned above, the Sti1p domain has a conserved function in eukaryotes, although the two domains from the C terminus of Tic40 and HsHip were only 35% homologous (Bédard et al., 2007). The TPR domain comprises three or more motifs that form a pair of antiparallel α-helices that interact with different target proteins. The target proteins that bind to the TPR domains rely on the primary sequences of the TPR domain (Lamb et al., 1995), implying that variation of the TPR primary sequences should contribute to changes in the target-binding proteins and result in the functional divergence of Tic40 genes.
Neofunctionalization of Tic40 in B. oleracea Lineages Caused Functional Divergence of Homologous Genes from Brassica spp. A, B, and C Genomes
Two copies of Tic40 are retained in diploid Brassica spp. genomes after the whole-genome triplication. In addition to gene loss, possible evolutionary fates of paralogous genes created by polyploidy followed by diploidization include maintaining the ancestral function, silencing, and functional divergence (Whittle and Krochko, 2009; Liu and Adams, 2010). Functional divergence after gene duplication can hypothetically result in two alternative evolutionary fates: neofunctionalization and subfunctionalization (Liu and Adams, 2010). A previous study with the paralogs SHORT SUSPENSOR (SSP) and Brassinosteroid Kinase1 (BSK1) formed by a polyploidy event in the Brassicaceae family also showed evidence of neofunctionalization after duplication, as described by BSK1 retaining the ancestral expression pattern and function and SSP gaining a new function but losing its original function (Liu and Adams, 2010). Within that scenario, our data suggest that BolC9.Tic40 and its ortholog BnaC9.Tic40 gained the novel function related to male fertility but also retained an ancestral function similar to AtTic40, whereas its paralogs and homologs in Brassica spp. only retained the ancestral function.
Sequence analysis of homologous Tic40 genes revealed that the distribution of the gain-of-function Tic40 copy was strictly restricted to the B. oleracea lineages and its derivatives, suggesting that the neofunctionalization occurred after the divergence of Brassica spp. A, B, and C genomes. Molecular dating supports that the B. rapa lineage (A genome) and the B. oleracea lineage (C genome) diverged from each other less than 3 million years ago (Inaba and Nishio, 2002; Navabi et al., 2013; Arias et al., 2014). The separation of the B. nigra (B genome) clade from that of the B. oleracea-B. rapa clade occurred much earlier, around 20 million years ago (Arias et al., 2014). Due to their close relationships, comparisons between the three Brassica spp. genomes revealed extensive conservation of gene content and sequence identity (Panjabi et al., 2008; Cheng et al., 2013). However, analysis of the tissue-specific alteration of gene expression mediated by transposable element insertions suggests a potential for rapid functional divergence of orthologous genes between the A and C genomes (Zhao et al., 2013). Although polyploidy events provide a large number of new genes that could potentially undergo functional divergence (Blanc and Wolfe, 2004; Chen et al., 2011), examples of substantial neofunctionalization or subfunctionalization of paralogous and homologous genes from the A, B, and C genomes are rare (Liu and Adams, 2010). Our results here suggest that BolC9.Tic40 diverged functionally from its homologous genes, BraA10.Tic40 and BniB.Tic40b, and the paralogous gene BolC2.Tic40 and that this divergence occurred recently, after the separation of the Brassica spp. A and C genomes.
Evolutionary Dynamics of the Gain-of-Function Tic40 Gene in Brassica spp. Lineages
Although the evolutionary novelty of Tic40 by neofunctionalization is well supported, the underlying causes for the emergence and maintenance of the novel Tic40 gene that provides the new function related to male fertility are unknown. Most analyzed diploid B. oleracea lineage accessions carried the gain-of-function Tic40 copies, suggesting its importance for the adaptive evolution of B. oleracea lineages. The novel function of Tic40 can restore the male fertility of the B. napus male-sterile line 7365A, the sterility of which results from the dominant gene BnRf. Thus, it seems likely that the emergence and maintenance of the novel function of Tic40 was coupled to the evolution of Rf in B. oleracea lineages. This scenario suggests intermolecular coevolution and that the BolC9.Tic40 protein might directly interact with the Rf protein.
Homologous Chromosomal Rearrangements Contribute to Allelic and Phenotypic Diversity
In the amphidiploid B. napus with merged homologous A and C genomes, cytological observations and genetic mapping with molecular markers demonstrated that the A and C genomes have retained sufficient homology to allow chromosome pairing, which may result in occasional homologous exchanges (Parkin et al., 1995; Nicolas et al., 2007). Chromosomal rearrangements caused by such homologous exchanges are prevalent in B. napus, as evidenced by genetic mapping analysis of four B. napus segregating double haploid populations (Udall et al., 2005). Further studies demonstrated that homologous nonreciprocal transpositions may cause qualitative changes in the expression of specific homologous genes and phenotypic variation in the resynthesized amphidiploid B. napus (Gaeta et al., 2007). Studies of the effects of homologous chromosomal rearrangements in polyploidy have revealed several causes of phenotypic variation: changes in the expression of parental genes can occur by altering methylation or regulation, for example, flowering time variation with the altered expression of parental FLOWERING LOCUS C genes (Pires et al., 2004), and some genomic exchanges exhibit a heterosis-like effect due to the formation of intergenome heterozygosity, which was interpreted by altering variation in seed yield coupled to homologous chromosomal rearrangements (Osborn et al., 2003).
In this study, we show that changes in protein function of homologous genes that evolved after duplication, followed by homologous exchange, gave rise to allelic and phenotypic diversity related to male sterility. Thus, homologous chromosomal rearrangements may be an important mechanism creating novel allele combinations and phenotypic diversity in polyploid species with highly syntenic genomes.
MATERIALS AND METHODS
Plant Materials
The plants used in this study were grown in soil under natural conditions. Genomic DNAs of Brassica oleracea genotypes T14, CGN06903, CGN18458, and T9, Brassica rapa genotypes R7, CGN06832, CGN13925, and Chiffu, and Brassica nigra genotypes CGN06620, CGN06625, and CGN06630 (Table I) were used for the isolation of diploid Brassica spp. Tic40 homologs by PCR. The self-cross homozygous wild-type progeny and the male-sterile individual from the 7365AB near-isogenic line were used to isolate Brassica napus Tic40 homologs. The Arabidopsis thaliana tic40 mutant (transfer DNA insertion mutant SALK_028413) was purchased from the Arabidopsis Biological Resource Center mutant collection (http://abrc.osu.edu/). Fifty-nine genotypes of nine Brassica spp. were collected from different regions of the globe that are considered to represent great genetic diversity (Supplemental Table S4).
Genome Synteny Analysis
Whole-genomic information for A. thaliana, Arabidopsis lyrata, Capsella rubella, Thellungiella halophila, B. rapa, and B. oleracea was downloaded from public databases (www.phytozome.com and http://www.arabidopsis.org/; Hu et al., 2011; Wang et al., 2011; Slotte et al., 2013; Yang et al., 2013; Liu et al., 2014). The genomic multiple sequences alignments were performed using the progressive alignment tool BLASTZ (http://www.bx.psu.edu/miller_lab/; Harris, 2007). The results were visualized using the VISTA tool, a synteny browser for comparative sequences from multiple species (http://genome.lbl.gov/vista/index.shtml). Gene annotation of the A. thaliana Tic40 region was produced using the Web-based gene prediction program FGENE-SH Arabidopsis (http://www.softberry.com/berry.phtml).
Cloning of Tic40 Genes from B. napus and Three Diploid Brassica spp.
The cDNA sequence of AtTic40 was used to query the Brassica spp. EST database in GenBank (National Center for Biotechnology Information) using the MEGABLAST program. Identified Brassica spp. Tic40 sequences were aligned together with cDNAs of several above-mentioned Brassicaceae spp. Tic40 homologs. Conserved regions in the exons identified in this alignment were selected to design degenerate primers to amplify Tic40 homologs from various Brassica spp. (for details of degenerate primers, see Supplemental Table S1). To validate the efficacy of these degenerate primers, multiple primer combinations were used to amplify diverse Tic40 homologs in three diploid Brassica spp. and A. thaliana. PCR products were separated on a 6% (w/v) denaturing polyacrylamide gel. Twenty clones from each PCR product were sequenced. A set of three primer combinations resulting in overlapping sequences was selected to amplify Brassica spp. Tic40 genes. Tic40 copies of homozygous B. napus genotypes 7365B and 7365A were amplified using the same set of primer combinations. The full-length genomic sequences of these Tic40 homologs were validated by PCR. Subsequently, partial sequences containing the TPR and Hop domains of Tic40 from 59 Brassica spp. genotypes were isolated using the primer combination 6L/14R1.
Sequence Alignment and Phylogenetic Analysis of Tic40 Homologs
For full-length cDNA amplification, total RNA prepared from young buds of 7365A, 7365B, Chiffu, T9, and CGN06620 were used in reverse transcription-PCR. Then, the full-length ORFs of Tic40 homologs were aligned using ClustalX (Thompson et al., 1997), and nucleotide identities between them were calculated simultaneously. Bootstrapped NJ phylogenetic trees were constructed using Kimura’s two-parameter model, and bootstrap values (1,000 replications) were calculated using MEGA4.0 (Tamura et al., 2007). Homologous protein sequences of Tic40 in flowering plants were identified by BLASTp from the nonredundant protein sequences databases at the National Center for Biotechnology Information. Multiple protein sequence alignments were performed by MUSCLE (http://www.ebi.ac.uk/Tools/msa/muscle/) and visualized by GeneDoc (http://www.nrbsc.org/gfx/genedoc/). Domain structures of Tic40 proteins were predicted according to the topology of AtTic40 as described (Chou et al., 2003).
Validation of Homologous Chromosomal Rearrangements
We obtained information of 23 flanking gene models around BolC9.Tic40 and BraA10.tic40 from the genomes of B. oleracea C9 and B. rapa A10, respectively, using orthologs in A. thaliana as controls. Conserved primer combinations spanning one or more introns in each flanking gene were designed to carry out the PCR assay in 7365A, 7365B, B. oleracea T9, and B. rapa Chiffu. The primer combinations with identical bands in 7365A and 7365B but different bands between B. rapa Chiffu and B. oleracea T9, or with stable codominant polymorphic bands between 7365A and 7365B, were considered to amplify actual flanking sequences around BnaC9.Tic40 and bnac9.Tic40. PCR products were separated on a 6% (w/v) denaturing polyacrylamide gel. Ten clones from each product were sequenced. Sequencing reads of 300 to 800 bp were assembled using DNAStar (http://www.dnastar.com/). Homologous sequences were aligned and sequence divergences were calculated by MEGA4.0 (Tamura et al., 2007). If successive flanking sequences in 7365B and 7365A originated from B. oleracea C9 and B. rapa A10, respectively, the homologous chromosomal rearrangement around BnaC9.Tic40 could be validated.
Genetic Complementation
The genomic fragments of 12 Tic40 homologs from B. napus 7365A and 7365B, B. nigra CGN06620, B. oleracea T9, B. rapa Chiffu, and A. thaliana Columbia were amplified using high-fidelity PCR (for details of primers, see Supplemental Table S1). The fragments were cloned into the pCAMBIA1305.1 binary vector. cDNA fragments and 2,000-bp promoter regions of BnaC9.Tic40 and bnac9.tic40 were used to generate chimeric complementation constructs. Constructs were assembled by gene splicing by overlap extension PCR (for details of primers, see Supplemental Table S1). Chimeric cDNA fragments were inserted downstream of the BnaC9.Tic40 and bnac9.tic40 promoters in pCAMBIA1305.1. These constructs were introduced into the host cells Agrobacterium tumefaciens GV3101. A. tumefaciens-mediated transformation of the attic40 mutant was performed using the floral dip method (Clough and Bent, 1998), and transformation of the B. napus male-sterile 7365A was performed as described previously by Dun et al. (2011).
Expression Patterns of Brassica spp. Tic40 Homologs
Total RNA isolated from various tissues including roots, stems, seedlings, inflorescences, and young siliques of heterozygous 7365B, B. rapa Chiffu, and B. oleracea T9 were used for real-time PCR analysis. First-stand cDNAs were diluted 20-fold and amplified according to the instructions for RealMasterMix (SYBR Green [FP202]; TIANGEN; for details of primers, see Supplemental Table S1). The assay was performed in triplicate with the CFX96 real-time system (Bio-Rad), and BnACT7 (EV220887.1) was used as a control to normalize the expression data. The results were analyzed using CFX Manager Software according to the 2−ddCt method (Livak and Schmittgen, 2001). Approximately 1,500- to 2,000-bp upstream regions of the five B. napus Tic40 genes were amplified from 7365AB and cloned into the binary vector pBI201. The promoter-GUS fusion constructs were introduced into A. thaliana wild-type plants by A. tumefaciens-mediated transformation. GUS activity was visualized by staining inflorescences from transgenic lines.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers Fragaria vesca Tic40, XP_004307173; Citrus clementina Tic40, ESR43817; Cucumis sativus Tic40, XP_004148914; Populus trichocarpa Tic40, XP_002305876; Ricinus communis Tic40, XP_002531917; Solanum lycopersicum Tic40, XP_004250413; Vitis vinifera Tic40, XP_002282574; Theobroma cacao Tic40, EOY03909; Glycine max Tic40, XP_003538352; Cicer arietinum Tic40, XP_004500418; Pisum sativum Tic40, CAB50925; Zea mays Tic40, DAA37691; Setaria italica Tic40, XP_004975683; Sorghum bicolor Tic40, XP_002447878.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. The BnaC.Tic40 gene structure, expected annealing positions of degenerate primers used to amplify Brassica spp. Tic40 fragments, and an alignment of deduced amino acid sequences of Tic40 homologs from several monocot and eudicot plant species.
Supplemental Table S1. Primers used for PCR amplification in this study.
Supplemental Table S2. Sequence identity analysis of the full-length ORFs of Tic40 homologs by ClustalW.
Supplemental Table S3. The orthologs of the 23 flanking genes analyzed in A. thaliana, B. oleracea, and B. rapa and their physical genomic locations in B. oleracea and B. rapa.
Supplemental Table S4. The information for 59 diverse Brassica spp. accessions from different global regions.
Supplementary Material
Acknowledgments
We thank Hanhui Kuang (Huazhong Agricultural University) for research guidance, Dr. Shengyi Liu (Olicrops Research Institute, Chinese Academy of Agricultural Sciences) for providing the complete genomic information for B. oleracea, and the Centre for Genetic Resources (The Netherlands; http://www.wageningenur.nl/) for providing the Brassicaceae germplasms.
Glossary
- LF
least fractionated
- MF1
medium fractionated
- MF2
most fractionated
- cDNA
complementary DNA
- ORF
open reading frame
- IP
intron polymorphism
- NJ
neighbor-joining
Footnotes
This work was supported by the National Natural Science Foundation of China (grant no. 31130040) and the High-Tech Research and Development Program 863 (grant no. 2011AA10A104).
The online version of this article contains Web-only data.
Articles can be viewed online without a subscription.
References
- Arias T, Beilstein MA, Tang M, McKain MR, Pires JC. (2014) Diversification times among Brassica (Brassicaceae) crops suggest hybrid formation after 20 million years of divergence. Am J Bot 101: 86–91 [DOI] [PubMed] [Google Scholar]
- Bédard J, Kubis S, Bimanadham S, Jarvis P. (2007) Functional similarity between the chloroplast translocon component, Tic40, and the human co-chaperone, Hsp70-interacting protein (Hip). J Biol Chem 282: 21404–21414 [DOI] [PubMed] [Google Scholar]
- Beilstein MA, Al-Shehbaz IA, Kellogg EA. (2006) Brassicaceae phylogeny and trichome evolution. Am J Bot 93: 607–619 [DOI] [PubMed] [Google Scholar]
- Blanc G, Hokamp K, Wolfe KH. (2003) A recent polyploidy superimposed on older large-scale duplications in the Arabidopsis genome. Genome Res 13: 137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc G, Wolfe KH. (2004) Functional divergence of duplicated genes formed by polyploidy during Arabidopsis evolution. Plant Cell 16: 1679–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Truksa M, Snyder CL, El-Mezawy A, Shah S, Weselake RJ. (2011) Three homologous genes encoding sn-glycerol-3-phosphate acyltransferase 4 exhibit different expression patterns and functional divergence in Brassica napus. Plant Physiol 155: 851–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZJ. (2007) Genetic and epigenetic mechanisms for gene expression and phenotypic variation in plant polyploids. Annu Rev Plant Biol 58: 377–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng F, Mandáková T, Wu J, Xie Q, Lysak MA, Wang X. (2013) Deciphering the diploid ancestral genome of the mesohexaploid Brassica rapa. Plant Cell 25: 1541–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung F, Trick M, Drou N, Lim YP, Park JY, Kwon SJ, Kim JA, Scott R, Pires JC, Paterson AH, et al. (2009) Comparative analysis between homoeologous genome segments of Brassica napus and its progenitor species reveals extensive sequence-level divergence. Plant Cell 21: 1912–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou ML, Chu CC, Chen LJ, Akita M, Li HM. (2006) Stimulation of transit-peptide release and ATP hydrolysis by a cochaperone during protein import into chloroplasts. J Cell Biol 175: 893–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou ML, Fitzpatrick LM, Tu SL, Budziszewski G, Potter-Lewis S, Akita M, Levin JZ, Keegstra K, Li HM. (2003) Tic40, a membrane-anchored co-chaperone homolog in the chloroplast protein translocon. EMBO J 22: 2970–2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Conant GC, Wolfe KH. (2008) Turning a hobby into a job: how duplicated genes find new functions. Nat Rev Genet 9: 938–950 [DOI] [PubMed] [Google Scholar]
- Cui L, Wall PK, Leebens-Mack JH, Lindsay BG, Soltis DE, Doyle JJ, Soltis PS, Carlson JE, Arumuganathan K, Barakat A, et al. (2006) Widespread genome duplications throughout the history of flowering plants. Genome Res 16: 738–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun X, Zhou Z, Xia S, Wen J, Yi B, Shen J, Ma C, Tu J, Fu T. (2011) BnaC.Tic40, a plastid inner membrane translocon originating from Brassica oleracea, is essential for tapetal function and microspore development in Brassica napus. Plant J 68: 532–545 [DOI] [PubMed] [Google Scholar]
- Flagel LE, Wendel JF. (2009) Gene duplication and evolutionary novelty in plants. New Phytol 183: 557–564 [DOI] [PubMed] [Google Scholar]
- Force A, Lynch M, Pickett FB, Amores A, Yan YL, Postlethwait J. (1999) Preservation of duplicate genes by complementary, degenerative mutations. Genetics 151: 1531–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaeta RT, Pires JC, Iniguez-Luy F, Leon E, Osborn TC. (2007) Genomic changes in resynthesized Brassica napus and their effect on gene expression and phenotype. Plant Cell 19: 3403–3417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RS (2007) Improved pairwise alignment of genomic DNA. PhD thesis. Pennsylvania State University, University Park, PA [Google Scholar]
- Hu TT, Pattyn P, Bakker EG, Cao J, Cheng JF, Clark RM, Fahlgren N, Fawcett JA, Grimwood J, Gundlach H, et al. (2011) The Arabidopsis lyrata genome sequence and the basis of rapid genome size change. Nat Genet 43: 476–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Chen Y, Yi B, Xiao L, Ma C, Tu J, Fu T. (2007) Fine mapping of the recessive genic male sterility gene (Bnms3) in Brassica napus L. Theor Appl Genet 115: 113–118 [DOI] [PubMed] [Google Scholar]
- Inaba R, Nishio T. (2002) Phylogenetic analysis of Brassiceae based on the nucleotide sequences of the S-locus related gene, SLR1. Theor Appl Genet 105: 1159–1165 [DOI] [PubMed] [Google Scholar]
- Lagercrantz U. (1998) Comparative mapping between Arabidopsis thaliana and Brassica nigra indicates that Brassica genomes have evolved through extensive genome replication accompanied by chromosome fusions and frequent rearrangements. Genetics 150: 1217–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagercrantz U, Lydiate DJ. (1996) Comparative genome mapping in Brassica. Genetics 144: 1903–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb JR, Tugendreich S, Hieter P. (1995) Tetratrico peptide repeat interactions: to TPR or not to TPR? Trends Biochem Sci 20: 257–259 [DOI] [PubMed] [Google Scholar]
- Liu S, Liu Y, Yang X, Tong C, Edwards D, Parkin IAP, Zhao M, Ma J, Yu J, Huang S, et al. (2014) The Brassica oleracea genome reveals the asymmetrical evolution of polyploid genomes. Nat Commun 5: 3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SL, Adams KL. (2010) Dramatic change in function and expression pattern of a gene duplicated by polyploidy created a paternal effect gene in the Brassicaceae. Mol Biol Evol 27: 2817–2828 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Lukens L, Quijada PA, Udall J, Pires JC, Schranz ME, Osborn T. (2004) Genome redundancy and plasticity within ancient and recent Brassica crop species. Biol J Linn Soc Lond 82: 675–688 [Google Scholar]
- Lysak MA, Koch MA, Pecinka A, Schubert I. (2005) Chromosome triplication found across the tribe Brassiceae. Genome Res 15: 516–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H. (2005) Molecular genetic analyses of microsporogenesis and microgametogenesis in flowering plants. Annu Rev Plant Biol 56: 393–434 [DOI] [PubMed] [Google Scholar]
- Mun JH, Kwon SJ, Yang TJ, Seol YJ, Jin M, Kim JA, Lim MH, Kim JS, Baek S, Choi BS, et al. (2009) Genome-wide comparative analysis of the Brassica rapa gene space reveals genome shrinkage and differential loss of duplicated genes after whole genome triplication. Genome Biol 10: R111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navabi ZK, Huebert T, Sharpe AGO, O’Neill CM, Bancroft I, Parkin IA. (2013) Conserved microstructure of the Brassica B genome of Brassica nigra in relation to homologous regions of Arabidopsis thaliana, B. rapa and B. oleracea. BMC Genomics 14: 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas SD, Le Mignon G, Eber F, Coriton O, Monod H, Clouet V, Huteau V, Lostanlen A, Delourme R, Chalhoub B, et al. (2007) Homeologous recombination plays a major role in chromosome rearrangements that occur during meiosis of Brassica napus haploids. Genetics 175: 487–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn TC, Butrulle DV, Sharpe AG, Pickering KJ, Parkin IAP, Parker JS, Lydiate DJ. (2003) Detection and effects of a homeologous reciprocal transposition in Brassica napus. Genetics 165: 1569–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panjabi P, Jagannath A, Bisht NC, Padmaja KL, Sharma S, Gupta V, Pradhan AK, Pental D. (2008) Comparative mapping of Brassica juncea and Arabidopsis thaliana using intron polymorphism (IP) markers: homoeologous relationships, diversification and evolution of the A, B and C Brassica genomes. BMC Genomics 9: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin IAP, Gulden SM, Sharpe AG, Lukens L, Trick M, Osborn TC, Lydiate DJ. (2005) Segmental structure of the Brassica napus genome based on comparative analysis with Arabidopsis thaliana. Genetics 171: 765–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin IAP, Lydiate DJ, Trick M. (2002) Assessing the level of collinearity between Arabidopsis thaliana and Brassica napus for A. thaliana chromosome 5. Genome 45: 356–366 [DOI] [PubMed] [Google Scholar]
- Parkin IAP, Sharpe AG, Keith DJ, Lydiate DJ. (1995) Identification of the A and C genomes of amphidiploid Brassica napus (oilseed rape). Genome 38: 1122–1131 [DOI] [PubMed] [Google Scholar]
- Pires JC, Zhao JW, Schranz ME, Leon EJ, Quijada PA, Lukens LN, Osborn TC. (2004) Flowering time divergence and genomic rearrangements in resynthesized Brassica polyploids (Brassicaceae). Biol J Linn Soc Lond 82: 675–688 [Google Scholar]
- Rodríguez-Trelles F, Tarrío R, Ayala FJ. (2003) Convergent neofunctionalization by positive Darwinian selection after ancient recurrent duplications of the xanthine dehydrogenase gene. Proc Natl Acad Sci USA 100: 13413–13417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schranz ME, Lysak MA, Mitchell-Olds T. (2006) The ABC’s of comparative genomics in the Brassicaceae: building blocks of crucifer genomes. Trends Plant Sci 11: 535–542 [DOI] [PubMed] [Google Scholar]
- Slotte T, Hazzouri KM, Ågren JA, Koenig D, Maumus F, Guo YL, Steige K, Platts AE, Escobar JS, Newman LK, et al. (2013) The Capsella rubella genome and the genomic consequences of rapid mating system evolution. Nat Genet 45: 831–835 [DOI] [PubMed] [Google Scholar]
- Soltis DE, Albert VA, Leebens-Mack J, Bell CD, Paterson AH, Zheng C, Sankoff D, Depamphilis CW, Wall PK, Soltis PS. (2009) Polyploidy and angiosperm diversification. Am J Bot 96: 336–348 [DOI] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24: 1596–1599 [DOI] [PubMed] [Google Scholar]
- Tang H, Lyons E. (2012) Unleashing the genome of Brassica rapa. Front Plant Sci 3: 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Woodhouse MR, Cheng F, Schnable JC, Pedersen BS, Conant G, Wang X, Freeling M, Pires JC. (2012) Altered patterns of fractionation and exon deletions in Brassica rapa support a two-step model of paleohexaploidy. Genetics 190: 1563–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. (1997) The CLUSTAL_X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25: 4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Town CD, Cheung F, Maiti R, Crabtree J, Haas BJ, Wortman JR, Hine EE, Althoff R, Arbogast TS, Tallon LJ, et al. (2006) Comparative genomics of Brassica oleracea and Arabidopsis thaliana reveal gene loss, fragmentation, and dispersal after polyploidy. Plant Cell 18: 1348–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udall JA, Quijada PA, Osborn TC. (2005) Detection of chromosomal rearrangements derived from homologous recombination in four mapping populations of Brassica napus L. Genetics 169: 967–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udall JA, Wendel JF. (2006) Polyploidy and crop improvement. Crop Sci (Suppl) 46: S3–S14 [Google Scholar]
- Van de Peer Y, Maere S, Meyer A. (2009) The evolutionary significance of ancient genome duplications. Nat Rev Genet 10: 725–732 [DOI] [PubMed] [Google Scholar]
- Wang X, Wang H, Wang J, Sun R, Wu J, Liu S, Bai Y, Mun JH, Bancroft I, Cheng F, et al. (2011) The genome of the mesopolyploid crop species Brassica rapa. Nat Genet 43: 1035–1039 [DOI] [PubMed] [Google Scholar]
- Whittle CA, Krochko JE. (2009) Transcript profiling provides evidence of functional divergence and expression networks among ribosomal protein gene paralogs in Brassica napus. Plant Cell 21: 2203–2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe KH. (2001) Yesterday’s polyploids and the mystery of diploidization. Nat Rev Genet 2: 333–341 [DOI] [PubMed] [Google Scholar]
- Xia S, Cheng L, Zu F, Dun X, Zhou Z, Yi B, Wen J, Ma C, Shen J, Tu J, et al. (2012) Mapping of BnMs4 and BnRf to a common microsyntenic region of Arabidopsis thaliana chromosome 3 using intron polymorphism markers. Theor Appl Genet 124: 1193–1200 [DOI] [PubMed] [Google Scholar]
- Yang R, Jarvis DE, Chen H, Beilstein MA, Grimwood J, Jenkins J, Shu S, Prochnik S, Xin M, Ma C, et al. (2013) The reference genome of the halophytic plant Eutrema salsugineum. Front Plant Sci 4: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Du J, Lin F, Tong C, Yu J, Huang S, Wang X, Liu S, Ma J. (2013) Shifts in the evolutionary rate and intensity of purifying selection between two Brassica genomes revealed by analyses of orthologous transposons and relics of a whole genome triplication. Plant J 76: 211–222 [DOI] [PubMed] [Google Scholar]
- Zhou Z, Dun X, Xia S, Shi D, Qin M, Yi B, Wen J, Shen J, Ma C, Tu J, et al. (2012) BnMs3 is required for tapetal differentiation and degradation, microspore separation, and pollen-wall biosynthesis in Brassica napus. J Exp Bot 63: 2041–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziolkowski PA, Kaczmarek M, Babula D, Sadowski J. (2006) Genome evolution in Arabidopsis/Brassica: conservation and divergence of ancient rearranged segments and their breakpoints. Plant J 47: 63–74 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.