Abstract
This white paper identifies knowledge gaps and new challenges in healthcare epidemiology research, assesses the progress made toward addressing research priorities, provides the Society for Healthcare Epidemiology of America (SHEA) Research Committee’s recommendations for high-priority research topics, and proposes a road map for making progress toward these goals. It updates the 2010 SHEA Research Committee document, “Charting the Course for the Future of Science in Healthcare Epidemiology: Results of a Survey of the Membership of SHEA,” which called for a national approach to healthcare-associated infections (HAIs) and a prioritized research agenda. This paper highlights recent studies that have advanced our understanding of HAIs, the establishment of the SHEA Research Network as a collaborative infrastructure to address research questions, prevention initiatives at state and national levels, changes in reporting and payment requirements, and new patterns in antimicrobial resistance.
I. BACKGROUND
Healthcare-associated infections (HAIs) continue to pose a major challenge to healthcare professionals in all healthcare settings. Research on prevention of HAIs remains essential, especially in light of new and emerging pathogens such as carbapenem-resistant Enterobacteriaceae (CRE), which pose major challenges for treatment. In 2010, a white paper by the Society for Healthcare Epidemiology of America (SHEA) Research Committee outlined priorities for “a national approach to HAIs: scrutinizing the science base, developing a prioritized research agenda, conducting studies that address the questions that have been identified, creating and deploying guidelines that are based on the outcomes of these studies, and then initiating new studies that assess the efficacy of the interventions.”1(p118)
In recent years, a number of studies have advanced further the understanding of HAIs as being largely preventable.2 Simultaneously, prevention of HAIs has attracted increasing visibility, and HAIs have come under enhanced scrutiny by healthcare personnel (HCP), patients, and regulatory agencies.3 Numerous initiatives have been put into place at state and national levels, including required reporting of certain HAIs, public availability of HAI rates, and tying prevention of HAIs to hospital reimbursement.4,5 In addition to achieving the desired outcome of putting HAI prevention front and center in the patient safety movement, these initiatives raised important issues regarding the standardization of measurement and reporting, resources needed to ensure accurate and comprehensive surveillance, and knowledge gaps in HAI epidemiology and prevention.6–9
This white paper identifies knowledge gaps and new challenges, assesses the progress made toward addressing research priorities, provides the SHEA Research Committee’s recommendations for high-priority research topics, and proposes a road map for making progress toward these goals.
II. KNOWLEDGE GAPS IN ASSESSMENT AND MEASUREMENT OF HAIS
A. Surveillance
For surveillance measurements to impact outcomes, collected data elements and reported rates must be valid, reliable, accurate, and actionable. Subjective elements in definitions are a primary problem with HAI surveillance. Additional limitations of current surveillance for several HAIs include variation in the timing with which “hospital-associated” is defined and variability in methods for case finding. The Centers for Disease Control and Prevention (CDC) National Healthcare Safety Network (NHSN) recently revised a number of surveillance definitions and is planning to implement additional revisions in the near future to address many of these limitations (Table 1).10–12 The NHSN also has proposed revisions to definitions for infections in long-term care.13 Despite these improvements, additional research is needed to address a number of remaining knowledge gaps as well as to assess the impact of these new definitions on surveillance processes and outcomes. Broadly, these research topics include:
TABLE 1.
Major National Healthcare Safety Network (NHSN) Modifications in Healthcare-Associated Infection (HAI) Definitions, 2013 and 2014
| HAI | NHSN terminology change | Criteria and/or criteria modifications | Justification for changes | Timing |
|---|---|---|---|---|
| VAP | VAE, divided into 3 tiers:
|
Under new definition algorithm, patient is identified as having VAE when mechanical ventilation is received for >2 calendar days with a combination of:
Criteria apply to:
|
Reduces:
Improves:
Includes potentially automatable component. |
2013 |
| CLABSI | MBI-LCBI | Under new definition, patient is identified as having MBI-LCBI (a primary BSI) when meeting criteria for: Presence of selected organisms (viridans group streptococci, Enterobacteriaceae, Candida species, certain anaerobes) AND neutropenia:
Allogeneic hematopoietic stem cell transplant:
|
January 2013 | |
| SSI | None | Modifications to NHSN operative procedure definition:
|
|
|
| CAUTI | None | Modifications to NHSN definition:
|
|
2013 |
NOTE. ANC, absolute neutrophil count; CAUTI, catheter-associated urinary tract infection; CLABSI, central-line associated bloodstream infection; HAI, healthcare-associated infection; IVAC, infection-related ventilator-associated complication; MBI-LCBI, mucosal barrier injury–laboratory-confirmed bloodstream infection; NHSN, National Health and Safety Network; SSI, surgical site infection; UTI, urinary tract infection; VAC, ventilator-associated condition; VAE, ventilator-associated event; VAP, ventilator-associated pneumonia.
How to use relatively objective criteria to improve the reliability of HAI surveillance definitions while retaining clinical relevance and credibility;
Best ways to improve the efficiency of HAI surveillance methods and how to better utilize existing health information technology (HIT) and provide guidance for future HIT developments;
How to improve performance of HAI surveillance across the continuum of healthcare, including ambulatory sites, long-term care facilities, and tracking of patients who seek care across multiple healthcare facilities;
How to improve performance of HAI surveillance for special populations, including pediatric patients;
How to improve methods to provide HAI surveillance data to healthcare colleagues, hospital administrators, payors, and patients to drive improvements in HAI prevention practices and how to effectively account for nonmodifiable risk factors and place emphasis on facilities that continue to need improvement.
1. Ventilator-Associated Pneumonia (VAP)
VAP historically has been difficult to standardize because of the nonspecific nature of the signs and symptoms, poor interrater reliability in interpretation of radiographic findings, and variation in lower respiratory tract sampling methods.14,15
Improvements in VAP rates have been difficult to link to improvements of more objective outcomes, including duration of mechanical ventilation, duration of intensive care unit (ICU) stay, and mortality.16,17 The NHSN has proposed new working definitions for ventilator-associated events (VAEs) for surveillance in adult populations (Table 1). As these new definitions become widely used for surveillance, additional research is needed to better understand the clinical relevance and preventability of the events detected and how best to utilize VAE surveillance to drive improvements in clinical outcomes.
2. Central Line–Associated Bloodstream Infection (CLABSI)
Despite tremendous strides in the prevention of CLABSI in the ICU in recent years,2 research is needed to address the reliability and validity of surveillance definitions, with the goal of revising CLABSI definitions to identify infections that are likely to be preventable through additional improvements in central line insertion and maintenance. A considerable number of ICU-acquired bacteremia may be related to mucosal translocation rather than catheter-derived, and prevention efforts related to catheter insertion and maintenance are not likely to impact bacteremia acquired by this mechanism.18 Another major challenge in CLABSI surveillance relates to the large proportion of bloodstream infections (BSIs) that are not reported because they are attributed to another site. Adjudicating primary versus secondary BSIs includes invoking subjective non-BSI infection criteria and may result in misclassification.19 Although “getting to zero” for CLABSI and other HAIs is a laudable goal, it is not realistic to achieve unless the prevention efforts align with pathogenesis and reliable, accurate, and valid metrics are used to gauge the effectiveness of prevention tailored to the patient population under consideration.20–24
As an example, the NHSN’s ongoing work to evaluate a modified definition, termed “Mucosal Barrier Injury–Laboratory Confirmed Bloodstream Infection” (MBI-LCBI; Table 1) can be used to exclude from the CLABSI definition episodes of bacteremia among oncology patients and hematopoietic stem cell transplantation recipients that are likely to represent translocation of gastrointestinal organisms rather than infections attributable to intravascular catheters. Additional research is needed to evaluate the reliability, variability, and impact of this and other possible modifications to CLABSI surveillance definitions and to assess methods to increase the efficiency of surveillance through use of automated data.
3. Surgical Site Infection (SSI)
SSI surveillance is challenging and complex because of the wide array of surgical procedures, the variety of settings in which these procedures take place, prolonged follow-up, and the need to collect detailed denominator data. Considerable interfacility variability also exists in SSI surveillance, particularly with respect to case finding.25–27
Advancements are already underway by the Centers for Medicaid and Medicare Services (CMS) and NHSN to recommend use of administrative data to identify medical records for SSI surveillance. This methodology has been shown to improve case capture and reporting of SSI while decreasing the labor needed for surveillance.28–31 Additional research is needed to identify strategies to standardize and enhance the application of SSI surveillance methods across facilities through use of automated data. In addition, research is needed to evaluate surveillance definitions and methods that can be used to detect SSIs associated with procedures that are performed in non–acute care settings and to track surgical complications for patients who seek healthcare at multiple facilities.
4. Catheter-Associated Urinary Tract Infection (CAUTI)
CAUTI definitions currently require a combination of laboratory and clinical criteria (Table 1); however, distinguishing asymptomatic bacteriuria from clinical infection remains problematic, particularly among critically ill patients. Research is needed to identify CAUTI definitions that rely on relatively objective criteria and are associated with meaningful clinical outcomes; also needed are novel interventions for reducing catheter use and CAUTI, evaluation of implementation strategies to disseminate best practices for CAUTI prevention, and antibiotic stewardship for reducing unnecessary antibiotic use in response to asymptomatic bacteriuria in catheterized patients.
B. Improving Risk Adjustment for HAIs
To make meaningful and fair comparisons across institutions, it is essential to adjust for factors other than the quality of care that may influence the risk of infection. These may be specific to the type of HAI under consideration, but most generally include case-mix, length of stay, and device use. Case-mix refers to the diversity, clinical complexity, and need for resources in the population of all the patients in the facility.
With hospitals now required to publicly report HAI data through the NHSN, risk adjustment becomes an even more important consideration. Publicly reported HAI data should account for variability in patient case mix, adjust for non-modifiable risk factors, and be based on consistent case detection systems, without surveillance becoming overly onerous to those conducting the surveillance. One example of recent NHSN efforts to improve risk adjustment is the current use of procedure-specific, multivariate risk models that incorporate additional weighted patient factors, which could calculate more credible, standardized, and reliable risk-adjusted SSI metrics than the previously used NHSN risk index.10 Additional research is needed to further improve the ability to meaningfully risk adjust HAI data without substantially increasing data collection efforts.32
C. Burden of HAIs in Settings across the Spectrum of Healthcare
Although a considerable body of literature has accumulated regarding HAI epidemiology, pathogenesis, and prevention in large tertiary care settings, data in settings other than acute care are limited. In particular, there is a paucity of data on the transmission dynamics of multidrug-resistant organisms (MDROs) at the intra- and interfacility level.33,34 Most studies that have addressed this issue have used point prevalence studies, which have limitations regarding determination of where and when acquisition of MDROs occurred. Even within acute care, most of the current evidence base has been generated solely or largely from ICU populations. As HAI incidence decreases in ICU settings, the focus on acute care, non-ICU inpatients rises, but the extrapolation of acute care ICU study findings to other healthcare settings remains problematic because of differences in healthcare system infrastructure, infection prevention staffing, patient/resident population, and acuity of illness.
A number of examples indicate the need for research focused on non-ICU settings. A considerable portion of CLABSIs now occur in non-ICU inpatients and outpatients. VAP data in patients who receive long-term mechanical ventilation are limited but urgently needed. Studies further suggest that long-term care facilities may be large, underappreciated reservoirs of MDROs, yet few studies have evaluated prevention efforts in long-term care.35 Similarly, little is known regarding the burden of HAIs in long-term acute care hospitals (LTACHs), critical access and small rural and community hospitals, emergency departments, and ambulatory care. Finally, the majority of C. difficile infection, although healthcare associated, begins outside of the acute care hospital.36 For example, the current paradigm of screening and isolation for containment of MDROs in acute care may not be tenable or desirable in long-term care settings where stays are indefinite and facilities serve as resident homes.37 Equally importantly, the financial impact of HAIs in non-ICU settings has not been adequately studied.
Understanding the transmission dynamics of MDROs and devising prevention strategies in non–acute care settings has taken on even more urgency as accumulating literature suggests that these settings may be major reservoirs of new and emerging MDROs, such as CRE and MDR Acinetobacter species.38–40 As healthcare settings continue to evolve and transitions of care become more complex and fluid, these gaps in our knowledge need to be addressed to effectively and cost-efficiently contain HAIs across the spectrum of healthcare.
III. RESEARCH GAPS IN THE PREVENTION OF HAIS
Despite recent advances in HAI prevention, a number of gaps remain in our ability to effectively prevent HAIs.
A. Technologies and Products
The last few years have seen an unprecedented increase in information technologies and devices to detect and prevent HAIs.
1. Technologies for Detection of HAIs
Traditional methods of surveillance are labor intensive and often limited in scope. Automated surveillance systems have the potential to streamline and facilitate efficient review of relevant data and detection of outbreaks. On the other hand, HAI surveillance data generated by institutions using electronic surveillance compared with those that use traditional chart review may differ considerably, and this variability may have implications for reported measures. Automated methods of surveillance may also require intensive information technology support for troubleshooting, validating, and updating the software or the incoming data.
Automated surveillance can expand and better define the scope of infection prevention and stewardship activities, reduce infection prevention time spent on surveillance and clerical tasks, and improve response to public health issues. As the burden of regulatory compliance grows, automated surveillance programs may have tools to facilitate reporting to external agencies. However, these systems are expensive.41 A recent Association for Professionals in Infection Control and Epidemiology (APIC) position paper describes the critical elements to evaluate when considering use of an automated surveillance system. Some currently available automated surveillance systems are being used for research purposes, and as this area grows in scope and the use of automated surveillance systems increases, it will lend itself to robust research studies designed to examine the reliability and validity of electronic surveillance, its impact on surveillance and infection preventionists’ workload and activities, and its impact on clinically relevant outcomes.42,43
2. Technologies for Prevention of HAIs
New technologies are playing an increasingly prominent role in infection prevention activities. For example, there are new devices to facilitate environmental decontamination at terminal cleaning to prevent C. difficile infection (CDI) and MDROs. These systems include hydrogen peroxide vapor, ultraviolet light, self-disinfecting surfaces, and sporicidal disinfectants. However, head-to-head comparisons of these products are lacking, and additional study on their practicality, efficacy, and cost effectiveness is urgently needed.42,43
Hand hygiene remains challenging in healthcare institutions and monitoring, and encouraging compliance with hand hygiene is very labor intensive. Using observers to monitor hand hygiene captures less than 1% of hand hygiene opportunities. Electronic monitoring tools for hand hygiene that use trigger devices and feedback to capture and promote compliance have become available.44
Although there have been several feasibility pilot studies that suggest that electronic monitoring tools may be both sensitive and specific, the cost of these systems may be prohibitive, their role in managing hand hygiene is unclear, and additional study is needed in this area.44,45
With the rapid implementation of electronic medical records, electronic orders are increasingly being used to generate reminders of appropriate urinary catheter use and to prompt automatic removal. Using these reminders and stop orders can significantly reduce CAUTI rates; intervention studies regarding urinary catheter reminders and stop orders have previously employed all levels of technology (ranging from paper and verbal orders to electronic stop orders).46
B. Pediatric-Specific Issues
Pediatric facilities face unique challenges in preventing HAIs. Contagious infectious diseases make up a large proportion of pediatric hospitalizations. Many hospitalized children are especially susceptible to these infections because of a lack of opportunity to be fully vaccinated and age-dependent immaturity of the immune system (potential lack of a robust innate immune response or a memory immune response). In addition, because of the developmental immaturity of children, close interaction with HCP and the environment is the norm, and family members are often integrated into care processes. Despite these challenges, less emphasis has been placed on exploring pediatric epidemiology of important emerging HAIs, such as infections due to C. difficile and MDROs, and critically appraising new technology or other infection prevention interventions in the pediatric setting.47,48 Future directions of pediatric HAI research ideally would focus on evaluating pediatric care delivery and how that might impact infection prevention strategies, defining pediatric epidemiology of important HAIs, examining the role of shared play areas and toys in transmission of pathogens, and critically assessing novel technology and prevention strategies in pediatric settings.
C. Practices in Infection Prevention
Substantial knowledge gaps exist in our grasp of the pathogenesis and epidemiology of HAIs, including MDRO transmission and the understanding of the effectiveness of specific infection prevention practices (Table 2). As an example, we discuss HCP vaccination and demonstrate the effect of improved practices in infection prevention.
TABLE 2.
Examples of High-Priority Topics in Infection Prevention Research Identified by the Society for Healthcare Epidemiology of America Research Committee
| Topic | Examples of specific areas for investigation |
|---|---|
| HAI | Evaluate HAI prevention across the spectrum of healthcare especially non-acute care settings; Evaluate approaches for dissemination and implementation of HAI prevention methods; Evaluate role of electronic monitoring tools in managing hand hygiene compliance. |
| Device-associated infections (CLABSI, CAUTI, VAE) | Examine the epidemiology of DAI in non-ICU settings; Test novel technology and strategies for DAI prevention such as impregnated devices and maintenance bundles; Examine the reliability and validity of surveillance definitions in different patient populations and their impact on outcomes and practices. |
| SSI | Compare various postoperative wound care strategies for reducing SSIs; Assess the impact of an operating room checklist on SSI rates; Evaluate patient-specific risk factor modification (such as smoking cessation) strategies for reducing SSIs. |
| MDROs and Clostridium difficile | Assess transmission dynamics and novel interventions to prevent transmission in acute and non-acute care settings; Evaluate the role of the environment and the impact of environmental disinfection on transmission; Examine the role of laboratory technology to identify MDROs and guide infection prevention measures. |
| Employee health | Identify approaches to improve influenza and other vaccinations in HCP in settings where mandatory vaccination is not feasible; Evaluate practices to prevent needlestick injuries and other bloodborne pathogen exposures in HCP and explore methods for post-exposure prophylaxis for prevention of HIV, HCV, and HBV; Assess the role of HCP in transmitting organisms including MDROs to patients. |
| Respiratory viruses | Evaluate the effects of barrier precautions on respiratory virus transmission; Assess the acceptability of N-95 masks for prevention of respiratory virus transmission; Evaluate the role of novel diagnostics in preventing nosocomial respiratory viruses and identifying emerging respiratory viruses. |
| Antimicrobial stewardship | Evaluate the impact of antimicrobial stewardship programs on emergence of resistance, patient outcomes, and cost; Explore the benefits of alternative methods for antimicrobial stewardship such as post-prescription review; Assess the use of performance metrics for antimicrobial stewardship. |
| Environment | Compare available touchless cleaning technologies for efficacy and acceptability; Assess favored methods for surveillance of environmental cleaning; Assess the role of hospital epidemiologists and infection preventionists in changing policy related to environmental cleaning. |
NOTE. CAUTI, catheter-associated urinary tract infection; CLABSI, central line-associated bloodstream infection; DAI, device-associated infection; HAI, healthcare-associated infection; HBV, hepatitis B virus; HCP, healthcare personnel; HCV, hepatitis C virus; ICU, intensive care unit; MDRO, multidrug-resistant organism; SSI, surgical site infection; VAE, ventilator-associated event.
1. HCP Vaccination
Vaccination of HCP for influenza is a cornerstone of an effective influenza control plan. Higher vaccination levels among staff have been associated with a lower risk of nosocomial influenza cases and outbreaks and a lower risk of influenza-related illness and deaths, especially in long-term care settings.49–51 Since July 2007, the Joint Commission has required accredited critical access hospitals, other hospitals, and long-term care centers to establish annual influenza vaccination programs. Unfortunately, despite tremendous efforts to promote HCP influenza vaccination by government agencies, regulatory groups, professional societies, and visible vaccination champions, influenza vaccination rates among HCP remain unacceptably low. SHEA endorses a policy in which annual influenza vaccination is a condition of both initial and continued HCP employment and/or professional privileges.52 However, only a minority of healthcare institutions have successfully implemented influenza vaccination as a condition of employment, and additional studies are needed to build the evidence-base and promote uptake and translation of this practice.53
The incidence of pertussis in the United States has been increasing in recent years.54,55 The transmission of pertussis in healthcare settings has important medical and economic consequences. HCP are a priority group for vaccination because of their increased risk of acquiring infection and the potential to transmit pertussis to high-risk patients; however, studies suggest that misconceptions regarding the pertussis vaccine are common in HCP, and in a recent Web-based survey, 2 factors were negatively associated with intent to receive vaccination: the presence of children in the HCP home (odds ratio [OR], 0.69), and employment as a nurse (OR, 0.59).56 Additional research is needed to determine the acceptability of pertussis vaccines among HCP, the duration of immunity after booster doses, methods of optimizing compliance with vaccination, and the impact of vaccination on the management of pertussis exposures in healthcare settings.
IV. EVOLVING RESEARCH AGENDA
The pressing issues in HAI prevention revolve around (1) improving our understanding of pathogenesis and epidemiology of HAIs, including the role of MDROs, risk factors, and HAI burden across different healthcare settings; (2) devising appropriate and widely generalizable strategies to prevent HAIs using knowledge generated through research on pathogenesis and epidemiology; (3) rigorously testing those strategies for efficacy, effectiveness, and cost-effectiveness; and (4) effectively and promptly meeting the challenge of containing new and emerging HAIs and MDROs (Table 2).
This agenda is ambitious but important. A well-planned and coordinated research infrastructure capable of addressing a variety of research needs and questions is essential to tackle these questions adequately in a multifaceted manner.
V. BUILDING THE INFRASTRUCTURE TO ADVANCE RESEARCH AGENDA
A. Advances in Study Design
Infection prevention research has undergone considerable evolution over the last decade, with a better application of quasi-experimental design. As a result, methods have improved from the original core literature, which was focused largely on interventions in response to outbreaks. Although many study designs are applicable to infection control research, the most robust method for testing infection control and prevention interventions is the randomized controlled trial (RCT). Generally, multisite studies are needed to achieve sufficient statistical power. A snapshot of major multisite infection prevention studies in the last few years is shown in Table 3. Cluster RCTs that randomize healthcare units or entire facilities provide an ideal and much needed method for comparing the effectiveness of quality improvement strategies that cannot be allocated at an individual level. In the last few years, an increase in cluster RCTs for testing of infection prevention interventions has occurred. Cluster RCTs are advantageous, because they allow comparisons of infection prevention strategies under conditions of actual use and account for confounding factors. When large cluster RCTs are performed across a variety of healthcare facilities, these trials can achieve broad generalizability and sufficient power to answer important questions that are unable to be answered by single center or small multicenter studies. They can also be considerably more cost-effective than RCTs, because they may be able to leverage existing quality improvement infrastructure. The ability to harness administrative capacities of healthcare systems to make data collection less cumbersome is needed as an important enhancement to the practicality of RTCs.
TABLE 3.
Recent Published Major Multisite Clinical Trials Evaluating Interventions to Prevent Healthcare-Associated Infection
| Study | Study design |
Intervention | Comparator | Outcome | Results | Populations | No. of centers/ sites |
|---|---|---|---|---|---|---|---|
| Bode et al66 | RCT | Rapid screening for S. aureus and decolonization with topical mupirocin and chlorhexidine in hospitalized patients | Placebo | Hospital-associated S. aureus infection | Rate of hospital-associated S. aureus infection was lower in the group that received intervention | Medical and surgical patients (majority were surgical patients) | 5 |
| Bennett-Guerrer et al67 | Multicenter RCT | Gentamicin-collagen sponge | Standard care | SSI in 60 days following procedure | Rate of SSI was higher with use of the sponge | Colorectal surgical patients | 39 sites |
| Bennett-Guerrer et al68 | Multicenter RCT | Gentamicin-collagen sponge | Standard care | Sternal wound infection in 60 days following procedure | No difference in rate of sternal wound infection | Cardiothoracic surgical patients | 48 sites |
| Derde et al69 | Interrupted time series RCT | 6 months universal CHG bathing and hand hygiene improvement for 6 months followed by rapid screening | Conventional screening | Acquisition of resistant bacteria per 100 patient-days at risk | Reduction in resistant bacteria with improved hand hygiene and unit-wide CHG bathing. No additional reduction in acquisition with rapid testing or conventional screening and isolation of carriers. | ICU patients | 13 ICUs |
| Harris et al59 | Multicenter Cluster RCT | Universal gloves and gowns in all ICU patient contacts and when entering rooms | CDC recommendation for gloves and gowns when patients have known MRSA or VRE | Colonization acquisition rates of MRSA and VRE | No decrease the combined acquisition of MRSA and VRE, but 40% decrease in MRSA acquisition without increasing the rate of adverse events | Medical and surgical ICU patients | 20 ICUs |
| Huskins et al70 | Cluster RCT | Active surveillance for MRSA and VRE | No active surveillance | Incidence of MRSA and VRE colonization or infection | incidence of colonization or infection with MRSA or VRE per 1000 patient-days, did not differ significantly between the intervention and control ICUs | Adult ICU patients | 19 ICUs |
| Loeb et al71 | RCT | N-95 respirator | Surgical mask | Laboratory confirmed influenza | Surgical mask noninferior to N-95 respirator | ED, medical and pediatric areas | 8 |
| Rabih et al72 | RCT | Chlorhexidine-alchohol skin scrub | Povidone-iodine scrub | SSI within 30 days of surgery | Lower rates of SSI in chlorhexidine-alcohol group | Adult patient undergoing clean contaminated surgery | 6 |
| Milstone et al47 | Cluster RCT with crossover | Daily chlorhexidine bathing | Standard bathing practices | Bacteremia | Lower incidence of bacteremia among patients receiving daily CHG bathing | Pediatric ICU | |
| Timsit et al73 | RCT | Chlorhexidine impregnated sponge dressing and frequency of dressing changes every 7 days | Standard dressing changed at 3 days | Catheter related infection | Chlorhexidine impregnated sponge dressings decreased the rates of major catheter-related infection; dressing change frequency every 7 days did not impact catheter colonization | Adult ICUs | 5 sites/7 ICUs |
| Timsit et al74 | RCT | Chlorhexidine impregnated gel dressing and highly adherent dressing | Standard dressing | Catheter related infection | Chlorhexidine impregnated gel dressings decreased the rates of major catheter-related infection; highly adherent dressings did not impact catheter-related infection | Adult ICUs | 12 ICUs |
| de Smet et al75 | Cluster RCT with crossover | Selective digestive tract decontamination (SDD) and selective oropharyngeal decontamination (SOD) | No SDD or SOD | Survival and bacteremia | improved 28 day survival and reduced incidence of ICU acquired bacteremia in SDD/SOD group | Adults ICUs | 13 ICUs |
| Climo et al76 | Cluster RCT with crossover | Daily chlorhexidine bathing | Standard bathing practices | MRSA, VRE and bacteremia | Decreased acquisition of VRE and decreased bacteremia (primarily contaminants) | Adult ICUs | 9 ICUs/6 sites |
| Huang et al77 | Cluster RCT | Daily chlorhexidine bathing and intranasal mupiricin | Active MRSA surveillance with isolation or universal decolonization | MRSA clinical cultures and all cause bacteremia | Decreased MRSA clinical cultures and decreased bacteremias (primarily contaminants) with universal daily chlorhexidine and mupiricin | Adult ICU | 74 ICUs |
| Pickard et al78 | Cluster RCT | Silver or nitrofural-coated urinary catheters | Standard PTFE catheterization. | Symptomatic CAUTI | No clinically relevant decrease in symptomatic CAUTI with either type of impregnated catheter | Hospitalized patients | 24 |
NOTE. CAUTI, catheter-associated urinary tract infection; ED, emergency department; ICU, intensive care unit; MRSA, methicillin-resistant Staphylococcus aureus; PTFE, polytetrafluoroethylene; RCT, randomized controlled trial; VRE, vancomycin-resistant enterococci; SDD, selective digestive tract decontamination; SOD, selective oropharyngeal decontamination.
A number of issues must be overcome before cluster RCTs become commonly used.57 First, institutions must reach agreement on the concept of group randomization, which may involve waiver of individual informed consent. Currently, institutions vary in their approach to and acceptance of trials that randomize entire units of individuals. Second, infrastructure must be developed to allow involvement of multiple facilities in the most streamlined way possible. Third, regulatory requirements, such as institutional review board (IRB) approval, must be streamlined, ideally by creating or using an existing central IRB to which participating institutions can defer. Fourth, study designs within cluster RCTs should be explored to better achieve balance of baseline covariates, such as use of stratified randomization or a crossover design.
B. Coordination of Research from Discovery to Dissemination
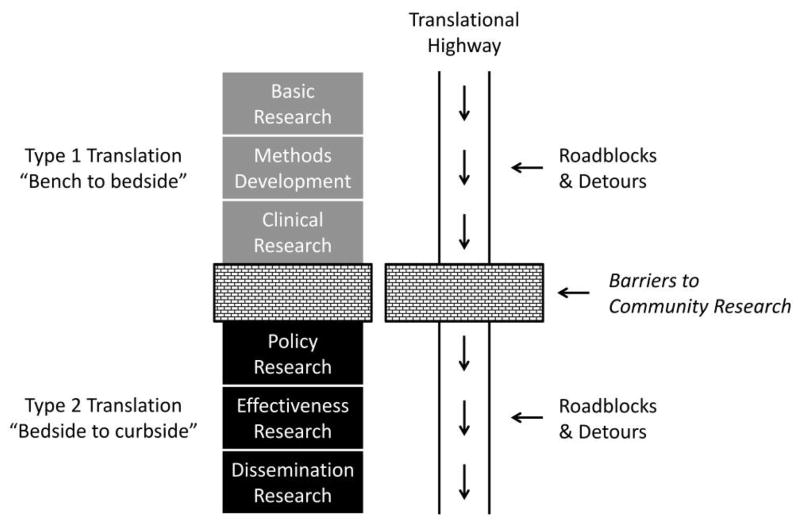
Research in HAI prevention is needed on all fronts and across the entire spectrum of basic and translational research. Transmission of MDROs serves as an example. Basic research elucidates the molecular basis of resistance and transmission, which leads to clinical and translational research to identify risk factors and mechanisms of transmission. Researchers should employ clinical trials (phases 1 to 3) to test interventions to reduce transmission in a variety of settings. Implementation and dissemination research would be used to examine effectiveness, feasibility, and fidelity of large-scale implementation across the entire spectrum of healthcare. Health services research would assess the outcomes of MDRO transmission and prevention of transmission as well as the institutional and societal economic cost of these infections. Finally, health policy research would examine the public health impact of governance strategies on HAI prevention. The spectrum of research is summarized in Figure 1.
FIGURE 1.
The continuum of translational research.
C. Collaborations to Facilitate Multisite Research
1. The SHEA Research Network
The previous SHEA white paper identified creation of a national research consortium as a priority. To achieve this aim, the SHEA Research Network (SRN) was established. The characteristics of the more than 200 participating institutions are summarized in Table 4.
TABLE 4.
Characteristics of Organizations in the Society for Healthcare Epidemiology of America Research Network
| Type of organization | hospitals |
|---|---|
| Participating hospitals | 244 |
| Teaching hospitals | 152 |
| Tertiary care hospitals | 118 |
| Public hospitals | 108 |
| Children’s hospitals | 26 |
| Government/Department of Veterans Affairs hospitals | 10 |
| Long-term acute care hospitals | 7 |
| Those with adult ICUs | ~185 |
| Those with pediatric ICUs (pediatric or NICU) | ~30 |
| Unique principal investigators | 265 |
| Electronic/IRB resources | 69 |
| Hospitals with access to electronic microbiology results | 79 |
| Access to demographic, admission and discharge information electronically | 77 |
| Those with local IRB | 75 |
NOTE. ICU, intensive care unit; IRB, institutional review board; NICU, neonatal intensive care unit.
An early study that employed the SRN collected data from 39 hospitals in 22 states (Figure 2) and provided an important contribution to the literature by demonstrating that the CMS nonpayment policy for hospital-acquired CAUTI did not result in overtesting to screen for and document a diagnosis of urinary tract infection as present at admission.58 Another major multicenter cluster RCT recently published using the SRN tested the effect of universal gowning and gloving in ICUs on transmission of methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE) as well as the impact on overall adverse events.59
FIGURE 2.
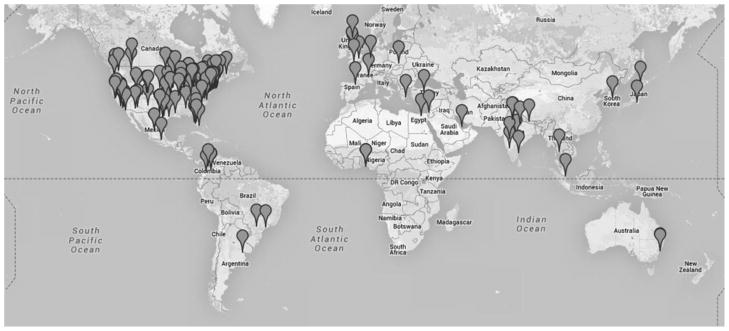
Geographic location of institutions participating in the Society for Healthcare Epidemiology of America (SHEA) Research Network.
These studies address the research priorities laid out by the SHEA Research Committee as identified by SHEA membership. The SRN has been used in over 8 studies on HAI prevention policy, influenza preparedness, public reporting of HAIs, surveillance of HAIs, antimicrobial susceptibility patterns, S. aureus infection prevention, and practices to prevent multidrug-resistant gram-negative infections.
The SRN has a number of strengths. Single-center studies are often not generalizable. For many unanswered questions in HAI prevention, single-center studies may have difficulty accruing enough subjects to provide conclusive answers. From this standpoint, the SRN serves as an easy-to-access collection of diverse healthcare institutions that are interested and able to participate in infection prevention studies. However, there are also a number of challenges that will need to be addressed: (1) SRN institutions are voluntary, and projects must have a limited scope without external funding; (2) variations in IRB requirements are common and pose challenges for approval across different universities and hospitals; (3) lack of standardization of data collection across institutions limits participation.
2. Promoting International Collaboration to Reduce HAIs
HAIs are an important public health problem in developing countries. The prevalence is generally higher in developing countries than in developed nations, and HAIs are a major cause of morbidity, mortality, and economic costs.60 Developing nations are classified by the World Bank by economy and gross national income (previously known as gross domestic product) per capita. These include approximately 147 countries in Latin America, Sub-Saharan Africa, Southeastern Europe, and major parts of Asia-Pacific. Major issues in HAI prevention in developing nations include lack of surveillance and infection control infrastructure and resources, unknown burden and epidemiology of HAIs, emergence of major antimicrobial resistance and MDROs, and unrestricted antibiotic use, often coupled with political instability and economic constraints. With the recognition that antibiotic resistance is a global problem, close collaboration between industrialized and developing nations is needed for capacity building, technology transfer, training resources, and surveillance and prevention activities.
3. Funding Sources
To close the gaps enumerated in this paper, the ability to conduct well-designed, large-scale studies is essential. To undertake this work, funding organizations must make HAI prevention research a priority. Historically, funding for HAI research has been very limited compared with that for many other disciplines of comparable magnitude and importance.61–63 The previous SHEA white paper highlighted this major obstacle to progress. With increasing visibility and calls to action for HAI research, federal organizations have begun to release funding opportunities for HAI research. For example, the Agency for Healthcare Research and Quality (AHRQ) and the CDC have released recent funding opportunities focused on HAI prevention. Although this is a welcome step, additional support in this area is needed urgently. In this regard, funding by industry offers potential opportunities but also necessitates careful, considered navigation of interactions and collaboration between industry and academia to ensure that research is free of bias and conflict of interest.
VI. RECOMMENDATIONS
A. Research Agenda
The previous white paper established the need to create a research infrastructure to address pressing questions in HAI prevention as designated by a SHEA membership survey. These priorities included (1) preventing the spread of multidrug-resistant aerobic gram-negative bacilli (eg, Acinetobacter species and Pseudomonas species) in healthcare settings; (2) implementing effective strategies to ensure antimicrobial stewardship in healthcare settings; (3) preventing the spread of and infections due to MRSA in healthcare settings; (4) developing effective strategies to ensure adherence to hand hygiene standards; and (5) developing strategies to prevent C. difficile in healthcare settings.
Since 2009, when the previous white paper was published, the groundwork has been laid and important landmark studies are underway to address some of these questions. However, the body of literature needed to close the gaps in these areas is still very much in its infancy. The SHEA Research Committee recommends that these same key areas remain priorities for the national research agenda.
B. Further Development and Maturation of Research Consortium
As mentioned above, the formative work in creating the SRN has been undertaken. Future steps should include (1) more international collaboration; (2) assuring a mix of institutions with adequate representation by nonacademic centers, long-term care facilities, LTACHs, ambulatory surgical centers, dialysis, pediatric hospitals, and large outpatient practices and VA hospitals; (3) streamlining IRB and other regulatory issues across the SRN to allow a rapid response and deployment of institutions for urgent emerging infections; (4) secure funds to create an infrastructure that can provide pilot funding for important questions and promising projects; (5) development of processes to allow efficient extraction of electronic medical record (EMR) data across varied types of EMR capability across institutions; (6) development of a process to collaborate effectively with industry and address potential conflicts of interest; (7) development of a Web portal where data regarding the characteristics of the SHEA member institutions can reside securely, so these data will be readily accessed for purposes of assessing feasibility of using the SRN for a certain project; and (8) exploration of the use of the SRN for health services research on HAIs, including financial and clinical outcomes, as well as for implementation and dissemination research. We anticipate that the next decade will be a productive time with generation of new knowledge critical to lead HAI prevention effectively.
Acknowledgments
N.S. reports research grants and/or contracts from the US Department of Veterans Affairs (VA) and the National Institutes of Health (NIH; AG40669). D.J.A. reports a financial relationship with UpToDate Online, a company involved in treatment of subjects, and having received research grants or contracts from the Centers for Disease Control and Prevention (CDC), Duke University/Duke Infection Control Outreach Network (U54CK000164), NIH/National Institute of Allergy and Infectious Diseases (NIAID) Duke University (K23AI095357), and CDC/Duke University (U54CK000172-01). B.I.B. reports having received research grants or contracts from The Joint Commission: Agency for Healthcare Research and Quality (AHRQ; 1R13HS022174-0112) and the CDC National Institute for Occupational Safety and Health National Personal Protective Technology Laboratory. P.C. reports being an advisor or consultant for Ecolab and holding patents, copyrights, or licenses from Steris. S.C. reports having received research grants or contracts from Viropharma, Merck, and Cubist for clinical trials in C. difficile infection. A.H. reports financial involvement with companies involved in the treatment of subjects and having served in an advisory or consultant role for Premier, Cubist, and UpToDate. S.S.H. reports leading a clinical trial in which participating hospitals receive contributed product from Sage. E.L. reports having received research grants or contracts from AHRQ, the CDC, and the state of Pennsylvania. D.R.L. reports having received research grants/contracts from Doris Duke Charitable Foundation, the University of Pennsylvania, and the Pennsylvania Department of Health. J.M. reports having received research grants or contracts from AHRQ, VA National Center for Patient Safety, and the NIH/National Institute of Nursing Research. L.G.M. reports having received research grant or contracts from Merck Harbor-UCLA and Cerexa and honoraria from Durata. A.M. reports a financial relationship with Sage Products, a company involved in the treatment of subjects, and past research support and research grants or contracts from the NIH. D.M. reports serving in an advisory or consultant role for Welch-Allyn. D.Y. reports having received research grants or contracts from the CDC (1U54 CK000172-01). D.M.Z. reports having received research grants or contracts from the NIH/NIAID and the NIH/National Cancer Institute.
Footnotes
Potential conflicts of interest. All other authors report no conflicts of interest relevant to this article.
References
- 1.Research Committee of the Society of Healthcare Epidemiology of America. Enhancing patient safety by reducing healthcare-associated infections: the role of discovery and dissemination. Infect Control Hosp Epidemiol. 2010;31:118–123. doi: 10.1086/650198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725–2732. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 3.Affordable Care Act S. [Accessed July 10, 2012];Payment Adjustment for Conditions Acquired in Hospitals No. 111–148, 124 Stat. Available. http://www.gpo.gov/fdsys/pkg/PLAW-111publ148/pdf/PLAW-111publ148.pdf.
- 4.Department of Health and Human Services. [Accessed March 12, 2014];Department of Health and Human Services Action Plan to Prevent Healthcare-Associated Infections 2012. 2009 http://www.hhs.gov/ash/initiatives/hai/actionplan/hhs_hai_action_plan_final_06222009.pdf.
- 5.Centers for Medicare and Medicaid Services. H. Medicare program; hospital inpatient prospective payment systems for acute care hospitals and the long term care hospital prospective payment system and proposed fiscal year 2014 rates. Federal Register. 2013;78:27622–27635. [PubMed] [Google Scholar]
- 6.Fakih MG, Greene MT, Kennedy EH, et al. Introducing a population-based outcome measure to evaluate the effect of interventions to reduce catheter-associated urinary tract infection. Am J Infect Control. 2012;40:359–364. doi: 10.1016/j.ajic.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saint S, Olmsted RN, Fakih MG, et al. Translating health care-associated urinary tract infection prevention research into practice via the bladder bundle. Jt Comm J Qual Patient Saf. 2009;35:449–455. doi: 10.1016/s1553-7250(09)35062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee GM, Hartmann CW, Graham D, et al. Perceived impact of the Medicare policy to adjust payment for health care-associated infections. Am J Infect Control. 2012;40:314–319. doi: 10.1016/j.ajic.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee GM, Kleinman K, Soumerai SB, et al. Effect of nonpayment for preventable infections in U.S. hospitals. N Engl J Med. 2012;367:1428–1437. doi: 10.1056/NEJMsa1202419. [DOI] [PubMed] [Google Scholar]
- 10.Mu Y, Edwards JR, Horan TC, Berrios-Torres SI, Fridkin SK. Improving risk-adjusted measures of surgical site infection for the national healthcare safety network. Infect Control Hosp Epidemiol. 2011;32:970–986. doi: 10.1086/662016. [DOI] [PubMed] [Google Scholar]
- 11.Klompas M. Advancing the science of ventilator-associated pneumonia surveillance. Crit Care. 2012;16:165. doi: 10.1186/cc11656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention (CDC) [Accessed March 12, 2014];CDC/National Healthcare Safety Network Surveillance Definitions for Specific Types of Infections. 2013 http://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf.
- 13.Stone ND, Ashraf MS, Calder J, et al. Surveillance definitions of infections in long-term care facilities: revisiting the McGeer criteria. Infect Control Hosp Epidemiol. 2012;33:965–977. doi: 10.1086/667743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klompas M. Prevention of ventilator-associated pneumonia. Expert Rev Anti Infect Ther. 2010;8:791–800. doi: 10.1586/eri.10.59. [DOI] [PubMed] [Google Scholar]
- 15.Klompas M. Interobserver variability in ventilator-associated pneumonia surveillance. Am J Infect Control. 2010;38:237–239. doi: 10.1016/j.ajic.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Bekaert M, Timsit JF, Vansteelandt S, et al. Attributable mortality of ventilator-associated pneumonia: a reappraisal using causal analysis. Am J Respir Crit Care Med. 2011;184:1133–1139. doi: 10.1164/rccm.201105-0867OC. [DOI] [PubMed] [Google Scholar]
- 17.Cook D, Guyatt G, Marshall J, et al. A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group. N Engl J Med. 1998;338:791–797. doi: 10.1056/NEJM199803193381203. [DOI] [PubMed] [Google Scholar]
- 18.Oostdijk EA, de Smet AM, Kesecioglu J, Bonten MJ Dutch SOD-SDD Trialists Group. The role of intestinal colonization with gram-negative bacteria as a source for intensive care unit-acquired bacteremia. Crit Care Med. 2011;39:961–966. doi: 10.1097/CCM.0b013e318208ee26. [DOI] [PubMed] [Google Scholar]
- 19.Thompson ND, Yeh LL, Magill SS, Ostroff SM, Fridkin SK. Investigating systematic misclassification of central line-associated bloodstream infection (CLABSI) to secondary bloodstream infection during health care-associated infection reporting. Am J Med Qual. 2013;28:56–59. doi: 10.1177/1062860612442565. [DOI] [PubMed] [Google Scholar]
- 20.Clancy CM. Getting to zero: our effort to eliminate infections nationwide. J Nurs Care Qual. 2010;25:189–192. doi: 10.1097/NCQ.0b013e3181dd9d2e. [DOI] [PubMed] [Google Scholar]
- 21.Clancy CM. Getting to zero: new resources aim to reduce health care-associated infections. Am J Med Qual. 2010;25:319–321. doi: 10.1177/1062860610370395. [DOI] [PubMed] [Google Scholar]
- 22.Edmond MB. Getting to zero: is it safe? Infect Control Hosp Epidemiol. 2009;30:74–76. doi: 10.1086/592411. [DOI] [PubMed] [Google Scholar]
- 23.Kaufman DA. “Getting to Zero”: preventing invasive Candida infections and eliminating infection-related mortality and morbidity in extremely preterm infants. Early Hum Dev. 2012;88:S45–S49. doi: 10.1016/S0378-3782(12)70014-2. [DOI] [PubMed] [Google Scholar]
- 24.Richards C. Getting to zero: an emerging policy framework for the elimination of hospital-associated infections. Infect Control Hosp Epidemiol. 2009;30:71–73. doi: 10.1086/595858. [DOI] [PubMed] [Google Scholar]
- 25.Anderson DJ, Kaye KS, Classen D, et al. Strategies to prevent surgical site infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29:S51–S61. doi: 10.1086/591064. [DOI] [PubMed] [Google Scholar]
- 26.Bolon MK, Hooper D, Stevenson KB, et al. Improved surveillance for surgical site infections after orthopedic implantation procedures: extending applications for automated data. Clin Infect Dis. 2009;48:1223–1229. doi: 10.1086/597584. [DOI] [PubMed] [Google Scholar]
- 27.Yokoe DS, Shapiro M, Simchen E, Platt R. Use of antibiotic exposure to detect postoperative infections. Infect Control Hosp Epidemiol. 1998;19:317–322. doi: 10.1086/647821. [DOI] [PubMed] [Google Scholar]
- 28.Calderwood MS, Ma A, Khan YM, et al. Use of Medicare diagnosis and procedure codes to improve detection of surgical site infections following hip arthroplasty, knee arthroplasty, and vascular surgery. Infect Control Hosp Epidemiol. 2012;33:40–49. doi: 10.1086/663207. [DOI] [PubMed] [Google Scholar]
- 29.Huang SS, Placzek H, Livingston J, et al. Use of Medicare claims to rank hospitals by surgical site infection risk following coronary artery bypass graft surgery. Infect Control Hosp Epidemiol. 2011;32:775–783. doi: 10.1086/660874. [DOI] [PubMed] [Google Scholar]
- 30.Olsen MA, Fraser VJ. Use of diagnosis codes and/or wound culture results for surveillance of surgical site infection after mastectomy and breast reconstruction. Infect Control Hosp Epidemiol. 2010;31:544–547. doi: 10.1086/652155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Platt R, Yokoe DS, Sands KE. Automated methods for surveillance of surgical site infections. Emerg Infect Dis. 2001;7:212–216. doi: 10.3201/eid0702.010212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moehring RW, Anderson DJ. “But my patients are different!”: risk adjustment in 2012 and beyond. Infect Control Hosp Epidemiol. 2011;32:987–989. doi: 10.1086/662202. [DOI] [PubMed] [Google Scholar]
- 33.Won SY, Munoz-Price LS, Lolans K, et al. Emergence and rapid regional spread of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae. Clin Infect Dis. 2011;53:532–540. doi: 10.1093/cid/cir482. [DOI] [PubMed] [Google Scholar]
- 34.Lin MY, Lyles-Banks RD, Lolans K, et al. The importance of long-term acute care hospitals in the regional epidemiology of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae. Clin Infect Dis. 2013;57:1246–1252. doi: 10.1093/cid/cit500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Fallon E, Kandel R, Schreiber R, D’Agata EM. Acquisition of multidrug-resistant gram-negative bacteria: incidence and risk factors within a long-term care population. Infect Control Hosp Epidemiol. 2010;31:1148–1153. doi: 10.1086/656590. [DOI] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention. Vital signs: preventing Clostridium difficile infections. MMWR Morb Mortal Wkly Rep. 2012;61:157–162. [PubMed] [Google Scholar]
- 37.Juthani-Mehta M, Quagliarello VJ. Infectious diseases in the nursing home setting: challenges and opportunities for clinical investigation. Clin Infect Dis. 2010;51:931–936. doi: 10.1086/656411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thom KA, Maragakis LL, Richards K, et al. Assessing the burden of Acinetobacter baumannii in Maryland: a statewide cross-sectional period prevalence survey. Infect Control Hosp Epidemiol. 2012;33:883–888. doi: 10.1086/667376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention. Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR Morb Mortal Wkly Rep. 2013;62:165–170. [PMC free article] [PubMed] [Google Scholar]
- 40.Gould CV, Rothenberg R, Steinberg JP. Antibiotic resistance in long-term acute care hospitals: the perfect storm. Infect Control Hosp Epidemiol. 2006;27:920–925. doi: 10.1086/507280. [DOI] [PubMed] [Google Scholar]
- 41.Furuno JP, Schweizer ML, McGregor JC, Perencevich EN. Economics of infection control surveillance technology: cost-effective or just cost? Am J Infect Control. 2008;36:S12–S17. doi: 10.1016/j.ajic.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McDonald LC, Arduino M. Editorial commentary: climbing the evidentiary hierarchy for environmental infection control. Clin Infect Dis. 2013;56:36–39. doi: 10.1093/cid/cis845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carling PC, Huang SS. Improving healthcare environmental cleaning and disinfection: current and evolving issues. Infect Control Hosp Epidemiol. 2013;34:507–513. doi: 10.1086/670222. [DOI] [PubMed] [Google Scholar]
- 44.Boyce JM. Measuring healthcare worker hand hygiene activity: current practices and emerging technologies. Infect Control Hosp Epidemiol. 2011;32:1016–1028. doi: 10.1086/662015. [DOI] [PubMed] [Google Scholar]
- 45.Morgan DJ, Pineles L, Shardell M, et al. Automated hand hygiene count devices may better measure compliance than human observation. Am J Infect Control. 2012;40:955–959. doi: 10.1016/j.ajic.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 46.Meddings J, Rogers MA, Macy M, Saint S. Systematic review and meta-analysis: reminder systems to reduce catheter-associated urinary tract infections and urinary catheter use in hospitalized patients. Clin Infect Dis. 2010;51:550–560. doi: 10.1086/655133. [DOI] [PubMed] [Google Scholar]
- 47.Milstone AM, Elward A, Song X, et al. Daily chlorhexidine bathing to reduce bacteraemia in critically ill children: a multicentre, cluster-randomised, crossover trial. Lancet. 2013;381:1099–1106. doi: 10.1016/S0140-6736(12)61687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sammons JS, Toltzis P, Zaoutis TE. Clostridium difficile infection in children. JAMA Pediatr. 2013;167:567–573. doi: 10.1001/jamapediatrics.2013.441. [DOI] [PubMed] [Google Scholar]
- 49.Salgado CD, Giannetta ET, Hayden FG, Farr BM. Preventing nosocomial influenza by improving the vaccine acceptance rate of clinicians. Infect Control Hosp Epidemiol. 2004;25:923–928. doi: 10.1086/502321. [DOI] [PubMed] [Google Scholar]
- 50.Lemaitre M, Meret T, Rothan-Tondeur M, et al. Effect of influenza vaccination of nursing home staff on mortality of residents: a cluster-randomized trial. J Am Geriatr Soc. 2009;57:1580–1586. doi: 10.1111/j.1532-5415.2009.02402.x. [DOI] [PubMed] [Google Scholar]
- 51.Shugarman LR, Hales C, Setodji CM, Bardenheier B, Lynn J. The influence of staff and resident immunization rates on influenza-like illness outbreaks in nursing homes. J Am Med Dir Assoc. 2006;7:562–567. doi: 10.1016/j.jamda.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 52.Talbot TR, Babcock H, Caplan AL, et al. Revised SHEA position paper: influenza vaccination of healthcare personnel. Infect Control Hosp Epidemiol. 2010;31:987–995. doi: 10.1086/656558. [DOI] [PubMed] [Google Scholar]
- 53.Miller BL, Ahmed F, Lindley MC, Wortley PM. Institutional requirements for influenza vaccination of healthcare personnel: results from a nationally representative survey of acute care hospitals–United States, 2011. Clin Infect Dis. 2011;53:1051–1059. doi: 10.1093/cid/cir633. [DOI] [PubMed] [Google Scholar]
- 54.Centers for Disease Control and Prevention. Pertussis epidemic–Washington, 2012. MMWR Morb Mortal Wkly Rep. 2012;61:517–522. [PubMed] [Google Scholar]
- 55.Centers for Disease Control and Prevention. [Accessed March 12, 2014];Pertussis (whooping cough) 2013 http://www.cdc.gov/pertussis/surv-reporting.html.
- 56.Goins WP, Schaffner W, Edwards KM, Talbot TR. Healthcare workers’ knowledge and attitudes about pertussis and pertussis vaccination. Infect Control Hosp Epidemiol. 2007;28:1284–1289. doi: 10.1086/521654. [DOI] [PubMed] [Google Scholar]
- 57.Platt R, Takvorian SU, Septimus E, et al. Cluster randomized trials in comparative effectiveness research: randomizing hospitals to test methods for prevention of healthcare-associated infections. Med Care. 2010;48:S52–S57. doi: 10.1097/MLR.0b013e3181dbebcf. [DOI] [PubMed] [Google Scholar]
- 58.Morgan DJ, Meddings J, Saint S, et al. Does nonpayment for hospital-acquired catheter-associated urinary tract infections lead to overtesting and increased antimicrobial prescribing? Clin Infect Dis. 2012;55:923–929. doi: 10.1093/cid/cis556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harris AD, Pineles L, Belton B, et al. Universal glove and gown use and acquisition of antibiotic-resistant bacteria in the ICU: a randomized trial. JAMA. 2013;310:1571–1580. doi: 10.1001/jama.2013.277815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosenthal VD, Maki DG, Salomao R, et al. Device-associated nosocomial infections in 55 intensive care units of 8 developing countries. Ann Intern Med. 2006;145:582–591. doi: 10.7326/0003-4819-145-8-200610170-00007. [DOI] [PubMed] [Google Scholar]
- 61.Rice LB. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J Infect Dis. 2008;197:1079–1081. doi: 10.1086/533452. [DOI] [PubMed] [Google Scholar]
- 62.Rice LB. Do we really need new anti-infective drugs? Curr Opin Pharmacol. 2003;3:459–463. doi: 10.1016/j.coph.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 63.Kwon S, Schweizer ML, Perencevich EN. National Institute of Allergy and Infectious Disease (NIAID) Funding for Studies of Hospital-Associated Bacterial Pathogens: Are Funds Proportionate to Burden of Disease? Antimicrob Resist Infect Control. 2012;1:5. doi: 10.1186/2047-2994-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klompas M, Kleinman K, Platt R. Development of an algorithm for surveillance of ventilator-associated pneumonia with electronic data and comparison of algorithm results with clinician diagnoses. Infect Control Hosp Epidemiol. 2008;29:31–37. doi: 10.1086/524332. [DOI] [PubMed] [Google Scholar]
- 65.Sexton DJ, Chen LF, Anderson DJ. Current definitions of central line-associated bloodstream infection: is the emperor wearing clothes? Infect Control Hosp Epidemiol. 2010;31:1286–1289. doi: 10.1086/657583. [DOI] [PubMed] [Google Scholar]
- 66.Bode LG, Kluytmans JA, Wertheim HF, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med. 2010;362:9–17. doi: 10.1056/NEJMoa0808939. [DOI] [PubMed] [Google Scholar]
- 67.Bennett-Guerrero E, Pappas TN, Koltun WA, et al. Gentamicin-collagen sponge for infection prophylaxis in colorectal surgery. N Engl J Med. 2010;363:1038–1049. doi: 10.1056/NEJMoa1000837. [DOI] [PubMed] [Google Scholar]
- 68.Bennett-Guerrero E, Ferguson TB, Jr, Lin M, et al. Effect of an implantable gentamicin-collagen sponge on sternal wound infections following cardiac surgery: a randomized trial. JAMA. A2010;304:755–762. doi: 10.1001/jama.2010.1152. [DOI] [PubMed] [Google Scholar]
- 69.Derde LP, Cooper BS, Goossens H, et al. Interventions to reduce colonisation and transmission of antimicrobial-resistant bacteria in intensive care units: an interrupted time series study and cluster randomised trial. Lancet Infect Dis. 2014;14:31–9. doi: 10.1016/S1473-3099(13)70295-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huskins WC, Huckabee CM, O’Grady NP, et al. Intervention to reduce transmission of resistant bacteria in intensive care. N Engl J Med. 2011:1407–1418. doi: 10.1056/NEJMoa1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Loeb M, Dafoe N, Mahony J, et al. Surgical mask vs N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA. 2009;302:1865–1871. doi: 10.1001/jama.2009.1466. [DOI] [PubMed] [Google Scholar]
- 72.Darouiche RO, Wall MJ, Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362:18–26. doi: 10.1056/NEJMoa0810988. [DOI] [PubMed] [Google Scholar]
- 73.Timsit JF, Schwebel C, Bouadma L, et al. Chlorhexidine-impregnated sponges and less frequent dressing changes for prevention of catheter-related infections in critically ill adults: a randomized controlled trial. JAMA. 2009;301:1231–1241. doi: 10.1001/jama.2009.376. [DOI] [PubMed] [Google Scholar]
- 74.Timsit JF, Mimoz O, Mourvillier B, et al. Randomized controlled trial of chlorhexidine dressing and highly adhesive dressing for preventing catheter-related infections in critically ill adults. Am J Resp Crit Care Med. 2012;186:1272–1278. doi: 10.1164/rccm.201206-1038OC. [DOI] [PubMed] [Google Scholar]
- 75.de Smet AM, Kluytmans JA, Blok HE, et al. Selective digestive tract decontamination and selective oropharyngeal decontamination and antibiotic resistance in patients in intensive-care units: an open-label, clustered group-randomised, crossover study. Lancet Infect Dis. 2011;11:372–380. doi: 10.1016/S1473-3099(11)70035-4. [DOI] [PubMed] [Google Scholar]
- 76.Climo MW, Yokoe DS, Warren DK, et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med. 2013;368:533–542. doi: 10.1056/NEJMoa1113849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med. 2013;368:2255–2265. doi: 10.1056/NEJMoa1207290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pickard R, Lam T, MacLennan G, et al. Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short-term catheterisation in hospital: a multicentre randomised controlled trial. Lancet. 2012;380:1927–1935. doi: 10.1016/S0140-6736(12)61380-4. [DOI] [PubMed] [Google Scholar]