An important, but often unrecognized issue is the metabolic capacity of crop weeds to resist herbicides which poses a threat to herbicide sustainability and world crop production.
Abstract
Weedy plant species that have evolved resistance to herbicides due to enhanced metabolic capacity to detoxify herbicides (metabolic resistance) are a major issue. Metabolic herbicide resistance in weedy plant species first became evident in the 1980s in Australia (in Lolium rigidum) and the United Kingdom (in Alopecurus myosuroides) and is now increasingly recognized in several crop-weed species as a looming threat to herbicide sustainability and thus world crop production. Metabolic resistance often confers resistance to herbicides of different chemical groups and sites of action and can extend to new herbicide(s). Cytochrome P450 monooxygenase, glycosyl transferase, and glutathione S-transferase are often implicated in herbicide metabolic resistance. However, precise biochemical and molecular genetic elucidation of metabolic resistance had been stalled until recently. Complex cytochrome P450 superfamilies, high genetic diversity in metabolic resistant weedy plant species (especially cross-pollinated species), and the complexity of genetic control of metabolic resistance have all been barriers to advances in understanding metabolic herbicide resistance. However, next-generation sequencing technologies and transcriptome-wide gene expression profiling are now revealing the genes endowing metabolic herbicide resistance in plants. This Update presents an historical review to current understanding of metabolic herbicide resistance evolution in weedy plant species.
Antibiotics and agricultural chemicals (herbicides, fungicides, insecticides, etc.) are of immense value in controlling pest organisms plaguing human health and agricultural production. These chemicals greatly contribute to human health and the abundant food production evident in many but not all parts of the world. However, there is a major threat looming for their continued efficacy posed by the evolution of resistant pest populations. The widespread evolution of resistant pest populations is a salutary example of evolution in action. Strong selection pressure on large, genetically diverse pest populations initially causes high mortality, but there are initially rare resistance genes present in populations that are selected, enriched, and result in resistance evolution.
Herbicide resistance in the grass weed Lolium rigidum in Australian cropping is one of the world’s most dramatic examples of resistance evolution. Genetically diverse L. rigidum, often at high densities, infests much of the vast Australian grain belt and is combated with herbicides. There has been a pattern of initial herbicide success on L. rigidum, followed by herbicide failure due to rapid resistance evolution. Particularly worrisome is the fact that resistant populations often exhibit cross-resistance to different herbicides, and this can even extend to resistance to experimental herbicides not yet commercialized.
Over the past 25 years, we have studied the biochemical and genetic bases of herbicide resistance and cross-resistance in L. rigidum and have established that resistant individuals can exhibit from one to several coexisting resistance mechanisms. There is both target-site and non-target-site resistance. Target-site resistance occurs by mutation within a gene coding for an herbicide target-site enzyme (limiting the herbicide binding) or by overproduction of the target enzyme (gene overexpression or amplification). Non-target-site resistance involves mechanisms that minimize the amount of active herbicide reaching the target site (e.g. reduced herbicide uptake or translocation, increased herbicide sequestration, or enhanced herbicide metabolism). It is essential to understand that the accumulation of several resistance mechanisms within resistant individuals is now the normal situation for L. rigidum across vast areas of Australia (Powles and Matthews, 1992; Hall et al., 1994; Powles and Yu, 2010; Han et al., 2014a). Herbicide target-site enzymes/molecules (Heap, 2014) and their genes are mostly well known, and target-site resistance is often documented in resistant weed populations (Tranel and Wright, 2002; Délye, 2005; Powles and Yu, 2010; Yu and Powles, 2014). As target-site resistance is relatively easy to study, then, when identified, researchers often fail to examine for other coexisting resistance mechanisms. This is unfortunate, as the evolutionary reality is that any and all gene traits that can endow survival to an herbicide will be selected (Powles and Matthews, 1992). Insufficiently appreciated is that the intensity of the herbicide selection (herbicide rate used) is an important factor determining the resistance mechanism(s) selected, especially in genetically diverse, cross-pollinated species like L. rigidum. A very effective (high) herbicide dose results in very high mortality, and among the few survivors in large treated populations there may be resistant individuals carrying an initially rare target-site gene mutation(s). However, herbicides frequently do not achieve very high mortality, due to a lower effective dose resulting from low herbicide application rate, poor application, large plants, adverse environmental factors, or plant stress, etc. Unfortunately, herbicides often have been used at low doses in Australia. When there is a low herbicide dose there is lower plant mortality, and some survive because they possess gene traits that confer survival at the prevailing low herbicide dose. Principal among the possible mechanisms enabling plant survival from a low herbicide dose is the capacity to metabolize (degrade or detoxify) enough herbicide for the plant to survive. Many herbicides can be metabolized by plants. Thus, especially where metabolizable herbicides are at low doses (low rate, suboptimal conditions, poor timing, etc.), individual plants survive because they possess sufficient capacity to metabolize the herbicide. Therefore, the genes coding for the enzymes conferring herbicide metabolism are selected at low herbicide doses and can be enriched (e.g. through cross-pollination) in the population, resulting in high levels of resistance within a few generations. In research on herbicide resistance, too few studies examine for enhanced herbicide metabolism capacity; thus, this topic is underresearched and underappreciated, yet it is very important. Here, we focus on herbicide resistance conferred by an enhanced capacity to metabolize herbicides (hereinafter defined as metabolic resistance). Metabolic herbicide resistance and cross-resistance are widespread in the grass weeds L. rigidum, Alopecurus myosuroides, and Echinochloa phyllopogon and increasingly prevalent in some other weed species.
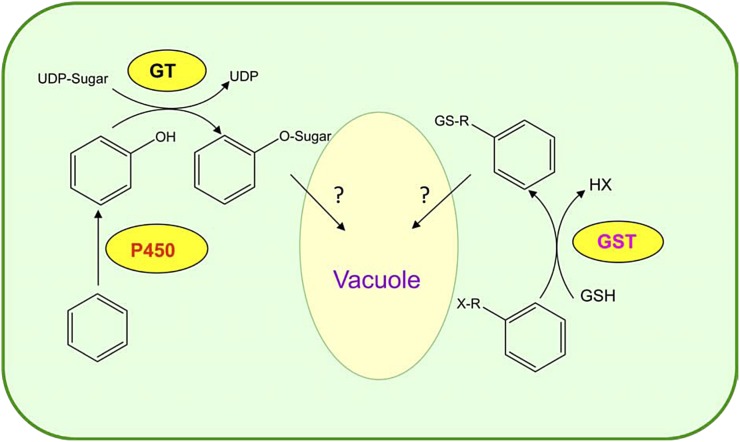
Metabolic resistance can be endowed by increased activity of endogenous cytochrome P450 monooxygenases (P450s), glucosyl transferases (GTs), glutathione S-transferases (GSTs), and/or other enzyme systems such as aryl acylamidase (Carey et al., 1997) that can metabolize herbicides. P450s, GTs, and GSTs belong to major enzyme superfamilies with many roles in primary and secondary metabolism, and, by chance, some of them achieve herbicide detoxification (Kreuz et al., 1996; Cole and Edwards, 2000; Edwards and Dixon, 2000; Werck-Reichhart et al., 2000; Morant et al., 2003; Siminszky, 2006; Yuan et al., 2007). For instance, some P450s can catalyze herbicide arylhydroxylation or alkylhydroxylation, which is followed by GT-catalyzed Glc conjugation (Fig. 1). Certain herbicides can also be directly inactivated by GST-catalyzed glutathione conjugation. Conjugated herbicides are subsequently transported into vacuoles for storage and/or further metabolism (Fig. 1). An important, potentially devastating characteristic of metabolic herbicide resistance is that the responsible enzymes can confer cross-resistance (for definitions, see Hall et al., 1994) to herbicides of different chemical groups and sites of action. Metabolic cross-resistance is determined by the ability of P450, GT, or GST to metabolize particular herbicide chemistries, irrespective of their sites of action. As discussed below, cross-resistance can be conferred to herbicides to which the plants have never been exposed. Thus, metabolism-based herbicide cross-resistance is a major threat, as it can automatically confer resistance to existing, new, or yet-to-be-discovered herbicides.
Figure 1.
Major superfamily enzymes involved in metabolic herbicide resistance (modified from De Prado and Franco, 2004). GSH, Reduced glutathione; X-R, the electrophile; GS-R, the glutathione conjugated product; HX, the unconjugated product.
HERBICIDE RESISTANCE AND CROSS-RESISTANCE IN L. RIGIDUM DUE TO ENHANCED CAPACITY FOR HERBICIDE METABOLISM
L. rigidum is by far the most widespread weed in Australian field cropping. Herbicides have long been employed for L. rigidum control, and resistance evolution quickly followed. Striking, and initially inexplicable, was that L. rigidum populations that evolved resistance to one herbicide (Heap and Knight, 1982) displayed cross-resistance to dissimilar herbicides (Heap and Knight, 1986). Subsequently, such cross-resistance became widespread in L. rigidum in Australia. Similarly, cross-resistance was early evident in A. myosuroides populations in the United Kingdom (Moss and Cussans, 1985). Since then, metabolic resistance and cross-resistance have been reported in some other resistant weed species (Coupland et al., 1990; Anderson and Gronwald, 1991; Gimenez-Espinosa et al., 1996; Hidayat and Preston, 1997, 2001; Maneechote et al., 1997; Singh et al., 1998; Fischer et al., 2000b; Veldhuis et al., 2000; Cocker et al., 2001; Fraga and Tasende, 2003; Park et al., 2004; Menendez et al., 2006; Owen et al., 2012; Ahmad-Hamdani et al., 2013; Ma et al., 2013; Iwakami et al., 2014c; for review, see De Prado and Franco, 2004; Preston, 2004; Yuan et al., 2007; Powles and Yu, 2010; Beckie and Tardif, 2012; Yu and Powles, 2014). As most research on metabolic resistance has focused on L. rigidum, A. myosuroides, and E. phyllopogon, we review metabolic resistance and cross-resistance in these three species while recognizing that metabolic resistance also occurs in other weedy species and is an increasingly observed phenomenon.
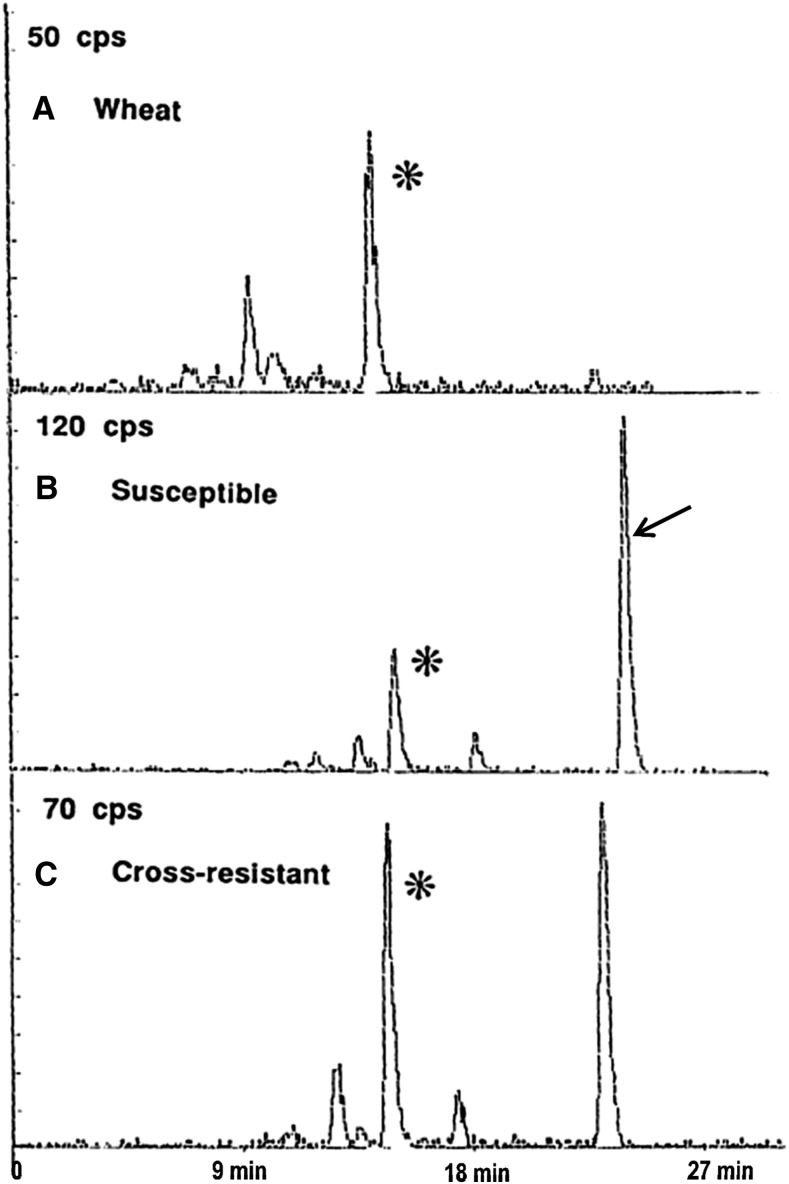
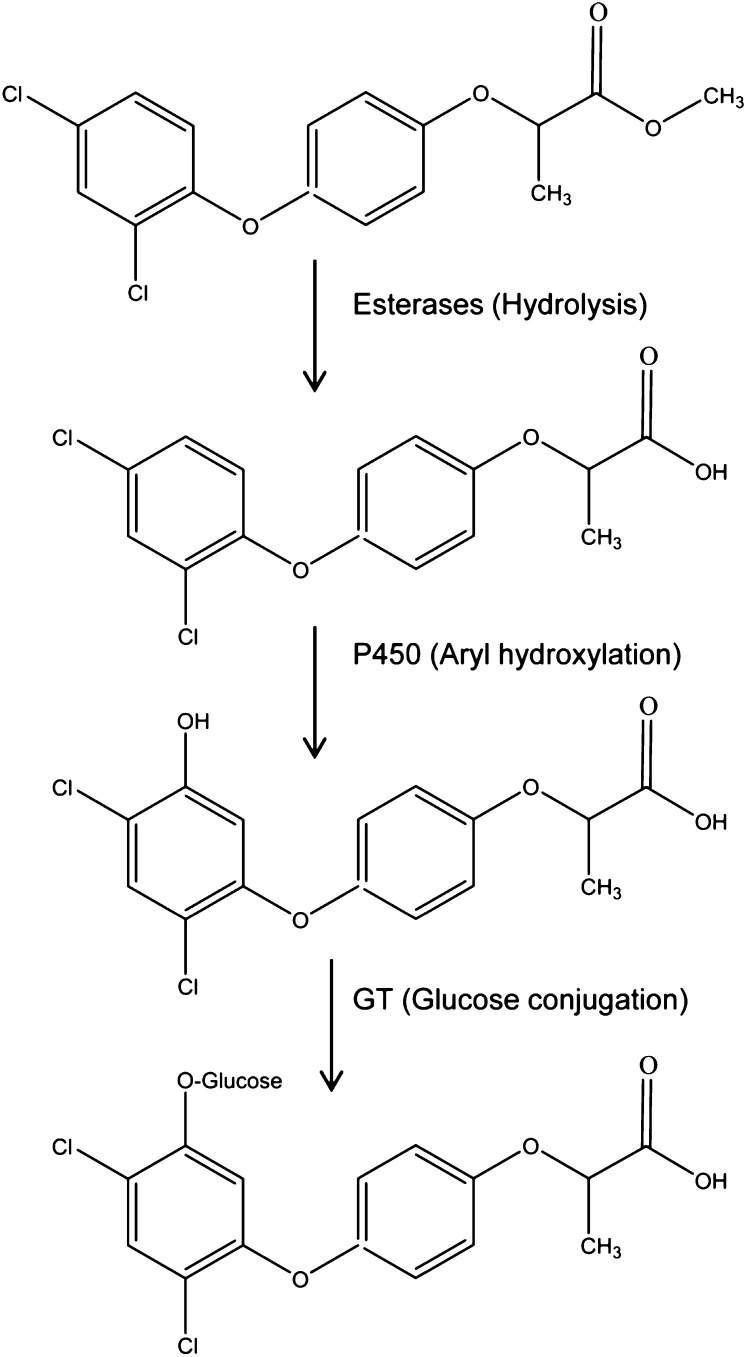
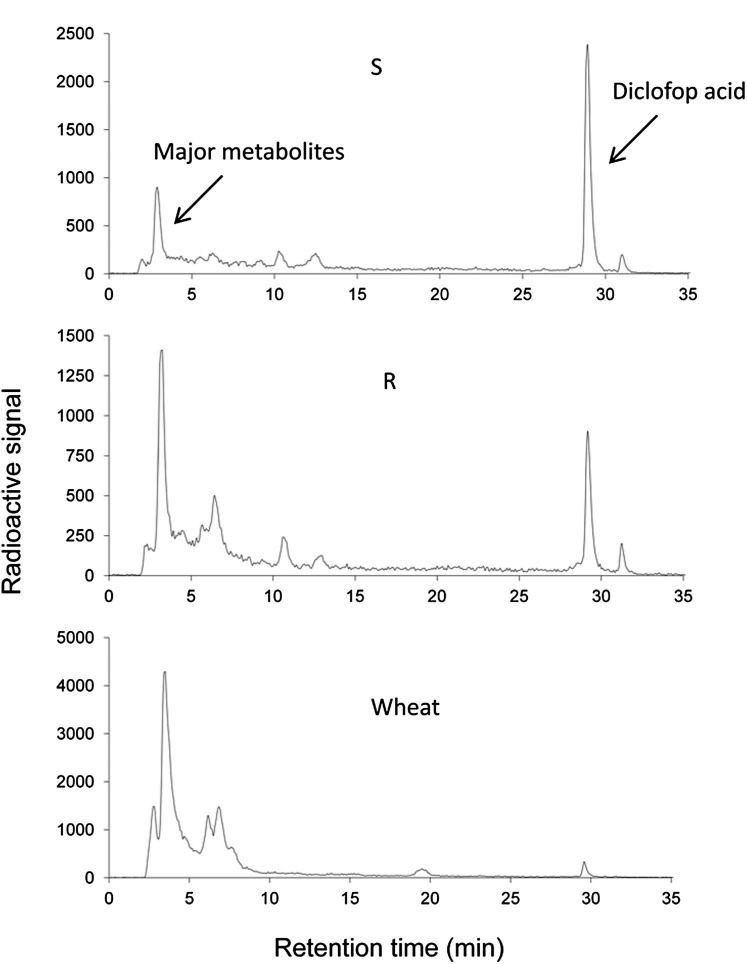
With one of the first multiply resistant L. rigidum populations identified (termed SLR31), we established that, as expected, cross-resistance was not target site based (Matthews et al., 1990; Christopher et al., 1991) but was due to enhanced rates of in vivo herbicide metabolism (Christopher et al., 1991, 1992; Holtum et al., 1991; Cotterman and Saari, 1992). We speculated that this metabolic resistance was likely due to enhanced activity of P450 enzymes (Powles et al., 1990), as the HPLC profile of herbicide metabolism in resistant L. rigidum is qualitatively similar to that in wheat (Triticum aestivum; Fig. 2) and wheat is known to metabolize herbicides such as chlorsulfuron and diclofop by P450 and GT (Shimabukuro et al., 1979, 1987; Sweetser et al., 1982; Zimmerlin and Durst, 1990; Fig. 3). P450 involvement was further indicated in cross-resistant L. rigidum, as the P450 inhibitor malathion inhibited the enhanced herbicide metabolism and reversed resistance to some specific herbicides (Christopher et al., 1994; Preston et al., 1996). Since then, we have established metabolic resistance in several L. rigidum populations resistant and cross-resistant to one or many herbicide chemical groups and sites of action (Table I).
Figure 2.
HPLC scans of [14C]chlorsulfuron metabolism in excised seedlings of wheat (A) and susceptible (B) and cross-resistant (C) L. rigidum (SLR31; modified from Christopher et al., 1991). The arrow indicates the parent herbicide chlorsulfuron. The major metabolites (asterisks) in wheat and both L. rigidum populations have the same retention time.
Figure 3.
Diclofop metabolism in wheat via esterase-mediated hydrolysis and P450-based arylhydroxylation followed by GT-catalyzed Glc conjugation (Shimabukuro et al., 1979, 1987; Zimmerlin and Durst, 1990).
Table I. L. rigidum populations with confirmed metabolic herbicide cross-resistance.
| Population | Selecting Herbicides | Metabolic Resistance to | Major References |
|---|---|---|---|
| Field-evolved populations | |||
| SLR31 | Trifluralin | Dinitroanilines | Tardif and Powles (1999) |
| Diclofop | ACCase inhibitors | Holtum et al. (1991) | |
| AHAS inhibitors | Christopher et al. (1991) | ||
| WLR1 | Chlorsulfuron | AHAS inhibitors | Christopher et al. (1992) |
| WLR2 | Amitrole | PSII inhibitors (including ureas) | Burnet et al. (1993a, 1993b); |
| Atrazine | Preston and Powles (1997) | ||
| VLR69 | Diuron | PSII inhibitors (including ureas) | Burnet et al. (1993a, 1993b); |
| Chlorsulfuron | AHAS inhibitors | Preston et al. (1996) | |
| Atrazine | PSII inhibitors | ||
| Diclofop | ACCase inhibitors | ||
| Low-herbicide-rate recurrent selection | |||
| VLR1 subset | Diclofop | ACCase inhibitors | Neve and Powles (2005a) |
| AHAS inhibitors | Yu et al. (2013b) | ||
| WALR1 subset | Diclofop | ACCase inhibitors | Manalil et al. (2011) |
| AHAS inhibitors | Yu et al. (2013b) |
An extreme example is L. rigidum population VLR69, with a 21-year field selection history by different herbicides resulting in resistance to at least nine herbicide groups across five different sites of action (Burnet et al., 1994). We established that metabolic resistance is a major mechanism in this population that likely involves multiple P450s, including some that can be reversed by certain P450 inhibitors (Burnet et al., 1993a, 1993b; Preston et al., 1996).
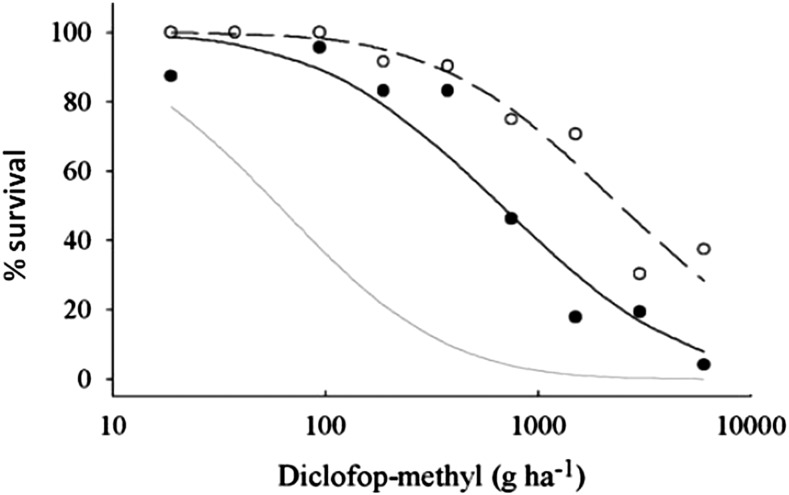
Further evidence for the importance of P450-mediated metabolic resistance comes from the deliberate selection of L. rigidum at low, sublethal herbicide rates. We established that herbicide-susceptible L. rigidum populations are capable of a low rate of metabolism of many herbicides and thus survive a low dose, whereas a full dose is lethal (Table II). Understandably, herbicide-susceptible populations of many weedy plant species have a basal level of herbicide metabolism (Hidayat and Preston, 1997; Maneechote et al., 1997; Veldhuis et al., 2000; Park et al., 2004; Yasuor et al., 2010; Ahmad-Hamdani et al., 2013; Ma et al., 2013). Of course, there will be genetic variability in this basal endogenous capacity to metabolize herbicides, and within large populations, some individuals will have a higher herbicide metabolism capacity. Thus, if a metabolizable herbicide is used at a low dose, some individuals within a population metabolize sufficient herbicide that they survive and reproduce. If there is continued herbicide selection at a low dose, the gene traits endowing this enhanced metabolism survival will be enriched and, especially in cross-pollinated species (like L. rigidum), the gene traits will be accumulated and resistance will become evident in the population. For example, we recurrently selected a small, herbicide-susceptible L. rigidum population at a low dose of the metabolizable herbicide diclofop. Survivors were allowed to cross-pollinate and produce seed, and the process was repeated for three successive generations. This resulted in diclofop resistance and, importantly, cross-resistance to certain other metabolizable but otherwise dissimilar herbicides (Neve and Powles, 2005a; Fig. 4). This result has since been confirmed in several different herbicide-susceptible L. rigidum populations recurrently selected at a low diclofop dose, always with the same rapid evolution of dioclofop resistance and cross-resistance evolution to other dissimilar but metabolizable herbicides (Neve and Powles, 2005b; Manalil et al., 2011). This low-diclofop-dose selected resistance is due to enhanced rates of diclofop metabolism, again mimicking that of wheat and thus suggestive of P450 involvement (Yu et al., 2013b; Fig. 5). Conversely, we did a reverse study, in which recurrent selection of herbicide-susceptible L. rigidum for the individuals most susceptible to diclofop resulted in a rapid shift toward diclofop supersensitivity (Manalil et al., 2012). Importantly, these plants also become supersensitive to some other herbicides metabolizable by P450s (Manalil et al., 2012).
Table II. Herbicide-susceptible L. rigidum populations with some capacity to metabolize herbicides.
| Susceptible Population | Herbicides with Some Initial Metabolism | References |
|---|---|---|
| VLR1 | Chlorsulfuron | Christopher et al. (1991, 1992); Preston et al. (1996) |
| Diclofop | Holtum et al. (1991); Tardif et al. (1996); Preston et al. (1996) | |
| Chlorotoluron | Burnet et al. (1993a); Preston et al. (1996) | |
| Simazine | Burnet et al. (1993b); Preston et al. (1996) | |
| Sethoxydim | Tardif et al. (1993); Tardif and Powles (1994) | |
| Tralkoxydim | Preston et al. (1996) | |
| Haloxyfop | Tardif et al. (1996) | |
| Low-rate-selected supersensitive VLR1 subset | Diclofop | Han et al. (2013) |
| VLR2 | Chlorotoluron | Preston and Powles (1997) |
| VLR6 | Chlorsulfuron | Christopher et al. (1991) |
| WALR1 | Diclofop | Yu et al. (2013b) |
Figure 4.
Dose-response curves for herbicide-susceptible (gray line) and twice-selected (solid line, black circles) and triple-selected (broken line, white circles) diclofop-resistant L. rigidum populations. The resistant populations were the result of selection at 0.1- and 0.5-fold (twice selected) or at 0.1-, 0.5-, and 2-fold (triple selected) of the recommended dose of 375 g ha−1 (adapted from Neve and Powles, 2005a).
Figure 5.
HPLC scans comparing diclofop metabolism between wheat and the unselected susceptible (S) and low-dose-selected resistant (R) L. rigidum populations (modified from Yu et al., 2013b).
Clearly, the above-mentioned studies establish that genetically variable, cross-pollinated L. rigidum exposed to metabolizable herbicides (especially at a low herbicide dose) results in resistance evolution that involves P450s and potentially other resistance genes. We emphasize that herbicides select for all possible resistance traits, so target-site resistance mutations and any other potential resistance mechanisms are also selected at low herbicide doses when populations are large. This is starkly evident in the L. rigidum population WLR1, which for 7 years was selected in large commercial wheat fields at a low chlorsulfuron dose (6 g ha−1 compared with the Australian recommended field rate of 20 g ha−1; Christopher et al., 1992). This poor commercial practice resulted in the selection and enrichment of genes endowing metabolic resistance to chlorsulfuron (Christopher et al., 1992) as well as at least six different target-site acetohydroxyacid synthase (AHAS) gene mutations (Yu et al., 2008).
Recently, we have shown that the auxinic herbicide 2,4-dichlorophenoxyacetic acid (2,4-D), a known P450 inducer (Adele et al., 1981; Hirose et al., 2007), can provide protection against metabolizable herbicides in susceptible L. rigidum. With 2,4-D pretreatment followed by diclofop treatment, there was a 10-fold increase in diclofop herbicide rates causing 50% plant mortality and herbicide rates causing 50% reduction in plant growth (Han et al., 2013). This occurred because the 2,4-D pretreatment induced a higher capacity for diclofop metabolism, with the HPLC profile of diclofop metabolites similar to that observed in wheat (Han et al., 2013), indicative of P450 involvement. These 2,4-D-pretreated susceptible L. rigidum plants in every way were transiently similar to field-evolved metabolic resistant L. rigidum. For instance, the 2,4-D pretreatment also induced cross-protection to the metabolizable but otherwise dissimilar sulfonylurea herbicide chlorsulfuron (and other herbicides that can be metabolized), and the P450 inhibitor malathion could reverse this effect. Therefore, protection against herbicides induced by 2,4-D pretreatment of susceptible L. rigidum is due to the induction of higher rates of herbicide metabolism, mirroring that identified in resistant L. rigidum populations. We hypothesize that the pretreatment with 2,4-D rapidly induces higher expression of herbicide-metabolizing genes, hence providing transient protection (safening) against the subsequently applied herbicide.
Our work on several well-characterized metabolic resistant L. rigidum populations shows that metabolic herbicide resistance can be endowed by one or several nuclear gene loci (Busi et al., 2011, 2013). For example, metabolic resistance in the L. rigidum population SLR31 was found to be controlled by two loci (Busi et al., 2011). In another resistant L. rigidum population, at least three resistance genes were enriched (Busi et al., 2013). A monogenic resistance trait was reported previously for metabolic resistance to chlorsulfuron in a multiple resistant L. rigidum population (VLR69; Preston, 2003), but our recent work indicates more complicated genetic control patterns (Han et al., 2014). We emphasize that each resistant population is a different evolutionary event, and it is to be expected for metabolic resistance involving P450 and other enzyme superfamilies that individuals and populations, particularly of genetically variable, cross-pollinated species such as L. rigidum, differ in the number of gene loci conferring herbicide resistance. Major influencing factors in the evolution of metabolic resistance are the herbicide chemistry, herbicide dose, duration of exposure, and environmental conditions, interacting with genetic diversity. What is unknown and intriguing is whether the expression of some metabolic resistance genes under herbicide selection is subject to epigenetic control. Given the complex genetic nature of metabolic herbicide resistance in cross-pollinated weed species, identifying all the P450s and other genes involved remains challenging. However, comprehensive genomic approaches such as next-generation transcriptome sequencing (RNA sequencing [RNA-seq]) opens up new research opportunities (see below).
In total, these studies with a range of L. rigidum populations show that resistance and cross-resistance to different metabolizable herbicides are due to an enhanced capacity for herbicide metabolism, which can be inhibited (and resistance thus reversed) in vivo by P450 inhibitors. However, it must be stated that, until recently, there was little direct evidence for the involvement of specific P450s or the identity of other genes responsible for this herbicide metabolic resistance. Despite much effort, our studies to isolate P450-active microsomes from L. rigidum have not been successful (S. Powles and D. Werck-Reichhart, unpublished data). Thus, until recently, progress had stalled on further characterizing herbicide-metabolizing enzymes and identifying the specific genes conferring resistance (see below).
RECENT PROGRESS ON THE IDENTIFICATION OF SPECIFIC GENES ENDOWING METABOLIC HERBICIDE RESISTANCE IN L. RIGIDUM
Until now, biochemical and other molecular approaches for the discovery of herbicide-metabolizing and resistance-endowing genes in Lolium spp. have been difficult and have yielded little (Preston and Powles, 1997; Fischer et al., 2001; Duhoux and Délye, 2013). Recently, utilizing global differential gene expression profiling (RNA-seq) technology, we have generated an L. rigidum reference transcriptome library using Roche 454 technology (Gaines et al., 2014). RNA-seq has been performed using Illumina HiSeq with resistant and susceptible individuals from a well-characterized, metabolism-based resistant L. rigidum population (Neve and Powles, 2005a; Yu et al., 2013b). Differentially expressed contigs (putatively annotated as P450s, nitronate monooxygenase [NMO], GST, and GT) were highly expressed in resistant versus susceptible plants and cosegregated with diclofop resistance in an F2 herbicide resistance segregating population (Table III). Supporting our previous study in which 2,4-D pretreatment induced protection against diclofop (Han et al., 2013), 2,4-D-treated susceptible L. rigidum individuals showed overexpression of the same transcripts (Table III). Furthermore, four of these transcripts (two P450s, NMO, and GT) were consistently highly expressed in nine unrelated L. rigidum populations with field-evolved metabolic resistance from both Europe and Australia (Table III). This suggests that these four genes collectively play critical roles in conferring metabolic herbicide resistance in L. rigidum (Gaines et al., 2014). While a role in endowing resistance is expected for the P450 and GT genes, the possible role of NMO in diclofop metabolic resistance is unknown and remains to be determined. This enzyme is a flavin-dependent monooxygenase and catalyzes an oxidative denitrification reaction (Gadda and Francis, 2010). Current research is functionally characterizing these four genes. Given the diversity and complexity of the genetic control of metabolic resistance, variation in the resistance genes involved is envisaged to differ among populations with distinct evolutionary selection histories (Table I). For example, the GST genes may contribute to metabolic resistance in some L. rigidum populations (Table III).
Table III. Candidate enzymes identified in metabolic resistant L. rigidum populations (data from Gaines et al., 2014).
| Candidate Enzymes | Highly Induced in Susceptible L. rigidum by 2,4-D Pretreatment | Higher Expression in Two Australian Metabolic Resistant L. rigidum Populations | Higher Expression in Eight French Metabolic Resistant L. rigidum Populations |
|---|---|---|---|
| P450s (two CYP72As) | Yes | Yes | Yes |
| NMO | Yes | Yes | Yes |
| GT | Yes | Yes | Yes (seven out of eight) |
| GST1 (τ class) | Yes | Yes | Yes (six out of eight) |
| GST2a (ϕ class) | Yes | No | Yes (five out of eight) |
| GST3 (τ class) | Yes | Yes | No |
Highly similar to LrGSTF1, a homolog of AmGSTF1 (Cummins et al., 2013).
Our recent success (Gaines et al., 2014) further confirms that global RNA-seq, when coupled with genetic and physiological validation (e.g. 2,4-D-induced gene expression changes), is powerful for metabolic herbicide resistance gene discovery. We are now focused on metabolic resistance gene discovery in several field-evolved resistant Lolium spp. populations and in other resistant weed species.
HERBICIDE RESISTANCE/CROSS-RESISTANCE AND RESISTANCE GENE DISCOVERY IN A. MYOSUROIDES
Similar to L. rigidum, an herbicide-resistant A. myosuroides population early reported in the United Kingdom (Moss and Cussans, 1985) displayed cross-resistance to herbicides of different chemical groups and sites of action (Kemp et al., 1990). Subsequent studies with these resistant A. myosuroides populations demonstrated that resistance is due to enhanced rates of herbicide metabolism that could be reduced by P450 inhibitors (Kemp et al., 1990; Hall et al., 1995, 1997; Hyde et al., 1996). Since then, metabolic herbicide resistance, likely involving P450s, has been identified in many other European A. myosuroides populations (Menendez and De Prado, 1997; Cocker et al., 1999; Letouzé and Gasquez, 2001, 2003; De Prado and Franco, 2004). In addition to P450s, a GST with glutathione peroxidase activity (specifically, the Phi class GSTF1 gene product) has been shown to play a role in resistance to some herbicides in some resistant A. myosuroides populations (Cummins et al., 1997, 1999, 2011). Transgenic Arabidopsis (Arabidopsis thaliana) expressing the A. myosuroides GSTF1 gene (AmGSTF1) has improved tolerance to some herbicides, which is reversible by the application of a specific GST inhibitor (Cummins et al., 2013). Resistance is due to increased accumulation of protective compounds (glutathione, anthocyanins, and flavonoids), which is mediated by the AmGSTF1 gene via a yet-unknown regulating mechanism, rather than direct herbicide detoxification activity by the GST itself (Cummins et al., 2013). In addition, higher expression of the GSTF1-like genes was recently found in at least 10 other resistant UK A. myosuroides populations (R. Edwards, personal communication). However, a similar GST-based resistance mechanism seems to be less evident in metabolic resistant Australian L. rigidum populations (Cummins et al., 2013; Table III; R. Edwards, personal communication). This likely reflects genetic diversity and different herbicide selection, as the resistant UK A. myosuroides populations had been selected with fenoxaprop, which is known to be detoxified by GST (Edwards and Cole, 1996), while many of the resistant Australian L. rigidum populations were selected with diclofop, which can be P450 detoxified (Zimmerlin and Durst, 1990).
METABOLIC HERBICIDE RESISTANCE AND RESISTANCE GENE DISCOVERY IN ECHINOCHLOA spp.
E. phyllopogon, a predominantly self-pollinated allotetraploid species, is a major global weed long selected with herbicides and prone to resistance evolution. More than 40 years of herbicide use has resulted in the evolution of resistance to several different herbicides in many populations of E. phyllopogon (Fischer et al., 2000a; Osuna et al., 2002; Yasuor et al., 2009). Cross-resistance to dissimilar herbicides was shown to be non-target-site based (Osuna et al., 2002; Yasuor et al., 2009), and studies involving in vivo herbicide metabolism, measurement of microsomal P450 content and activity, and use of P450 inhibitors/inducers all strongly indicate that resistance is due to P450-based enhanced herbicide metabolism (Fischer et al., 2000b; Osuna et al., 2002; Yun et al., 2005; Yasuor et al., 2009, 2010). With recent advances in P450 gene discovery in herbicide-tolerant rice (Oryza sativa; Pan et al., 2006; Saika et al., 2014), progress has been made in the identification, cloning, and characterization of the P450 genes responsible for metabolic resistance in E. phyllopogon. For example, two P450 genes (CYP71AK2 and CYP72A) were recently found to be highly induced by bispyribac-sodium in multiple herbicide-resistant plants (Iwakami et al., 2014b). More recently, the two P450 genes (CYP81A12 and CYP81A21) identified in a resistant E. phyllopogon population conferred resistance to certain metabolizable herbicides when expressed in Arabidopsis, and yeast (Saccharomyces cerevisiae WAT11 strain)-expressed CYP81A12 and CYP81A21 enzymes metabolized herbicide through O-demethylation (Iwakami et al., 2014a). These two highly similar P450 genes (likely homologs) are likely to be up-regulated simultaneously by a single trans-acting element in the resistant individuals (Iwakami et al., 2014a). Therefore, based on available studies on metabolic resistance genetics and P450 gene discovery in weedy species, a single P450 can confer resistance to a few herbicides, as has been reported in herbicide-tolerant crops (e.g. Pan et al., 2006; Dam et al., 2007). However, it is more likely that the expression of several existing P450s (and other genes) contributing to the basal level of herbicide metabolism in a weedy plant could be simultaneously up-regulated through a regulatory cascade, endowing resistance to a wider range of herbicides.
In addition, it has long been known that metabolic resistance to the rice-selective herbicide propanil in Echinochloa crus-galli and Echinochloa colona is due to rapid propanil hydrolysis catalyzed by the enzyme aryl acylamidase (Leah et al., 1995; Carey et al., 1997), similar to that occurring in tolerant rice. Several organophosphate insecticides/herbicides can be used as synergists to combat the aryl acylamidase-endowed resistance (for review, see Hoagland et al., 2004).
Compared with cross-pollinated diploid species (e.g. L. rigidum and A. myosuroides), resistance gene enrichment and thus resistance evolution in self-pollinated polyploid species (e.g. E. phyllopogon and Avena fatua) are expected to be slower (Yu et al., 2013a). Nevertheless, metabolic resistance gene discovery may be made easier in E. phyllopogon due to the ease of generating self-pollinated true-breeding lines and the likely fewer resistance genes involved.
METABOLIC HERBICIDE CROSS-RESISTANCE IN WEED SPECIES: A VERY IMPORTANT BUT UNDERSTUDIED THREAT
Metabolic herbicide cross-resistance, ranging from resistance to a few through many metabolizable herbicides, is most evident in the economically important and damaging grass weeds L. rigidum, A. myosuroides, and E. phyllopogon. However, such resistance has also been identified in populations of at least 12 other weed species (Owen et al., 2012; Ma et al., 2013; Iwakami et al., 2014c; for review, see Preston, 2004; Powles and Yu, 2010; Beckie and Tardif, 2012). A very recent development is the discovery of metabolic resistance to atrazine and 4-hydroxyphenylpyruvate dioxygenase-inhibiting herbicides in Amaranthus tuberculatus (Ma et al., 2013). Therefore, herbicide resistance (and cross-resistance) due to an enhanced capacity to metabolize (detoxify) herbicides is becoming increasingly reported and should be recognized as a significant threat to global herbicide efficacy and thus food production (Preston, 2004; Powles and Yu, 2010; Délye et al., 2011; Beckie and Tardif, 2012; Délye, 2013; Yu and Powles, 2014). Despite this threat, metabolic resistance has been severely underinvestigated, likely because it is difficult to study and can co-occur along with easily identified target-site mutations that provide higher levels of resistance and mask the presence of metabolic resistance. For example, a large survey of the target-site acetyl-coenzyme A carboxylase (ACCase) gene mutations in A. myosuroides in France (over 10,000 seedlings in 243 populations) established that 75% of the resistant plants did not have target-site resistance and thus must have non-target-site resistance, although the mechanistic basis was not characterized (Délye et al., 2007).
Similarly, in Australia, there is much, albeit indirect, evidence that L. rigidum across vast areas exhibits both target-site resistance and metabolic cross-resistance to many herbicides (Llewellyn and Powles, 2001; Owen et al., 2007, 2014; Malone et al., 2014). Our most recent work analyzing the in vivo metabolism of [14C]diclofop in 33 multiply resistant L. rigidum populations collected in a random field survey (Owen et al., 2014) provides direct evidence that metabolic resistance is common (H.P. Han, Q. Yu, M. Owen, G.R. Cawthray, and S.B. Powles, unpublished data). This work revealed that nearly 80% of resistant L. rigidum populations showed metabolic herbicide resistance. While target-site resistance co-occurs (91% of the populations) in these resistant L. rigidum populations, 70% of the populations exhibit both target-site resistance (ACCase mutations) and non-target-site enhanced herbicide metabolism. Direct evidence of metabolic resistance in large numbers of resistant plants is currently being obtained using an automated 14C-labeled herbicide metabolism screen at Bayer CropScience in Frankfurt, Germany. This work, conducted with hundreds of resistant populations from many geographies, has established that metabolic resistance in L. rigidum is widespread. For example, analysis of more than 2,000 individuals from 301 different resistant L. rigidum populations from France revealed that 72% of the populations displayed metabolic resistance (to ACCase-inhibiting herbicides), with only 28% of the populations possessing solely target-site resistance (R. Beffa, personal communication).
CAN METABOLIC HERBICIDE CROSS-RESISTANCE BE MITIGATED?
The dire threat of metabolic herbicide resistance is that it can endow resistance across herbicides of different chemical groups. For example, in many L. rigidum populations selected with ACCase-inhibiting herbicides, there is concomitant cross-resistance to AHAS-inhibiting herbicides (Table I). This has also been reported in A. myosuroides (Letouzé and Gasquez, 2001) and Digitaria sanguinalis (Hidayat and Preston, 1997, 2001). Similarly, in Phalaris minor, selection with a phenylurea herbicide resulted in cross-resistance to ACCase-inhibiting herbicides (Singh et al., 1998). In addition, selection with the very-long-chain fatty acid synthesis inhibitor herbicide pyroxasulfone in L. rigidum resulted in cross-resistance to the thiocarbamate herbicides prosulfocarb and triallate (Busi and Powles, 2013). As discussed above, such metabolic cross-resistance occurs because the P450 and/or other metabolism genes (e.g. GT and GST) responsible for resistance can serendipitously metabolize a range of herbicide chemical structures. The degree of metabolic cross-resistance in a given weed population will be dependent on the particular resistance gene selected and the substrate specificity of the enzymes encoded by the resistance genes. Environmental conditions also play a role in metabolic resistance evolution, as the enzymes involved (e.g. P450s and GSTs) can respond to biotic or abiotic stresses (Marrs, 1996; Schuler and Werck-Reichhart, 2003).
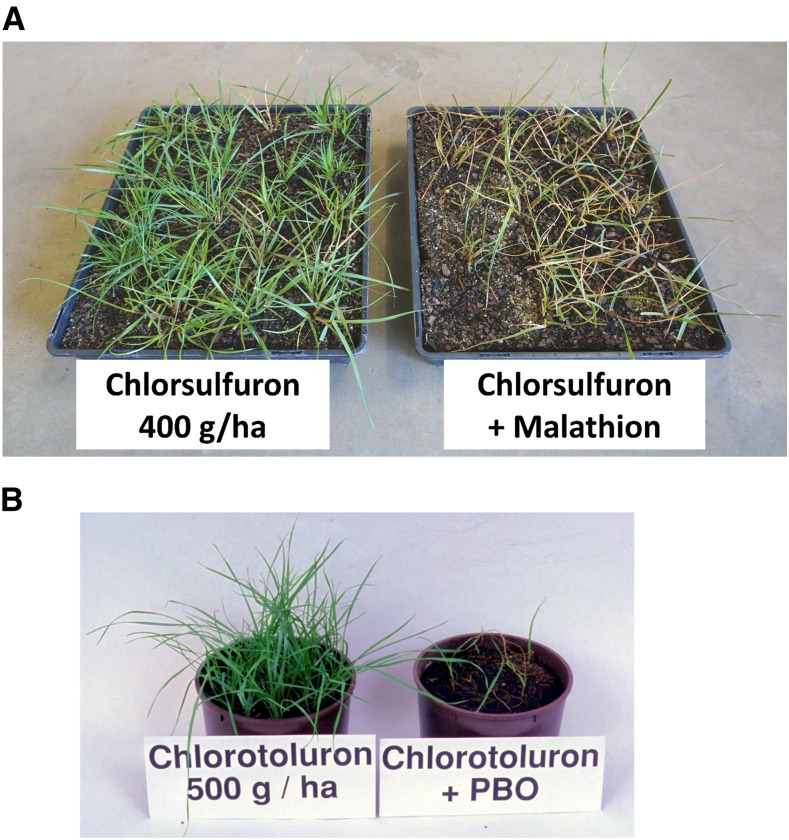
Resistance management strategies based on herbicide rotation are confounded by the fact that metabolic resistance can confer resistance across different herbicide groups, and thus the efficacy of herbicide mixtures or rotations can be compromised. Therefore, once metabolic resistance has evolved, how can it be mitigated? One possibility is to use chemical synergists to inhibit the enzymes responsible for resistance. Insecticide synergists have long been successfully used for combating insecticide metabolic resistance (for review, see Bernard and Philogène, 1993). As discussed in previous sections, certain P450 or GST inhibitors can reverse metabolic resistance in weeds if the resistance is solely metabolism based. For instance, the P450 inhibitors malathion, aminobenzotriazole, and piperonyl butoxide can inhibit the in vivo metabolism of certain AHAS-inhibiting herbicides, chlorotoluron, and simazine, respectively, thus reversing resistance (Burnet et al., 1993a; Christopher et al., 1994; Preston et al., 1996; Fig. 6). Similarly, a specific inhibitor of AmGSTF1 can enhance the efficacy of some herbicides in A. myosuroides (Cummins et al., 2013). The crop selectivity and any other impacts of these inhibitors are challenges remaining to be addressed. Alternatively, once key genes endowing metabolic resistance are identified, specific double-stranded RNAs can be designed and delivered to plants to accurately silence or suppress the gene(s) conferring resistance. For example, silencing of P450 or other metabolic genes in insects by RNA interference (RNAi) has been shown to increase insect susceptibility to insecticides or inhibitory plant metabolites (Mao et al., 2007; Liu et al., 2014). RNAi can simultaneously target several genes; therefore, it is especially suitable for studying (and likely combating) metabolic herbicide resistance, as this is often under polygenic control (Busi et al., 2011). Pioneering work (Sammons et al., 2011) shows that RNAi plus herbicides has the potential to achieve a renaissance of important existing herbicides.
Figure 6.
Synergistic effects of P450 inhibitors (malathion and piperonyl butoxide) applied 1 h before herbicide treatment. A, Metabolic herbicide-resistant L. rigidum plants were treated with 400 g ha−1 chlorsulfuron alone (left) or with chlorsulfuron plus 1 kg ha−1 malathion (right). B, Plants were treated with 500 g ha−1 chlorotoluron (left) or with chlorotoluron plus 2.1 kg ha−1 piperonyl butoxide (PBO; right). Application of the P450 inhibitor alone had no visual effect on plant growth.
Interestingly, we have shown that metabolism-based resistant L. rigidum plants have reduced fitness (Vila-Aiub et al., 2005a, 2005b, 2009). At least in the resistant L. rigidum population examined, for unknown reasons, the presence of up-regulated metabolic resistance genes may come at a fitness cost. This fitness cost may be explored by agronomic practices such as crop competition and pasture phases to moderate resistance evolution in the field.
CONCLUSION
(1) Metabolic resistance, conferring resistance potentially to many herbicides, is a particular threat to herbicide sustainability and thus global crop production, but it is underinvestigated.
(2) Metabolic resistance evolution can be rapid, as the responsible genes can be at high initial frequencies. Many weedy plant species are genetically diverse, including genetic diversity in their capacity to metabolize herbicides. Under persistent herbicide selection, especially if at reduced herbicide rates, weed individuals with higher metabolic capacity will be rapidly selected, resulting in resistance evolution. High survival frequencies at relatively low herbicide use rates in Australia have been revealed in many L. rigidum populations (Neve and Powles, 2005b). We emphasize that metabolic resistance evolution will be much faster in cross-pollinated weed species, because there is easy pollen-mediated resistance gene exchange and enrichment, in comparison with self-pollinated species.
(3) Metabolic resistance is often due to enhancement of a plant’s existing capacity for herbicide metabolism, mimicking the metabolism occurring in herbicide-tolerant crops. In most studies, herbicide metabolism between resistant and susceptible weed plants has been found to be quantitatively but not qualitatively different. The metabolic resistance in the weed mimics that of tolerant crops in being based on similar (unidentified) P450 and other metabolic enzyme families (Christopher et al., 1991; Veldhuis et al., 2000; Park et al., 2004; Yasuor et al., 2010; Ma et al., 2013; Yu et al., 2013b). Some cereal crops (e.g. wheat, maize [Zea mays], barley [Hordeum vulgare], etc.) have a high capacity to metabolize certain herbicides; thus, these herbicides are used to control weeds across huge areas. This is a selection pressure for the evolution in weeds of the same capacity to metabolize herbicides.
(4) Most previous studies on metabolic resistance have been on grass species; however, metabolic resistance is beginning to be reported in dicot weed species, such as Amaranthus tuberculatus resistant to mesotrione and atrazine (Ma et al., 2013). This indicates that herbicide metabolism capacity exists in both grass and dicot weed species. Therefore, most weedy plant species and not just grass species are at risk of evolving metabolic herbicide resistance to selective herbicides.
(5) Herbicides must be used cautiously and at full rates. In addition, new crop-selective herbicide discoveries should be examined early to ascertain whether they are active on metabolic resistant weed populations (e.g. L. rigidum and A. myosuroides), so that the incidence of metabolic resistance can be managed proactively. Furthermore, care should be taken when tank mixing or applying herbicides sequentially, so that herbicides that can induce metabolic gene expression and hence cause herbicide antagonism are not used together (e.g. 2,4-D and ACCase- or AHAS-inhibiting herbicides).
(6) Compared with the high level of biochemical and molecular understanding of metabolic insecticide resistance (Li et al., 2007; Hoi et al., 2014), there is thus far limited progress in understanding and tackling plant metabolic herbicide resistance. Identifying the genes endowing metabolic resistance in weedy plants, and their regulation, are challenging but now underway in L. rigidum (Gaines et al., 2014), A. myosuroides (Cummins et al., 2013), and E. phyllopogon (Iwakami et al., 2014a, 2014b). A collaborative effort across Australia and Europe is beginning to tackle metabolic resistance in major crop weeds. Over the next few years, much should be revealed about the specific genes endowing metabolic resistance and the regulation of these genes. This information is essential for strategies to biochemically or genetically overcome metabolic resistance.
Acknowledgments
We thank Dr. Danica Goggin for valuable comments on and proofreading of the article.
Glossary
- P450
cytochrome P450 monooxygenases
- GT
glucosyl transferase
- GST
glutathione S-transferase
- AHAS
acetohydroxyacid synthase
- 2,4-D
2,4-dichlorophenoxyacetic acid
- RNA-seq
RNA sequencing
- NMO
nitronate monooxygenase
- ACCase
acetyl-coenzyme A carboxylase
- RNAi
RNA interference
Footnotes
This work was supported by the Grains Research and Development Corporation of Australia and the Australian Research Council.
References
- Adele P, Reichhart D, Salaün JP, Benveniste I, Durst F. (1981) Induction of cytochrome P-450 and monooxygenase activity by 2,4-dichlorophenoxyacetic acid in higher plant tissue. Plant Sci Lett 22: 39–46 [Google Scholar]
- Ahmad-Hamdani MS, Yu Q, Han HP, Cawthray G, Wang SF, Powles SB. (2013) Herbicide resistance endowed by enhanced rates of herbicide metabolism in wild oat (Avena spp.). Weed Sci 61: 55–62 [Google Scholar]
- Anderson MP, Gronwald JW. (1991) Atrazine resistance in a velvetleaf (Abutilon theophrasti) biotype due to enhanced glutathione S-transferase activity. Plant Physiol 96: 104–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckie HJ, Tardif FJ. (2012) Herbicide cross resistance in weeds. Crop Prot 35: 15–28 [Google Scholar]
- Bernard CB, Philogène BJ. (1993) Insecticide synergists: role, importance, and perspectives. J Toxicol Environ Health 38: 199–223 [DOI] [PubMed] [Google Scholar]
- Burnet MWM, Holtum JAM, Powles SB. (1994) Resistance to nine herbicide classes in a Lolium rigidum biotype. Weed Sci 42: 369–377 [Google Scholar]
- Burnet MWM, Loveys BR, Holtum JAM, Powles SB. (1993a) A mechanism of chlorotoluron resistance in Lolium rigidum. Planta 190: 182–189 [Google Scholar]
- Burnet MWM, Loveys BR, Holtum JAM, Powles SB. (1993b) Increased detoxification is a mechanism of simazine resistance in Lolium rigidum. Pestic Biochem Physiol 46: 207–218 [Google Scholar]
- Busi R, Neve P, Powles S. (2013) Evolved polygenic herbicide resistance in Lolium rigidum by low-dose herbicide selection within standing genetic variation. Evol Appl 6: 231–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busi R, Powles SB. (2013) Cross-resistance to prosulfocarb and triallate in pyroxasulfone-resistant Lolium rigidum. Pest Manag Sci 69: 1379–1384 [DOI] [PubMed] [Google Scholar]
- Busi R, Vila-Aiub MM, Powles SB. (2011) Genetic control of a cytochrome P450 metabolism-based herbicide resistance mechanism in Lolium rigidum. Heredity (Edinb) 106: 817–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey VF, Hoagland RE, Talbert RE. (1997) Resistance mechanism of propanil-resistant barnyardgrass. II. In vivo metabolism of the propanil molecule. Pestic Sci 49: 333–338 [Google Scholar]
- Christopher JT, Powles SB, Holtum JAM. (1992) Resistance to acetolactate synthase-inhibiting herbicides in annual ryegrass (Lolium rigidum) involves at least two mechanisms. Plant Physiol 100: 1909–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher JT, Powles SB, Liljegren DR, Holtum JA. (1991) Cross-resistance to herbicides in annual ryegrass (Lolium rigidum). II. Chlorsulfuron resistance involves a wheat-like detoxification system. Plant Physiol 95: 1036–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher JT, Preston C, Powles SB. (1994) Malathion antagonises metabolism-based chlorsulfuron resistance in Lolium rigidum. Pestic Biochem Physiol 49: 172–182 [Google Scholar]
- Cocker KM, Moss SR, Coleman JOD. (1999) Multiple mechanisms of resistance to fenoxaprop-P-ethyl in United Kingdom and other European populations of herbicide-resistant Alopecurus myosuroides (black grass). Pestic Biochem Physiol 65: 169–180 [Google Scholar]
- Cocker KM, Northcroft DS, Coleman JOD, Moss SR. (2001) Resistance to ACCase-inhibiting herbicides and isoproturon in UK populations of Lolium multiflorum: mechanisms of resistance and implications for control. Pest Manag Sci 57: 587–597 [DOI] [PubMed] [Google Scholar]
- Cole DJ, Edwards R (2000) Secondary metabolism of agrochemicals in plants. In TR Robert, ed, Agrochemicals and Plant Protection. John Wiley & Sons, Chichester, UK, pp 107–154 [Google Scholar]
- Cotterman JC, Saari LL. (1992) Rapid metabolic inactivation is the basis for cross-resistance to chlorsulfuron in diclofop-methyl-resistant rigid ryegrass (Lolium rigidum) biotype SR4/84. Pestic Biochem Physiol 43: 182–192 [Google Scholar]
- Coupland D, Lutman PJW, Heath C. (1990) Uptake, translocation and metabolism of mecaprop in a sensitive and resistant biotype of Stellaria media. Pestic Biochem Physiol 36: 61–67 [Google Scholar]
- Cummins I, Cole DJ, Edwards R. (1999) A role for glutathione transferases functioning as glutathione peroxidases in resistance to multiple herbicides in black-grass. Plant J. 18: 285–292 [DOI] [PubMed] [Google Scholar]
- Cummins I, Dixon DP, Freitag-Pohl S, Skipsey M, Edwards R. (2011) Multiple roles for plant glutathione transferases in xenobiotic detoxification. Drug Metab Rev 43: 266–280 [DOI] [PubMed] [Google Scholar]
- Cummins I, Moss S, Cole DJ, Edwards R. (1997) Glutathione transferases in herbicide-resistant and herbicide-susceptible black-grass (Alopecurus myosuroides). Pestic Sci 51: 244–250 [Google Scholar]
- Cummins I, Wortley DJ, Sabbadin F, He Z, Coxon CR, Straker HE, Sellars JD, Knight K, Edwards L, Hughes D, et al. (2013) Key role for a glutathione transferase in multiple-herbicide resistance in grass weeds. Proc Natl Acad Sci USA 110: 5812–5817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dam T, Guida AD, Hazel CB, Li B, Williams ME, inventors. September 13, 2007. Polynucleotide encoding a maize herbicide resistance gene and methods for use. US Patent Application No. 2007/0214515 A1
- Délye C. (2005) Weed resistance to acetyl coenzyme A carboxylase inhibitors: an update. Weed Sci 53: 728–746 [Google Scholar]
- Délye C. (2013) Unravelling the genetic bases of non-target-site-based resistance (NTSR) to herbicides: a major challenge for weed science in the forthcoming decade. Pest Manag Sci 69: 176–187 [DOI] [PubMed] [Google Scholar]
- Délye C, Gardin JAC, Boucansaud K, Chauvel B, Petit C. (2011) Non-target-site-based resistance should be at the centre of herbicide resistance research attention: black grass (Aleppcurus myosuroides) as an illustration. Weed Res 51: 433–437 [Google Scholar]
- Délye C, Menchari Y, Guillemin JP, Matéjicek A, Michel S, Camilleri C, Chauvel B. (2007) Status of black grass (Alopecurus myosuroides) resistance to acetyl-coenzyme A carboxylase inhibitors in France. Weed Res 47: 95–105 [Google Scholar]
- De Prado RA, Franco AR. (2004) Cross-resistance and herbicide metabolism in grass weeds in Europe: biochemical and physiological aspects. Weed Sci 52: 441–447 [Google Scholar]
- Duhoux A, Délye C. (2013) Reference genes to study herbicide stress response in Lolium sp.: up-regulation of P450 genes in plants resistant to acetolactate-synthase inhibitors. PLoS ONE 8: e63576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R, Cole DJ. (1996) Glutathione transferases in wheat (Triticum) with activity toward fenoxaprop-ethyl and other herbicides. Pestic Biochem Physiol 54: 96–104 [Google Scholar]
- Edwards R, Dixon DP (2000) The role of glutathione transferases in herbicides metabolism. In AH Cobb, RC Kirkwood eds, Herbicides and Their Mechanisms of Action. Sheffield Academic Press, Sheffield, UK, pp 38–71 [Google Scholar]
- Fischer AJ, Ateh CM, Bayer DE, Hill JE. (2000a) Herbicide-resistant early (Echinochloa orizoides) and later (E. phyllopogon) watergrass in California rice fields. Weed Sci 48: 225–230 [Google Scholar]
- Fischer AJ, Bayer DE, Carriere MD, Ateh CM, Yim KO. (2000b) Mechanisms of resistance to bispyribac-sodium in an Echinochloa phyllopogon accession. Pestic Biochem Physiol 28: 156–165 [Google Scholar]
- Fischer TC, Klattig JT, Gierl A. (2001) A general cloning strategy for divergent plant cytochrome P450 genes and its application in Lolium rigidum and Ocimum basilicum. Theor Appl Genet 103: 1014–1021 [Google Scholar]
- Fraga MI, Tasende MG. (2003) Mechanism of resistance to simazine in Sonchus oleraceus. Weed Res 43: 333–340 [Google Scholar]
- Gadda G, Francis K. (2010) Nitronate monooxygenase, a model for anionic flavin semiquinone intermediates in oxidative catalysis. Arch Biochem Biophys 493: 53–61 [DOI] [PubMed] [Google Scholar]
- Gaines TA, Lorentz L, Figge A, Herrmann J, Maiwald F, Ott MC, Han H, Busi R, Yu Q, Powles SB, et al. (2014) RNA-Seq transcriptome analysis to identify genes involved in metabolism-based diclofop resistance in Lolium rigidum. Plant J 78: 865–876 [DOI] [PubMed] [Google Scholar]
- Gimenez-Espinosa R, Romera E, Tena M, De Prodo R. (1996) Fate of atrazine in treated and pristine accessions of three Setaria species. Pestic Biochem Physiol 56: 196–207 [Google Scholar]
- Hall LM, Holtum JAM, Powles SB (1994) Mechanisms responsible for cross resistance and multiple resistance. In SB Powles, JAM Holtum, eds, Herbicide Resistance in Plants: Biology and Biochemistry. CRC Press, Boca Raton, FL, pp 243–261 [Google Scholar]
- Hall LM, Moss SR, Powles SB. (1995) Mechanism of resistance to chlorotoluron in two biotypes of the grass weed Alopecurus myosuroides. Pestic Biochem Physiol 53: 180–182 [Google Scholar]
- Hall LM, Moss SR, Powles SB. (1997) Mechanisms of resistance to aryloxyphenoxypropionate herbicides in two resistant biotypes of Alopecurus myosuroides (blackgrass): herbicide metabolism as a cross-resistance mechanism. Pestic Biochem Physiol 57: 87–98 [Google Scholar]
- Han H, Yu Q, Cawthray GR, Powles SB. (2013) Enhanced herbicide metabolism induced by 2,4-D in herbicide susceptible Lolium rigidum provides protection against diclofop-methyl. Pest Manag Sci 69: 996–1000 [DOI] [PubMed] [Google Scholar]
- Han HP, Yu Q, Vila-Aiub M, Powles SB. (2014) Genetic inheritance of cytochrome P450-mediated metabolic resistance to chlorsulfuron in a multiple herbicide resistant Lolium rigidum population. Crop Prot 65: 57–63 [Google Scholar]
- Heap I (2014) International Survey of Herbicide Resistant Weeds. www.weedscience.com (May 1, 2014)
- Heap I, Knight R. (1982) A population of ryegrass tolerant to the herbicide diclofop-methyl. J Aust Inst Agric Sci 48: 156–157 [Google Scholar]
- Heap I, Knight R. (1986) The occurrence of herbicide cross-resistance in a population of annual ryegrass, Lolium-rigidum, resistant to diclofop-methyl. Aust J Agric Res 37: 149–156 [Google Scholar]
- Hidayat I, Preston C. (1997) Enhanced metabolism of fluazifop acid in a biotype of Digitaria sanguinalis resistant to the herbicide fluazifop-P-butyl. Pestic Biochem Physiol 57: 137–146 [Google Scholar]
- Hidayat I, Preston C. (2001) Cross-resistance to imazethapyr in a fluazifop-P-butyl-resistant population of Digitaria sanguinalis. Pestic Biochem Physiol 71: 190–195 [Google Scholar]
- Hirose S, Kawahigashi H, Tagiri A, Imaishi H, Ohkawa H, Ohkawa Y. (2007) Tissue-specific expression of rice CYP72A21 induced by auxins and herbicides. Plant Biotechnol Rep 1: 27–36 [Google Scholar]
- Hoagland RE, Norsworthy JK, Carey F, Tallbert RE. (2004) Metabolically based resistance to the herbicide propanil in Echinochloa species. Weed Sci 52: 475–486 [Google Scholar]
- Hoi KK, Daborn PJ, Battlay P, Robin C, Batterham P, O’Hair RAJ, Donald WA. (2014) Dissecting the insect metabolic machinery using twin ion mass spectrometry: a single P450 enzyme metabolizing the insecticide imidacloprid in vivo. Anal Chem 86: 3525–3532 [DOI] [PubMed] [Google Scholar]
- Holtum JAM, Matthews JM, Häusler RE, Liljegren DR, Powles SB. (1991) Cross-resistance to herbicides in annual ryegrass (Lolium rigidum). III. On the mechanism of resistance to diclofop-methyl. Plant Physiol 97: 1026–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde RJ, Hallahan DL, Bowyer JR. (1996) Chlorotoluron metabolism in leaves of resistant and susceptible biotypes of the grass weed Alopecurus myosuroides. Pestic Sci 47: 185–190 [Google Scholar]
- Iwakami S, Endo M, Saika H, Okuno J, Nakamura N, Yokoyama M, Watanabe H, Toki S, Uchino A, Inamura T. (2014a) Cytochrome P450 CYP81A12 and CYP81A21 are associated with resistance to two acetolactate synthase inhibitors in Echinochloa phyllopogon. Plant Physiol 165: 618–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakami S, Uchino A, Kataoka Y, Shibaike H, Watanabe H, Inamura T. (2014b) Cytochrome P450 genes induced by bispyribac-sodium treatment in a multiple-herbicide-resistant biotype of Echinochloa phyllopogon. Pest Manag Sci 70: 549–558 [DOI] [PubMed] [Google Scholar]
- Iwakami S, Watanabe H, Miura T, Matsumoto H, Uchino A. (2014c) Occurrence of sulfonylurea resistance in Sagittaria trifolia, a basal monocot species, based on target-site and non-target-site resistance. Weed Biol Manage 14: 43–49 [Google Scholar]
- Kemp MS, Moss SR, Thomas TH (1990) Herbicide resistance in Alopecurus myosuroides In MB Green, H LeBaron, W Moberg, eds, Managing Resistance to Agrochemicals: From Fundamentals to Practical Strategies. American Chemical Society, Washington, DC, pp 376–393 [Google Scholar]
- Kreuz KK, Tommasini R, Martinoia E. (1996) Old enzyme for a new job: herbicide detoxification in plants. Plant Physiol 111: 349–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leah JM, Caseley JC, Riches CR, Valverde B. (1995) Age-related mechanisms of propanil tolerance in jungle-rice, Echinochloa colona. Pestic Sci 43: 347–354 [Google Scholar]
- Letouzé A, Gasquez J. (2001) Inheritance of fenoxaprop-P-ethyl resistance in a blackgrass (Alopecurus myosuroides Huds.) population. Theor Appl Genet 103: 288–296 [Google Scholar]
- Letouzé A, Gasquez J. (2003) Enhanced activities of several herbicide-degrading enzymes: a suggested mechanism responsible for multiple resistance in blackgrass (Alopecurus myosuroides Huds.). Agronomie 23: 601–608 [Google Scholar]
- Li X, Schuler MA, Berenbaum MR. (2007) Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu Rev Entomol 52: 231–253 [DOI] [PubMed] [Google Scholar]
- Liu S, Liang QM, Zhou WW, Jiang YD, Zhu QZ, Yu H, Zhang CX, Gurr GM, Zhu ZR. (2014) RNA interference of NADPH-cytochrome P450 reductase of the rice brown planthopper Nilaparvata lugens, increases susceptibility to insecticides. Pest Manag Sci (in press) 10.1002/ps.3760 [DOI] [PubMed] [Google Scholar]
- Llewellyn R, Powles SB. (2001) High levels of herbicide resistance in rigid ryegrass (Lolium rigidum) across the western Australian wheatbelt. Weed Technol 15: 242–248 [Google Scholar]
- Ma R, Kaundun SS, Tranel PJ, Riggins CW, McGinness DL, Hager AG, Hawkes T, McIndoe E, Riechers DE. (2013) Distinct detoxification mechanisms confer resistance to mesotrione and atrazine in a population of waterhemp. Plant Physiol 163: 363–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone JM, Boutsalis P, Baker J, Preston C. (2014) Distribution of herbicide-resistant acetyl-coenzyme A carboxylase alleles in Lolium rigidum across rain cropping areas of South Australia. Weed Res 54: 78–86 [Google Scholar]
- Manalil S, Busi R, Renton M, Powles SB. (2011) Rapid evolution of herbicide resistance by low herbicide dosages. Weed Sci 59: 210–217 [Google Scholar]
- Manalil S, Busi R, Renton M, Powles SB. (2012) An herbicide-susceptible rigid ryegrass (Lolium rigidum) population made even more susceptible. Weed Sci 60: 101–105 [Google Scholar]
- Maneechote C, Preston C, Powles SB. (1997) A diclofop-methyl-resistant Avena sterilis biotype with a herbicide-resistant acetyl-coenzyme A carboxylase and enhanced metabolism of diclofop-methyl. Pestic Sci 49: 105–114 [Google Scholar]
- Mao YB, Cai WJ, Wang JW, Hong GJ, Tao XY, Wang LJ, Huang YP, Chen XY. (2007) Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat Biotechnol 25: 1307–1313 [DOI] [PubMed] [Google Scholar]
- Marrs KA. (1996) The function and regulation of glutathione S-transferases in plants. Annu Rev Plant Physiol Plant Mol Biol 47: 127–158 [DOI] [PubMed] [Google Scholar]
- Matthews JM, Holtum JAM, Liljegren DR, Furness B, Powles SB. (1990) Cross-resistance to herbicides in annual ryegrass (Lolium rigidum). I. Properties of the herbicide target enzymes acetyl-coenzyme A carboxylase and acetolactate synthase. Plant Physiol 94: 1180–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez J, Bastida F, De Prado R. (2006) Resistance to chlorotoluron in a downy brome (Bromus tectorum) biotype. Weed Sci 54: 237–245 [Google Scholar]
- Menendez J, De Prado R. (1997) Diclofop-methyl cross-resistance in a chlorotoluron-resistant biotype of Alopecurus myosuroides. Pestic Biochem Physiol 56: 123–133 [Google Scholar]
- Morant M, Bak S, Møller BL, Werck-Reichhart D. (2003) Plant cytochromes P450: tools for pharmacology, plant protection and phytoremediation. Curr Opin Biotechnol 14: 151–162 [DOI] [PubMed] [Google Scholar]
- Moss SR, Cussans GW. (1985) Variability in the susceptibility of Alopecurus myosuroides (black-grass) to chlorotoluron and isoproturon. Asp Appl Biol 9: 91–98 [Google Scholar]
- Neve P, Powles S. (2005a) Recurrent selection with reduced herbicide rates results in the rapid evolution of herbicide resistance in Lolium rigidum. Theor Appl Genet 110: 1154–1166 [DOI] [PubMed] [Google Scholar]
- Neve P, Powles S. (2005b) High survival frequencies at low herbicide use rates in populations of Lolium rigidum result in rapid evolution of herbicide resistance. Heredity (Edinb) 95: 485–492 [DOI] [PubMed] [Google Scholar]
- Osuna MD, Vidotto F, Fischer AJ, Bayer DE, De Prado R, Ferrero A. (2002) Cross-resistance to bispyribac-sodium and bensulfuron-methyl in Echinochloa phyllopogon and Cyperus difformis. Pestic Biochem Physiol 73: 9–17 [Google Scholar]
- Owen M, Walsh M, Llewellyn R, Powles SB. (2007) Widespread occurrence of multiple herbicide resistance in Western Australian annual ryegrass (Lolium rigidum) populations. Aust J Agric Res 58: 711–718 [Google Scholar]
- Owen MJ, Goggin DE, Powles SB. (2012) Non-target-site-based resistance to ALS-inhibiting herbicides in six Bromus rigidus populations from Western Australian cropping fields. Pest Manag Sci 68: 1077–1082 [DOI] [PubMed] [Google Scholar]
- Owen MJ, Martinez NJ, Powles SB. (2014) Multiple herbicide-resistant Lolium rigidum (annual ryegrass) now dominates across the Western Australia grain belt. Weed Res 54: 314–324 [Google Scholar]
- Pan G, Zhang X, Liu K, Zhang J, Wu X, Zhu J, Tu J. (2006) Map-based cloning of a novel rice cytochrome P450 gene CYP81A6 that confers resistance to two different classes of herbicides. Plant Mol Biol 61: 933–943 [DOI] [PubMed] [Google Scholar]
- Park KW, Fandrich L, Mallory-Smith CA. (2004) Absorption, translocation, and metabolism of propoxycarbazone-sodium in ALS-inhibitor resistant Bromus tectorum biotypes. Pestic Biochem Physiol 79: 18–24 [Google Scholar]
- Powles SB, Holtum JAM, Matthews JM, Liljegren DR (1990) Herbicide cross-resistance in annual ryegrass (Lolium rigidum Gaud). In MB Green, H LeBaron, W Moberg, eds, Managing Resistance to Agrochemicals: From Fundamentals to Practical Strategies. American Chemical Society, Washington, DC, pp 394–406 [Google Scholar]
- Powles SB, Matthews JM (1992) Multiple herbicide resistance in annual ryegrass (Lolium rigidum): a driving force for the adoption of integrated weed management strategies. In I Denholm, A Devonshire, D Holloman, eds, Resistance 91: Achievements and Developments in Combating Pesticide Resistance. Elsevier Press, London, pp 75–87 [Google Scholar]
- Powles SB, Yu Q. (2010) Evolution in action: plants resistant to herbicides. Annu Rev Plant Biol 61: 317–347 [DOI] [PubMed] [Google Scholar]
- Preston C. (2003) Inheritance and linkage of metabolism-based herbicide cross-resistance in rigid ryegrass (Lolium rigidum). Weed Sci 51: 4–12 [Google Scholar]
- Preston C. (2004) Herbicide resistance in weeds endowed by enhanced detoxification: complication for management. Weed Sci 52: 448–453 [Google Scholar]
- Preston C, Powles SB. (1997) Light-dependent enhanced metabolism of chlorotoluron in a substituted urea herbicide-resistant biotype of Lolium rigidum Gaud. Planta 201: 202–208 [Google Scholar]
- Preston C, Tardif FJ, Christopher JT, Powles SB. (1996) Multiple resistance to dissimilar herbicide chemistries in a biotype of Lolium rigidum due to enhanced activity of several herbicide degrading enzymes. Pestic Biochem Physiol 54: 123–134 [Google Scholar]
- Saika H, Horita J, Taguchi-Shiobara F, Nonaka S, Ayako NY, Iwakami S, Hori K, Matsumoto T, Tanaka T, Itoh T, et al. (2014) A novel rice cytochrome P450 gene, CYP72A31, confers tolerance to acetolactate synthase-inhibiting herbicides in rice and Arabidopsis. Plant Physiol 166: 1232–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammons RD, Ivashuta S, Liu H, Wang D, Feng PCC, Kouranov AY, Andersen SE inventors. December 1, 2011. Polynucleotide molecules for gene regulation in plants. US Patent Application No. US2011/0296556 A1
- Schuler MA, Werck-Reichhart D. (2003) Functional genomics of P450s. Annu Rev Plant Biol 54: 629–667 [DOI] [PubMed] [Google Scholar]
- Shimabukuro RH, Walsh WC, Hoerauf RA. (1979) Metabolism and selectivity of diclofop-methyl in wild oat and wheat. J Agric Food Chem 27: 615–623 [DOI] [PubMed] [Google Scholar]
- Shimabukuro RH, Walsh WC, Jacobson A. (1987) Aryl-O-glucoside of diclofop: a detoxification product in wheat shoots and wild oat-cell suspension-culture. J Agric Food Chem 35: 393–397 [Google Scholar]
- Siminszky B. (2006) Plant cytochrome P450-mediated herbicide metabolism. Phytochem Rev 5: 445–458 [Google Scholar]
- Singh S, Kirkwood RC, Marshall G. (1998) Effect of the monooxygenase inhibitor piperonyl butoxide on the herbicidal activity and metabolism of isoproturon on herbicide resistant and susceptible biotypes of Phalaris minor and wheat. Pestic Biochem Physiol 59: 143–153 [Google Scholar]
- Sweetser PB, Schow GS, Hutchison JM. (1982) Metabolism of chlorsulfuron by plants: biological basis for selectivity of a new herbicide for cereals. Pestic Biochem Physiol 17: 18–23 [Google Scholar]
- Tardif FJ, Holtum JAM, Powles SB. (1993) Occurrence of a herbicide resistant acetyl-coenzyme A carboxylase mutant in annual ryegrass (Lolium rigidum) selected by sethoxydim. Planta 190: 176–181 [Google Scholar]
- Tardif FJ, Powles SB. (1994) Herbicide multiple-resistance in a Lolium rigidum biotype is endowed by multiple mechanisms: isolation of a subset with resistant acetyl-CoA carboxylase. Physiol Plant 91: 488–494 [Google Scholar]
- Tardif FJ, Powles SB. (1999) Effect of malathion on resistance to soil-applied herbicides in a population of rigid ryegrass (Lolium rigidum). Weed Sci 47: 258–261 [Google Scholar]
- Tardif FJ, Preston C, Holtum JAM, Powles SB. (1996) Resistance to acetyl-coenzyme A carboxylase inhibiting herbicides endowed by a single major gene encoding a resistant target site in a biotype of Lolium rigidum. Aust J Plant Physiol 23: 15–23 [Google Scholar]
- Tranel PJ, Wright TR. (2002) Resistance of weeds to ALS inhibiting herbicides: what have we learned? Weed Sci 50: 700–712 [Google Scholar]
- Veldhuis LJ, Hall LM, O’Donovan JT, Dyer W, Hall JC. (2000) Metabolism-based resistance of a wild mustard (Sinapis arvensis L.) biotype to ethametsulfuron-methyl. J Agric Food Chem 48: 2986–2990 [DOI] [PubMed] [Google Scholar]
- Vila-Aiub MM, Neve P, Powles SB. (2005a) Resistance cost of a cytochrome P450 herbicide metabolism mechanism but not an ACCase target site mutation in a multiple resistant Lolium rigidum population. New Phytol 167: 787–796 [DOI] [PubMed] [Google Scholar]
- Vila-Aiub MM, Neve P, Powles SB. (2009) Evidence for an ecological cost of enhanced herbicide metabolism in Lolium rigidum. J Ecol 97: 772–780 [Google Scholar]
- Vila-Aiub MM, Neve P, Steadman KJ, Powles SB. (2005b) Ecological fitness of a multiple herbicide-resistant Lolium rigidum population: dynamics of seed germination and seedling emergence of resistant and susceptible phenotypes. J Appl Ecol 42: 288–298 [Google Scholar]
- Werck-Reichhart D, Hehn A, Didierjean L. (2000) Cytochromes P450 for engineering herbicide tolerance. Trends Plant Sci 5: 116–123 [DOI] [PubMed] [Google Scholar]
- Yasuor H, Osuna MD, Ortiz A, Saldain NE, Eckert JW, Fisher A. (2009) Mechanism of resistance to penoxsulam in late watergrass (Echinochloa phyllopogon (Stapf) Koss). J Agric Food Chem 57: 3653–3660 [DOI] [PubMed] [Google Scholar]
- Yasuor H, Zou W, Tolstikov VV, Tjeerdema RS, Fischer AJ. (2010) Differential oxidative metabolism and 5-ketoclomazone accumulation are involved in Echinochloa phyllopogon resistance to clomazone. Plant Physiol 153: 319–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Ahmad-Hamdani MS, Han H, Christoffers MJ, Powles SB. (2013a) Herbicide resistance-endowing ACCase gene mutations in hexaploid wild oat (Avena fatua): insights into resistance evolution in a hexaploid species. Heredity (Edinb) 110: 220–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Han H, Cawthray GR, Wang SF, Powles SB. (2013b) Enhanced rates of herbicide metabolism in low herbicide-dose selected resistant Lolium rigidum. Plant Cell Environ 36: 818–827 [DOI] [PubMed] [Google Scholar]
- Yu Q, Han H, Powles SB. (2008) Mutations of the ALS gene endowing resistance to ALS-inhibiting herbicides in Lolium rigidum populations. Pest Manag Sci 64: 1229–1236 [DOI] [PubMed] [Google Scholar]
- Yu Q, Powles SB. (2014) Resistance to AHAS inhibitor herbicides: current understanding. Pest Manag Sci 70: 1340–1350 [DOI] [PubMed] [Google Scholar]
- Yuan JS, Tranel PJ, Stewart CN., Jr (2007) Non-target-site herbicide resistance: a family business. Trends Plant Sci 12: 6–13 [DOI] [PubMed] [Google Scholar]
- Yun MS, Yogo Y, Miura R, Yamasue Y, Fischer A. (2005) Cytochrome P-450 monooxygenase activity in herbicide-resistant and susceptible late watergrass (Echinochloa phyllopogon). Pestic Biochem Physiol 83: 107–114 [Google Scholar]
- Zimmerlin A, Durst F. (1990) Xenobiotic metabolism in plants: aryl hydroxylation of diclofop by a cytochrome-P-450 enzyme from wheat. Phytochemistry 29: 1729–1732 [Google Scholar]