Abstract
Mast cell progenitors (MCp) leave the bone marrow and migrate to peripheral tissues where they mature. Although the existence of committed MCp in adult mouse and human blood has been postulated, they have never been found. We have isolated a rare population of cells in adult mouse blood, committed to the mast cell lineage. These were identified as lineage− c-kithi ST2+ integrin β7hi CD16/32hi cells. Moreover, a major difference in maturity of these cells based on FcεRI expression was observed between the Th2-prone BALB/c strain and the Th1-prone C57BL/6 strain (66% vs 25% FcεRI+, respectively). Therefore, the choice of mouse strain is critical when studying disease models such as experimental asthma where mast cells and their progenitors are involved.
Keywords: blood, mast cell progenitors, mast cells
To cite this article: Dahlin JS, Heyman B, Hallgren J. Committed mast cell progenitors in mouse blood differ in maturity between Th1 and Th2 strains. Allergy 2013; 68: 1333–1337.
In allergic individuals, IgE-sensitized mast cells are activated by allergens, leading to the release of pro-inflammatory mediators that participate in the allergic response. In patients with allergic asthma, mast cell numbers are increased in the lung, which likely worsen the symptoms 1,2. This increase in mast cells is explained by the recruitment of mast cell progenitors (MCp) into the tissue 3. Even though these MCp need to migrate through the blood, committed MCp in adult mouse and human blood have not previously been found 4,5. However, they are present in fetal mouse blood and decline in frequency from gestation day 15.5 until birth 6. The first identification of committed MCp in adult mice was done in 2005 by three different laboratories. Arinobu et al. 4 found committed MCp in the intestine and bipotent basophil/mast cell progenitors in the spleen, whereas the other studies 5,7 identified committed MCp in the bone marrow.
Here, we isolate an extremely rare population of committed MCp in naïve mouse blood using gradient centrifugation and flow cytometry with double sorting followed by culture in a myeloerythroid cytokine cocktail. The MCp constituted 0.0045% of the mononuclear cells (MNC) in adult BALB/c blood and were identified as lineage− (Lin−) c-kithi ST2+ integrin β7hi CD16/32hi cells. Using FcεRI expression as a maturation marker, these cells were less mature in C57BL/6 mice than in BALB/c mice.
Materials and methods
Animals
BALB/c mice from Bommice (Ry, Denmark) were maintained at the National Veterinary Institute (Uppsala, Sweden). C57BL/6 mice were from Scanbur AB (Sollentuna, Sweden). All experiments were approved by the Ethics Committee in Uppsala.
Preparation of blood MNC
Six- to twenty-week-old mice were euthanized with isoflurane (Schering Plough A/S, Farum, Denmark), and approximately 700 μl of blood was withdrawn into 300 μl 2 mg/ml EDTA in PBS, pH 7.4. The blood was diluted 1 : 1 in RPMI 1640 supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, 10 mM HEPES, 1 mM sodium pyruvate, and 50 μM 2-ME (all from Sigma-Aldrich, St. Louis, MO, USA). MNC were isolated using Ficoll-Paque PREMIUM (GE Healthcare Bio-sciences AB, Uppsala, Sweden). Platelets were removed by centrifugation.
Analyses of blood MCp
To quantify MCp in a limiting dilution assay, the isolated MNC were plated and cultured with mast cell growth factors 8. The frequency of MCp was estimated as described 8. For FACS sorting and flow cytometric analysis, MNC were resuspended in PBS with 2% FCS (Sigma-Aldrich), washed, and stained. Cells were doubly sorted on a FACS Aria III (BD Biosciences, Franklin Lakes, NJ, USA) into 96-well plates for multiple cells or 60-well Terasaki plates for single cells. Doublet cells were excluded by forward and side scatter properties. The sorted cells were cultured in a myeloerythroid cytokine cocktail 4 (Peprotech, Rocky Hill, NJ, USA) with 10 ng/ml erythropoietin (R&D Systems, Minneapolis, MN, USA). Flow cytometry was performed on a LSR II (BD), and the data were analyzed using FlowJo (Tree Star Inc., Ashland, OR, USA). Photographs were taken with a Nikon Eclipse 90i microscope using a Nikon DS-Fi1 camera and NIS-Elements AR 3.2 software (Nikon, Melville, NY, USA). The objective used was a Plan Apo 40x, numerical aperture 0.95 (Nikon).
Antibodies
MNC were stained with PE–cyanine 7 anti-c-kit (2B8), PE or V450 Horizon anti-CD16/32 (2.4G2), FITC anti-integrin β7 (FIB504), PE–cyanine 5 antilineage antibodies CD3 (17A2), CD4 (GK1.5), CD8b (eBioH35-17.2), CD11b (M1/70), CD19 (ebio1D3), Gr-1 (RB6-8C5), B220 (RA3-6B2), TER-119 (TER-119), PE anti-FcεRI (MAR-1), and biotinylated anti-T1/ST2 (DJ8) followed by streptavidin–allophycocyanin or allophycocyanin–cyanine 7. Cultured cells were pre-incubated with unlabeled anti-CD16/32 and stained with antibodies against c-kit and FcεRI. The antibodies were from BD Biosciences, eBioscience (Hatfield, UK) or MD Bioproducts (Zürich, Switzerland).
Results and discussion
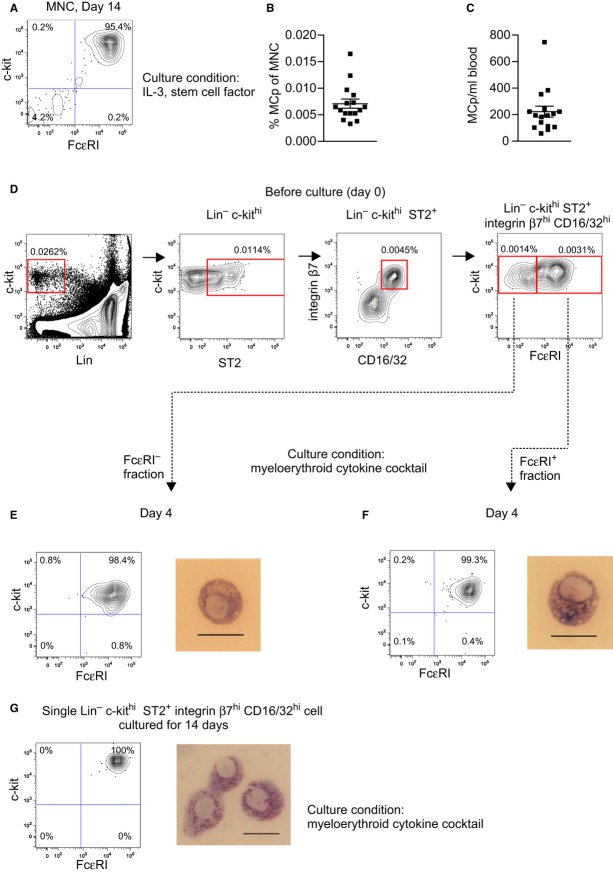
To determine the mast cell-forming capacity in blood from BALB/c mice, MNC were cultured using a limiting dilution assay with IL-3 and stem cell factor, known to selectively induce mast cells from bone marrow 9. After 14 days, 25 randomly selected wells containing colonies were analyzed and they all consisted of c-kit+ FcεRI+ mast cells (Fig.1A). Thus, the number of MCp was estimated to 0.0071 ± 0.0009% of the MNC (Fig.1B), equivalent to 223 ± 42 MCp/ml blood (Fig.1C). This was comparable to the results by Sonoda et al. 10, where the frequency of MCp was estimated by injection of limitedly diluted wild-type blood cells into the skin of mast cell-deficient mice.
Figure 1.
Lin− c-kithi ST2+ integrin β7hi CD16/32hi cells in the blood are committed to the mast cell lineage. (A) MNC were extracted from blood of BALB/c mice and cultured in IL-3 and stem cell factor in a limiting dilution assay. Twenty-five randomly selected wells with colonies from 3 experiments were analyzed with flow cytometry on day 14. A representative graph is shown. (B) The frequency of MCp per MNC and (C) the number of MCp per milliliter blood was estimated in 16 individual mice pooled from three independent experiments. Mean ± SEM is shown. (D) Blood MNC from BALB/c mice were analyzed with flow cytometry, and the gating strategy of Lin− c-kithi ST2+ integrin β7hi CD16/32hi cells is shown. The Lin− c-kithi ST2+ integrin β7hi CD16/32hi fraction was subdivided into FcεRI− and FcεRI+ cells, sorted into different wells, and cultured in a myeloerythroid cytokine cocktail. The graphs represent flow cytometric analysis of Lin− c-kithi ST2+ integrin β7hi CD16/32hi FcεRI− cells (E left) or Lin− c-kithi ST2+ integrin β7hi CD16/32hi FcεRI+ cells (F left) cultured for 4 days. Lin− c-kithi ST2+ integrin β7hi CD16/32hi FcεRI− cells (E right) or Lin− c-kithi ST2+ integrin β7hi CD16/32hi FcεRI+ cells (F right) were cultured for 4 days and stained with May–Grünwald–Giemsa. Graphs and photographs from day 4 are representative of 2 experiments. The scale bars correspond to 20 μm. (G) Single cells from the Lin− c-kithi ST2+ integrin β7hi CD16/32hi fraction were sorted into separate wells of Terasaki plates and cultured for 14 days in a myeloerythroid cytokine cocktail. Random wells containing at least 200 cells were analyzed with flow cytometry (G left). One representative graph is shown out of 38. May–Grünwald–Giemsa staining of representative day 14 cells is shown (G right). The scale bar corresponds to 20 μm. The experiment was performed three times with similar results.
Next, we wanted to identify which individual MNC in blood were committed to the mast cell lineage. Sorted cells were cultured in a myeloerythroid cytokine cocktail, which has previously been used to define committed MCp in other organs 4. As mast cells can have segmented nuclei when cultured in vitro 11, it is difficult to distinguish mast cells and basophils by morphology. Thus, flow cytometry was used to verify lineage commitment. A rare population of Lin− c-kithi ST2+ integrin β7hi CD16/32hi cells was identified (Fig.1D). These cells were subdivided into FcεRI− and FcεRI+ cells, which were sorted and cultured (Fig.1D). On average, three cells of the FcεRI− and 11 cells of the FcεRI+ phenotype could be isolated per milliliter blood after sorting. After 4 days, both fractions gave rise to more than 98% c-kit+ FcεRI+ mast cells, with little or no contamination of other cells (Fig.1E left, F left). The analysis was performed at this relatively early time point, since short-lived basophils would still be present 4. May–Grünwald–Giemsa staining showed that the mast cells were about to fill up their granules (Fig.1E right, F right). Possibly, the cells cultured from the original FcεRI+ fraction had a higher extent of filled granules than the FcεRI− fraction (Fig.1E right, F right). In two additional experiments, all Lin− c-kithi ST2+ integrin β7hi CD16/32hi cells irrespective of FcεRI expression were sorted, cultured for 4 days, and analyzed with flow cytometry with similar results as in Fig.1E, F (data not shown). To verify that the blood MCp developed into mature mast cells, single Lin− c-kithi ST2+ integrin β7hi CD16/32hi cells were sorted into individual Terasaki wells and cultured for 14 days. Consistent with the data from day 4, these cells gave rise to 100% c-kit+ FcεRI+ mast cells (Fig.1G left), which had now developed fully metachromatic granules (Fig.1G right). Collectively, our data suggest that Lin− c-kithi ST2+ integrin β7hi CD16/32hi are committed MCp. Earlier attempts to identify MCp in blood of naïve animals have failed 4,5,12. Though, in rats, immature cells phenotypically similar to bone marrow MCp and peritoneal mast cells have been found in blood after transient depletion of mast cells in the peritoneum 12. The MCp that we have identified are present in naïve mouse blood, and they are committed to the mast cell lineage. The majority of the blood MCp expressed FcεRI suggesting that they are more mature than the committed MCp found in bone marrow, which completely lack FcεRI expression 5.
Previous studies aiming to elucidate the mechanisms behind the recruitment of MCp to the lung upon antigen-induced allergic lung inflammation have identified differences in the pathways of C57BL/6 and BALB/c mice 13. Therefore, we investigated whether those two strains had the same population of blood MCp. The C57BL/6 and the BALB/c mice had similar frequencies of Lin− c-kithi ST2+ integrin β7hi CD16/32hi cells in their blood (Fig.2A). However, on average 66% of the MCp were FcεRI+ in BALB/c mice, whereas only 25% were positive in C57BL/6 mice (Fig.2B). This indicates that the blood MCp are less mature in C57BL/6 mice. Nonetheless, the majority of the Lin− c-kithi ST2+ integrin β7hi CD16/32hi cells from C57BL/6 blood had developed into c-kit+ FcεRI+ mast cells after 4 days (Fig.2C left). The percentage of c-kit+ FcεRI+ mast cells was slightly lower than that in BALB/c mice (compare Fig.1E,F with Fig.2C left), again suggesting that the blood MCp in C57BL/6 mice are more immature. Though, after 9 days in culture, the Lin− c-kithi ST2+ integrin β7hi CD16/32hi cells from the C57BL/6 mice gave rise to 99.8% c-kit+ FcεRI+ mast cells (Fig.2C right).
Figure 2.
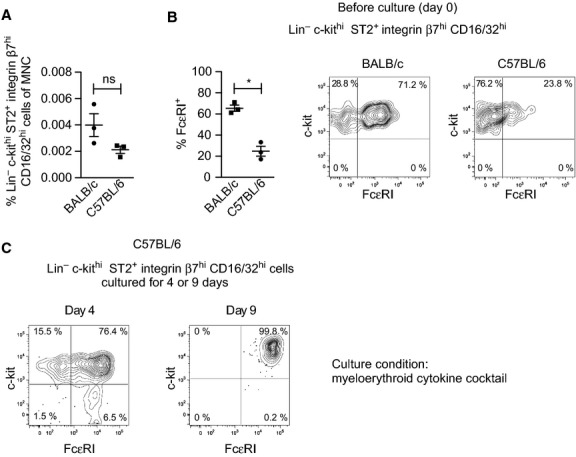
Blood MCp in C57BL/6 mice are less mature than those in BALB/c mice. (A, B) Blood MNC were stained, analyzed with flow cytometry, and Lin− c-kithi ST2+ integrin β7hi CD16/32hi cells were gated as described in Fig.1D. The frequency of Lin− c-kithi ST2+ integrin β7hi CD16/32hi cells per MNC (A) and the percentage of FcεRI+ MCp (B) were quantified in three independent experiments where BALB/c and C57BL/6 mice were analyzed in parallel. Each dot represents blood pooled from 5 to 6 male mice with an age of 6–9 weeks. The graphs represent means ± SEM. Two-tailed Student's t-tests were used to compare the groups. ns = not significant (P > 0.05), *P < 0.01. (C) Lin− c-kithi ST2+ integrin β7hi CD16/32hi cells from C57BL/6 mice were sorted, cultured for 4 or 9 days in a myeloerythroid cytokine cocktail, and analyzed by flow cytometry. The day 4 graph is representative of two independent experiments, and the day 9 graph is from one experiment.
In conclusion, we have identified a rare population of committed MCp in adult mouse blood. These Lin− c-kithi ST2+ integrin β7hi CD16/32hi cells constituted 0.0045% of the blood MNC, comparable to the total number of cells with mast cell–forming capacity in the blood (compare Fig.1B with D). We also found that the MCp in the Th1-prone C57BL/6 mice were less mature than that in Th2-prone BALB/c mice, which may explain why the migratory properties of MCp differ between these two strains 13. In analogy, it is possible that MCp from allergic patients are more mature than those of healthy people. This needs to be considered in the search for committed MCp from human peripheral blood.
Acknowledgments
We thank Zhoujie Ding for technical help. Flow cytometry was performed at the BioVis platform, SciLifeLab, Uppsala.
Author contributions
J.S.D. and J.H. designed the research; J.S.D. performed the research; J.S.D., B.H., and J.H. analyzed the data; and J.S.D., B.H., and J.H. wrote the article.
Funding
This work was supported by grants from the Swedish Research Council (J.H. and B.H.) and Malin and Lennart Philipson Foundation (J.H.).
Conflicts of interest
The authors declare no competing financial interests.
References
- Andersson CK, Bergqvist A, Mori M, Mauad T, Bjermer L, Erjefalt JS. Mast cell-associated alveolar inflammation in patients with atopic uncontrolled asthma. J Allergy Clin Immunol. 2011;127:905–912. doi: 10.1016/j.jaci.2011.01.022. [DOI] [PubMed] [Google Scholar]
- Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346:1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- Hallgren J, Gurish MF. Mast cell progenitor trafficking and maturation. Adv Exp Med Biol. 2011;716:14–28. doi: 10.1007/978-1-4419-9533-9_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arinobu Y, Iwasaki H, Gurish MF, Mizuno S, Shigematsu H, Ozawa H, et al. Developmental checkpoints of the basophil/mast cell lineages in adult murine hematopoiesis. Proc Natl Acad Sci USA. 2005;102:18105–18110. doi: 10.1073/pnas.0509148102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamur MC, Grodzki AC, Berenstein EH, Hamawy MM, Siraganian RP, Oliver C. Identification and characterization of undifferentiated mast cells in mouse bone marrow. Blood. 2005;105:4282–4289. doi: 10.1182/blood-2004-02-0756. [DOI] [PubMed] [Google Scholar]
- Rodewald HR, Dessing M, Dvorak AM, Galli SJ. Identification of a committed precursor for the mast cell lineage. Science. 1996;271:818–822. doi: 10.1126/science.271.5250.818. [DOI] [PubMed] [Google Scholar]
- Chen CC, Grimbaldeston MA, Tsai M, Weissman IL, Galli SJ. Identification of mast cell progenitors in adult mice. Proc Natl Acad Sci USA. 2005;102:11408–11413. doi: 10.1073/pnas.0504197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlin JS, Feinstein R, Cui Y, Heyman B, Hallgren J. CD11c+ cells are required for antigen-induced increase of mast cells in the lung. J Immunol. 2012;189:3869–3877. doi: 10.4049/jimmunol.1201200. [DOI] [PubMed] [Google Scholar]
- Tsai M, Takeishi T, Thompson H, Langley KE, Zsebo KM, Metcalfe DD, et al. Induction of mast cell proliferation, maturation, and heparin synthesis by the rat c-kit ligand, stem cell factor. Proc Natl Acad Sci USA. 1991;88:6382–6386. doi: 10.1073/pnas.88.14.6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda T, Ohno T, Kitamura Y. Concentration of mast-cell progenitors in bone marrow, spleen, and blood of mice determined by limiting dilution analysis. J Cell Physiol. 1982;112:136–140. doi: 10.1002/jcp.1041120120. [DOI] [PubMed] [Google Scholar]
- Gurish MF, Friend DS, Webster M, Ghildyal N, Nicodemus CF, Stevens RL. Mouse mast cells that possess segmented/multi-lobular nuclei. Blood. 1997;90:382–390. [PubMed] [Google Scholar]
- Jamur MC, Moreno AN, Mello LF, Souza Junior DA, Campos MR, Pastor MV, et al. Mast cell repopulation of the peritoneal cavity: contribution of mast cell progenitors versus bone marrow derived committed mast cell precursors. BMC Immunol. 2010;11:32. doi: 10.1186/1471-2172-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TG, Finkelman FD, Austen KF, Gurish MF. T regulatory cells control antigen-induced recruitment of mast cell progenitors to the lungs of C57BL/6 mice. J Immunol. 2010;185:1804–1811. doi: 10.4049/jimmunol.1001146. [DOI] [PMC free article] [PubMed] [Google Scholar]