Phytochrome-mediated phototropic enhancement depends on PINOID family kinases.
Abstract
Several members of the AGCVIII kinase subfamily, which includes PINOID (PID), PID2, and WAVY ROOT GROWTH (WAG) proteins, have previously been shown to phosphorylate PIN-FORMED (PIN) auxin transporters and control the auxin flow in plants. PID has been proposed as a key component of the phototropin signaling pathway that induces phototropic responses, although the responses were not significantly impaired in the pid single and pid wag1 wag2 triple mutants. This raises questions about the functional roles of the PID family in phototropic responses. Here, we investigated hypocotyl phototropism in the pid pid2 wag1 wag2 quadruple mutant in detail to clarify the roles of the PID family in Arabidopsis (Arabidopsis thaliana). The pid quadruple mutants exhibited moderate responses in continuous light-induced phototropism with a decrease in growth rates of hypocotyls and normal responses in pulse-induced phototropism. However, they showed serious defects in enhancements of pulse-induced phototropic curvatures and lateral fluorescent auxin transport by red light pretreatment. Red light pretreatment significantly reduced the expression level of PID, and the constitutive expression of PID prevented pulse-induced phototropism, irrespective of red light pretreatment. This suggests that the PID family plays a significant role in phytochrome-mediated phototropic enhancement but not the phototropin signaling pathway. Red light treatment enhanced the intracellular accumulation of PIN proteins in response to the vesicle-trafficking inhibitor brefeldin A in addition to increasing their expression levels. Taken together, these results suggest that red light preirradiation enhances phototropic curvatures by up-regulation of PIN proteins, which are not being phosphorylated by the PID family.
Sessile organisms, such as plants, develop many adaptational mechanisms. Phototropism is one such acclimation response in plants to light environments, by which they can advantageously obtain light energy for photosynthesis. So far, phototropic responses, which have been well studied for many years, are separated mainly into two types according to their characters: the first positive phototropism and the second positive phototropism (Iino, 2001; Whippo and Hangarter, 2006; Briggs, 2014). The first positive phototropism is induced by a pulse of blue light, and the magnitudes of the responses are dependent totally on the total light fluence. However, the second positive phototropism is induced by a prolonged irradiation of blue light, and the extent of the curvature responses depends on the duration of the blue light illumination and not on the total light fluences.
Classical physiological analysis has revealed that plant photoreceptor phytochromes influence phototropin (phot)-mediated phototropic responses in several aspects (Iino, 2001; Whippo and Hangarter, 2006; Sakai and Haga, 2012; Briggs, 2014). For the first positive pulse-induced phototropism, phytochromes are involved in the enhancement of phototropic curvatures in addition to desensitization (Liu and Iino, 1996a; Janoudi et al., 1997a; Whippo and Hangarter, 2004; Haga and Sakai, 2012). For the second positive phototropism, phytochromes participate in the establishment of a phototropic system, which is necessary to respond to a prolonged irradiation of blue light, the promotion of phototropic curvature rates, and the reduction of the time required to induce curvature responses (Janoudi et al., 1992; Liu and Iino, 1996b; Hangarter, 1997; Haga and Sakai, 2012). All such regulation is controlled by multiple phytochromes (Parks et al., 1996; Hangarter, 1997; Janoudi et al., 1997a, 1997b; Whippo and Hangarter, 2004). However, the molecular mechanisms underlying the phytochrome-mediated regulation of phototropism remain largely unknown (Briggs, 2014).
Recent studies indicate that red light treatment, which activates phytochrome, induces several cellular events related to phototropic enhancement. Han et al. (2008) reported that red light pretreatment inhibits the blue light-dependent loss of phot1 from the plasma membrane. Nagashima et al. (2008a) showed that red light irradiation reduces the expression of ATP-BINDING CASSETTE subfamily B19 (ABCB19), which is an auxin efflux transporter known to be a negative regulator for phototropism (Noh et al., 2003; Nagashima et al., 2008b). Furthermore, ABCB19 is directly phosphorylated by phot1, resulting in the reduction of auxin transport (Christie et al., 2011). Kami et al. (2012) showed that the red light-induced nuclear import of phytochrome A is necessary for the acceleration of phototropic curvature rates by red light pretreatment in continuous light-induced phototropism. Although these cellular events may be required to establish phototropic enhancement, how they are associated with the enhancement has not been elucidated.
The PINOID (PID) family belongs to the AGC kinase family, which is a group of serine/threonine protein kinases sharing sequence similarity in their catalytic kinase domains with cAMP-dependent protein kinase A, cGMP-dependent protein kinase G, and phospholipid-dependent protein kinase C, and consists of four members (PID, WAVY ROOT GROWTH1 [WAG1], WAG2, and PID2) in Arabidopsis (Arabidopsis thaliana; Galván-Ampudia and Offringa, 2007). The PID family is known to be involved in the localization of auxin efflux carrier PIN-FORMED (PIN) proteins (Friml et al., 2004; Michniewicz et al., 2007; Huang et al., 2010). However, the functions of PID2 have not been well elucidated. Careful analysis with the pid single mutant showed that PID is partially involved in both the pulse-induced first positive phototropism and the time-dependent second positive phototropism (Haga and Sakai, 2012). Furthermore, the pid wag1 wag2 triple mutant showed partial impairment of the continuous light-induced second positive phototropism (Ding et al., 2011; Preuten et al., 2013). Therefore, the PID family seemed to be one of the key components for the regulation of phototropic responses.
Recently, it was proposed that phot-mediated transcriptional down-regulation of PID is involved in the asymmetrical distribution of PIN3 during continuous light-induced phototropism (Ding et al., 2011). However, it was reported that transcriptional down-regulation of the PID family is also induced by overhead red light treatment (Haga and Sakai, 2012). Furthermore, we also showed that PID participates in the phytochrome-mediated regulation of phototropism, because the pid single mutant showed partial impairment of phototropic enhancement by red light pretreatment (Haga and Sakai, 2012). Therefore, it is possible that the PID family functions in not only the phot-mediated regulation of the localization of PIN proteins but also the phytochrome-mediated regulation of phototropism.
In this study, we investigated hypocotyl phototropism in the pid quadruple mutant, which had not been used for phototropic analysis, using a precise physiological method (Haga and Sakai, 2012) to elucidate the physiological functions of the PID family in phototropic responses. Intriguingly, phytochrome-mediated phototropic enhancement was severely impaired in the pid quadruple mutant, indicating that the PID family is a critical component for phytochrome regulation. In addition, our analysis suggests that the PID family is not necessary for continuous light-induced phototropism, because the pid quadruple mutant showed nearly normal phototropism when induced by continuous blue light irradiation.
RESULTS
The PID Family Is Not Necessary for Continuous Light-Induced Second Positive Phototropism
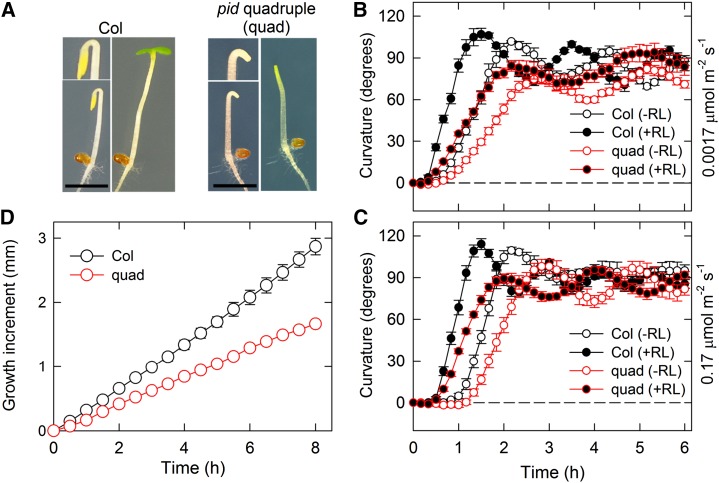
To examine a functional redundancy among the PID family members in hypocotyl phototropism of Arabidopsis, we prepared the pid pid2 wag1 wag2 quadruple mutant by crossing pid-14 (Salk_049736) with wag1 (Salk_002056C), wag2 (Salk_070240), and pid2 (Sail_269_G07) mutants. The genetic combination of the pid quadruple mutant that we generated was the same as that produced by Cheng et al. (2008). The pid quadruple mutant lacked cotyledons as reported previously (Cheng et al., 2008) but showed the apical hook formation under darkness and the hook opening under overhead white light without cotyledons (Fig. 1A). We investigated continuous light-induced second positive phototropism in the pid quadruple mutant using two different fluence rates of blue light: very low (0.0017 μmol m−2 s−1) and low (0.17 μmol m−2 s−1; Fig. 1, B and C). Although the steady-state levels of phototropic curvatures of the mutant were similar to those of the wild type, irrespective of fluence rates, curvature rates and the oscillation were slightly attenuated compared with the wild type (Fig. 1, B and C). The mutant showed a decrease in the hypocotyl growth rates (Fig. 1D), suggesting that its reduction is involved in the attenuation of phototropic curvature rates in the pid quadruple mutant. Overhead red light pretreatment for 2 min before 2 h of phototropic stimulation reduced the lag time to induce phototropic responses and accelerated phototropic curvature rates in wild-type hypocotyls (Fig. 1, B and C) as described previously (Hangarter, 1997; Haga and Sakai, 2012). Similar responses were also observed in the mutant (Fig. 1, B and C). These results indicated that the PID family does not play any significant role in continuous light-induced hypocotyl phototropism.
Figure 1.
Continuous light-induced second positive hypocotyl phototropism. A, Phenotypes of the pid-14 (Salk_049736) wag1 (Salk_002056C) wag2 (Salk_070240) pid2 (Sail_269_G07) quadruple (quad) mutant. Two-day-old dark-grown seedlings of Col-0 (left) and the quad mutant (right) are shown. The magnified images are also shown. The seedlings were incubated under continuous white light at 30 μmol m−2 s−1 for 18 h. The quad mutant lacked cotyledons. Bar = 2 mm. B and C, Hypocotyl phototropism in Col-0 and the quad mutant. Two-day-old dark-grown seedlings were pretreated with (black circles) or without (white circles) overhead red light (RL). After 2 h, the hypocotyls were stimulated with unilateral blue light at 0.0017 μmol m−2 s−1 (B) or 0.17 μmol m−2 s−1 (C) for 6 h. The hypocotyl curvatures were determined at 10-min intervals. The data shown are the means ± se from eight seedlings. D, Elongation growth of hypocotyls. Hypocotyl lengths of 2-d-old dark-grown Col-0 and quad seedlings were monitored for 8 h at 30-min intervals. The data shown are the means ± se from eight seedlings.
The PID Family Is Necessary for Phytochrome-Mediated Enhancement of Pulse-Induced First Positive Phototropism
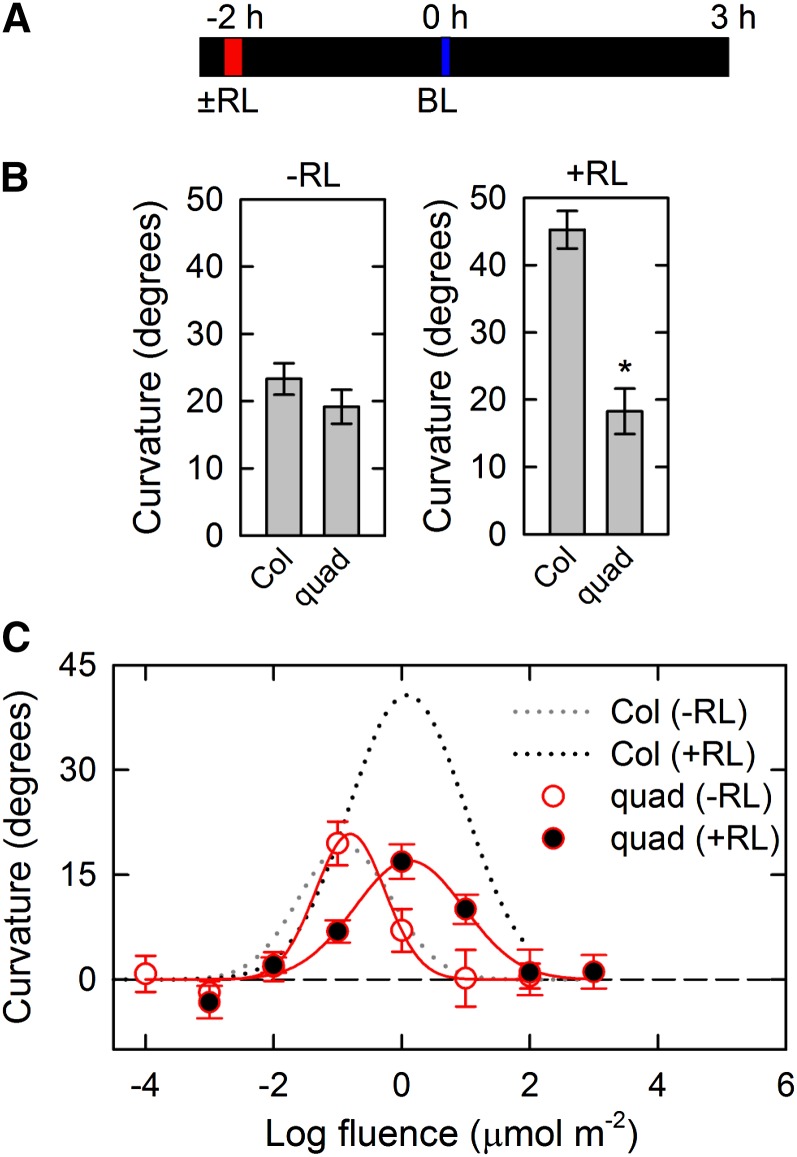
Next, we evaluated the roles of the PID family in pulse-induced first positive phototropism. Dark-grown seedlings were stimulated with unilateral blue light for 1 min at optimum total fluence (0.1 μmol m−2), and phototropic curvatures were determined 3 h after the onset of blue light irradiation (Haga and Sakai, 2012; Fig. 2A). Because oscillation responses observed in the continuous light-induced phototropism (Fig. 1) were not induced by a pulse of blue light (Haga and Sakai, 2012), time-course analysis was not conducted for the pulse-induced phototropism. Wild-type seedlings showed phototropic curvatures of more than 20°, and the pid quadruple mutant also exhibited similar phototropic curvatures (Fig. 2B, left). The effects of red light pretreatment were also examined in pulse-induced phototropism. Dark-grown seedlings were pretreated with overhead red light for 2 min before 2 h of phototropic stimulation, and the hypocotyls were then stimulated with unilateral blue light for 1 min at optimum total fluence (1 μmol m−2). Although red light pretreatment enhanced phototropic responses in the wild type, the pid quadruple mutant did not show such phototropic enhancement (Fig. 2B, right). Although the previous study had already reported that the pid single mutant exhibits a reduction of red light effects on pulse-induced phototropism (Haga and Sakai, 2012), the pid quadruple mutant showed the more severe phenotype in this response.
Figure 2.
Pulse-induced first positive hypocotyl phototropism in the pid quadruple (quad) mutant. A, Experimental scheme for pulse-induced phototropism. Two-day-old dark-grown seedlings were pretreated with or without overhead red light (RL). After 2 h, the hypocotyls were stimulated with unilateral blue light (BL) for 60 s. The hypocotyl curvatures were determined 3 h after the onset of BL. B, Effects of RL pretreatment. The dark-grown seedlings were stimulated with unilateral BL at 0.0017 μmol m−2 s−1 (left) or 0.017 μmol m−2 s−1 (right) for 60 s. The hypocotyl curvatures were determined 3 h after the onset of BL. The data shown are the means ± se from 11 to 16 seedlings. *, Statistically significant difference between the wild type and quad mutant (Student’s t test, P < 0.001). C, Fluence-response curves of pulse-induced phototropism. Hypocotyls were stimulated with unilateral BL at various fluences with (black symbols) or without (gray symbols) RL pretreatment. The data shown are the means ± se from 16 seedlings. The fitted curves obtained from Col-0 data (Haga and Sakai, 2012) were reproduced as dotted lines for comparison.
To better understand the roles of the PID family in the enhancement of phototropic responses, we next analyzed the fluence-response relationship of pulse-induced phototropism with or without red light pretreatment (Fig. 2C). When red light pretreatment was not used, the fluence-response curve in the pid quadruple mutant was very similar to that in the wild type. It is known that red light pretreatment causes two major alterations of fluence-response curves: enhancement of phototropic curvatures and a shift in the optimum total fluence of blue light to higher fluences (so-called desensitization; Liu and Iino, 1996a; Haga and Sakai, 2012). Although the optimum total fluence was shifted to higher fluences of blue light in the pid quadruple mutant, the enhancement of phototropic curvatures was severely impaired in the mutant (Fig. 2C). Therefore, these results indicated that the PID family plays a significant role in phytochrome-mediated enhancement of pulse-induced phototropism but not in the phot signaling pathway.
The PID Family Is Required for Phytochrome-Mediated Enhancement of Time-Dependent Second Positive Phototropism
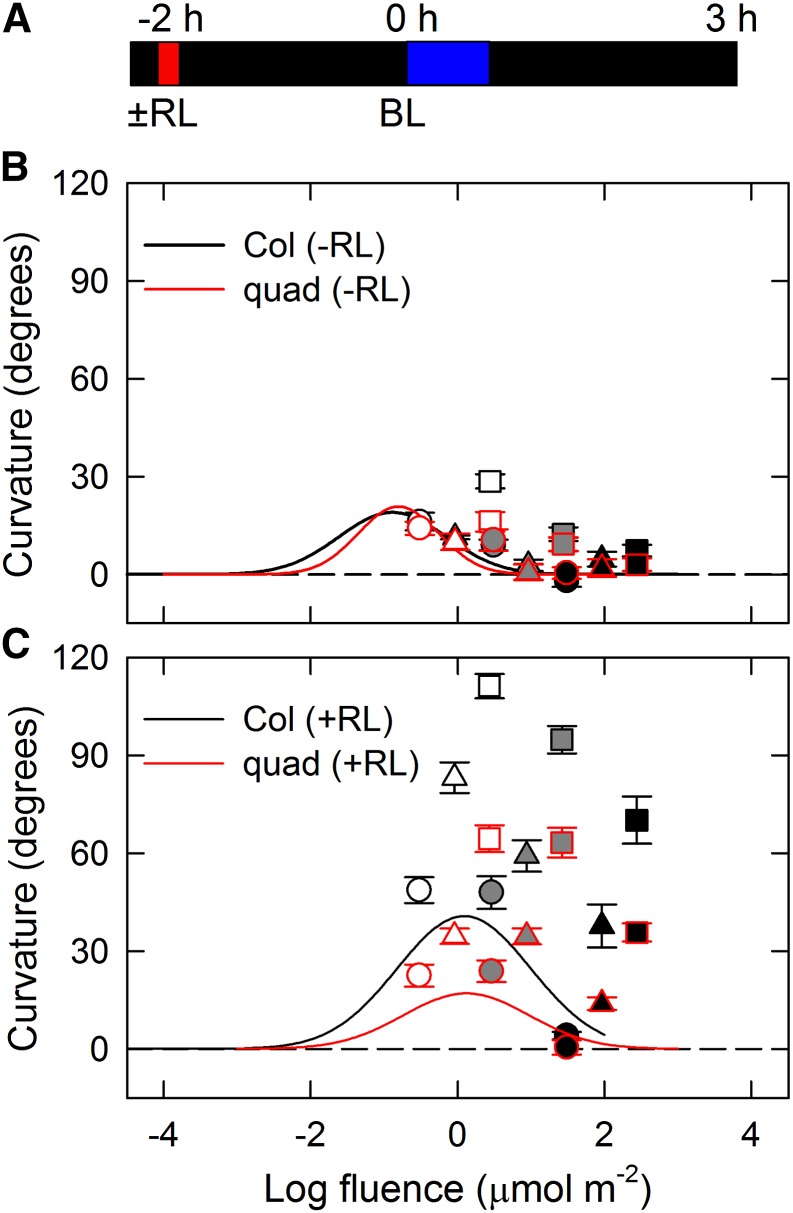
We subsequently investigated time-dependent second positive phototropism (Fig. 3). Without red light pretreatment, phototropic curvatures deviated slightly from the fluence-response curve of pulse-induced phototropism after 27 min of irradiation but not after 9 min of irradiation in the wild type (Fig. 3B). However, the red light pretreatment significantly promoted the deviation in phototropic responses, and 9 min of irradiation were enough for second positive phototropism to appear (Fig. 3C). The results indicated that red light pretreatment, presumably through phytochromes, is necessary for the induction of time-dependent phototropism as reported previously (Janoudi et al., 1992; Liu and Iino, 1996b; Haga and Sakai, 2012). The pid quadruple mutant showed obvious responses of time-dependent phototropism with red light pretreatment and no clear responses without red light pretreatment similar to wild-type response, although the magnitudes of the curvature responses were partially impaired in the mutant (Fig. 3, B and C). Therefore, the results indicate that the PID family is necessary for amplification of time-dependent phototropic responses by red light pretreatment and that the occurrence of phototropic response itself is independent of PID function.
Figure 3.
Fluence-response relationship of time-dependent second positive phototropism in the pid quadruple (quad) mutant. A, Experimental scheme for time-dependent phototropism. Dark-grown seedlings were pretreated with or without red light (RL), and the hypocotyls were irradiated with unilateral blue light (BL) at fixed fluence rates for 3 to 27 min. Phototropic curvatures were determined 3 h after the onset of BL. B, Time-dependent phototropism without RL pretreatment. The hypocotyls were irradiated with unilateral BL at fixed fluence rates (white symbols, 0.0017 μmol m−2 s−1; gray symbols, 0.017 μmol m−2 s−1; and black symbols, 0.17 μmol m−2 s−1) for 3 (circles), 9 (triangles), and 27 (squares) min. Black-edged symbols show data obtained from Col-0 seedlings, and red-edged symbols show data obtained from the quad mutant. The data shown are the means ± se from 11 to 16 seedlings. The solid lines represent the fitted curves obtained from Figure 2B. C, Effects of RL pretreatment on time-dependent phototropism. Other details are as described above.
Constitutive Expression of PID Attenuates Hypocotyl Phototropism
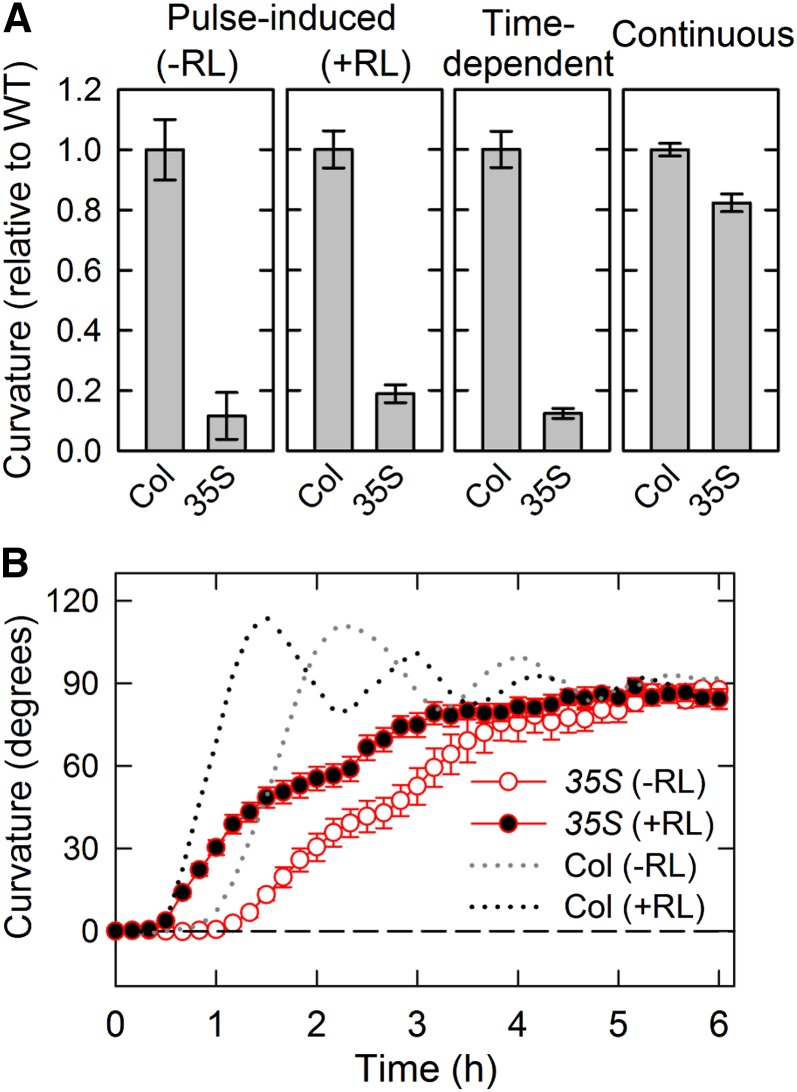
We also analyzed the effects of constitutive expression of PID on hypocotyl phototropism using transgenic plants harboring the cauliflower mosaic virus 35S promoter-driven PID complementary DNA gene (35S::PID). Pulse-induced phototropism and time-dependent phototropism were severely impaired in the transgenic plants, whereas continuous light-induced phototropism was slightly attenuated (Fig. 4A). Time-course analysis of continuous light-induced phototropism indicated that phototropic curvature rates were reduced and oscillation responses were severely impaired in the transgenic plants, although the steady-state levels of phototropic curvatures were very similar to those of the wild type (Fig. 4B). The results suggest that excessive amounts of PID proteins prevent phototropic responses, especially pulse-induced and time-dependent phototropisms.
Figure 4.
Effects of constitutive expression of PID on hypocotyl phototropism. Dark-grown transgenic plants harboring the cauliflower mosaic virus 35S promoter driven PID gene (35S::PID) were pretreated with or without red light (RL), and the hypocotyls were stimulated with unilateral blue light (BL). A, Hypocotyl phototropism of the transgenic plant. For pulse-induced phototropism with or without RL pretreatment, hypocotyls were stimulated with BL at optimal fluence (Fig. 2). For time-dependent phototropism, hypocotyls were stimulated with BL at 0.17 μmol m−2 s−1 for 27 min. For phototropism induced by continuous irradiation, seedlings were irradiated with BL at 0.17 μmol m−2 s−1 for 3 h. Phototropic curvatures were determined 3 h after the onset of stimulation. The data shown as relative values to the wild type (WT) are the means ± se from 15 to 16 seedlings. B, Time course of continuous light-induced phototropism. Dark-grown transgenic plants were pretreated with (black circles) or without (white circles) RL, and the hypocotyls were stimulated with unilateral BL at 0.17 μmol m−2 s−1 for 6 h. The hypocotyl curvatures were determined at 10-min intervals. The data shown are the means ± se from eight seedlings. The phototropic responses of Col-0 seedlings obtained from Figure 1C are reproduced as dotted lines for comparison.
Phytochrome Reduces PID Protein Levels
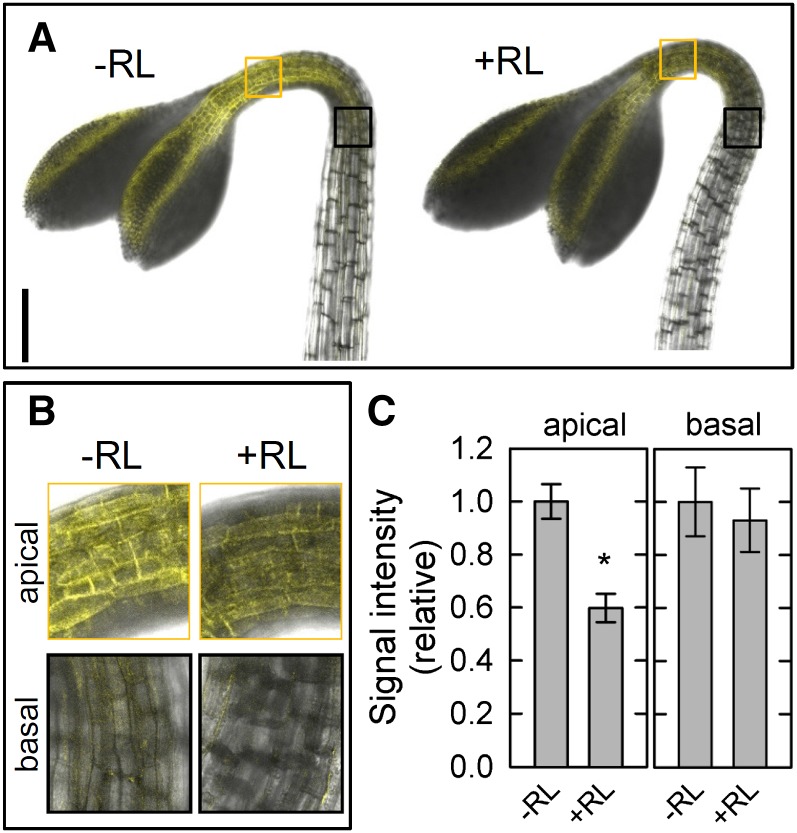
Although this study clearly showed that the PID family has a critical role in phytochrome-mediated enhancement of phototropic responses, it remains unclear how PID kinases are involved in the enhancement. Because it has been reported that red light treatment reduces the transcriptional levels of PID genes in Arabidopsis hypocotyls (Haga and Sakai, 2012), such regulation may participate in the enhancement. To investigate this possibility, we examined the effects of red light pretreatment on the expression of PID using a yellow fluorescent protein (YFP) VENUS (Fig. 5). When dark-grown transgenic plants harboring the VENUS-fused PID gene driven by its own promoter (PID::PID-VENUS) were not treated with overhead red light, the fluorescent signals derived from PID-VENUS were detected in cell peripheral regions of the epidermis around the apical shoots but not in the hypocotyls (Fig. 5, A and B). This suggests that PID proteins are localized at the plasma membrane of epidermal cells as described previously (Michniewicz et al., 2007; Kleine-Vehn et al., 2009) and that PID functions mainly in the apical parts, at least in dark-grown seedlings. Therefore, we focused on the apical regions for additional analysis. Red light treatment reduced the fluorescent signals significantly 2 h after the treatment (Fig. 5C), whereas clear attenuation was not observed at an earlier time, such as 15 min after the pretreatment (data not shown). The results support the previous view that transcriptional levels of PID are attenuated by red light irradiation (Haga and Sakai, 2012). This suggests that PID protein levels are also reduced through transcriptional down-regulation of PID. Therefore, we concluded that a reduction of PID protein levels is probably involved in phytochrome-mediated phototropic enhancement.
Figure 5.
Effects of red light (RL) treatment on changes in levels of PID proteins. Dark-grown transgenic plants harboring PID::PID-VENUS (Michniewicz et al., 2007) were pretreated with or without RL. After 2 h, VENUS signals were observed around the apical shoots. A, Representative pictures of the apical part in the transgenic plants. Bar = 200 μm. B, Magnified images of the apical region (orange squares) and the basal region (black squares) shown in A. C, Quantification of fluorescent signals in the transgenic plants. The VENUS signals were measured at the apical and basal parts with or without RL pretreatment. The data shown as relative values to averaged data obtained from seedlings without RL pretreatment are the means ± se from seven to eight seedlings. *, Statistically significant difference (Student’s t test, P < 0.001).
Phytochrome Enhances Expression Levels of PIN Proteins
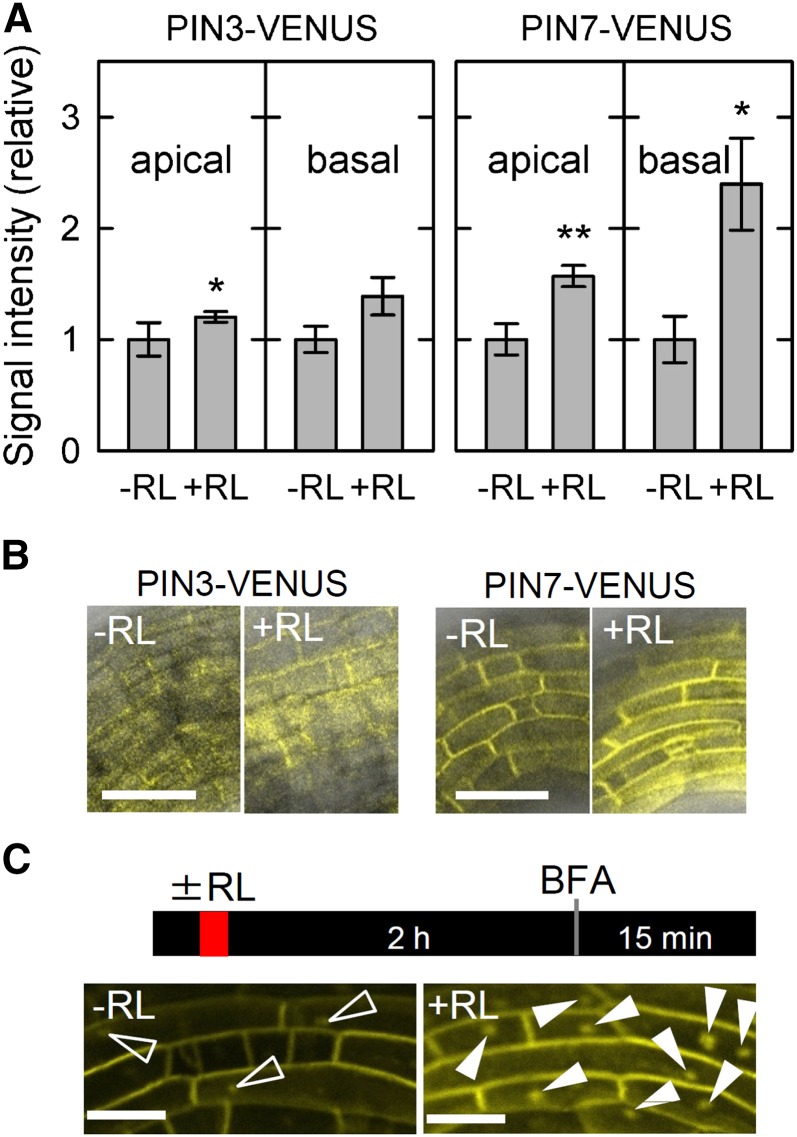
Previously, we reported that red light pretreatment enhances transcriptional levels of the PIN family (Haga and Sakai, 2012), suggesting that accumulation of PIN proteins is induced by the pretreatment. To investigate the possibility, we generated the transgenic pin3 and pin7 mutants harboring PIN3::PIN3-VENUS and PIN7::PIN7-VENUS (the VENUS-fused PIN gene driven by its own promoter), respectively, and examined the effects of red light treatment (Fig. 6). When expression patterns of PIN-VENUS were observed around the apical hook regions where the expression level of PID-VENUS (Fig. 5) was relatively high, the light treatment enhanced the fluorescent signals derived from PIN-VENUS, and the enhancement was more significant in the pin7 transgenic plants harboring PIN7::PIN7-VENUS (Fig. 6A). The promotional effects were also observed in the hypocotyls just below the hook regions. However, both PIN3-VENUS and PIN7-VENUS did not show clear polarity, and the red light treatment did not alter the distribution of the fluorescence (Fig. 6B). The results indicate that red light pretreatment enhances protein levels of the PIN family, whereas it does not affect the distribution of PIN proteins.
Figure 6.
Effects of red light (RL) treatment on PIN proteins. Dark-grown transgenic pin3 mutant harboring PIN3::PIN3-VENUS and the pin7 mutant harboring PIN7::PIN7-VENUS were pretreated with or without RL. The fluorescent signals were analyzed in the apical shoot regions similar to Figure 5. A, Quantification of fluorescent signals in the transgenic plants. The VENUS signals were measured at the apical parts 2 h after RL treatment. The data shown as relative values to averaged data obtained from seedlings without RL pretreatment are the means ± se from 10 to 11 seedlings. *, Statistically significant difference (Student’s t test, P < 0.05); **, Statistically significant difference (Student’s t test, P < 0.01). B, Effects of RL treatment on the distribution of fluorescent signals in the transgenic plants. Representative pictures of the apical part in the transgenic plants are shown. Bar = 50 μm. C, Effects of RL treatment on formation of the BFA compartment. After 2 h of RL treatment, the pin7 transgenic plants were incubated into the growth medium containing BFA at 250 μm for 15 min. White arrowheads indicate BFA compartments. Bar = 25 μm.
PIN proteins are known to undergo constitutive cycles of clathrin-mediated endocytosis and recycling to the plasma membrane through a brefeldin A (BFA) -sensitive exocytosis pathway or a BFA-independent pathway (Dhonukshe et al., 2007; Kleine-Vehn et al., 2009). BFA is a fungal toxin that inhibits a subset of guanine nucleotide exchange factors for ADP-ribosylation factors, including GNOM, and its treatment inhibits PIN recycling and leads to PIN accumulation into so-called BFA compartments (Steinmann et al., 1999; Geldner et al., 2001). Because the PID-dependent PIN phosphorylation decreases affinity of PIN proteins for the BFA-sensitive exocytosis pathway (Kleine-Vehn et al., 2009), down-regulation of PID by red light pretreatment seemed to suppress PID-dependent PIN phosphorylation and stimulate PIN accumulation into BFA compartments under BFA treatment conditions. When dark-grown pin7 transgenic plants harboring PIN7::PIN7-VENUS were treated with the inhibitor, VENUS signals were mainly observed around cell peripheral regions, and intracellular BFA compartments were rarely observed (Fig. 6C). However, red light treatment enhanced the accumulation of PIN7-VENUS into BFA compartments significantly (Fig. 6C). Although it is possible that reduction of PIN proteins by red light treatment influences formation of BFA compartments, the results suggested that red light irradiation increases the population of PIN proteins, which are not being phosphorylated by PID and are recycled to the plasma membrane through a BFA-sensitive exocytosis pathway.
The PID Family Is Necessary for the Promotion of the Auxin Gradient during the Enhanced Pulse-Induced Phototropism
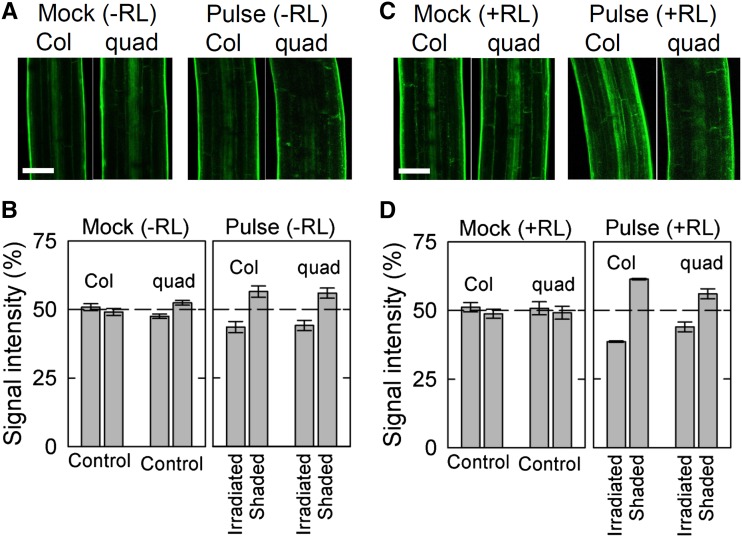
We also examined the distribution of auxin during pulse-induced phototropism in the pid quadruple mutant. Recently, a fluorescent auxin analog (7-nitro-2,1,3-benzoxadiazole [NBD] -conjugated naphthalene-1-acetic acid [NAA]) has been developed to visualize auxin distribution (Hayashi et al., 2014). When dark-grown seedlings were treated with NBD-NAA, fluorescent signals were evenly distributed in the epidermis of hypocotyls of both the wild type and the pid quadruple mutant without phototropic stimulation (Fig. 7, A, left and B, left). When the seedlings were stimulated with a pulse of blue light at an optimum total fluence, fluorescent signals on the shaded side were stronger than those on the irradiated side in both the wild type and the mutant (Fig. 7, A, right and B, right). The uneven distribution of the fluorescent signals observed in the wild type was very similar to that in the wild type harboring DR5rev::GFP as previously reported (Haga and Sakai, 2012). The results strongly suggested that gradients of the fluorescent signals reflect an asymmetrical distribution of auxin. However, red light pretreatment enhanced the uneven distribution of fluorescent signals in the wild type but not in the pid quadruple mutant (Fig. 7, C and D). The results suggest that phytochrome-mediated promotion of the auxin gradient is specifically impaired in the pid quadruple mutant.
Figure 7.
Effects of red light (RL) treatment on the distribution of auxin during pulse-induced phototropism in the pid quadruple (quad) mutant using a fluorescent auxin analog. Dark-grown seedlings pretreated with or without RL were irradiated with a pulse of blue light (BL). After 2.5 h, the seedlings were incubated into growth medium containing a fluorescent auxin analog (NBD-NAA; Hayashi et al., 2014) at 5 μm for 15 min. The fluorescent signals derived from NBD-NAA were then quantified. A, Representative confocal micrographs during pulse-induced hypocotyl phototropism. Seedlings were stimulated with or without a pulse of BL from the left side at the optimum total fluence (Fig. 2). The images were obtained around 3 h after stimulation. Bar = 100 μm. B, Distribution of fluorescence derived from NBD-NAA between the irradiated and shaded sides of the hypocotyls during pulse-induced phototropism. The distribution was calculated as the percentage of the signal intensity obtained from the two sides. The data shown are the means ± se from nine seedlings. C, Representative confocal micrographs during the enhanced pulse-induced phototropism by RL pretreatment. Seedlings pretreated with RL were stimulated with or without a pulse of BL from the left side at the optimum total fluence (Fig. 2). Other details are described above. D, Distribution of fluorescence derived from NBD-NAA between the irradiated and shaded sides of the hypocotyls during the enhanced pulse-induced phototropism. The data shown are the means ± se from nine seedlings.
DISCUSSION
It is well known that phytochrome affects many aspects of phot-mediated phototropic responses in plants. Major aspects of phytochrome regulation are phototropic adaptation and enhancement (Iino, 2001; Whippo and Hangarter, 2006; Briggs, 2014). Our previous study suggested the partial involvement of PID in the phytochrome-mediated enhancement of phototropism (Haga and Sakai, 2012). This study extends the previous results, showing that phototropic enhancement is dependent totally on the PID family, especially in pulse-induced first positive phototropism (Fig. 2). In addition, the promotive effect of red light pretreatment on the auxin gradient was severely impaired in the pid quadruple mutant (Fig. 7). It has been reported that PIN proteins are necessary for the asymmetrical distribution of auxin during pulse-induced phototropism (Haga and Sakai, 2012). Furthermore, it is known that PID kinases regulate the functions of PIN proteins through changes in their phosphorylation status (Friml et al., 2004; Michniewicz et al., 2007; Huang et al., 2010). Therefore, it seems that the PID family is involved in phytochrome-mediated phototropic enhancement through regulation of the phosphorylation status of PIN proteins.
Red light treatment reduced the levels of PID proteins largely (Fig. 5) because of the transcriptional down-regulation of the PID family (Haga and Sakai, 2012), although protein turnover may be also involved. However, red light enhanced the accumulation of PIN proteins into BFA compartments (Fig. 6C). Because it has been reported that nonphosphorylated PIN proteins are transported to the plasma membrane preferentially through a GNOM-dependent exocytosis pathway that is severely impaired by BFA treatment (Kleine-Vehn et al., 2009), it is most likely that reduced levels of PID proteins increase the population of nonphosphorylated PIN proteins when dark-grown seedlings are stimulated with red light. Therefore, it is possible that nonphosphorylated PIN proteins are more sensitive to the phot-mediated regulation of auxin transport activity and/or subcellular localization than phosphorylated PIN proteins (Fig. 8), resulting in enhancement of the auxin gradient. In addition, PID-mediated regulation of auxin transport activity of PIN proteins may be also involved in the enhancement of phototropic responses directly or indirectly, because a recent study has indicated that PID regulates not only the subcellular localization but also the auxin transport activity of PIN proteins (Zourelidou et al., 2014). It should be noted, however, that both the pid quadruple mutant and the PID overexpression line exhibit an impairment of phototropic curvatures. It suggests that the PID family not only negatively but also positively functions in the phototropic responses. The balance of PID levels and/or function of PID under darkness may be necessary for expression of the phytochrome-mediated phototropic enhancement.
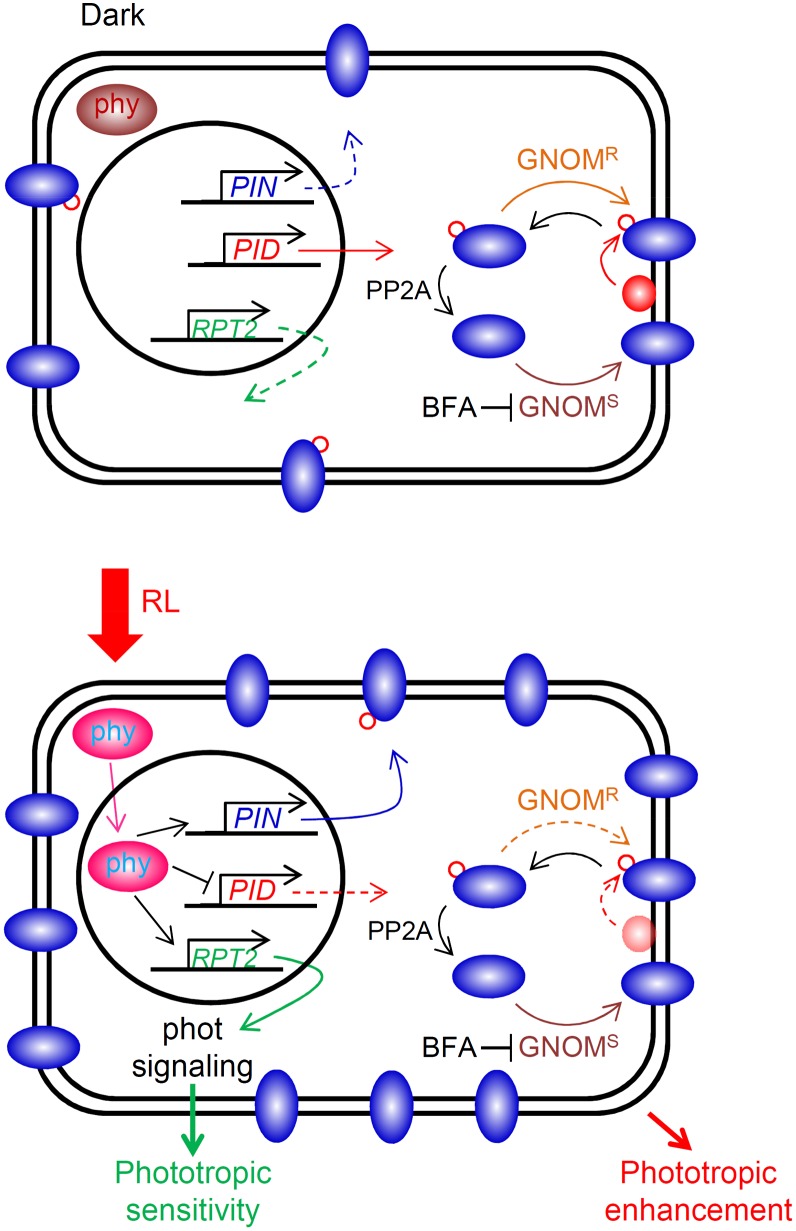
Figure 8.
Cellular events underlying phytochrome (phy)-mediated phototropic enhancement in the apical regions of the shoots. Under dark conditions (top), phytochromes localize at the cytosol. Therefore, transcriptional up-regulation of the PIN family does not appear, resulting in a relatively lower abundance of membrane-localized PIN proteins. When irradiated with red light (RL; bottom), activated phytochromes (i.e. phytochrome far red-absorbing forms) move to the nuclear membrane and promote transcript levels of the PIN family, resulting in increases in PIN proteins at the plasma membrane. Furthermore, the expression levels of the PID family are reduced by RL treatment. Under such conditions, it is expected that a population of nonphosphorylated PIN proteins is increased at the plasma membrane through a GNOM-sensitive (GNOMS) pathway rather than a GNOM-resistant (GNOMR) pathway. However, it is expected that RPT2 is the most likely candidate involved in phytochrome-mediated regulation of phototropic sensitivity, because the rpt2 mutant can respond to only limited ranges of light fluence rates and the transcriptional levels are up-regulated by light treatment, including RL (Sakai et al., 2000). This model proposes molecular events just before phototropic stimulation, because this study focused on the molecular mechanisms involved in phototropic enhancement by RL pretreatment. Blue elliptical shapes indicate PIN proteins, and red circles show PID proteins. Red-outlined circles indicate phosphorylation of PIN proteins. PP2A, Protein phosphatase 2A.
Because red light treatment enhanced protein levels of the PIN family (Fig. 6), phytochrome-mediated phototropic enhancement may result from the promotion of PIN activity through transcriptional up-regulation of the PIN family (Haga and Sakai, 2012) in addition to the emergence of greater asymmetry of PIN activity and/or the localization by down-regulation of the PID family (Fig. 8). Because it has been reported that PID functions as a negative regulator for auxin transport activity of PIN proteins interacting with ABCB1 proteins (Wang et al., 2012), it seems that such a regulatory mechanism also participates in phototropic enhancement. Additional studies are necessary to clarify the molecular mechanisms underlying phytochrome-mediated phototropic enhancement.
This study indicates that the PID family is not required for phytochrome-mediated phototropic adaptation (desensitization). Our previous study suggested that ROOT PHOTOTROPISM2 (RPT2), a phot adaptor protein, is the most likely candidate for photosensory adaptation in phototropic responses (Fig. 8; Sakai et al., 2000). This is because the rpt2 mutant is responding to a very limited range of light fluence rates, and the transcriptional levels of RPT2 are up-regulated by light treatment (Sakai et al., 2000). However, it has been proposed that phytochrome-mediated stabilization of the membrane localization of phot is involved in phytochrome-mediated phototropic regulation. This is because red light pretreatment prevents blue light-induced internalization of phot proteins from the plasma membrane (Han et al., 2008). However, blue light irradiation at lower fluences, which is still effective in inducing hypocotyl phototropism in Arabidopsis, could not attenuate the membrane localization of phot proteins significantly (Supplemental Fig. S1; Supplemental Methods S1). Therefore, it seems that phytochrome-mediated stabilization of phot membrane localization functions in phototropic responses induced by higher intensities of blue light (e.g. 20 μmol m−2 s−1) but not by lower intensities of the light.
In dark-grown seedlings, PID proteins were mainly localized at the epidermal cell peripheral region of shoot apical parts (Fig. 5). Very recently, it has been reported that the upper parts of Arabidopsis shoots are very important for the perception of phototropic signals and in the production of auxin asymmetrical distribution (Preuten et al., 2013; Yamamoto et al., 2014). Furthermore, severe impairment of phototropic responses in the phot1 mutant was complemented by the expression of phot1 in epidermal cells (Preuten et al., 2013). Taken together with this study, critical events involved in hypocotyl phototropism may occur at the most apical epidermal cells in Arabidopsis.
The pid quadruple mutant showed that its apical hook opens slightly when 2-d-old dark-grown seedlings were observed (Fig. 1A). It has been reported that an open-hook phenotype appears in 3- and 4-d-old wag2 single mutants but not in 2-d-old seedlings (Willige et al., 2012). Therefore, it is possible that the PID family is involved in a negative regulation of hook opening. However, the hook appeared to open evenly in the pid quadruple mutant when the dark-grown seedlings were incubated under overhead white light (Fig. 1A). The result indicates that the PID family is not necessary for light-inducible hook-opening responses.
During continuous light-induced phototropism, it is proposed for the establishment of the auxin gradient that phot-mediated transcriptional down-regulation of the PID gene is an important mechanism for producing the asymmetrical localization of PIN3 (Ding et al., 2011). To support the proposed model, the pid triple mutant showed defects of continuous light-induced phototropism (Ding et al., 2011). However, the pid quadruple mutant exhibited attenuation of growth rate, causing partial impairment of phototropic curvature rates in the continuous light-induced phototropism (Fig. 1). Furthermore, pulse-induced phototropism was not impaired in the pid quadruple mutant when the seedlings were not pretreated with red light (Fig. 2). Therefore, we concluded that the PID family members are not critical components for phot-mediated phototropic responses. That is, the proposed model for establishing auxin asymmetry by transcriptional down-regulation of PID is unlikely a main pathway, and another regulatory mechanism seems to be involved in the production of the auxin gradient during continuous light-induced phototropism. Recently, Willige et al. (2013) reported that D6 PROTEIN KINASE (D6PK) participates in the phototropism of Arabidopsis hypocotyls. The D6PK family belongs to the AGC kinase family and phosphorylates PIN proteins to regulate auxin transport activity (Zourelidou et al., 2009). Furthermore, continuous light-induced phototropism was severely impaired in the d6pk multiple mutant (Willige et al., 2013), indicating that the D6PK family is required to establish the auxin gradient through regulation of PIN activity. Precise physiological analysis will be necessary to clarify the functional roles of the D6PK family in phototropic responses in the near future.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) seeds of pid-14 (Salk_049736), wag1 (Salk_002056C), wag2 (Salk_070240), and pid2 (Sail_269_G07) mutants (Columbia-0 [Col-0] background) were obtained from the Arabidopsis Biological Resource Center. The pid quadruple mutant was produced by crossing the above mutants and genotyped using the primer sets listed in Supplemental Table S1. Because the pid null mutant was completely sterile (Bennett et al., 1995), PID/pid heterozygous wag1 wag2 pid2 homozygous seedlings were prepared. Seedlings lacking cotyledons were selected for analysis, because all of the mutant seedlings showing such phenotypes were the pid wag1 wag2 pid2 quadruple mutant, although we confirmed the genotypes of the pid homozygous plants after the experiments; 35S::PID and PID::PID-VENUS transgenic plants were obtained from Remko Offringa (Benjamins et al., 2001) and Marcus Heisler (Michniewicz et al., 2007), respectively. For cloning of PIN3::PIN3-YFP and PIN7::PIN7-YFP, the inserts were amplified by PCR (Supplemental Table S2) and cloned as follows. The 5′ region of PIN3 genomic DNA (from −2.0 to +0.7 kb from the start of transcription) and the 5′ region of PIN7 genomic DNA (from −2.1 to +0.8 kb from the start of transcription) were cloned in pDONR221P1-P4 (Life Technologies). The 3′ region of PIN3 genomic DNA (from +0.7 kb to the 3′ untranslated region) and the 3′ region of PIN7 genomic DNA (from +0.8 kb to the 3′ untranslated region) were cloned in pDONR221P3-P2 (Life Technologies). The VENUS complementary DNAs were cloned in pDONR221P4r-P3r (Life Technologies). The three different pDONR constructs were recombined with pDEST17 by Multi-Gateway LR reaction (Life Technologies) to obtain PIN3::PIN3-VENUS and PIN7::PIN7-VENUS. These fusion genes were subcloned into pDONR/ZEO by BP reaction (Life Technologies) and then subcloned into binary vector pMDC99 by LR reaction. pMDC99 was obtained from the Arabidopsis Biological Resource Center. By Agrobacterium tumefaciens-mediated transformation (Clough and Bent, 1998), PIN3::PIN3-VENUS and PIN7::PIN7-VENUS constructs were transformed into pin3-4 and pin7 (Salk_044687) mutants, respectively. All mutant and transgenic plants were from a Col-0 background.
Etiolated Arabidopsis seedlings were prepared as described previously (Haga and Sakai, 2012). Briefly, seeds of Arabidopsis were sown in 0.2-mL plastic tubes filled with 1.5% (w/v) agar medium (Sakai et al., 2000), placed in a black plastic box, and kept at 4°C for 3 to 5 d. After the induction of germination, the prepared seeds were incubated for 2 d under complete darkness. Seedlings were selected by hypocotyl length (3–5 mm) and kept in the black plastic box during the experiments to maintain high humidity until required for treatments. Experimental manipulations were carried out under dim green light.
Induction of Phototropism
For phototropic stimulation, selected seedlings were irradiated using a blue light-emitting diode light source (470 ± 30 nm; LED-B; Eyela) through two layers of a blue filter (no. 72 film; Tokyo Butai Shomei). The fluence rate was controlled with neutral density plastic filters (Fujifilm) and by changing the distance from the light source to the seedlings. The direction of phototropic stimulation was perpendicular to the plane of the hook (Haga and Sakai, 2012). For pretreatment with red light, the seedlings were irradiated with overhead red light (660 ± 20 nm; LED-R; Eyela) at 20 μmol m−2 s−1 for 2 min.
Measurement of Curvature and Growth
Images of dark-grown seedlings were recorded just before phototropic stimulation and at 3 h after the onset of stimulation with a digital camera (D5000; Nikon), from which a UV/IR light cut filter was removed (ICAS Enterprises IDAS Division) under IR illumination (IRDR-110; Nissen Electronics). For time-course experiments, images of the seedlings were captured at 10-min intervals using the same equipment. The angles and lengths of the hypocotyls were measured with an e-Ruler (Haga and Sakai, 2012).
Chemical Treatment
BFA was purchased from Nacalai-Tesque, and a fluorescent auxin analog, NBD-NAA, was prepared as described previously (Hayashi et al., 2014). Both chemicals were dissolved with dimethyl sulfoxide. Two hours after red light treatment, the dark-grown pin7 transgenic plants harboring PIN7::PIN7-VENUS were incubated in the growth medium containing BFA at 200 μm for 15 min. For the fluorescent auxin analog, the dark-grown wild type and the pid quadruple mutant were incubated in the growth medium containing NBD-NAA at 5 μm for 15 min.
Laser-Scanning Confocal Microscopy
Fluorescent signals were detected with a Leica TCS-SP5 confocal laser-scanning microscope (Leica Microsystems). VENUS signals were excited with an argon laser at 514 nm, and the spectral detector was set at 525 to 560 nm. The fluorescent signals derived from NBD-NAA were excited at 488 nm, and the spectral detector was set at 500 to 530 nm. GFP signals were detected as described previously (Haga and Sakai, 2012). All scans were carried out at a 2,048 × 2,048-pixels resolution with repeated scanning of two lines.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Effects of blue light irradiation on localization of PHOT1 proteins.
Supplemental Table S1. Gene-specific primers used for genotyping.
Supplemental Table S2. Primers used for cloning of PIN3::PIN3-YFP and PIN7::PIN7-YFP.
Supplemental Methods S1. Cloning of 35S::PHOT1-GFP.
Supplementary Material
Acknowledgments
We thank the Arabidopsis Biological Resource Center for providing the pid-14 (Salk_049736), wag1 (Salk_002056C), wag2 (Salk_070240), and pid2 (Sail_269_G07) mutants and the pMDC99 vector and Remko Offringa (Leiden University), Marcus Heisler (European Molecular Biology Laboratory), and Dr. Atsushi Miyawaki (RIKEN Brain Science Institute) for providing the 35S::PID seeds, PID::PID-VENUS seeds, and the VENUS complementary DNA, respectively.
Glossary
- BFA
brefeldin A
- Col-0
Columbia-0
- NAA
naphthalene-1-acetic acid
- NBD
7-nitro-2,1,3-benzoxadiazole
Footnotes
This work was supported by the Japan Society for the Promotion of Science KAKENHI (grant no. 22570058 to T.S.), KAKENHI (Grant-in-Aid for Challenging Exploratory Research no. 24657027 to K.H.), and the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Grant-in-Aid for Scientific Research on Innovative Areas “Plant Environmental Sensing” no. 23120510 to T.S.).
The online version of this article contains Web-only data.
Articles can be viewed online without a subscription.
References
- Benjamins R, Quint A, Weijers D, Hooykaas P, Offringa R. (2001) The PINOID protein kinase regulates organ development in Arabidopsis by enhancing polar auxin transport. Development 128: 4057–4067 [DOI] [PubMed] [Google Scholar]
- Bennett SRM, Alvarez J, Bossinger G, Smyth DR. (1995) Morphogenesis in pinoid mutants of Arabidopsis thaliana. Plant J 8: 505–520 [Google Scholar]
- Briggs WR. (2014) Phototropism: some history, some puzzles, and a look ahead. Plant Physiol 164: 13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Qin G, Dai X, Zhao Y. (2008) NPY genes and AGC kinases define two key steps in auxin-mediated organogenesis in Arabidopsis. Proc Natl Acad Sci USA 105: 21017–21022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie JM, Yang H, Richter GL, Sullivan S, Thomson CE, Lin J, Titapiwatanakun B, Ennis M, Kaiserli E, Lee OR, et al. (2011) phot1 inhibition of ABCB19 primes lateral auxin fluxes in the shoot apex required for phototropism. PLoS Biol 9: e1001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Dhonukshe P, Aniento F, Hwang I, Robinson DG, Mravec J, Stierhof YD, Friml J. (2007) Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. Curr Biol 17: 520–527 [DOI] [PubMed] [Google Scholar]
- Ding Z, Galván-Ampudia CS, Demarsy E, Łangowski Ł, Kleine-Vehn J, Fan Y, Morita MT, Tasaka M, Fankhauser C, Offringa R, et al. (2011) Light-mediated polarization of the PIN3 auxin transporter for the phototropic response in Arabidopsis. Nat Cell Biol 13: 447–452 [DOI] [PubMed] [Google Scholar]
- Friml J, Yang X, Michniewicz M, Weijers D, Quint A, Tietz O, Benjamins R, Ouwerkerk PBF, Ljung K, Sandberg G, et al. (2004) A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science 306: 862–865 [DOI] [PubMed] [Google Scholar]
- Galván-Ampudia CS, Offringa R. (2007) Plant evolution: AGC kinases tell the auxin tale. Trends Plant Sci 12: 541–547 [DOI] [PubMed] [Google Scholar]
- Geldner N, Friml J, Stierhof YD, Jürgens G, Palme K. (2001) Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413: 425–428 [DOI] [PubMed] [Google Scholar]
- Haga K, Sakai T. (2012) PIN auxin efflux carriers are necessary for pulse-induced but not continuous light-induced phototropism in Arabidopsis. Plant Physiol 160: 763–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han IS, Tseng TS, Eisinger W, Briggs WR. (2008) Phytochrome A regulates the intracellular distribution of phototropin 1-green fluorescent protein in Arabidopsis thaliana. Plant Cell 20: 2835–2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangarter RP. (1997) Gravity, light and plant form. Plant Cell Environ 20: 796–800 [DOI] [PubMed] [Google Scholar]
- Hayashi K, Nakamura S, Fukunaga S, Nishimura T, Jenness MK, Murphy AS, Motose H, Nozaki H, Furutani M, Aoyama T. (2014) Auxin transport sites are visualized in planta using fluorescent auxin analogs. Proc Natl Acad Sci USA 111: 11557–11562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Zago MK, Abas L, van Marion A, Galván-Ampudia CS, Offringa R. (2010) Phosphorylation of conserved PIN motifs directs Arabidopsis PIN1 polarity and auxin transport. Plant Cell 22: 1129–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M. (2001) Phototropism in higher plants. In Häder D, Lebert M, eds, Photomovement: ESP Comprehensive Series in Photosciences, Vol 1 Elsevier, Amsterdam, pp 659–811 [Google Scholar]
- Janoudi AK, Gordon WR, Wagner D, Quail P, Poff KL. (1997a) Multiple phytochromes are involved in red-light-induced enhancement of first-positive phototropism in Arabidopsis thaliana. Plant Physiol 113: 975–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoudi AK, Konjevic R, Apel P, Poff KL, Poff KL. (1992) Time threshold for second positive phototropism is decreased by a preirradiation with red light. Plant Physiol 99: 1422–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoudi AK, Konjević R, Whitelam G, Gordon W, Poff KL. (1997b) Both phytochrome A and phytochrome B are required for the normal expression of phototropism in Arabidopsis thaliana seedlings. Physiol Plant 101: 278–282 [Google Scholar]
- Kami C, Hersch M, Trevisan M, Genoud T, Hiltbrunner A, Bergmann S, Fankhauser C. (2012) Nuclear phytochrome A signaling promotes phototropism in Arabidopsis. Plant Cell 24: 566–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine-Vehn J, Huang F, Naramoto S, Zhang J, Michniewicz M, Offringa R, Friml J. (2009) PIN auxin efflux carrier polarity is regulated by PINOID kinase-mediated recruitment into GNOM-independent trafficking in Arabidopsis. Plant Cell 21: 3839–3849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ, Iino M. (1996a) Effects of red light on the fluence-response relationship for pulse-induced phototropism of maize coleoptiles. Plant Cell Environ 19: 609–614 [DOI] [PubMed] [Google Scholar]
- Liu YJ, Iino M. (1996b) Phytochrome is required for the occurrence of time-dependent phototropism in maize coleoptiles. Plant Cell Environ 19: 1379–1388 [DOI] [PubMed] [Google Scholar]
- Michniewicz M, Zago MK, Abas L, Weijers D, Schweighofer A, Meskiene I, Heisler MG, Ohno C, Zhang J, Huang F, et al. (2007) Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell 130: 1044–1056 [DOI] [PubMed] [Google Scholar]
- Nagashima A, Suzuki G, Uehara Y, Saji K, Furukawa T, Koshiba T, Sekimoto M, Fujioka S, Kuroha T, Kojima M, et al. (2008a) Phytochromes and cryptochromes regulate the differential growth of Arabidopsis hypocotyls in both a PGP19-dependent and a PGP19-independent manner. Plant J 53: 516–529 [DOI] [PubMed] [Google Scholar]
- Nagashima A, Uehara Y, Sakai T. (2008b) The ABC subfamily B auxin transporter AtABCB19 is involved in the inhibitory effects of N-1-naphthyphthalamic acid on the phototropic and gravitropic responses of Arabidopsis hypocotyls. Plant Cell Physiol 49: 1250–1255 [DOI] [PubMed] [Google Scholar]
- Noh B, Bandyopadhyay A, Peer WA, Spalding EP, Murphy AS. (2003) Enhanced gravi- and phototropism in plant mdr mutants mislocalizing the auxin efflux protein PIN1. Nature 423: 999–1002 [DOI] [PubMed] [Google Scholar]
- Parks BM, Quail PH, Hangarter RP. (1996) Phytochrome A regulates red-light induction of phototropic enhancement in Arabidopsis. Plant Physiol 110: 155–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuten T, Hohm T, Bergmann S, Fankhauser C. (2013) Defining the site of light perception and initiation of phototropism in Arabidopsis. Curr Biol 23: 1934–1938 [DOI] [PubMed] [Google Scholar]
- Sakai T, Haga K. (2012) Molecular genetic analysis of phototropism in Arabidopsis. Plant Cell Physiol 53: 1517–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Wada T, Ishiguro S, Okada K. (2000) RPT2: A signal transducer of the phototropic response in Arabidopsis. Plant Cell 12: 225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann T, Geldner N, Grebe M, Mangold S, Jackson CL, Paris S, Gälweiler L, Palme K, Jürgens G. (1999) Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF. Science 286: 316–318 [DOI] [PubMed] [Google Scholar]
- Wang B, Henrichs S, Geisler M. (2012) The AGC kinase, PINOID, blocks interactive ABCB/PIN auxin transport. Plant Signal Behav 7: 1515–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whippo CW, Hangarter RP. (2004) Phytochrome modulation of blue-light-induced phototropism. Plant Cell Environ 27: 1223–1228 [Google Scholar]
- Whippo CW, Hangarter RP. (2006) Phototropism: bending towards enlightenment. Plant Cell 18: 1110–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willige BC, Ahlers S, Zourelidou M, Barbosa ICR, Demarsy E, Trevisan M, Davis PA, Roelfsema MRG, Hangarter R, Fankhauser C, et al. (2013) D6PK AGCVIII kinases are required for auxin transport and phototropic hypocotyl bending in Arabidopsis. Plant Cell 25: 1674–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willige BC, Ogiso-Tanaka E, Zourelidou M, Schwechheimer C. (2012) WAG2 represses apical hook opening downstream from gibberellin and PHYTOCHROME INTERACTING FACTOR 5. Development 139: 4020–4028 [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Suzuki T, Aihara Y, Haga K, Sakai T, Nagatani A. (2014) The phototropic response is locally regulated within the topmost light-responsive region of the Arabidopsis thaliana seedling. Plant Cell Physiol 55: 497–506 [DOI] [PubMed] [Google Scholar]
- Zourelidou M, Absmanner B, Weller B, Barbosa IC, Willige BC, Fastner A, Streit V, Port SA, Colcombet J, de la Fuente van Bentem S, et al. (2014) Auxin efflux by PIN-FORMED proteins is activated by two different protein kinases, D6 PROTEIN KINASE and PINOID. eLife 3: e02860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zourelidou M, Müller I, Willige BC, Nill C, Jikumaru Y, Li H, Schwechheimer C. (2009) The polarly localized D6 PROTEIN KINASE is required for efficient auxin transport in Arabidopsis thaliana. Development 136: 627–636 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.