Natural products are an important resource in the discovery of herbicides.
Abstract
Herbicides with new modes of action (MOAs) are badly needed due to the rapidly evolving resistance to commercial herbicides, but a new MOA has not been introduced in over 20 years. The greatest pest management challenge for organic agriculture is the lack of effective natural product herbicides. The structural diversity and evolved biological activity of natural phytotoxins offer opportunities for the development of both directly used natural compounds and synthetic herbicides with new target sites based on the structures of natural phytotoxins. Natural phytotoxins are also a source for the discovery of new herbicide target sites that can serve as the focus of traditional herbicide discovery efforts. There are many examples of strong natural phytotoxins with MOAs other than those used by commercial herbicides, which indicates that there are molecular targets of herbicides that can be added to the current repertoire of commercial herbicide MOAs.
The evolutionary forces driving the survival of species include chemical interactions between organisms, which function in positive interactions such as mutualistic and symbiotic relationships and negative interactions such as competitive and parasitic relationships. These processes have led to the emergence of novel secondary metabolic pathways (often through gene duplication), producing a vast array of structurally diverse and biologically active molecules (Moore and Purugganan, 2005; Ober, 2005; Flagel and Wendel, 2009; Jiang et al., 2013). This evolutionary process is similar to a high-throughput screen. However, unlike conventional in vitro screens, which test many compounds on a single biochemical target over a very short period of time, this natural high-throughput process selects molecules based on their whole-organism activities, involving numerous chemical interactions between countless organisms and target sites over millions of years. To date, approximately 200,000 secondary metabolites have been identified (Tulp and Bohlin, 2005), with many more expected to be discovered. Few of these compounds have been examined for phytotoxicity, and the modes or mechanisms of action (MOAs) of even fewer known phytotoxins have been elucidated.
The negative chemical interactions between organisms are often characterized using anthropomorphic language, such as chemical warfare, referring to the production of phytotoxins used by plant pathogens to invade their host plants (Maor and Shirasu, 2005), and the novel weapons hypothesis, which is associated with the chemical-based advantage of some invasive plant species over native plant populations (Callaway and Aschehoug, 2000; Callaway and Ridenour, 2004; Callaway and Maron, 2006; Cappuccino and Arnason, 2006; Callaway et al., 2008). While simplistic, this terminology illustrates how these toxin-based interactions exploit biochemical weaknesses between an organism and its host or enemy/competitor to enhance its own survival (Verhoeven et al., 2009). In fact, these interactions can even be multitrophic, such as when exotic plants enhance their invasiveness by promoting the growth of certain native soil pathogens noxious to native plants (Mangla et al., 2008; Barto et al., 2011).
As humans evolved from a nomadic hunter-gatherer subsistence existence to an agricultural lifestyle, they learned to utilize certain biologically active secondary metabolites to manage agricultural pests. Indeed, the concept that nature is an excellent source of natural pesticides is captured in the following ancient Lithica poem (circa 400 B.C.): “All the pests that out of earth arise, the earth itself the antidote supplies” (Ibn et al., 1781). Less than a century later, Greek and Roman treatises described practices to control agricultural pests that include the use of essential oils. Similar documents are found in Chinese literature, such as a survey describing plant species used to control plant pests (Yang and Tang, 1988). The mid-20th century ushered in the use of synthetic pesticides, which have revolutionized agriculture. Like pharmaceuticals (Harvey, 1999, 2008; Newman and Cragg, 2012), many pesticides are based on natural compounds. However, natural products have not played a major role in herbicide discovery (Copping and Duke, 2007; Hüter, 2011).
CURRENT IMPACT OF NATURAL PRODUCTS ON HERBICIDE DISCOVERY AND DEVELOPMENT
While almost 70% of all newly registered active pesticide ingredients have their origins in natural products research, only 8% of conventional herbicides are derived from natural compounds and only 7% of biochemical biopesticides (natural compounds) approved by the U.S. Environmental Protection Agency are bioherbicides (Cantrell et al., 2012). This is remarkable, because weeds have the largest negative impact on crop productivity among pests (Pimentel et al., 2005), and the lack of weed control is the most pressing concern expressed by farmers (Stokstad, 2013). Furthermore, in the United States, herbicides are used in far larger volumes than insecticides and fungicides combined (Köhler and Triebskorn, 2013).
There is a growing need for new herbicides with safer toxicological and environmental profiles and new MOAs. This need is driven by both the loss of older herbicides due to safety issues and to the rapidly increasing evolution of resistance to herbicides and herbicide classes that remain on the market (Heap, 2014). Natural product-based herbicides are considered by the public to be generally safer than conventional synthetic herbicides, although this assumption remains to be validated. Furthermore, there is a strong rationale for examining natural products to uncover novel MOAs (Dayan et al., 2012; Gerwick and Sparks, 2014).
The U.S. Environmental Protection Agency has three categories of biopesticides: (1) microbial pesticides used as biocontrol organisms; (2) plant-incorporated protectants (PIPs), which are natural pesticides produced by crops due to the presence of transgenes (e.g. Bacillus thuringiensis toxin production in transformed crops); and (3) biochemical pesticides, which are naturally occurring materials (Environmental Protection Agency, 2014). This Update covers the current status of the use of natural compounds as herbicides and as starting molecules for the production of synthetic herbicides. We also discuss the promise of natural compounds for future herbicide and herbicide MOA discovery. We preface this with a brief discussion of the potential use of PIP bioherbicides.
POTENTIAL USE OF NATURAL PRODUCTS AS PIP BIOHERBICIDES
After glyphosate-resistant crops, the second most successful transgenic crops are those transformed to produce B. thuringiensis toxins (Duke, 2011), demonstrating that the PIP approach to biopesticides has great potential. While there are currently no successful examples of bioherbicide PIPs, the approach of enhancing the allelopathy of crops with the use of transgenes to enhance or impart allelochemical production for weed control is promising and requires further study (Duke et al., 2001; Duke, 2003; Bertin et al., 2008).
Allelopathy has a controversial past due to the plethora of less than robust studies about this topic and the often questionable claims about the role of allelochemicals in plant-plant interactions (Duke, 2010). For decades, attempts have been made to enhance the allelopathic properties of crops by conventional breeding and variety selection (Batish et al., 2011; Bertholdsson et al., 2012; Worthington and Reberg-Horton, 2013). However, to our knowledge, rice (Oryza sativa) is the only crop for which there are allelopathic germplasm releases produced by conventional breeding (Kong et al., 2011; Gealy and Yan, 2012; Gealy et al., 2013). Much, if not all, of the allelopathy of rice is due to momilactones that are exuded from rice roots (Kato-Noguchi, 2004; Kong et al., 2004). Rice lines with an RNA interference knockout of a gene in the momilactone pathway are unable to produce momilactones and lose their allelopathic properties (Xu et al., 2012). Allelopathic rice varieties do not provide the same level of weed control as herbicides, but the allelopathic suppression of weeds enables reductions in the use of synthetic herbicides (Gealy et al., 2003). A higher level of allelopathy might be obtained by imparting or increasing the production of allelochemicals in crops using more advanced genetic manipulation. Such an approach can be facilitated by identifying genes that encode enzymes involved in the synthesis of potent allelochemicals and elucidating how the expression of these genes is regulated (for review, see Duke et al., 2009). Importantly, allelochemicals such as momilactone B in rice (Kato-Noguchi and Ino, 2004) and sorgoleone in Sorghum spp. (Dayan et al., 2010), which are produced by roots and released to the rhizosphere, reach target plants more quickly than shoot-localized phytotoxins.
POTENTIAL FOR THE DISCOVERY OF NEW MOAs OF HERBICIDES BASED ON NATURAL COMPOUNDS
Natural products offer an unparalleled source of structural diversity, with little overlap with synthetic compounds generated by traditional organic synthesis in the laboratory (Koch et al., 2005; Harvey, 2007; Lipkus et al., 2008). This wider structural diversity may enable natural products to have unique MOAs (Duke and Dayan, 2013).
While commercial herbicides have only approximately 20 MOAs (Duke, 2012), evidence from the natural phytotoxin literature suggests that there are many more viable MOAs. Table I summarizes the known molecular target sites of highly effective phytotoxins, along with examples of their natural compound inhibitors. Sites targeted by both synthetic and natural inhibitors are indicated in italics. Some natural compounds, such as Alternaria alternata ssp. lycopersici (AAL)-toxin and tentoxin, are active at lower concentrations than many commercial herbicides (Duke, 1993; Abbas et al., 1994). This analysis provides strong evidence that there are herbicide target site alternatives to those utilized by current commercial herbicides. The natural products discussed below illustrate the diversity of the MOAs of natural products with potential use as herbicides.
Table I. Molecular target sites of selected natural phytotoxins.
Target sites in italics are also target sites of synthetic commercial herbicides.
| MOA | Molecule | Source | Reference |
|---|---|---|---|
| Amino acid synthesis | |||
| GS | Phosphinothricin | Pseudomonas syringae and Streptomyces spp. | Lydon and Duke (1999) |
| Phosalacine | Omura et al. (1984a) | ||
| Tabtoxinine-β-lactam | P. syringae pv tabaci | Thomas et al. (1983) | |
| Trp synthase | 5-Methyl-Trp | Cantharellus cibarius | Hsiao et al. (2007) |
| Asp transaminase (aminotransferase) | Gostatin | Streptomyces sumanensis | Nishino et al. (1984) |
| Cornexistin | Paecilomyces variotii | Amagasa et al. (1994) | |
| Orn carbamoyl transferase | Phaseolotoxin | P. syringae pv phaseolicola | Templeton et al. (2005) |
| β-Cystathionase | Rhizobitoxine | Bradyrhizobium spp. | Giovanelli et al. (1973) |
| Energy transfer | |||
| CF1 ATPase | Tentoxin | Alternaria tenuis | Groth (2002); Meiss et al. (2008) |
| Photophosphorylation uncoupler | Nigericin | Streptomyces hygroscopicus | Shavit and San Pietro (1967) |
| PSI electron diverter | Pyridazocidin | Streptomyces spp. | Gerwick et al. (1997) |
| PSII electron transport | Sorgoleone | Sorghum bicolor | Gonzalez et al. (1997) |
| Cyanobacterin | Scytonema hofmanni | Lee and Gleason (1994) | |
| Fischerellin A | Fischerella muscicola | Hagmann and Juettner (1996) | |
| Stigmatellin | Stigmatella aurantica | Oettmeier et al. (1985) | |
| Aurachins | S. aurantica | Oettmeier et al. (1990) | |
| Photosynthetic pigment synthesis | |||
| Tyr aminotransferase | Cineole analog | Grossmann et al. (2012) | |
| p-Hydroxyphenylpyruvate dioxygenase | Leptospermone | Leptospermum scoparium | Dayan et al. (2007a) |
| Deoxyxylulose-5-phosphate reductase | Fosmidomycin | Streptomyces lavendulae | Kuzuyama et al. (1998) |
| Glu-1-semialdehyde aminotransferase | Gabaculine | Streptomyces toyacaenis | Kahn and Kannangara (1987) |
| Aminolevulinic dehydratase | Gabaculine | S. toyacaenis | Kedy et al. (1994) |
| Protoporphyrinogen oxidase | Cyperin | Preussia fleischhakii and others | Harrington et al. (1995) |
| Lipid synthesis | |||
| ENR | Cyperin | P. fleischhakii and others | Dayan et al. (2008) |
| β-Ketoacyl-ACP synthase | Thiolactomycin | Norcardia and Streptomyces spp. | Price et al. (2001) |
| Cerulenin | Cephalosporium cerulens | Feld et al. (1989) | |
| Ceramide synthase | AAL-toxins | Alternaria alternata | Abbas et al. (1994) |
| Fumonisins | Fusarium spp. | Abbas et al. (1994) | |
| Membrane functions and lipid stability | |||
| H+-ATPase | Sorgoleone | S. bicolor | Hejl and Koster (2004b) |
| Juglone | Juglans spp. | Hejl and Koster (2004a) | |
| Prehelminthosporol | Bipolaris sorokiniana | Olbe et al. (1995) | |
| NADH oxidase | Glaucarubolone | Castela polyandra | Morré and Grieco (1999) |
| Fusicoccin | Fusicoccum amygdali | Gomarasca et al. (1993) | |
| Simalikalactone D | Quassia africana | Morré and Grieco (1999) | |
| Membrane destabilizers | Syringomycin | P. syringae | Schagina et al. (1998) |
| Beticolins | Cercospora beticola | Goudet et al. (1998) | |
| T-toxins | Bipolaris maydis | Levings et al. (1995) | |
| Cercosporin | Cercospora kikuchii | Daub (1982) | |
| Cuticle destabilizers | Pelargonic acid | Pelargonium spp. | Coleman and Penner (2006) |
| Sarmentine | Piper longum | Huang et al. (2010) | |
| Gene expression and regulation | |||
| Adenylosuccinate synthase | Hydantocidin | Streptomyces hygroscopicus | Siehl et al. (1996) |
| Ribofuranosyl triazolone | Schmitzer et al. (2000) | ||
| Isoleucyl tRNA synthase | Pseudomonic acids | Pseudomonas fluorescens | Clinch (1996) |
| Peptide deformylase | Actinonin | Actinomyces sp. MG848-hF6 | Hou et al. (2007) |
| Ser/Thr protein phosphatases | Cantharidin | Epicauta spp. | Bajsa et al. (2011a) |
| RNA polymerase | Tagetitoxin | P. syringae pv tagetis | Mathews and Durbin (1990) |
| Aminopeptidase | Bestatin | Actinomycetes spp. | Umezawa et al. (1976) |
| Lys deacetylases | Helminthosporium carbonum-toxin | Cochliobolus carbonum | Meeley and Walton (1991) |
| AMP deaminase | Carbocyclic coformycin | Saccharothrix spp. | Dancer et al. (1997) |
| Calmodulin | Ophiobolin A | Helminthosporium oryzae | Leung et al. (1985) |
| Hormonal regulation | |||
| Jasmonate mimic | Coronatine | P. syringae | Block et al. (2005) |
| Cinnacidin | Nectria sp. DA060097 | Irvine et al. (2008) | |
| Auxin signaling | Toyocamycin | Streptomyces toyocansis | Hayashi et al. (2009) |
| Terfestatin A | Streptomyces sp. F40 | Hayashi et al. (2008) | |
| ACC synthase | Rhizobitoxine | Bradyrhizobium elkanii | Yasuta et al. (1999) |
| GA mimic | GA3 | Gibberella fujikuroi | Hedden et al. (2001) |
| GA oxidase | Myrigalone | Myrica gale | Oracz et al. (2012) |
| Cytokinin mimic | Cytokinin | Agrobacterium tumefaciens | Jameson (2000) |
| Macrostructure | |||
| Microtubule polymerization | Citral | Cymbopogon citratus | Chaimovitsh et al. (2010) |
| Cellulose synthesis | Thaxtomin | Streptomyces scabies | Scheible et al. (2003); Bischoff et al. (2009) |
| Golgi assembly | 7-Dehydrobrefeldin A | Alternaria carfhami | Driouich et al. (1997) |
| Plant cell cycle | |||
| DNA polymerase α and δ | Aphidicolin | Phoma betae | Ikegami et al. (1978) |
| Ribonucleotide reductase | Mimosine | Mimosa pudica | Perennes et al. (1993) |
| Proteasome interference | Lactacystin | Streptomyces spp. | Planchais et al. (2000) |
AMINO ACID SYNTHESIS
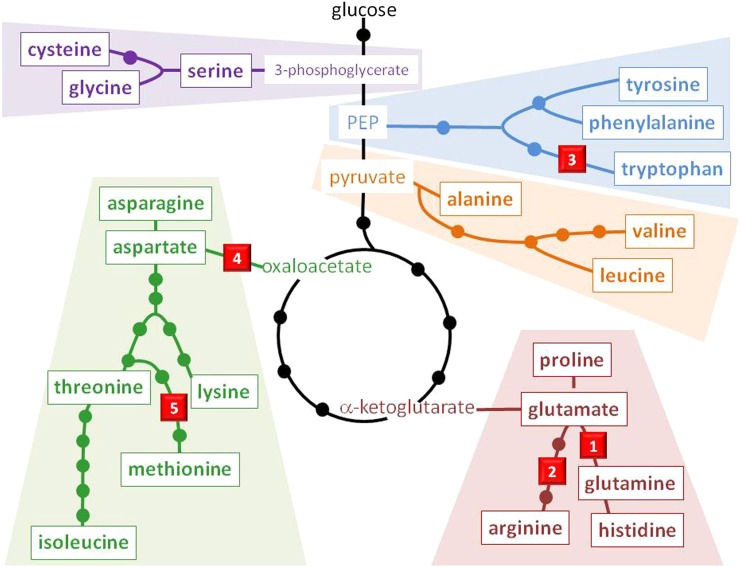
Plants synthesize all essential amino acids used to build the proteins responsible for myriad biochemical and structural functions. Some of the most successful commercial herbicides target enzymes involved in amino acid synthesis, and these pathways are also the targets of natural phytotoxins (Table I; Fig. 1).
Figure 1.
Amino acid biosynthesis pathways showing the enzymatic target sites of natural compound phytotoxins. 1, GS, the target site of phosphinothricin; 2, Orn carbamoyl transferase, the target site of phaseolotoxin; 3, Trp synthase, the target site of 5-methyl-Trp; 4, Asp transaminase, the target site of gostatin; 5, β-cystathionase, the target site of rhizobitoxine. PEP, Phosphoenolpyruvate.
Gln Synthetase
Gln synthetase (GS) catalyzes the ATP-dependent condensation of Glu with ammonia to yield Gln. GS holds a special place with regard to the use of natural products as herbicides. Indeed, GS is the target site of l-phosphinothricin [homoalanin-4-yl(methyl)phosphinate; Fig. 1], a natural peptide produced by several Streptomyces spp. (Leason et al., 1982). l-Phosphinothricin is the active ingredient of glufosinate (sold under several trade names, including Basta, Ignite, and Liberty; Lydon and Duke, 1999). Glufosinate also contains an equivalent amount of the inactive d-enantiomer. Glufosinate is the only commercial herbicide with this MOA.
Inhibition of GS causes the accumulation of toxic levels of ammonia as well as the inhibition of photorespiration due to reduced levels of amino acid donors (for review, see Lydon and Duke, 1999; Duke and Dayan, 2015). Glufosinate is a broad-spectrum herbicide that kills weeds more quickly than glyphosate [N-(phosphonomethyl)glycine], the most widely used herbicide worldwide (Duke and Powles, 2008). Transgenic, glufosinate-resistant crops have been commercialized (for review, see Duke, 2014), but they are not as widely used as glyphosate-resistant crops. However, the rapidly increasing evolution of glyphosate-resistant weeds has intensified the adoption of glufosinate-resistant crops and the introduction of crops that have transgenes for resistance to both herbicides in the same crop variety.
Streptomyces hygroscopicus and Streptomyces viridochromogenes also synthesize the tripeptide l-alanyl-l-alanyl-phosphinothricin (bialaphos, also known as bilanofos), a proherbicide that releases l-phosphinothricin in planta (Tachibana and Kaneko, 1986; Tachibana et al., 1986; Wild and Ziegler, 1989). Bialaphos is sold in Japan as a minor bioherbicide. Other natural product GS inhibitors such as tabtoxinine-β-lactam, phosalacine, and oxetin have not been commercialized (Thomas et al., 1983; Omura et al., 1984a, 1984b; Duke and Dayan, 2015).
Orn Carbamoyl Transferase
Orn carbamoyl transferase, a key enzyme in the urea cycle that converts Orn and carbamoyl phosphate to citrulline, is the target site of phaseolotoxin, a sulfodiaminophosphinyl peptide produced by P. syringae pathovars that are responsible for halo blight (Fig. 1). While phaseolotoxin is a competitive inhibitor of Orn carbamoyl transferase, it is converted in planta to octicidine, which in turn is an irreversible inhibitor of Orn carbamoyl transferase and is the predominant form of the toxin in infected tissues. No commercial herbicides have been developed to target this enzyme (Turner, 1986; Bender et al., 1999; Templeton et al., 2005).
Trp Synthase
Trp synthase catalyzes the last two steps in the synthesis of Trp (Fig. 1). This pathway does not exist in the animal kingdom, making it an interesting target site for herbicide development. 5-Methyl-Trp is an indole compound in the fruiting bodies and mycelia of fungi such as C. cibarius (Muszyńska et al., 2013). The levels of this compound are generally low (approximately 1 mg 100 g−1 dry weight). Initial studies on the bacteriostatic activity of 5-methyl-Trp have suggested that this antimetabolite inhibits an early step of Trp synthesis (Moyed, 1960), and it was later confirmed that it inhibits Trp synthase in plants (Hsiao et al., 2007).
Asp Transaminase
Asp transaminase, also called Asp aminotransferase, is an important pyridoxal phosphate-dependent enzyme in amino acid metabolism (Fig. 1). This enzyme catalyzes the reversible transfer of an α-amino group interconverting Asp and α-ketoglutarate to oxaloacetate and Glu. A number of phytotoxic natural products such as gostatin (5-amino-2-carboxy-4-oxo-1,4,5,6-tetrahydro-pyridine-3-acetic acid), produced by S. sumanensis (Nishino et al., 1984), and cornexistin, produced by P. variotii (Nakajima et al., 1991; Amagasa et al., 1994), target this enzyme. Gostatin is a slow-binding inhibitor (time dependent) that acts as a suicide substrate (mechanism-based inhibitor). On the other hand, the mechanism of inhibition of Asp aminotransferase by cornexistin is not well characterized, but it appears that cornexistin may undergo in planta metabolic bioactivation.
β-Cystathionase
β-Cystathionase, also called cystathionine β-lyase, catalyzes a pyridoxal phosphate-dependent elimination reaction where l-cystathionine and water are converted to l-homocysteine, NH3, and pyruvate (Fig. 1). This reaction is important for several metabolic pathways (i.e. Met metabolism, Cys metabolism, selenoamino acid metabolism, nitrogen metabolism, and sulfur metabolism). Therefore, its inhibition by the microbial metabolite rhizobitoxine [2-amino-4-(2-amino-3-hydropropoxy)-transbut-3-enoic acid] causes phytotoxicity, resulting in the accumulation of homoserine, and plants become chlorotic (Giovanelli et al., 1971, 1973; Okazaki et al., 2007).
ENERGY TRANSFER
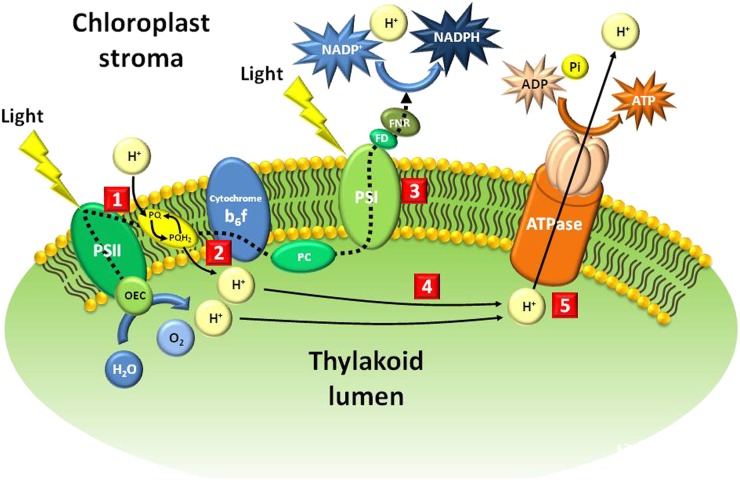
Coupling Factor1 ATPase
Coupling Factor1 (CF1) ATPase, the chloroplastic ATP synthase producing the ATP required for the light-independent reactions, is located within the thylakoid membrane, with its CF1 part extending into the stroma (Fig. 2). Tentoxin, a cyclic tetrapeptide from the plant pathogen A. tenuis (Saad et al., 1970), inhibits chloroplast development (Halloin et al., 1970), interacting directly with chloroplast CF1 ATPase (Pinet et al., 1996; Groth, 2002; Meiss et al., 2008). This compound acts like a selective herbicide (Durbin and Uchytil, 1977; Lax et al., 1988) and has been the focus of a combinatorial synthesis program based on its unique structural scaffold (Jiménez et al., 2003).
Figure 2.
Various target sites of natural products that interfere with various aspects of the light reaction of photosynthesis within the thylakoid membrane. 1, The QB binding site of plastoquinone, the target site of sorgoleone; 2, electron flow between PSII and the cytochrome b6/f complex, the target site of aurachins; 3, pyridazocidin diverts electrons from PSI; 4, the target site of nigericin, which uncouples photophosphorylation; 5, chloroplast CF1 ATPase, the target site of tentoxin. The dotted black line illustrates the flow of electrons from the oxygen-evolving complex to ferredoxin-NADP+ reductase. Solid black lines illustrate the accumulation of the proton gradient inside the lumen and its use by CF1 ATPase to synthesize ATP. FD, Ferredoxin; FNR, ferredoxin-NADP+ reductase; OEC, oxygen-evolving complex; PC, plastocyanin; Pi, inorganic phosphate; PQ, plastoquinone; PQH2, reduced PQ.
Photophosphorylation Uncoupler
The electron transport chain and oxidative phosphorylation are coupled by the proton gradient across the thylakoid membrane of the chloroplast. This proton gradient, which is generated by the splitting of water by the light reaction of photosynthesis, is required for oxidative phosphorylation of ADP to ATP through the action of ATP synthase (Fig. 2), and uncoupling these processes is herbicidal. Nigericin, a metabolite from S. hygroscopicus, is a strong uncoupler of photophosphorylation (Shavit and San Pietro, 1967).
PSII
PSII catalyzes the energy-demanding, light-driven splitting of water, which releases oxygen and provides the reducing equivalents (electrons) required for the conversion of CO2 into chemical energy (Fig. 2). A key step in this process that is sensitive to inhibition is the transfer of electrons from the secondary electron-acceptor (Qb) binding site to plastoquinone. Sorgoleone, a plastoquinone analog produced by sorghum roots, is a very potent inhibitor of photosynthesis (Gonzalez et al., 1997; Rimando et al., 1998; Dayan et al., 2003, 2007b; Dayan, 2006). Several microbial metabolites, such as cyanobacterin from S. hofmanni (Lee and Gleason, 1994), fischerellin A from F. muscicola (Hagmann and Juettner, 1996; Srivastava et al., 1998), and stigmatellin from S. aurantica (Oettmeier et al., 1985), also inhibit PSII. Aurachins are metabolites from S. aurantica that act downstream from the QB binding site by interfering with the photosynthetic electron flow between PSII and the cytochrome b6/f complex (Oettmeier et al., 1990).
PSI
PSI is a key component of the photosynthetic electron transport chain (Fig. 2). A number of synthetic herbicides (e.g. bipyridiliums) divert electrons from PSI and prevent the subsequent conversion of NADP into NADPH (Hess, 2000; Trebst, 2007). In addition, these herbicides are dications that become highly reactive free radicals upon acceptance of electrons from PSI, generating reactive oxygen species that cause extensive and very rapid desiccation of foliage. Pyridazocidin is a cationic phytotoxin produced by some Streptomyces spp. that acts by the same mechanism as bipyridilium herbicides in both broadleaf and grass weeds (Gerwick et al., 1997).
PHOTOSYNTHETIC PIGMENT SYNTHESIS
Carotenoids and chlorophylls are essential for photosynthesis and other important biochemical processes (Cazzonelli, 2011; Dayan and Dayan, 2011). These pathways are targeted by several classes of herbicides (Dayan and Duke, 2003, 2010). While no natural products that directly interfere with the carotenoid and chlorophyll synthesis pathways have been developed as herbicides, several products act by targeting key enzymes in these pathways.
Carotenoid Synthesis
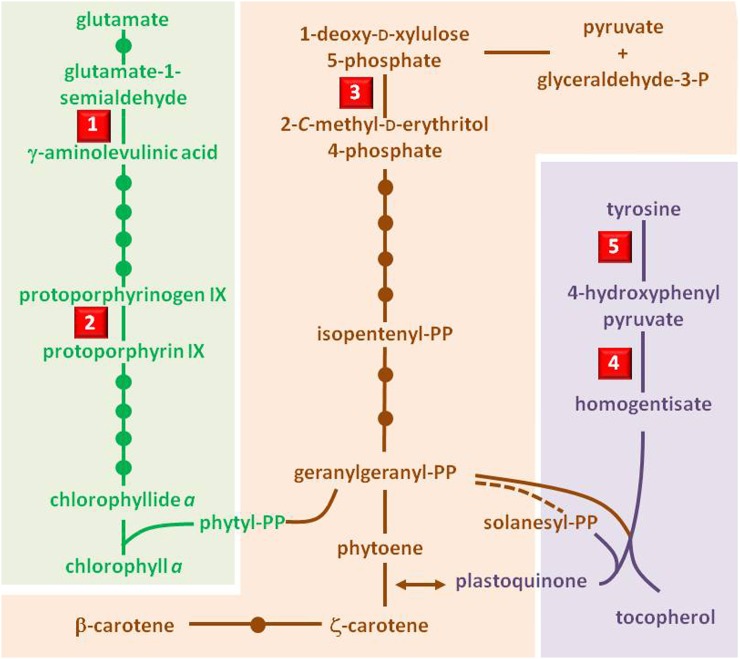
Tyr Aminotransferase
Tyr aminotransferase (or Tyr transaminase) catalyzes the conversion of Tyr to p-hydroxyphenylpyruvate (the first step in plastoquinone and tocopherol synthesis; Fig. 3). Inhibition of Tyr aminotransferase ultimately affects carotenoid synthesis because plastoquinone is a necessary cofactor of the enzyme phytoene desaturase (PDS; Norris et al., 1995). The herbicidal activity of cinmethylin, a 1,4-cineole derivative, is reported to be due to its inhibition of Tyr aminotransferase (Grossmann et al., 2012). If this finding is confirmed, it would represent the most recent discovery of a new commercial herbicide target site (Grossmann et al., 2012). While many cineoles (e.g. 1,4-cineole and 1,8-cineole) are phytotoxic (Romagni et al., 2000a), their MOA has not been elucidated. The presence of an epoxide ring is required for the biological activity of these monoterpenes.
Figure 3.
Simplified pathways of chlorophyll (green) and carotenoid (orange) biosynthesis. These two pathways are connected via the phytyl tail, originating from the chloroplastic isoprenoid geranylgeranyl pyrophosphate (PP). The pathway of prenylquinones, shown in purple, is also important for carotenoid biosynthesis because plastoquinone is an essential cofactor for PDS activity. Solid lines represent single reactions, and the dotted line represents many steps. 1, Glu-1-semialdehyde aminotransferase, the target site of gabaculine; 2, protoporphyrinogen oxidase, one of the target sites of cyperin; 3, deoxyxylulose-5-phosphate reductase, the target site of fosmidomycin; 4, p-hydroxyphenylpyruvate dioxygenase, the target site of natural β-triketones such as leptospermone; 5, tyrosine aminotransferase, the target site of the 1,4-cineole-derived herbicide cinmethylin.
p-Hydroxyphenylpyruvate Dioxygenase
p-Hydroxyphenylpyruvate dioxygenase (HPPD) is a ubiquitous Fe(II)-containing nonheme oxygenase that catalyzes the conversion of p-hydroxyphenylpyruvate into homogentisate (Fig. 3). Inhibition of HPPD leads to the depletion of plastoquinone, a cofactor for PDS. The bleaching symptoms caused by inhibition of PDS are similar to those caused by herbicides that inhibit PDS directly. The triketone HPPD inhibitor class of herbicides was derived from a natural β-triketone, leptospermone. The clue leading to the discovery of the triketones came from the observation that leptospermone, a natural compound from the allelopathic bottlebrush plant (Callistemon citrinus), is phytotoxic (Lee et al., 1997). The herbicidal activity of leptospermone is also due to its inhibition of p-hydroxyphenylpyruvate dioxygenase (Dayan and Duke, 2003; Dayan et al., 2007a; Owens et al., 2013). Inhibition of HPPD was the last herbicide MOA introduced for major commercial herbicides in the past 25 years (Duke, 2012). Other natural products from plants, such as usnic acid from lichens and sorgoleone from Sorghum spp., are also HPPD inhibitors (Romagni et al., 2000b; Meazza et al., 2002), although sorgoleone has other target sites that appear to be more important for its phytotoxicity (Dayan et al., 2010).
Deoxyxylulose-5-Phosphate Reductase
Deoxyxylulose-5-phosphate reductase catalyzes the second step of the nonmevalonate pathway to terpenoid synthesis (Fig. 3). Inhibition of deoxyxylulose-5-phosphate reductase causes bleaching of green tissues due to a reduction in carotenoid content. Fosmidomycin, a phytotoxic metabolite from S. lavendulae, is a potent inhibitor of this enzyme, with I50 in the nanomolar range (Kuzuyama et al., 1998).
Chlorophyll Synthesis
Glu-1-Semialdehyde Aminotransferase
Glu-1-semialdehyde aminotransferase (also called Glu-1-semialdehyde 2,1-aminomutase) catalyzes the last step of γ-aminolevulinic acid synthesis, a key step in chlorophyll synthesis (Fig. 3). Therefore, the inhibition of this enzyme is lethal to plants. Gabaculine (3-aminobenzoic acid), a toxin produced by S. toyacaenis, is a potent inhibitor of Glu-1-semialdehyde aminotransferase (Kannangara and Schouboe, 1985) and represses ALA synthesis in plants (Flint, 1984). Gabaculine-resistant green algae have overcome the effect of this phytotoxin by overexpressing the target enzyme (Kahn and Kannangara, 1987), whereas Synechococcus spp. have developed resistance following the deletion of a tripeptide near the NH2 terminus of the enzyme and an Met-248-to-Ile substitution, which reduces the specific activity of the enzyme but increases resistance to gabaculine 100-fold (Grimm et al., 1991). Gabaculine-resistant Glu-1-semialdehyde aminotransferases from both microbial and plant sources have been used as selectable markers in plants (Gough et al., 2001; Ferradini et al., 2011).
Aminolevulinic Acid Synthesis
Aminolevulinic dehydratase, the enzyme catalyzing the step following Glu-1-semialdehyde aminotransferase activity in chlorophyll synthesis, is affected by gabaculine, but it was later shown that this is only a secondary effect associated with the inhibition of the synthesis of aminolevulinic acid (Kedy et al., 1994).
Protoporphyrinogen Oxidase
Protoporphyrinogen oxidase (PPO) catalyzes the last step in common between heme and chlorophyll synthesis in plants (Fig. 3). PPO is a well-known target site for herbicides whose action results in the rapid light-dependent peroxidation of membranes. Many of the commercial herbicides affecting this enzyme are diphenyl ethers. The natural diphenyl ether cyperin, produced by a number of plant pathogens, is moderately active against PPO (Harrington et al., 1995), but its phytotoxicity is light independent, suggesting that it has a different MOA. This notion was later confirmed (see below).
LIPID SYNTHESIS
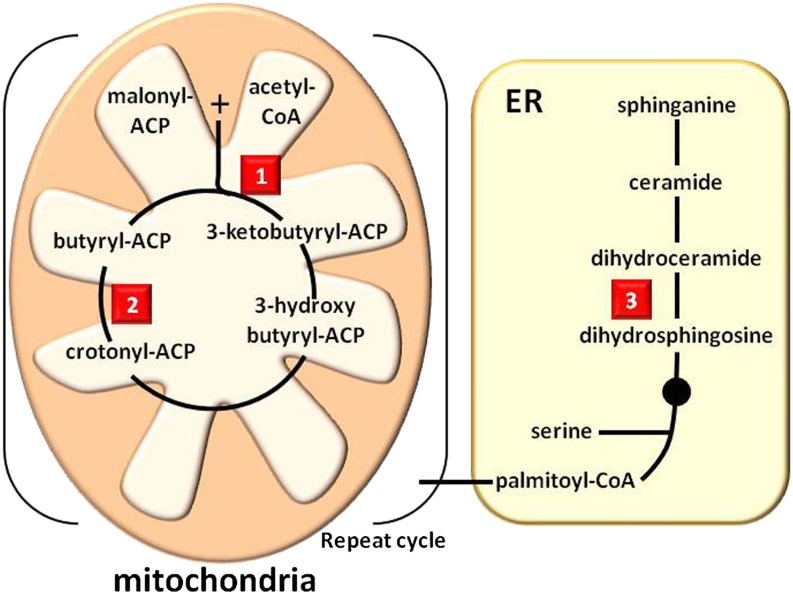
β-Ketoacyl-Acyl Carrier Protein Synthase
β-Ketoacyl-acyl carrier protein (ACP) synthase (also called 3-oxoacyl-ACP synthase) is a key enzyme of the dissociated fatty acid biosynthesis complex in plants and bacteria (Fig. 4). Thiolactomycin, a metabolite from Norcardia and Streptomyces spp., and cerulenin, a metabolite from C. cerulens, are potent inhibitors of the plant enzyme involved in de novo fatty acid synthesis (Nishida et al., 1986; Feld et al., 1989). A study elucidating the structure of bacterial β-ketoacyl-ACP synthase crystallized with thiolactomycin revealed the essential enzyme-ligand binding interactions and established the existence of hydrophobic and pantetheine-binding pockets (Price et al., 2001).
Figure 4.
Simplified pathways of lipid biosynthesis, showing target sites of natural phytotoxins. Fatty acid synthesis initiates in the mitochondria. The biosynthesis of ceramide, which leads to the production of cerebrosides, is localized in the endoplasmic reticulum (ER). 1, β-Ketoacyl-ACP synthase, the target site of thiolactomycin; 2, ENR, one of the target sites of cyperin; 3, ceramide synthase, the target site of AAL-toxins and fumonisins.
Enoyl-ACP Reductase
Enoyl-ACP reductase (ENR), a component of type II fatty acid synthase in plants and prokaryotes, catalyzes a critical step in fatty acid elongation (Fig. 4). This enzyme is sensitive to triclosan, a synthetic diphenyl ether (McMurry et al., 1998; Roujeinikova et al., 1999) commonly used in antibacterial soaps. As mentioned above (inhibitors of PPO), cyperin is a natural diphenyl ether produced by several plant pathogens that has some inhibitory activity against PPO, but its phytotoxicity is not light dependent. Inhibition of ENR alters fatty acid synthesis and leads to a rapid light-independent destabilization of membrane integrity. Cyperin’s mechanism is consistent with that of triclosan, as it inhibits plant ENR (Dayan et al., 2008).
Ceramide Synthase
Ceramide synthases are integral membrane proteins in the endoplasmic reticulum that catalyze the synthesis of ceramide via the acylation of sphinganine to a long-chain fatty acid (Fig. 4). AAL-toxins and fumonisins, produced by A. alternata and Fusarium spp., respectively, inhibit ceramide synthases at submicromolar concentrations (Abbas et al., 1994, 1998). These toxins cause the rapid accumulation of sphingolipid precursors and the subsequent rapid loss of plasma membrane integrity. Phytosphingosine, one of the precursors that accumulate after AAL-toxin exposure, is highly phytotoxic (Tanaka et al., 1993) and may account for most of the herbicidal activity. A survey of the responses of 88 plant species to AAL-toxin revealed that some plants are highly sensitive to this toxin, whereas others are unaffected (Abbas et al., 1995). Unfortunately, no AAL-toxin or fumonisin analog with high phytotoxicity and low mammalian toxicity has been found (Abbas et al., 2002).
MEMBRANE FUNCTIONS AND LIPID STABILITY
H+-ATPase
Plasma membrane H+-ATPase and associated membrane proteins play essential roles in maintaining water uptake and cell turgor, which are essential for plant growth and development (Fig. 5). Inhibition of H+-ATPase reduces mineral and water uptake by the roots, resulting in stomata closure, which consequently negatively affects respiration, photosynthesis, and other processes. A number of natural plant products, such as sorgoleone from Sorghum spp. (Hejl and Koster, 2004b), juglone from Juglans spp. (Hejl and Koster, 2004a), and prehelminthosporol from B. sorokiniana (Olbe et al., 1995), interfere with plant growth by inhibiting H+-ATPase.
Figure 5.
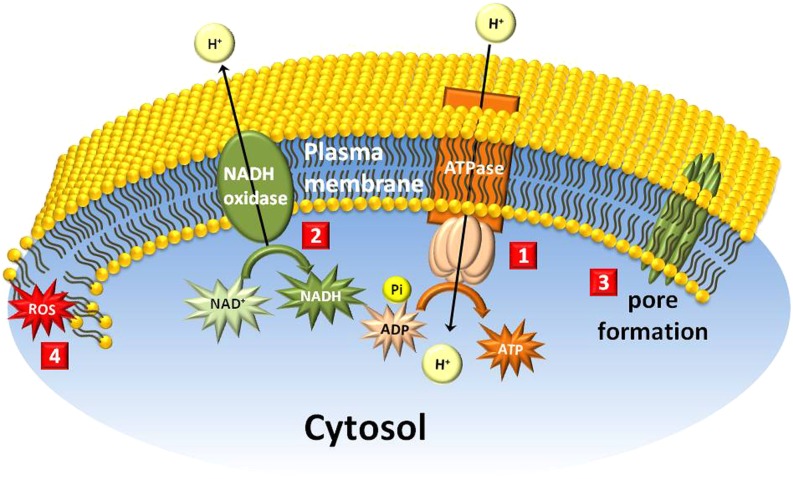
Membrane functions and lipid stability are affected by natural products. 1, Plasma membrane H+-ATPase, the target site of sorgoleone and juglone; 2, auxin-induced and constitutive NADH oxidases, the target sites of fusicoccin and simalikalactone D, respectively; 3, loss of membrane integrity by the self-assembly of large amphiphilic molecules (such as syringomycin) to form pores; 4, loss of membrane integrity by natural products such as cercosporin that generate large amounts of reactive oxygen species (ROS). Pi, Inorganic phosphate.
NADH Oxidase
Auxin-induced NADH oxidase is responsible for proton extrusion associated with cell elongation in response to auxin (Fig. 5). This process is also accompanied by the acidification of the cytoplasm (Morré et al., 1986). Stimulation of elongation by auxin normally occurs after a lag of several minutes. However, fusicoccin, a product from F. amygdali, triggers the same response without delay (Cleland, 1976). As a consequence, plants exposed to fusicoccin quickly wilt due to their inability to close their stomata (Gomarasca et al., 1993). Glaucarubolone, a quassinoid extracted from the root bark of C. polyandra, inhibits auxin-induced plasma membrane NADH oxidase in many plant species when used at nanomolar concentrations (Morré and Grieco, 1999). The quassinoid simalikalactone D, on the other hand, inhibits constitutive NADH oxidase activity. From a structural standpoint, quassinoids that possess an oxymethylene bridge tend to have higher phytotoxic activities than those without this structure (Dayan et al., 1999).
Membrane and Cuticle Destabilizers
In addition to the cell wall, the plasma membrane plays a critical role in keeping the entire cellular structure intact, providing an environment suitable for all physiological and biochemical processes that occur in the cytoplasm. The plasma membrane is also at the interface between the cell and its environment. Destabilization of the plasma membrane has deleterious effects on plants, and a number of natural products act by interfering with the integrity of membranes. Syringomycin, a metabolite produced by P. syringae, is an example of a large amphiphilic lipodepsinonapeptide that creates pores in the plasma membrane when assembled into large macromolecules. The loss of membrane integrity results in a loss of electrolytes and rapid necrosis in plant tissues (Backman and DeVay, 1971; Schagina et al., 1998; Bender et al., 1999; Malev et al., 2001). The nonpeptide fungal toxin beticolin is produced by C. beticola (Ducrot et al., 1996). This toxin can also assemble into channel-like structures, which leads to the loss of electrolytes and rapid cell death (Goudet et al., 1998; Fig. 5). Similarly, the B. maydis metabolite T-toxins cause rapid loss of membrane integrity by binding to the plant mitochondrial receptor URF13, thus changing its conformation and forming a pore comprising at least six transmembrane α-helices (Levings et al., 1995).
Cercosporin, produced by C. kikuchii, as with many perylenequinones, can be photoactivated and generate large amounts of reactive oxygen species (both singlet oxygen and superoxide ions), leading to the peroxidation of membrane lipids (Fig. 5; Daub, 1982; Daub and Hangarter, 1983; Daub et al., 2005).
Pelargonic acid (nonanoic acid) occurs naturally as esters in the oil of Pelargonium spp. This compound is used as a burndown herbicide, which acts by stripping the cuticle from the leaf surface, resulting in rapid, uncontrolled tissue desiccation (Lederer et al., 2004; Coleman and Penner, 2006, 2008). Sarmentine is a lipophilic pyrrolidine (isolated from P. longum) that has a similar MOA to that of pelargonic acid (Huang et al., 2010).
GENE EXPRESSION AND REGULATION
Adenylosuccinate Synthase
Adenylosuccinate is a ubiquitous enzyme that plays a key role in purine biosynthesis. This enzyme catalyzes the GTP-dependent conversion of IMP and l-Asp to GDP, phosphate, and N(6)-(1,2-dicarboxyethyl)-AMP. Hydantocidin, a metabolite produced by S. hygroscopicus, is activated in planta via phosphorylation to form an IMP analog, which is a potent inhibitor of adenylosuccinate synthetase. The use of hydantocidin and its structural analogs as an herbicide has been studied extensively (Heim et al., 1995; Cseke et al., 1996; Fonné-Pfister et al., 1996; Poland et al., 1996; Siehl et al., 1996). Ribofuranosyl triazolone, another natural phytotoxin that targets adenylosuccinate synthetase upon phosphorylation, is readily obtained by synthetic means, making it a more suitable starting backbone for developing new herbicides than hydantocidin (Schmitzer et al., 2000).
Isoleucyl tRNA Synthetase
Pseudomonic acids A and C, produced by P. fluorescens, are inhibitors of isoleucyl tRNA synthetase. Isoleucyl tRNA synthetase, a class I aminoacyl tRNA synthetase, catalyzes the attachment of Ile to its cognate tRNA molecule. The mechanism of inhibition of pseudomonic acids involves the interaction of its long side chains with both the Ile and ATP binding sites on the enzyme (Hughes and Mellows, 1978; Clinch, 1996).
Peptide Deformylase
Peptide deformylase is a critical enzyme that initiates protein translation in prokaryotes by removing the N-formyl group from N-formyl Met. This enzyme is the target of actinonin, a peptide-like hydroxamic acid produced by soil actinomycetes. Actinonin has been patented for herbicide use but has not been developed as a commercial product. Actinonin functions in plants by inhibiting the prokaryote-like plastid peptide deformylase, leading to stunting, bleaching, and necrosis in a wide range of agriculturally relevant weed species (Hou et al., 2006; Fernández-San Millán et al., 2011).
Ser/Thr Protein Phosphatases
Protein phosphatases act in concert with their protein kinase counterparts to modulate the phosphorylation status of proteins involved in such functions as signal transduction pathways and the regulation of gene expression. Therefore, the inhibition of protein phosphatases affects a large number of molecular and physiological processes. Cantharidin is a potent toxin produced by Epicauta spp. and Lytta vesicatoria insects. Cantharidin and its analogs are strong inhibitors of plant Ser/Thr protein phosphatases (Bajsa et al., 2011b). Endothall is a commercial herbicide that is structurally similar to cantharidin and also inhibits plant Ser/Thr protein phosphatases. This herbicide is primarily used for aquatic weed control (Netherland et al., 2000; Bajsa et al., 2011a).
RNA Polymerase
Tagetitoxin, a metabolite produced by P. syringae pv tagetis, inhibits RNA synthesis directed by RNA polymerases in both chloroplasts and Escherichia coli (Langston-Unkefer et al., 1984; Mathews and Durbin, 1990). Plants treated with tagetitoxin do not accumulate plastid 70S ribosomes. Consequently, none of the plastid-encoded polypeptides translated by chloroplastic ribosomes are produced (Lukens et al., 1987).
Aminopeptidase
Aminopeptidases are ubiquitous proteolytic enzymes that hydrolyze single amino acids at the N termini of peptidic substrates. These enzymes are involved in physiological processes such as mitosis, angiogenesis, and regulation of the cell oxidation state (Lowther and Matthews, 2002). Natural inhibitors of plant aminopeptidases, such as bestatin produced by actinomycetes (Umezawa et al., 1976), may provide relatively simple structural backbones for new herbicides (Oszywa et al., 2013). Bestatin was recently used to dissect aspects of jasmonate signaling in Arabidopsis (Arabidopsis thaliana; Zheng et al., 2006).
Lys Deacetylases
H. carbonum (HC)-toxin, a host-selective cyclic peptide from C. carbonum (Meeley and Walton, 1991), acts as an inhibitor of Lys deacetylase (previously known as histone deacetylase; Walton, 2006). Lys deacetylase removes acetyl groups from ε-N-acetyl-Lys amino acids on histones, allowing the histones to wrap the DNA more tightly, while inhibition of this step destabilizes DNA (Abbas et al., 2001; Walton, 2006).
AMP Deaminase
AMP deaminase catalyzes the deamination of AMP to produce IMP and NH3. This irreversible reaction essentially removes AMP from the adenylate pool and drives the equilibrium of the reaction catalyzed by adenylate kinase toward ATP synthesis. Carbocyclic coformycin is a potent phytotoxin whose primary MOA involves the inhibition of AMP deaminase following phosphorylation of the 5′-hydroxyl group (Dancer et al., 1997; Lindell et al., 1999; Riley et al., 1999).
Calmodulin
Calmodulin is a multifunctional, calcium-binding, intermediate messenger protein found in all eukaryotic cells. Calmodulin transduces calcium signals by binding calcium ions and then modifying its interactions with various target proteins. Ophiobolin A is a fungal phytotoxin produced by H. oryzae that inactivates calmodulin by reacting with Lys residues in calmodulin (Leung et al., 1985; Kong Au and Chow Leung, 1998).
HORMONAL REGULATION
Plant hormones affect virtually all aspects of plant growth and development. Consequently, many plant pathogens produce compounds that either mimic plant hormones or interfere with endogenous hormone synthesis in order to gain the upper hand in natural plant-pathogen interactions. Synthetic auxin mimics such as 2,4-dichlorophenoxyacetic acid are widely used as herbicides.
Jasmonate Mimics
Certain pathovars of P. syringae produce coronatine, a jasmonic acid mimic (Ichihara et al., 1977; Koda et al., 1996; Block et al., 2005). Coronatine suppresses natural salicylic acid-dependent plant defense mechanisms. This compound also induces the opening of stomata, which may also help the invading organism gain access to the apoplast (Jones and Dangl, 2006). Cinnacidin, a microbial product isolated from a fungal fermentation extract of Nectria sp. DA060097, has a promising herbicidal activity profile. Foliar application of cinnacidin causes stunting and chlorosis. Both coronatine and cinnacidin act by mimicking the role of jasmonic acid (Irvine et al., 2008).
Auxin Signaling
Toyocamycin, produced by S. toyocansis, inhibits auxin-responsive gene expression and blocks the auxin-enhanced degradation of the auxin/indole-3-acetic acid (IAA) repressor modulated by the SCFTIR1 ubiquitin proteasome pathway (Hayashi et al., 2009). However, toyocamycin does not affect the proteolytic activity of the proteasome. Toyocamycin acts on the ubiquitination process regulated by SCFTIR1. Terfestatin A, a molecule produced by Streptomyces sp. F40, also interferes with auxin signaling by inhibiting the expression of auxin-inducible genes (Hayashi et al., 2008).
Auxin Functions
IAA, the primary auxin in plants, is an important hormone that regulates a large number of growth and developmental processes. Patten and Glick (1996) estimated that up to 80% of microbes isolated from the rhizosphere produce IAA, and many of these microorganisms highjack the functions of IAA to promote their interactions with plant tissues (Duca et al., 2014).
Ethylene Synthesis
Ethylene is a potent modulator of plant growth and development, affecting many aspects of the plant life cycle, including seed germination, root hair development, root nodulation, flower senescence, abscission, and fruit ripening. In higher plants, 1-aminocyclopropane-1-carboxylase (ACC) synthase is the rate-limiting enzyme that functions in the biosynthesis of ethylene from Met. Rhizobitoxine, a B. elkanii product, acts as a competitive inhibitor of ACC synthase (Yasuta et al., 1999). This pathway is related to β-cystathionase, the other known target site of rhizobitoxine.
GA Overload
GAs are tetracyclic diterpenoid acid plant hormones that regulate growth and developmental processes such as stem elongation, germination, dormancy, flowering, and leaf and fruit senescence. To date, 126 GAs have been identified. The first identified GA was isolated from the fungal pathogen G. fujikuroi and is responsible for foolish seedling disease in rice. The synthesis of GAs may be advantageous to pathogens, and these compounds have been developed as plant growth regulators, but whether GAs can serve as structural leads for potential herbicides is unclear.
GA 3-Oxidase
GA biosynthesis is usually restricted to actively growing and elongating tissues, and GA 3-oxidase catalyzes the final step of the biosynthetic pathway that produces physiologically active GAs. Myrigalone, a natural β-triketone produced by the plant M. gale, interferes with GA metabolism and signaling by inhibiting GA 3-oxidase and by disrupting the GA signaling pathway. These processes are important for endosperm weakening and embryo growth (Oracz et al., 2012).
Cytokinins
Cytokinins, another class of phytohormones that participate in the complex regulatory network of plant hormones, promote cell division, or cytokinesis. Cytokinins are primarily involved in cell growth and differentiation. Cytokinin is produced by many pathogens to promote their infection by retarding senescence in infected leaf tissue. For example, the virulence of A. tumefaciens, the organism responsible for crown gall formation, is associated with the integration of the fungal genes for cytokinin and auxin production into the plant genome (Jameson, 2000). Like GAs, cytokinins are unlikely to serve as effective herbicides.
MACROSTRUCTURE
Cell wall formation requires cellulose synthesis. This process is complex and is still not fully understood. However, it is known that the direction of cellulose deposition by the cellulose synthase complex, which originates from the Golgi bodies, is intimately connected to the direction of skeletal microtubules. These processes are the targets of a number of phytotoxins.
Microtubule Polymerization
Microtubule polymerization is an essential process in all eukaryotic plants that is involved in cell growth and cellulose deposition, cytokinesis, mitosis, and vesicular transport (Fig. 6; Hashimoto, 2013). Some of the most effective mitotic inhibitors discovered to date are natural products, such as colchicine from crocus (Colchicum spp.) bulbs, vinblastine from Vinca rosea (Catharanthus roseus), and taxol from Taxus spp. (Vaughn and Vaughan, 1988). These compounds are growth inhibitors that either destabilize or hyperstabilize microtubules. Citral, a monoterpene produced by many plants such as C. citratus, disrupts microtubule polymerization within minutes after exposure. The inhibition of microtubule polymerization by citral has been confirmed by in vitro assays (Chaimovitsh et al., 2010).
Figure 6.
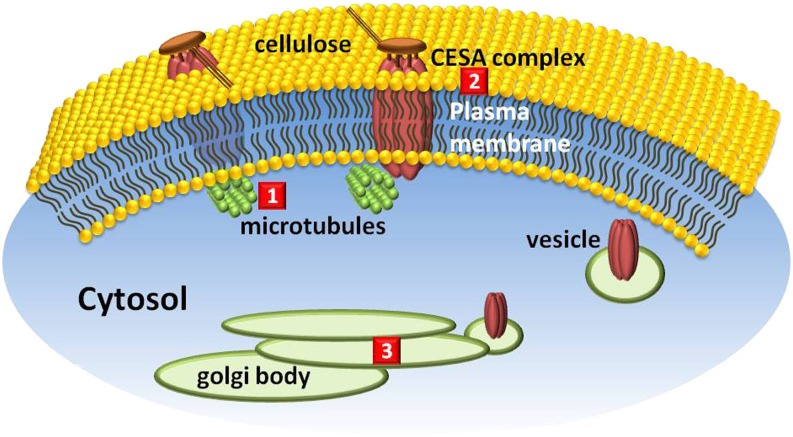
Cell macrostructures affected by natural products. 1, Microtubule polymerization is affected by citral; 2, cellulose synthase (CESA) is the target site of thaxtomin; 3, Golgi body structural integrity is disrupted by brefeldin A.
Cellulose Synthesis
Cellulose is the major component of the plant cell wall, providing strength to the plant architecture and protecting cells against pathogens, dehydration, and other abiotic factors. Cellulose deposition is carried out by the cellulose synthase complex (at least three different cellulose synthase enzymes and other associated proteins), which is tightly associated with the cortical microtubule cytoskeleton (Fig. 6). Thaxtomin A, a phytotoxic cyclic dipeptide analog produced by S. scabies and other species, inhibits cellulose synthesis by interfering with the formation of the cellulose synthase complexes on the outside of the cell (King et al., 2001; Scheible et al., 2003; Bischoff et al., 2009; King and Calhoun, 2009). Thaxtomin has been developed as a bioherbicide.
Golgi Assembly
Brefeldin A and its hydroxylated 7-dehydrobrefeldin A analog are produced by A. carfhami, a fungal pathogen of safflower (Carthamus tinctorius). Both of these compounds cause a cis-to-trans breakdown of the Golgi stacks in plant cells (Driouich et al., 1997) and block the secretion of cell wall polysaccharides and proteins as well as the transport of soluble proteins to the vacuole (Fig. 6; Ritzenthaler et al., 2002). The molecular target of these compounds is unknown.
PLANT CELL CYCLE
DNA Polymerase α and δ
Several natural products interfere with the plant cell cycle (Planchais et al., 2000). For example, aphidicolin analogs are phytotoxins from P. betae (Ichihara et al., 1984) that reversibly inhibit DNA polymerase α and δ and interrupt the G1/S progression (Ikegami et al., 1978).
Ribonucleotide Reductase
Ribonucleotide reductase catalyzes the formation of deoxyribonucleotides from ribonucleotides, which in turn are used in the synthesis of DNA. Mimosine is a nonprotein amino acid (β-3-hydroxy-4 pyridone) phytotoxin produced by M. pudica (Reigosa and Malvido-Pazos, 2007; Williams and Hoagland, 2007). Mimosine inhibits ribonucleotide reductase at the G1 stage, before the initiation of replication (Perennes et al., 1993).
Proteasome Interference
A major step in proteolysis by the proteasome is catalyzed by the anaphase-promoting complex (a multimeric ubiquitin ligase). This complex targets B-type cyclins and other regulatory proteins to the 26S proteasome for degradation, allowing exit from mitosis. Some Streptomyces spp. produce lactacystin (Omura et al., 1991). This bacterial metabolite interferes with the function of the proteasome by specifically inhibiting the anaphase-promoting complex, thus preventing the destruction of cyclins and causing the cells to escape from mitosis (Planchais et al., 2000).
SUMMARY
Herbicides with new MOAs that can be used as biochemical bioherbicides are badly needed for both conventional and organic agriculture. The structural diversity and evolved biological activities of natural compounds offer opportunities for the development of biochemical bioherbicides and synthetic herbicides based on the structures of natural phytotoxins. Natural phytotoxins are also a source of discovery of new herbicide target sites that can serve as the focus of traditional herbicide discovery efforts. The array of target sites of potent phytotoxins listed in Table I indicates that there may be no preferred target sites for phytotoxins in nature. The currently available information is probably skewed toward known target sites that were more easily determined. For example, it is very simple to determine if a compound inhibits PSII, but it is usually quite challenging to discover a new MOA. There are many natural phytotoxins for which the MOA is unknown. For example, the metabolomic profile in plants elicited by ascaulitoxin aglycone does not match any of the profiles elicited by phytotoxins with a broad array of known targets, indicating that this phytotoxin has a different, still unknown target (Duke et al., 2011). Thus, without considerably more information, no conclusions can be drawn about whether phytotoxins for particular targets are more common in nature.
MOA work with natural products can lead to the production of new tools for studying plant physiology and biochemistry (Dayan et al., 2010). For example, natural phytotoxins that interfere with different targets in mitosis have been invaluable for probing different aspects of this process (Vaughn and Vaughan, 1988; Planchais et al., 2000). Few natural phytotoxins have physicochemical properties that are optimal for direct application to weeds or soil in which weed seeds germinate (Tice, 2001), but there are exceptions, such as l-phosphinothricin and leptospermone. Structural modification of a natural compound can often improve its activity at the target site as well as the physicochemical properties required for adequate uptake, translocation, and environmental half-life. In most cases, the natural phytotoxins that we have discussed will kill plants at low doses, making it clear that there are herbicide target sites that can be added to the current repertoire of commercial herbicide molecular targets, provided that safe, efficacious, economical compounds can be found that target these sites.
Glossary
- MOA
mechanisms of action
- PIP
plant-incorporated protectant
- GS
Gln synthetase
- QB
secondary electron-accepting plastoquinone of PSII
- HPPD
4-hydroxyphenylpyruvate dioxygenase
- PDS
phytoene desaturase
- PPO
protoporphyrinogen oxidase
- ACP
acyl carrier protein
- ENR
enoyl-acyl carrier protein reductase
- IAA
indole-3-acetic acid
- ACC
1-aminocyclopropane-1-carboxylase
References
- Abbas HK, Duke SO, Shier WT, Duke MV (2002) Inhibition of ceramide synthesis in plants by phytotoxins. In RK Upadhyay, ed, Advances in Microbial Toxin Research and Its Biotechnological Exploitation. Kluwer Academic/Plenum, London, pp 211–229 [Google Scholar]
- Abbas HK, Gronwald JW, Plaisance KL, Paul RN, Lee YW. (2001) Histone deacetylase activity and phytotoxic effects following exposure of Duckweed (Lemna pausicostata L.) to apicidin and HC-toxin. Phytopathology 91: 1141–1148 [DOI] [PubMed] [Google Scholar]
- Abbas HK, Paul RN, Riley RT, Tanaka T, Shier WT. (1998) Ultrastructural effects of AAL-toxin TA from the fungus Alternaria alternata on black nightshade (Solanum nigrum L.) leaf discs and correlation with biochemical measures of toxicity. Toxicon 36: 1821–1832 [DOI] [PubMed] [Google Scholar]
- Abbas HK, Tanaka T, Duke SO, Boyette CD. (1995) Susceptibility of various crop and weed species to AAL-toxin, a natural herbicide. Weed Technol 9: 125–130 [Google Scholar]
- Abbas HK, Tanaka T, Duke SO, Porter JK, Wray EM, Hodges L, Sessions AE, Wang E, Merrill AH, Jr, Riley RT. (1994) Fumonisin- and AAL-toxin-induced disruption of sphingolipid metabolism with accumulation of free sphingoid bases. Plant Physiol 106: 1085–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amagasa T, Paul RN, Heitholt JJ, Duke SO. (1994) Physiological effects of cornexistin on Lemna pausicostata. Pestic Biochem Physiol 49: 37–42 [Google Scholar]
- Backman PA, DeVay JE. (1971) Studies on the mode of action and biogenesis of the phytotoxin syringomycin. Plant Pathol 1: 215–234 [Google Scholar]
- Bajsa J, Pan Z, Dayan FE, Owens DK, Duke SO. (2011a) Validation of serine-threonine protein phosphatase as the herbicide target site of endothall. Pestic Biochem Physiol 102: 38–44 [Google Scholar]
- Bajsa J, Pan Z, Duke SO. (2011b) Transcriptional responses to cantharidin, a protein phosphatase inhibitor, in Arabidopsis thaliana reveal the involvement of multiple signal transduction pathways. Physiol Plant 143: 188–205 [DOI] [PubMed] [Google Scholar]
- Barto EK, Hilker M, Müller F, Mohney BK, Weidenhamer JD, Rillig MC. (2011) The fungal fast lane: common mycorrhizal networks extend bioactive zones of allelochemicals in soils. PLoS ONE 6: e27195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batish DR, Kohli RK, Singh HP. (2011) Allelopathy: concept, chemicals involved and possible utilization for weed management. J Plant Biol 38: 33–42 [Google Scholar]
- Bender CL, Alarcón-Chaidez F, Gross DC. (1999) Pseudomonas syringae phytotoxins: mode of action, regulation, and biosynthesis by peptide and polyketide synthetases. Microbiol Mol Biol Rev 63: 266–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholdsson NO, Andersson SC, Merker A. (2012) Allelopathic potential of Triticum spp., Secale spp. and Triticosecale spp. and use of chromosome substitutions and translocations to improve weed suppression ability in winter wheat. Plant Breed 131: 75–80 [Google Scholar]
- Bertin C, Weston LA, Kaur H. (2008) Allelopathic crop development: molecular and traditional plant breeding approaches. Plant Breed Rev 30: 231–258 [Google Scholar]
- Bischoff V, Cookson SJ, Wu S, Scheible WR. (2009) Thaxtomin A affects CESA-complex density, expression of cell wall genes, cell wall composition, and causes ectopic lignification in Arabidopsis thaliana seedlings. J Exp Bot 60: 955–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block A, Schmelz EA, Jones JB, Klee HJ. (2005) Coronatine and salicylic acid: the battle between Arabidopsis and Pseudomonas for phytohormone control. Mol Plant Pathol 6: 79–83 [DOI] [PubMed] [Google Scholar]
- Callaway RM, Aschehoug ET. (2000) Invasive plants versus their new and old neighbors: a mechanism for exotic invasion. Science 290: 521–523 [DOI] [PubMed] [Google Scholar]
- Callaway RM, Cipollini D, Barto K, Thelen GC, Hallett SG, Prati D, Stinson K, Klironomos J. (2008) Novel weapons: invasive plant suppresses fungal mutualists in America but not in its native Europe. Ecology 89: 1043–1055 [DOI] [PubMed] [Google Scholar]
- Callaway RM, Maron JL. (2006) What have exotic plant invasions taught us over the past 20 years? Trends Ecol Evol 21: 369–374 [DOI] [PubMed] [Google Scholar]
- Callaway RM, Ridenour WM. (2004) Novel weapons: invasive success and the evolution of increased competitive ability. Front Ecol Environ 2: 436–443 [Google Scholar]
- Cantrell CL, Dayan FE, Duke SO. (2012) Natural products as sources for new pesticides. J Nat Prod 75: 1231–1242 [DOI] [PubMed] [Google Scholar]
- Cappuccino N, Arnason JT. (2006) Novel chemistry of invasive exotic plants. Biol Lett 2: 189–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzonelli C. (2011) Carotenoids in nature: insights from plants and beyond. Funct Plant Biol 38: 833–847 [DOI] [PubMed] [Google Scholar]
- Chaimovitsh D, Abu-Abied M, Belausov E, Rubin B, Dudai N, Sadot E. (2010) Microtubules are an intracellular target of the plant terpene citral. Plant J 61: 399–408 [DOI] [PubMed] [Google Scholar]
- Cleland RE. (1976) Kinetics of hormone-induced H+ secretion. Plant Physiol 58: 210–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinch K. (1996) Synthesis of analogues of monic acids A and C: potential herbicides and inhibitors of isoleucyl tRNA synthetase. Bioorg Med Chem Lett 6: 467–472 [Google Scholar]
- Coleman R, Penner D. (2006) Desiccant activity of short chain fatty acids. Weed Technol 20: 410–415 [Google Scholar]
- Coleman R, Penner D. (2008) Organic acid enhancement of pelargonic acid. Weed Technol 22: 38–41 [Google Scholar]
- Copping LG, Duke SO. (2007) Natural products that have been used commercially as crop protection agents. Pest Manag Sci 63: 524–554 [DOI] [PubMed] [Google Scholar]
- Cseke C, Gerwick BC, Crouse GD, Murdoch MG, Green SB, Heim DR. (1996) 2a-Phosphohydantocidin: the in vivo adenylosuccinate synthetase inhibitor responsible for hydantocidin phytotoxicity. Pestic Biochem Physiol 55: 210–217 [Google Scholar]
- Dancer JE, Hughes RG, Lindell SD. (1997) Adenosine-5′-phosphate deaminase: a novel herbicide target. Plant Physiol 114: 119–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daub ME. (1982) Peroxidation of tobacco membrane lipids by the photosensitizing toxin, cercosporin. Plant Physiol 69: 1361–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daub ME, Hangarter RP. (1983) Light-induced production of singlet oxygen and superoxide by the fungal toxin, cercosporin. Plant Physiol 73: 855–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daub ME, Herrero S, Chung KR. (2005) Photoactivated perylenequinone toxins in fungal pathogenesis of plants. FEMS Microbiol Lett 252: 197–206 [DOI] [PubMed] [Google Scholar]
- Dayan FE. (2006) Factors modulating the levels of the allelochemical sorgoleone in Sorghum bicolor. Planta 224: 339–346 [DOI] [PubMed] [Google Scholar]
- Dayan FE, Dayan EA. (2011) Porphyrins: one ring in the colors of life. Am Sci 99: 236–243 [Google Scholar]
- Dayan FE, Duke SO (2003) Herbicides: carotenoid biosynthesis inhibitors. In JR Plimmer, DW Gammon, NN Ragsdale, eds, Encyclopedia of Agrochemicals, Vol 2. John Wiley & Sons, New York, pp 744–749 [Google Scholar]
- Dayan FE, Duke SO (2010) Protoporphyrinogen oxidase-inhibiting herbicides. In R Krieger, J Doull, E Hodgson, H Maibach, L Reiter, L Ritter, J Ross, WJ Slikker, J Van Hemmen, eds, Haye’s Handbook of Pesticide Toxicology, Ed 3, Vol 2. Academic Press, Elsevier, San Diego, pp 1733–1751 [Google Scholar]
- Dayan FE, Duke SO, Sauldubois A, Singh N, McCurdy C, Cantrell CL. (2007a) p-Hydroxyphenylpyruvate dioxygenase is a herbicidal target site for β-triketones from Leptospermum scoparium. Phytochemistry 68: 2004–2014 [DOI] [PubMed] [Google Scholar]
- Dayan FE, Ferreira D, Wang YH, Khan IA, McInroy JA, Pan Z. (2008) A pathogenic fungi diphenyl ether phytotoxin targets plant enoyl (acyl carrier protein) reductase. Plant Physiol 147: 1062–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan FE, Kagan IA, Rimando AM. (2003) Elucidation of the biosynthetic pathway of the allelochemical sorgoleone using retrobiosynthetic NMR analysis. J Biol Chem 278: 28607–28611 [DOI] [PubMed] [Google Scholar]
- Dayan FE, Owens DK, Duke SO. (2012) Rationale for a natural products approach to herbicide discovery. Pest Manag Sci 68: 519–528 [DOI] [PubMed] [Google Scholar]
- Dayan FE, Rimando AM, Pan Z, Baerson SR, Gimsing AL, Duke SO. (2010) Sorgoleone. Phytochemistry 71: 1032–1039 [DOI] [PubMed] [Google Scholar]
- Dayan FE, Watson SB, Galindo JCG, Hernández A, Dou J, McChesney JD, Duke SO. (1999) Phytotoxicity of quassinoids: physiological responses and structural requirements. Pestic Biochem Physiol 65: 15–24 [Google Scholar]
- Dayan FE, Watson SB, Nanayakkara NPD. (2007b) Biosynthesis of lipid resorcinols and benzoquinones in isolated secretory plant root hairs. J Exp Bot 58: 3263–3272 [DOI] [PubMed] [Google Scholar]
- Driouich A, Jauneau A, Staehelin LA. (1997) 7-Dehydrobrefeldin A, a naturally occurring brefeldin A derivative, inhibits secretion and causes a cis-to-trans breakdown of Golgi stacks in plant cells. Plant Physiol 113: 487–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duca D, Lorv J, Patten CL, Rose D, Glick BR. (2014) Indole-3-acetic acid in plant-microbe interactions. Antonie van Leeuwenhoek 106: 85–125 [DOI] [PubMed] [Google Scholar]
- Ducrot PH, Einhorn J, Kerhoas L, Lallemand JY, Milat ML, Blein JP, Neuman A, Prange T. (1996) Cercospora beticola toxins. Part XI. Isolation and structure of beticolin 0. Tetrahedron Lett 37: 3121–3124 [Google Scholar]
- Duke SO. (1993) Tentoxin effects on variable fluorescence and P515 electrochromic absorbance changes in tentoxin-sensitive and -resistant plant species. Plant Sci 90: 119–126 [Google Scholar]
- Duke SO. (2003) Weeding with transgenes. Trends Biotechnol 21: 192–195 [DOI] [PubMed] [Google Scholar]
- Duke SO. (2010) Allelopathy: current status of research and future of the discipline. A commentary. Allelo J 25: 17–30 [Google Scholar]
- Duke SO. (2011) Comparing conventional and biotechnology-based pest management. J Agric Food Chem 59: 5793–5798 [DOI] [PubMed] [Google Scholar]
- Duke SO. (2012) Why have no new herbicide modes of action appeared in recent years? Pest Manag Sci 68: 505–512 [DOI] [PubMed] [Google Scholar]
- Duke SO (2014) Biotechnology: herbicide-resistant crops. In N Van Alfen, ed, Encyclopedia of Agriculture and Food Systems, Vol. 2. Elsevier, San Diego, pp 94–116 [Google Scholar]
- Duke SO, Baerson SR, Gressel J (2009) Genomics and weeds: a synthesis. In CN Steward, ed, Weedy and Invasive Plant Genomics. Blackwell Publishing, Singapore, pp 221–247 [Google Scholar]
- Duke SO, Dayan FE. (2013) Clues to new herbicide mechanisms of action from natural sources. ACS Symp Ser 1141: 203–215 [Google Scholar]
- Duke SO, Dayan FE (2015) Natural toxins that affect plant amino acid metabolism. In F D’Mello, ed, Amino Acids in Higher Plants. CAB International, Wallingford, UK (in press) [Google Scholar]
- Duke SO, Evidente A, Fiore M, Rimando AM, Dayan FE, Vurro M, Christiansen N, Looser R, Hutzler J, Grossmann K. (2011) Effects of the aglycone of ascaulitoxin on amino acid metabolism in Lemna paucicostata. Pestic Biochem Physiol 100: 41–50 [Google Scholar]
- Duke SO, Powles SB. (2008) Glyphosate: a once-in-a-century herbicide. Pest Manag Sci 64: 319–325 [DOI] [PubMed] [Google Scholar]
- Duke SO, Scheffler BE, Dayan FE, Weston LA, Ota E. (2001) Strategies for using transgenes to produce allelopathic crops. Weed Technol 15: 826–834 [Google Scholar]
- Durbin RD, Uchytil TF. (1977) A survey of plant insensitivity to tentoxin. Phytopathology 67: 602–603 [Google Scholar]
- Environmental Protection Agency (2014) What are biopesticides? www.epa.gov/oppbppd1/biopesticides/whatarebiopesticides.htm (January 14, 2014)
- Feld A, Kobek K, Lichtenthaler HK. (1989) Inhibition of fatty-acid biosynthesis in isolated chloroplasts by the antibiotics cerulenin and thiolactomycin. Brighton Crop Protection Conference Weeds 2: 479–486 [Google Scholar]
- Fernández-San Millán A, Obregón P, Veramendi J. (2011) Over-expression of peptide deformylase in chloroplasts confers actinonin resistance, but is not a suitable selective marker system for plastid transformation. Transgenic Res 20: 613–624 [DOI] [PubMed] [Google Scholar]
- Ferradini N, Nicolia A, Capomaccio S, Veronesi F, Rosellini D. (2011) A point mutation in the Medicago sativa GSA gene provides a novel, efficient, selectable marker for plant genetic engineering. J Biotechnol 156: 147–152 [DOI] [PubMed] [Google Scholar]
- Flagel LE, Wendel JF. (2009) Gene duplication and evolutionary novelty in plants. New Phytol 183: 557–564 [DOI] [PubMed] [Google Scholar]
- Flint DH. (1984) Gabaculine inhibits δ-ALA synthesis in chloroplasts abstract no. S-965). Plant Physiol 75: 17016663565 [Google Scholar]
- Fonné-Pfister R, Chemla P, Ward E, Girardet M, Kreuz KE, Honzatko RB, Fromm HJ, Schär H-P, Grütter MG, Cowan-Jacob SW. (1996) The mode of action and the structure of a herbicide in complex with its target: binding of activated hydantocidin to the feedback regulation site of adenylosuccinate synthetase. Proc Natl Acad Sci USA 93: 9431–9436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gealy DR, Moldenhauer KAK, Jia MH. (2013) Field performance of STG06L-35-061, a new genetic resource developed from crosses between weed-suppressive indica rice and commercial southern U.S. long-grains. Plant Soil 370: 277–293 [Google Scholar]
- Gealy DR, Wailes EJ, Estorninos LE, Jr, Chavez RSC. (2003) Rice cultivar differences in suppression of barnyardgrass (Echinochloa crus-galli) and economics of reduced propanil rates. Weed Sci 51: 601–609 [Google Scholar]
- Gealy DR, Yan W. (2012) Weed suppression potential of ‘Rondo’ and other indica rice germplasm lines. Weed Technol 26: 524–527 [Google Scholar]
- Gerwick BC, Fields SS, Graupner PR, Gray JA, Chapin EL, Cleveland JA, Heim DR. (1997) Pyridazocidin, a new microbial phytotoxin with activity in the Mehler reaction. Weed Sci 45: 654–657 [Google Scholar]
- Gerwick BC, Sparks TC. (2014) Natural products for pest control: an analysis of their role, value and future. Pest Manag Sci 70: 1169–1185 [DOI] [PubMed] [Google Scholar]
- Giovanelli J, Owens LD, Mudd SH. (1971) Mechanism of inhibition of spinach β-cystathionase by rhizobitoxine. Biochim Biophys Acta 227: 671–684 [DOI] [PubMed] [Google Scholar]
- Giovanelli J, Owens LD, Mudd SH. (1973) β-Cystathionase in vivo inactivation by rhizobitoxine and role of the enzyme in methionine biosynthesis in corn seedlings. Plant Physiol 51: 492–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomarasca S, Vannini C, Venegoni A, Talarico A, Marre MT, Soave C. (1993) A mutant of Arabidopsis thaliana with a reduced response to fusicoccin. Plant Physiol 103: 165–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez VM, Kazimir J, Nimbal C, Weston LA, Cheniae GM. (1997) Inhibition of a photosystem II electron transfer reaction by the natural product sorgoleone. J Agric Food Chem 45: 1415–1421 [Google Scholar]
- Goudet C, Véry AA, Milat ML, Ildefonse M, Thibaud JB, Sentenac H, Blein JP. (1998) Magnesium ions promote assembly of channel-like structures from beticolin 0, a non-peptide fungal toxin purified from Cercospora beticola. Plant J 14: 359–364 [DOI] [PubMed] [Google Scholar]
- Gough KCG, Hawes WSH, Kilpatrick J, Whitelam GC. (2001) Cyanobacterial GR6 glutamate-1-semialdehyde aminotransferase: a novel enzyme-based selectable marker for plant transformation. Plant Cell Rep 20: 296–300 [Google Scholar]
- Grimm B, Smith AJ, Kannangara CG, Smith M. (1991) Gabaculine-resistant glutamate 1-semialdehyde aminotransferase of Synechococcus: deletion of a tripeptide close to the NH2 terminus and internal amino acid substitution. J Biol Chem 266: 12495–12501 [PubMed] [Google Scholar]
- Grossmann K, Hutzler J, Tresch S, Christiansen N, Looser R, Ehrhardt T. (2012) On the mode of action of the herbicides cinmethylin and 5-benzyloxymethyl-1,2-isoxazolines: putative inhibitors of plant tyrosine aminotransferase. Pest Manag Sci 68: 482–492 [DOI] [PubMed] [Google Scholar]
- Groth G. (2002) Structure of spinach chloroplast F1-ATPase complexed with the phytopathogenic inhibitor tentoxin. Proc Natl Acad Sci USA 99: 3464–3468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann L, Juettner F. (1996) Fischerellin A, a novel photosystem-II-inhibiting allelochemical of the cyanobacterium Fischerella muscicola with antifungal and herbicidal activity. Tetrahedron Lett 37: 6539–6542 [Google Scholar]
- Halloin JM, De Zoeten GA, Walker JC. (1970) The effects of tentoxin on chlorophyll synthesis and plastid structure in cucumber and cabbage. Plant Physiol 45: 310–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington PM, Singh BK, Szamosi IT, Birk JH. (1995) Synthesis and herbicidal activity of cyperin. J Agric Food Chem 43: 804–808 [Google Scholar]
- Harvey AL. (1999) Medicines from nature: are natural products still relevant to drug discovery? Trends Pharmacol Sci 20: 196–198 [DOI] [PubMed] [Google Scholar]
- Harvey AL. (2007) Natural products as a screening resource. Curr Opin Chem Biol 11: 480–484 [DOI] [PubMed] [Google Scholar]
- Harvey AL. (2008) Natural products in drug discovery. Drug Discov Today 13: 894–901 [DOI] [PubMed] [Google Scholar]
- Hashimoto T. (2013) Dissecting the cellular functions of plant microtubules using mutant tubulins. Cytoskeleton (Hoboken) 70: 191–200 [DOI] [PubMed] [Google Scholar]
- Hayashi KI, Kamio S, Oono Y, Townsend LB, Nozaki H. (2009) Toyocamycin specifically inhibits auxin signaling mediated by SCFTIR1 pathway. Phytochemistry 70: 190–197 [DOI] [PubMed] [Google Scholar]
- Hayashi KI, Yamazoe A, Ishibashi Y, Kusaka N, Oono Y, Nozaki H. (2008) Active core structure of terfestatin A, a new specific inhibitor of auxin signaling. Bioorg Med Chem 16: 5331–5344 [DOI] [PubMed] [Google Scholar]
- Heap I (2014) The International Survey of Herbicide Resistant Weeds. www/weedscience.org (February 27, 2014)
- Hedden P, Phillips AL, Rojas MC, Carrera E, Tudzynski B. (2001) Gibberellin biosynthesis in plants and fungi: a case of convergent evolution? J Plant Growth Regul 20: 319–331 [DOI] [PubMed] [Google Scholar]
- Heim DR, Cseke C, Gerwick BC, Murdoch MG, Green SB. (1995) Hydantocidin: a possible proherbicide inhibiting purine biosynthesis at the site of adenylosuccinate synthetase. Pestic Biochem Physiol 53: 138–145 [Google Scholar]
- Hejl AM, Koster KL. (2004a) Juglone disrupts root plasma membrane H+-ATPase activity and impairs water uptake, root respiration, and growth in soybean (Glycine max) and corn (Zea mays). J Chem Ecol 30: 453–471 [DOI] [PubMed] [Google Scholar]
- Hejl AM, Koster KL. (2004b) The allelochemical sorgoleone inhibits root H+-ATPase and water uptake. J Chem Ecol 30: 2181–2191 [DOI] [PubMed] [Google Scholar]
- Hess FD. (2000) Light-dependent herbicides: an overview. Weed Sci 48: 160–170 [Google Scholar]
- Hou CX, Dirk LMA, Goodman JP, Williams MA. (2006) Metabolism of the peptide deformylase inhibitor actinonin in tobacco. Weed Sci 54: 246–254 [Google Scholar]
- Hou CX, Dirk LMA, Pattanaik S, Das NC, Maiti IB, Houtz RL, Williams MA. (2007) Plant peptide deformylase: a novel selectable marker and herbicide target based on essential cotranslational chloroplast protein processing. Plant Biotechnol J 5: 275–281 [DOI] [PubMed] [Google Scholar]
- Hsiao P, Sanjaya, Su RC, Teixeira da Silva JA, Chan MT. (2007) Plant native tryptophan synthase beta 1 gene is a non-antibiotic selection marker for plant transformation. Planta 225: 897–906 [DOI] [PubMed] [Google Scholar]
- Huang H, Morgan CM, Asolkar RN, Koivunen ME, Marrone PG. (2010) Phytotoxicity of sarmentine isolated from long pepper (Piper longum) fruit. J Agric Food Chem 58: 9994–10000 [DOI] [PubMed] [Google Scholar]
- Hughes J, Mellows G. (1978) Inhibition of isoleucyl-transfer ribonucleic acid synthetase in Escherichia coli by pseudomonic acid. Biochem J 176: 305–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüter OF. (2011) Use of natural products in the crop protection industry. Phytochem Rev 10: 185–194 [Google Scholar]
- Ibn H, Tyrwhitt T, Orpheus (1781) Peri Lithôn de Lapidibus, Poema Orpheo a Quibusdam Adscriptum. Payne, White, and Elmsly, London [Google Scholar]
- Ichihara A, Oikawa H, Hayashi K, Hashimoto M, Sakamura S, Ryutaro S. (1984) 3-Deoxyaphidicolin and aphidicolin analogues as phytotoxins from Phoma betae. Agric Biol Chem 48: 1687–1689 [Google Scholar]
- Ichihara A, Shiraishi K, Sato H, Sakamura S, Nishiyama K, Sakai R, Furusaki A, Matsumoto T. (1977) The structure of coronatine. J Am Chem Soc 99: 636–637 [Google Scholar]
- Ikegami S, Taguchi T, Ohashi M, Oguro M, Nagano H, Mano Y. (1978) Aphidicolin prevents mitotic cell division by interfering with the activity of DNA polymerase-α. Nature 275: 458–460 [DOI] [PubMed] [Google Scholar]
- Irvine NM, Yerkes CN, Graupner PR, Roberts RE, Hahn DR, Pearce C, Gerwick BC. (2008) Synthesis and characterization of synthetic analogs of cinnacidin, a novel phytotoxin from Nectria sp. Pest Manag Sci 64: 891–899 [DOI] [PubMed] [Google Scholar]
- Jameson P. (2000) Cytokinins and auxins in plant-pathogen interactions: an overview. Plant Growth Regul 32: 369–380 [Google Scholar]
- Jiang WK, Liu YL, Xia EH, Gao LZ. (2013) Prevalent role of gene features in determining evolutionary fates of whole-genome duplication duplicated genes in flowering plants. Plant Physiol 161: 1844–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez JC, Chavarría B, López-Macià A, Royo M, Giralt E, Albericio F. (2003) Tentoxin as a scaffold for drug discovery: total solid-phase synthesis of tentoxin and a library of analogues. Org Lett 5: 2115–2118 [DOI] [PubMed] [Google Scholar]
- Jones JDG, Dangl JL. (2006) The plant immune system. Nature 444: 323–329 [DOI] [PubMed] [Google Scholar]
- Kahn A, Kannangara CG. (1987) Gabaculine-resistant mutants of Chlamydomonas reinhardtii with elevated glutamate 1-semialdehyde aminotransferase activity. Carlsberg Res Commun 52: 73–81 [Google Scholar]
- Kannangara CG, Schouboe A. (1985) Biosynthesis of Δ-aminolevulinate in greening barley leaves. VII. Glutamate 1-semialdehyde accumulation in gabaculine treated leaves. Carlsberg Res Commun 50: 179–191 [DOI] [PubMed] [Google Scholar]
- Kato-Noguchi H. (2004) Allelopathic substance in rice root exudates: rediscovery of momilactone B as an allelochemical. J Plant Physiol 161: 271–276 [DOI] [PubMed] [Google Scholar]
- Kato-Noguchi H, Ino I. (2004) Release level of momilactone B from rice plants. Plant Prod Sci 7: 189–190 [Google Scholar]
- Kedy P, Bruyant P, Balangé AP. (1994) Inhibition of 5-aminolevulinic acid dehydratase activity by gabaculine. Phytochemistry 36: 1169–1175 [Google Scholar]
- King RR, Calhoun LA. (2009) The thaxtomin phytotoxins: sources, synthesis, biosynthesis, biotransformation and biological activity. Phytochemistry 70: 833–841 [DOI] [PubMed] [Google Scholar]
- King RR, Lawrence CH, Gray JA. (2001) Herbicidal properties of the thaxtomin group of phytotoxins. J Agric Food Chem 49: 2298–2301 [DOI] [PubMed] [Google Scholar]
- Koch MA, Schuffenhauer A, Scheck M, Wetzel S, Casaulta M, Odermatt A, Ertl P, Waldmann H. (2005) Charting biologically relevant chemical space: a structural classification of natural products (SCONP). Proc Natl Acad Sci USA 102: 17272–17277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koda Y, Takahashi K, Kikuta Y, Greulich F, Toshima H, Ichihara A. (1996) Similarities of the biological activities of coronatine and coronafacic acid to those of jasmonic acid. Phytochemistry 41: 93–96 [Google Scholar]
- Köhler HR, Triebskorn R. (2013) Wildlife ecotoxicology of pesticides: can we track effects to the population level and beyond? Science 341: 759–765 [DOI] [PubMed] [Google Scholar]
- Kong C, Liang W, Xu X, Hu F, Wang P, Jiang Y. (2004) Release and activity of allelochemicals from allelopathic rice seedlings. J Agric Food Chem 52: 2861–2865 [DOI] [PubMed] [Google Scholar]
- Kong CH, Chen XH, Hu F, Zhang SZ. (2011) Breeding of commercially acceptable allelopathic rice cultivars in China. Pest Manag Sci 67: 1100–1106 [DOI] [PubMed] [Google Scholar]
- Kong Au T, Chow Leung P. (1998) Identification of the binding and inhibition sites in the calmodulin molecule for ophiobolin A by site-directed mutagenesis. Plant Physiol 118: 965–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzuyama T, Shimizu T, Takahashi S, Seto H. (1998) Fosmidomycin, a specific inhibitor of 1-deoxy-D-xylulose 5-phosphate reductoisomerase in the nonmevalonate pathway for terpenoid biosynthesis. Tetrahedron Lett 39: 7913–7916 [Google Scholar]
- Langston-Unkefer PL, Macy PA, Durbin RD. (1984) Inactivation of glutamine synthetase by tabtoxinine-β-lactam. Plant Physiol 76: 71–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lax AR, Shepherd HS, Edwards JV. (1988) Tentoxin, a chlorosis-inducing toxin from Alternaria as a potential herbicide. Weed Technol 2: 540–544 [Google Scholar]
- Leason M, Cunliffe D, Parkin D, Lea PJ, Miflin BJ. (1982) Inhibition of pea leaf glutamine synthetase by methionine sulphoximine, phosphinothricin and other glutamine analogues. Phytochemistry 21: 855–857 [Google Scholar]
- Lederer B, Fujimori T, Tsujino Y, Wakabayashi K, Böger P. (2004) Phytotoxic activity of middle-chain fatty acids. II. Peroxidation and membrane effects. Pestic Biochem Physiol 80: 151–156 [Google Scholar]
- Lee DL, Prisbylla MP, Cromartie TH, Dagarin DP, Howard SW, Provan WM, Ellis MK, Fraser T, Mutter LC. (1997) The discovery and structural requirements of inhibitors of p-hydroxyphenylpyruvate dioxygenase. Weed Sci 45: 601–609 [Google Scholar]
- Lee ESJ, Gleason FK. (1994) A second algicidal natural product from the cyanobacterium, Scytonema hofmanni. Plant Sci 103: 155–160 [Google Scholar]
- Leung PC, Taylor WA, Wang JH, Tipton CL. (1985) Role of calmodulin inhibition in the mode of action of ophiobolin a. Plant Physiol 77: 303–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levings SS, Rhoads DM, Siedow JN. (1995) Molecular interactions of Bipolaris maydis T-toxin and maize. Can J Bot 73: S483–S489 [Google Scholar]
- Lindell SD, Moloney BA, Hewitt BD, Earnshaw CG, Dudfield PJ, Dancer JE. (1999) The design and synthesis of inhibitors of adenosine 5′-monophosphate deaminase. Bioorg Med Chem Lett 9: 1985–1990 [DOI] [PubMed] [Google Scholar]
- Lipkus AH, Yuan Q, Lucas KA, Funk SA, Bartelt WF, III, Schenck RJ, Trippe AJ. (2008) Structural diversity of organic chemistry: a scaffold analysis of the CAS Registry. J Org Chem 73: 4443–4451 [DOI] [PubMed] [Google Scholar]
- Lowther WT, Matthews BW. (2002) Metalloaminopeptidases: common functional themes in disparate structural surroundings. Chem Rev 102: 4581–4608 [DOI] [PubMed] [Google Scholar]
- Lukens JH, Mathews DE, Durbin RD. (1987) Effect of tagetitoxin on the levels of ribulose 1,5-bisphosphate carboxylase, ribosomes, and RNA in plastids of wheat leaves. Plant Physiol 84: 808–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydon J, Duke SO (1999) Inhibitors of glutamine synthesis. In BK Singh, ed, Plant Amino Acids. Marcel Dekker, New York, pp 445–464 [Google Scholar]
- Malev VV, Kaulin YA, Bezrukov SM, Gurnev PA, Takemoto JY, Shchagina LV. (2001) Kinetics of opening and closure of syringomycin E channels formed in lipid bilayers. Membr Cell Biol 14: 813–829 [PubMed] [Google Scholar]
- Mangla S, Inderjit, Callaway RM. (2008) Exotic invasive plant accumulates native soil pathogens which inhibit native plants. J Ecol 96: 58–67 [Google Scholar]
- Maor R, Shirasu K. (2005) The arms race continues: battle strategies between plants and fungal pathogens. Curr Opin Microbiol 8: 399–404 [DOI] [PubMed] [Google Scholar]
- Mathews DE, Durbin RD. (1990) Tagetitoxin inhibits RNA synthesis directed by RNA polymerases from chloroplasts and Escherichia coli. J Biol Chem 265: 493–498 [PubMed] [Google Scholar]
- McMurry LM, Oethinger M, Levy SB. (1998) Triclosan targets lipid synthesis. Nature 394: 531–532 [DOI] [PubMed] [Google Scholar]
- Meazza G, Scheffler BE, Tellez MR, Rimando AM, Romagni JG, Duke SO, Nanayakkara D, Khan IA, Abourashed EA, Dayan FE. (2002) The inhibitory activity of natural products on plant p-hydroxyphenylpyruvate dioxygenase. Phytochemistry 60: 281–288 [DOI] [PubMed] [Google Scholar]
- Meeley RB, Walton JD. (1991) Enzymatic detoxification of HC-toxin, the host-selective cyclic peptide from Cochliobolus carbonum. Plant Physiol 97: 1080–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiss E, Konno H, Groth G, Hisabori T. (2008) Molecular processes of inhibition and stimulation of ATP synthase caused by the phytotoxin tentoxin. J Biol Chem 283: 24594–24599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RC, Purugganan MD. (2005) The evolutionary dynamics of plant duplicate genes. Curr Opin Plant Biol 8: 122–128 [DOI] [PubMed] [Google Scholar]
- Morré DJ, Grieco PA. (1999) Glaucarubolone and simalikalactone D, respectively, preferentially inhibit auxin-induced and constitutive components of plant cell enlargement and the plasma membrane NADH oxidase. Int J Plant Sci 160: 291–297 [Google Scholar]
- Morré DJ, Navas P, Penel C, Castillo FJ. (1986) Auxin-stimulated NADH oxidase (semidehydroascorbate reductase) of soybean plasma membrane: role in acidification of cytoplasm? Protoplasma 133: 195–197 [Google Scholar]
- Moyed HS. (1960) False feedback inhibition: inhibition of tryptophan biosynthesis by 5-methyltryptophan. J Biol Chem 235: 1098–1102 [PubMed] [Google Scholar]
- Muszyńska B, Sułkowska-Ziaja K, Ekiert H. (2013) Analysis of indole compounds in methanolic extracts from the fruiting bodies of Cantharellus cibarius (the Chanterelle) and from the mycelium of this species cultured in vitro. J Food Sci Technol 50: 1233–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M, Itoi K, Takamatsu Y, Sato S, Furukawa Y, Furuya K, Honma T, Kadotani J, Kozasa M, Haneishi T. (1991) Cornexistin: a new fungal metabolite with herbicidal activity. J Antibiot (Tokyo) 44: 1065–1072 [DOI] [PubMed] [Google Scholar]
- Netherland MD, Skogerboe JD, Owens CS, Madsen JD. (2000) Influence of water temperature on the efficacy of diquat and endothall versus curlyleaf pondweed. J Aquat Plant Manage 38: 25–32 [Google Scholar]
- Newman DJ, Cragg GM. (2012) Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod 75: 311–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida I, Kawaguchi A, Yamada M. (1986) Effect of thiolactomycin on the individual enzymes of the fatty acid synthase system in Escherichia coli. J Biochem 99: 1447–1454 [DOI] [PubMed] [Google Scholar]
- Nishino T, Murao S, Wada H. (1984) Mechanism of inactivation of pyridoxal phosphate-linked aspartate transaminase by gostatin. J Biochem 95: 1283–1288 [DOI] [PubMed] [Google Scholar]
- Norris SR, Barrette TR, DellaPenna D. (1995) Genetic dissection of carotenoid synthesis in Arabidopsis defines plastoquinone as an essential component of phytoene desaturation. Plant Cell 7: 2139–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ober D. (2005) Seeing double: gene duplication and diversification in plant secondary metabolism. Trends Plant Sci 10: 444–449 [DOI] [PubMed] [Google Scholar]
- Oettmeier W, Dostatni R, Majewski C, Hoefle G, Fecker T, Kunze B, Reichenbach H. (1990) The aurachins, naturally occurring inhibitors of photosynthetic electron flow through photosystem II and cytochrome b6/f-complex. Z Naturforsch 45C: 322–328 [Google Scholar]
- Oettmeier W, Godde D, Kunze B, Hoefle G. (1985) Stigmatellin: a dual type inhibitor of photosynthetic electron transport. Biochim Biophys Acta 807: 216–219 [Google Scholar]
- Okazaki S, Sugawara M, Yuhashi KI, Minamisawa K. (2007) Rhizobitoxine-induced chlorosis occurs in coincidence with methionine deficiency in soybeans. Ann Bot (Lond) 100: 55–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olbe M, Sommarin M, Gustafsson M, Lundborg T. (1995) Effect of the fungal pathogen Bipolaris sorokiniana toxin prehelminthosporol on barley root plasma membrane vesicles. Plant Pathol 44: 625–635 [Google Scholar]
- Omura S, Matsuzaki K, Fujimoto T, Kosuge K, Furuya T, Fujita S, Nakagawa A. (1991) Structure of lactacystin, a new microbial metabolite which induces differentiation of neuroblastoma cells. J Antibiot (Tokyo) 44: 117–118 [DOI] [PubMed] [Google Scholar]
- Omura S, Murata M, Hanaki H, Hinotozawa K, Oiwa R, Tanaka H. (1984a) Phosalacine, a new herbicidal antibiotic containing phosphinothricin: fermentation, isolation, biological activity and mechanism of action. J Antibiot (Tokyo) 37: 829–835 [DOI] [PubMed] [Google Scholar]
- Omura S, Murata M, Imamura N, Iwai Y, Tanaka H, Furusaki A, Matsumoto H. (1984b) Oxetin, a new antimetabolite from an actinomycete: fermentation, isolation, structure and biological activity. J Antibiot (Tokyo) 37: 1324–1332 [DOI] [PubMed] [Google Scholar]
- Oracz K, Voegele A, Tarkowská D, Jacquemoud D, Turecková V, Urbanová T, Strnad M, Sliwinska E, Leubner-Metzger G. (2012) Myrigalone A inhibits Lepidium sativum seed germination by interference with gibberellin metabolism and apoplastic superoxide production required for embryo extension growth and endosperm rupture. Plant Cell Physiol 53: 81–95 [DOI] [PubMed] [Google Scholar]
- Oszywa B, Makowski M, Pawełczak M. (2013) Purification and partial characterization of aminopeptidase from barley (Hordeum vulgare L.) seeds. Plant Physiol Biochem 65: 75–80 [DOI] [PubMed] [Google Scholar]
- Owens DK, Nanayakkara NPD, Dayan FE. (2013) In planta mechanism of action of leptospermone: impact of its physico-chemical properties on uptake, translocation, and metabolism. J Chem Ecol 39: 262–270 [DOI] [PubMed] [Google Scholar]
- Patten CL, Glick BR. (1996) Bacterial biosynthesis of indole-3-acetic acid. Can J Microbiol 42: 207–220 [DOI] [PubMed] [Google Scholar]
- Perennes C, Qin LX, Glab N, Bergounioux C. (1993) Petunia p34cdc2 protein kinase activity in G2/M cells obtained with a reversible cell cycle inhibitor, mimosine. FEBS Lett 333: 141–145 [DOI] [PubMed] [Google Scholar]
- Pimentel D, Zuniga R, Morrison D. (2005) Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol Econ 52: 273–288 [Google Scholar]
- Pinet E, Cavelier F, Verducci J, Girault G, Dubart L, Haraux F, Sigalat C, André F. (1996) Synthesis, structure, and properties of MeSer1-tentoxin, a new cyclic tetrapeptide which interacts specifically with chloroplast F1 H+-ATPase differentiation of inhibitory and stimulating effects. Biochemistry 35: 12804–12811 [DOI] [PubMed] [Google Scholar]
- Planchais S, Glab N, Inzé D, Bergounioux C. (2000) Chemical inhibitors: a tool for plant cell cycle studies. FEBS Lett 476: 78–83 [DOI] [PubMed] [Google Scholar]
- Poland BW, Lee SF, Subramanian MV, Siehl DL, Anderson RJ, Fromm HJ, Honzatko RB. (1996) Refined crystal structure of adenylosuccinate synthetase from Escherichia coli complexed with hydantocidin 5′-phosphate, GDP, HPO42−, Mg2+, and hadacidin. Biochemistry 35: 15753–15759 [DOI] [PubMed] [Google Scholar]
- Price AC, Choi KH, Heath RJ, Li Z, White SW, Rock CO. (2001) Inhibition of β-ketoacyl-acyl carrier protein synthases by thiolactomycin and cerulenin: structure and mechanism. J Biol Chem 276: 6551–6559 [DOI] [PubMed] [Google Scholar]
- Reigosa MJ, Malvido-Pazos E. (2007) Phytotoxic effects of 21 plant secondary metabolites on Arabidopsis thaliana germination and root growth. J Chem Ecol 33: 1456–1466 [DOI] [PubMed] [Google Scholar]
- Riley DA, Simpkins NS, Moffat D. (1999) Synthesis of novel carbocyclic analogues of indolocarbazole natural products. Tetrahedron Lett 40: 3929–3932 [Google Scholar]
- Rimando AM, Dayan FE, Czarnota MA, Weston LA, Duke SO. (1998) A new photosystem II electron transfer inhibitor from Sorghum bicolor. J Nat Prod 61: 927–930 [DOI] [PubMed] [Google Scholar]
- Ritzenthaler C, Nebenführ A, Movafeghi A, Stussi-Garaud C, Behnia L, Pimpl P, Staehelin LA, Robinson DG. (2002) Reevaluation of the effects of brefeldin A on plant cells using tobacco Bright Yellow 2 cells expressing Golgi-targeted green fluorescent protein and COPI antisera. Plant Cell 14: 237–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagni JG, Allen SN, Dayan FE. (2000a) Allelopathic effects of volatile cineoles on two weedy plant species. J Chem Ecol 26: 303–313 [Google Scholar]
- Romagni JG, Meazza G, Nanayakkara NPD, Dayan FE. (2000b) The phytotoxic lichen metabolite, usnic acid, is a potent inhibitor of plant p-hydroxyphenylpyruvate dioxygenase. FEBS Lett 480: 301–305 [DOI] [PubMed] [Google Scholar]
- Roujeinikova A, Levy CW, Rowsell S, Sedelnikova S, Baker PJ, Minshull CA, Mistry A, Colls JG, Camble R, Stuitje AR, et al. (1999) Crystallographic analysis of triclosan bound to enoyl reductase. J Mol Biol 294: 527–535 [DOI] [PubMed] [Google Scholar]
- Saad SM, Halloin JM, Hagedorn DJ. (1970) Production, purification, and bioassay of tentoxin. Phytopathology 60: 415–418 [DOI] [PubMed] [Google Scholar]
- Schagina LV, Kaulin YA, Feigin AM, Takemoto JY, Brand JG, Malev VV. (1998) Properties of ionic channels formed by the antibiotic syringomycin E in lipid bilayers: dependence on the electrolyte concentration in the bathing solution. Membr Cell Biol 12: 537–555 [PubMed] [Google Scholar]
- Scheible WR, Fry B, Kochevenko A, Schindelasch D, Zimmerli L, Somerville S, Loria R, Somerville CR. (2003) An Arabidopsis mutant resistant to thaxtomin A, a cellulose synthesis inhibitor from Streptomyces species. Plant Cell 15: 1781–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitzer PR, Graupner PR, Chapin EL, Fields SC, Gilbert JR, Gray JA, Peacock CL, Gerwick BC. (2000) Ribofuranosyl triazolone: a natural product herbicide with activity on adenylosuccinate synthetase following phosphorylation. J Nat Prod 63: 777–781 [DOI] [PubMed] [Google Scholar]
- Shavit N, San Pietro A. (1967) K+-dependent uncoupling of photophosphorylation by nigericin. Biochem Biophys Res Commun 28: 277–283 [DOI] [PubMed] [Google Scholar]
- Siehl DL, Subramanian MV, Walters EW, Lee SF, Anderson RJ, Toschi AG. (1996) Adenylosuccinate synthetase: site of action of hydantocidin, a microbial phytotoxin. Plant Physiol 110: 753–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A, Jüttner F, Strasser RJ. (1998) Action of the allelochemical, fischerellin A, on photosystem II. Biochim Biophys Acta 1364: 326–336 [DOI] [PubMed] [Google Scholar]
- Stokstad E. (2013) Infographic: pesticide planet. Science 341: 730–731 [DOI] [PubMed] [Google Scholar]
- Tachibana K, Kaneko K. (1986) Development of a new herbicide, bialaphos. J Pestic Sci 11: 297–304 [Google Scholar]
- Tachibana K, Watanabe W, Sekizawa Y, Takematsu T. (1986) Accumulation of ammonia in plants treated with bialaphos. J Pestic Sci 11: 33–37 [Google Scholar]
- Tanaka T, Abbas HK, Duke SO. (1993) Structure-dependent phytotoxicity of fumonisins and related compounds in a duckweed bioassay. Phytochemistry 33: 779–785 [Google Scholar]
- Templeton MD, Reinhardt LA, Collyer CA, Mitchell RE, Cleland WW. (2005) Kinetic analysis of the L-ornithine transcarbamoylase from Pseudomonas savastanoi pv. phaseolicola that is resistant to the transition state analogue (R)-Nδ-(N′-sulfodiaminophosphinyl)-L-ornithine. Biochemistry 44: 4408–4415 [DOI] [PubMed] [Google Scholar]
- Thomas MD, Langston-Unkefer PJ, Uchytil TF, Durbin RD. (1983) Inhibition of glutamine synthetase from pea by tabtoxinine-β-lactam. Plant Physiol 71: 912–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tice CM. (2001) Selecting the right compounds for screening: does Lipinski’s rule of 5 for pharmaceuticals apply to agrochemicals? Pest Manag Sci 57: 3–16 [DOI] [PubMed] [Google Scholar]
- Trebst A. (2007) Inhibitors in the functional dissection of the photosynthetic electron transport system. Photosynth Res 92: 217–224 [DOI] [PubMed] [Google Scholar]
- Tulp M, Bohlin L. (2005) Rediscovery of known natural compounds: nuisance or goldmine? Bioorg Med Chem 13: 5274–5282 [DOI] [PubMed] [Google Scholar]
- Turner JG. (1986) Effect of phaseolotoxin on the synthesis of arginine and protein. Plant Physiol 80: 760–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa H, Aoyagi T, Suda H, Hamada M, Takeuchi T. (1976) Bestatin, an inhibitor of aminopeptidase B, produced by actinomycetes. J Antibiot (Tokyo) 29: 97–99 [DOI] [PubMed] [Google Scholar]
- Vaughn KC, Vaughan MA. (1988) Mitotitic disruptors from higher plants: effects on plant cells. ACS Symp Ser 380: 273–293 [Google Scholar]
- Verhoeven KJF, Biere A, Harvey JA, van der Putten WH. (2009) Plant invaders and their novel natural enemies: who is naïve? Ecol Lett 12: 107–117 [DOI] [PubMed] [Google Scholar]
- Walton JD. (2006) HC-toxin. Phytochemistry 67: 1406–1413 [DOI] [PubMed] [Google Scholar]
- Wild A, Ziegler C. (1989) The effect of bialaphos on ammonium-assimilation and photosynthesis. I. Effect on the enzymes of ammonium-assimilation. Z Naturforsch 44C: 97–102 [Google Scholar]
- Williams RD, Hoagland RE. (2007) Phytotoxicity of mimosine and albizziine on seed germination and seedling growth of crops and weeds. Allelo J 19: 423–430 [Google Scholar]
- Worthington M, Reberg-Horton C. (2013) Breeding cereal crops for enhanced weed suppression: optimizing allelopathy and competitive ability. J Chem Ecol 39: 213–231 [DOI] [PubMed] [Google Scholar]
- Xu M, Galhano R, Wiemann P, Bueno E, Tiernan M, Wu W, Chung IM, Gershenzon J, Tudzynski B, Sesma A, et al. (2012) Genetic evidence for natural product-mediated plant-plant allelopathy in rice (Oryza sativa). New Phytol 193: 570–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang RZ, Tang CS. (1988) Plants used for pest control in China: a literature review. Econ Bot 42: 376–406 [Google Scholar]
- Yasuta T, Satoh S, Minamisawa K. (1999) New assay for rhizobitoxine based on inhibition of 1-aminocyclopropane-1-carboxylate synthase. Appl Environ Microbiol 65: 849–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Zhai Q, Sun J, Li CB, Zhang L, Li H, Zhang X, Li S, Xu Y, Jiang H, et al. (2006) Bestatin, an inhibitor of aminopeptidases, provides a chemical genetics approach to dissect jasmonate signaling in Arabidopsis. Plant Physiol 141: 1400–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]