Abstract
The adult brain was thought to be a slowly decaying organ, a sophisticated but flawed machine condemned to inevitable decline. Today we know that the brain is more plastic than previously assumed, as most prominently demonstrated by the constitutive birth of new neurons that occurs in selected regions of the adult brain, even in humans. However, the overall modest capacity for endogenous repair of the central nervous system (CNS) has sparked interest in understanding the barriers to neuronal regeneration and in developing novel approaches to enable neuronal and circuit repair for therapeutic benefit in neurodegenerative disorders and traumatic injuries. Scientists recently assembled in Baeza, a picturesque town in the south of Spain, to discuss aspects of CNS regeneration. The picture that emerged shows how an integrated view of developmental and adult neurogenesis may inform the manipulation of neural progenitors, differentiated cells, and pluripotent stem cells for therapeutic benefit and foster new understanding of the inner limits of brain plasticity.
Keywords: neural stem cells, regeneration, adult neurogenesis
INTRODUCTION
The extreme diversity of neuron types that populate the nervous system poses a fundamental challenge to a field that seeks to regenerate specific neurons in different diseases. Elegant work over decades has fundamentally contributed to our understanding of how the mammalian CNS, with its millions of cell types and billions of connections develops. During developmental neurogenesis, the “portrait” of each neuronal population is achieved through the combinatorial effects of cell intrinsic programs of fate specification (often acting through master transcription factors), and cell extrinsic signals that pattern the extracellular environment (the “niche”) where each neuronal population develops. In rodents, neurons are mostly generated before birth with connectivity being completed and refined during early stages of postnatal life. However, in selected regions of the mammalian brain, namely the subventricular zone (SVZ) of the lateral ventricle and the subgranular zone (SGZ) of the dentate gyrus (DG), remnants of embryonic neurogenesis persist throughout life to perpetuate the production of selected types of neurons, which in turn contribute to specific adult function and behavior (reviewed in Aimone et al., 2010). These permissive environments for the birth of new neurons are normally referred to as adult neurogenic niches. The mechanisms governing adult neural stem cell (NSC) maintenance and fate-specification, and those instructing neuronal survival, maturation, and integration in the adult have been the subjects of extensive investigation (reviewed in Kriegstein and Alvarez-Buylla, 2009). Last Fall, in the town of Baeza in the south of Spain, at the workshop entitled “cell replacement for regeneration in the nervous system: lessons from adult neurogenesis,” scientists presented new evidence that concepts and mechanisms of developmental neurogenesis are “reused” to maintain and pattern neurogenic niches of the adult brain. At a time when the boundaries of cell identity expand to enable lineage and nuclear reprogramming, new data were also presented on the generation of new neurons from both pluripotent stem cells and unconventional sources of differentiated cells, namely astroglia. The exciting new data encouraged a lively dialog among a diversified pool of developmental neurobiologists and experts of adult neurogenesis and CNS repair, all interested in ultimately generating desired neurons for therapeutic benefit.
Generating Diversity of Neurons and Connections: A Lesson from Development
It is reasonable to postulate that some of the same signals that instruct the establishment (and possibly the maintenance) of neuronal diversity and connectivity during development may also play a role during the generation and integration of new neurons within permissive neurogenic niches in the adult brain. There are of course no trivial solutions to the generation and integration of new neurons in a system that is fully built, and thus may not provide the plastic molecular environment of the embryonic CNS, however, some signals may have been “borrowed” from embryogenesis. Similarly to what occurs during development, adult NSC in the SVZ and SGZ must decide between maintaining a multipotent state (self renew) or commit to a neuronal fate. This occurs in response to both transcriptional/epigenetic programs that are cell intrinsic to adult NSC and signals coming from the neurogenic niche. It is generally thought that developmental signals governing fate choices are deregulated or turned-off in the adult. However, this is not always the case. Francois Guillemot (National Institute for Medical Research, London, UK) presented new evidence at the meeting suggesting that Mash 1, a proneural gene well-known for its function in controlling the differentiation of neural progenitors in the developing forebrain (Bertrand et al., 2002; Schuurmans and Guillemot, 2002) is also expressed in adult progenitors in both the hippocampus and the SVZ of the lateral ventricle, where it plays critical functional roles. Indeed, loss of Mash1 causes defective progenitor divisions in the adult neurogenic regions, demonstrating a novel role for this transcription factor in promoting the expansion of neuronal lineages in the adult brain. Data was also shown on the identification of Mash1 transcriptional target genes in the embryo (Gohlke et al., 2008; Pacary et al., 2011), which, given the parallel roles of this transcription factors in embryonic and adult NSC, may very well be involved in mediating Mash1 cell-autonomous function over neurogenic programs in the adult brain.
One could argue that the adult neurogenic niches are cellularly and structurally more complex than those supporting developmental neurogenesis. It is known that the adult SVZ contains multiple cell types, including migrating neuroblasts and putative intermediate precursor cells (Fig. 1). These cells differ in their date of birth and cell fate potential, with neural precursors positioned at distinct locations producing different types of neurons (Merkle et al., 2007). In addition, the structure and cellular composition of the SVZ varies in different species. The human SVZ exhibits a very peculiar organization with a gap between the ependymal zone and the astroglia ribbon, as shown at the meeting in elegant electromicroscopy (EM) work by Jose Manuel Garcia Verdugo (Centro de Investigación Príncipe Felipe, Spain). Adult NSC are in contact with endothelial cells, astrocytes, ependymal cells, more committed progenitors and neurons, which collectively provide extracellular signals that control NSC fate choices. Adult NSC are associated with blood vessels (Palmer et al., 2000; Mirzadeh et al., 2008; Shen et al., 2008; Tavazoie et al., 2008), and endothelium-derived factors have been identified (i.e., vascular endothelial growth factor, VEGF; and pigment epithelium-derived factor, PEDF) that control NSC self-renewal (Cao et al., 2004; Ramirez-Castillejo et al., 2006). In addition, ependymal cells produce Noggin, which has been shown to be a modulator of neurogenesis and gliogenesis from NSC (Lim et al., 2000; Colak et al., 2008). Most importantly, ependymal cells have a beating cilium, which is necessary to direct the flow of cerebrospinal fluid (CSF) and for generating gradients of choroid plexus-derived chemorepulsive factors in the CFS (i.e., Slit 2) (Sawamoto et al., 2006). Recent work has shown that primary cilia are essential for Hedgehog (Hh) signaling during mammalian development, and it is well established that extracellular signals in the form of morphogens are centrally important for patterning and fate specification of the embryonic CNS. Intriguing work by the laboratory of Arturo Alvarez-Buylla (University of California, San Francisco, USA) was presented at the meeting showing that Hedgehog signaling plays a central role in the adult SVZ by controlling the fate specification of a defined ventral subdomain of the adult SVZ. The work suggests for the first time that morphogens that pattern the neuroepithelium early in embryonic life also play related roles in the adult brain where they affect the types of neurons that adult NSC produce.
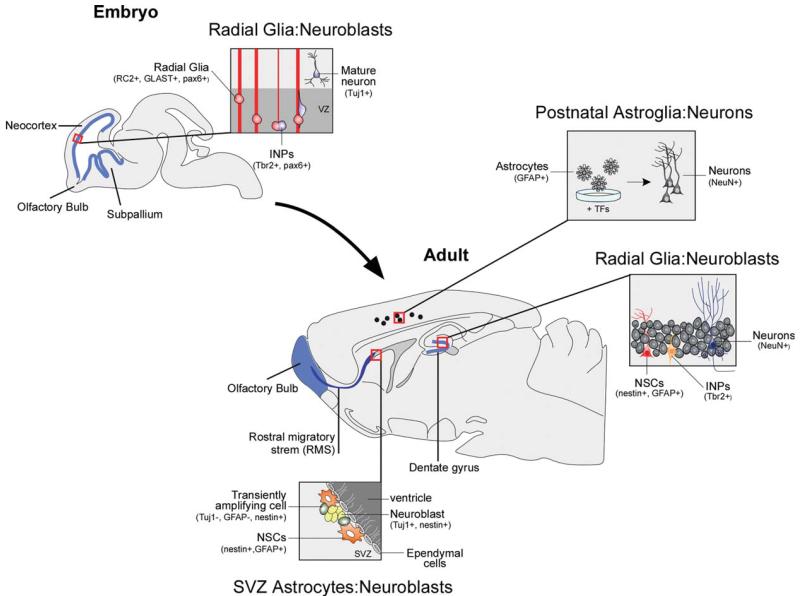
Figure 1.
Schema representing an integrated view of developmental and adult neurogenesis. During embryonic neurogenesis of the cerebral cortex, radial glial cells (RC2+, GLAST+, and Pax6+) give rise to neuroblasts that migrate to the cortex and differentiate into excitatory cortical projection neurons. In the adult brain, two regions retain the capacity to constitutively generate neurons (highlighted in blue): the subventricular zone (SVZ) of the lateral ventricles and the subgranular zone (SGZ) of the dentate gyrus. In the SVZ, slowly dividing NSC can give rise to rapidly dividing immature precursors. These actively proliferate and generate immature neuroblasts, which migrate in chains along the rostral migratory stream to the olfactory bulb (OB), where they differentiate into interneurons. Similarly, SGZ NSC in the dentate gyrus undergo proliferation, generate rapid amplifying intermediate progenitors, which then differentiate into dentate granule cells. Postnatal astroglia (GFAP+) may be efficiently converted into functional neurons in vitro under specific culture conditions and the use of neurogenic transcription factors (TFs), possibly offering an alternative cell source to pluripotent stem cells.
Regulation of Adult Neurogenesis
Newly generated neurons in the adult hippocampus contribute to learning and memory processes and to the regulation of mood. Recent evidence indicates that the number of newborn neurons and the timing of their differentiation and integration have to be precisely matched to the needs of the hippocampal network in order to optimize hippocampal function (Aimone et al., 2010; Deng et al., 2010; Mongiat and Schinder, 2011). This process could be regulated at different stages of NSC self-renewal, fate specification, and differentiation, as well as during neuroblast/neuron survival, maturation, and integration. The group of Chichung Lie (Helmholtz Zentrum München, Germany) presented data showing that a regulated interplay between Wnt and Notch signaling affects the choice of neuronal differentiation versus proliferation of NSC in the adult hippocampus. It was shown that the Notch-induced transcription factor Sox2 acts as a central regulator of NSC maintenance in the adult hippocampus, where it counteracts Wnt-induced neuronal differentiation (Kuwabara et al., 2009; Ehm et al., 2010). The data contribute to the understanding of the signaling network that ensures maintenance of hippocampal neurogenesis at a rate necessary for proper hippocampal function.
Once generated, adult-born neuroblasts and neurons need to put in place mechanisms that guarantee their survival, often over long periods of neuroblast migration before integration into circuitry (as it occurs for SVZ derived neurobalsts destined for the olfactory bulb). Glutamate, through selective receptors, stimulate the production of immature neurons, their survival during migration, and their long-term activity-dependent survival in a synaptic network (Platel et al., 2008; Platel et al., 2010). Angelique Bordey (Yale University School of Medicine, New Haven, USA) showed elegant data that single-cell knockout of NMDA receptors using neonatal electroporation resulted in neuroblast apoptosis at the time of NMDA receptor expression. This caused a 40% loss of neuroblasts along their migratory route from the SVZ to the olfactory bulb, demonstrating that NMDA receptor acquisition is critical for neuroblast survival prior to entering a synaptic network. In addition, it was shown that miRNAs play a central role in NMDA receptor mediated survival. Thus, it is reasonable to think that understanding the mechanisms that control the specification of axonal and dendritic arbors in newly generated neurons is crucial for their integration into mature neuronal circuits. Yet, how intracellular components are directed to dendrites or accumulate in axons, and how extracellular factors regulate polarity remain outstanding questions in neurogenesis. The group of Marco Canossa (Italian Institute of Technology, Italy) reported that the panneurotrophin receptor (p75NTR) localizes asymmetrically in the growing axon to polarize neurons such that neurotrophins signals can specify the future axon among different neurites.
It is known that newborn neurons in the hippocampus, before reaching a mature stage display very unique properties compared to developmentally generated neuronal counterparts (Doetsch and Hen, 2005; Piatti et al., 2006; Mongiat and Schinder, 2011). Immature neurons exhibit high excitability, reduced inhibition, and enhanced synaptic plasticity, which would allow them to process information differently from the mature neurons in the preexisting circuit. If maturation state matters, then it is reasonable to imagine that combinations of newly generated neurons at different stages of maturation may affect hippocampal network differently and uniquely. It is well known that the mammalian hippocampus exhibits a marked anatomical and functional segregation along its longitudinal (septo-temporal) axis (Piatti et al., 2006; Toni et al., 2008; Fanselow and Dong, 2010), however, the neurogenic niche of the dentate gyrus has traditionally been treated as homogeneous. Using Arc immunofluorescence the group of Alejandro Schinder (Leloir Institute, Buenos Aires, Argentina), reported at the meeting the exciting discovery that basal levels of circuit activity are higher in the septal than in the temporal dentate gyrus. Since activity can regulate neuronal development (Ge et al., 2007), they took advantage of this heterogeneous activity to investigate development and integration of newborn granule cells along the septo-temporal axis of the hippocampus. They found that newborn granule cells of the temporal pole displayed a slower rate of functional maturation, which could in turn be modulated by experimental manipulations that modified activity. For example, voluntary exercise in the running wheel enhanced network activity in the temporal dentate gyrus and accelerated integration. Maturation of newly generated neurons in the dentate thus occurs at a different pace in the ventral and dorsal hippocampus. Intriguingly, in work presented by Guo-li Ming (Johns Hopkins University, Baltimore, USA) knockdown of the Disrupted in Schizophrenia 1 (DISC1) gene during adult neurogenesis results in dramatic acceleration of maturation as exemplified by prominently enlarged cell bodies, accelerated morphogenesis and maturation of newborn granule neurons in the dentate gyrus of the hippocampus (Duan et al., 2007; Kim et al., 2009).
All together, the data provide mechanistic insight into the survival and maturation of adult-born neurons and demonstrate the presence of hippocampal areas with different sensitivity to the activity-dependent timing of neuronal integration. This further expands the degree of plasticity of the adult hippocampal network and contributes to our understanding of what makes newly generated neurons functionally relevant.
Cell Replacement Strategies for Nervous System Repair: Generating Specific Neuron Types
While constitutive adult neurogenesis occurs in selected areas of the brain (Fig. 1), neural stem cells are also present within non-neurogenic niches, where it is believed that the extracellular environment of the brain precludes their differentiation into neurons(Gage et al., 1995; Palmer et al., 1995; Suhonen et al., 1996; Palmer et al., 1999). Work presented by the group of José López-Barneo (University of Sevilla, Spain) provides evidence for the existence within the carotid body (CB) of a population of neural crestderived progenitors that can form multipotent and self-renewing colonies, in vitro. CB stem cells can differentiate to form glomus cells with normal histological, biochemical and electrophysiological characteristics in vitro (Lopez-Barneo et al., 2009) Cell fate mapping experiments have suggested that the CB stem cells are a subpopulation of glia-like cells, thus resembling in this respect NSC identified in the neurogenic regions of the brain.
While endogenous neural stem cells certainly could be a prime source of multipotent progenitors for the generation of new neurons, their limited numbers outside of the neurogenic niches poses a limitation to their use for regenerative therapy in the CNS. Other starting sources of cells have, of course, been considered. Pluripotent stem cells, like embryonic stem (ES) cells and induced pluripotent stem (iPS) cells, offer numerous advantages, not the least of which their ability to extensively proliferate without loosing fate potential, thus enabling the generation of large numbers of differentiated progeny, something that is necessary for highthroughput chemical screenings and transplantation. Several important experimental precedents reinforce the general idea that guided differentiation of ES/iPS cells into clinically relevant neuronal populations can be realized by emulating molecular mechanisms responsible for shaping embryonic neuronal diversity. Successful differentiation of ES and iPS cells into spinal motor neuron, and more recently into specific spinal motor neuron subtypes, has been achieved by treatment with select morphogens (Wichterle et al., 2002; Miles et al., 2004; Dimos et al., 2008; Peljto et al., 2010). Using cell-intrinsic signals, over-expression of the transcription factors Lmx1a and Msx1, leads to the directed differentiation of ES cells into midbrain dopaminergic neurons (Andersson et al., 2006). Aimed at directing the differentiation of ES cells into cortical projection neuron populations, Pierre Vanderhaeghen (Université Libre de Bruxelles, Belgium) presented data showing that under specific culture conditions, ES cells have the potential to generate cortical projection neuron progeny. Neurons are generated following the same temporal sequence of developmental corticogenesis (Gaspard et al., 2008). Upon transplantation into early postnatal or lesioned adult brain, ES-derived neurons were able to connect to distal targets of cortical projection neurons. The data indicate that ES cells have the potential to generate cortical projection neurons, paving the way for studies aimed at instructing the differentiation of cortical projection neuron types, should proper signals be identified. In this regard, Paola Arlotta (Harvard University, Cambridge, USA), presented data indicating that cell autonomous transcription factor signals may be used to instruct the generation of specific types of cortical projection neurons. The work focused on corticofugal projection neurons (CFuPN), and, in particular, corticospinal motor neurons (CSMN), a clinically relevant class of projection neurons of the neocortex that selectively dies in Amyotrophic Lateral Sclerosis (ALS, Lou Gehrig’s disease), and that is permanently injured in spinal cord injury (SCI). Using in vivo electroporation, the Arlotta’s group demonstrated that expression of a single transcription factor, Fezf2, is sufficient to direct the neuron type-specific differentiation of CFuPN when expressed in neural progenitors that would normally be fated to become medium spiny neurons in the intact, developing striatum (Rouaux and Arlotta, 2010). Intriguingly, new data suggest that it may even be possible to convert the identity of early postmitotic neuroblasts. Fezf2-expressing neurons were able to extend axonal projection to subcerebral targets, indicating that a cascade of gene expression downstream of Fezf2 likely activated CfuPN-specific axon guidance molecules to instruct proper connectivity. This may be the case in other brain regions. Indeed, Guillermina López-Bendito (Instituto de Neurociencias, Spain) presented recent data showing that the transcription factor Lhx2 regulates the acquisition of proper topographic connectivity of another major axonal path in the CNS, the thalamocortical system. It was shown that expression of Lhx2 protein is very dynamically regulated in distinct pools of postmitotic thalamic neurons suggesting a function in regulating distinct aspects of axon connectivity by these neuronal subtypes. Altogether, the data strongly suggest that cell autonomous programs that govern embryonic neurogenesis of individual types of neurons might be used to direct pluripotent stem cells to generate individual neuronal populations in the dish and possibly the establishment of correct connectivity upon transplantation in the brain. In addition, it may not be necessary to recapitulate the complex sequence and combination of signals that shape neuronal diversity during development. Rather, a small number of “master” signals may serve as switches to sufficiently drive the formation of distinct neuron types.
Unconventional Sources of Neurons: Astrocytes
Recent discoveries in the field of reprogramming have challenged the dogma that the identity of differentiated cell types may not be changed (reviewed in Plath and Lowry, 2011). Lineage reprogramming (also referred to as trans-differentiation) of one differentiated cell type into another without the generation of a multipotent/pluripotent stem cell intermediate has now been demonstrated to be possible for many cell types in the body, starting with seminal work in the pancreas (Zhou et al., 2008). The successful generation of clinically relevant cell types within complex organ systems could clearly have implications for regenerative medicine and tissue repair. There are now reasons to believe that the central nervous system (CNS) may be no exception, and that lineage reprogramming aimed at regenerating new neurons may be possible in the brain. Pioneer work presented by Magdalena Götz (Institute of Stem Cell Research, Helmholtz Center, Munich) showed that cell intrinsic programs that instruct neurogenesis from adult NSC may be used to direct the differentiation of postnatal astrocytes that are located outside of the neurogenic niches (Fig. 1). A condition sine qua non of “functional neurogenesis” is the ability of new neurons to make functional synapses. The group of Benedikt Berninger (Ludwig-Maximilians Universität München, Germany) showed that persistent expression of neurogenic fate determinants using silencing-resistant retroviral vectors instructs astroglia from the postnatal cortex to mature into fully functional, synapseforming neurons in vitro. Importantly, the neurotransmitter fate choice of astroglia-derived neurons can be controlled by selective expression of distinct neurogenic transcription factors, such that expression of the dorsal telencephalic fate determinant Ngn2 directs cortical astroglia to generate synapse-forming glutamatergic neurons, while expression of the ventral telencephalic fate determinant Dlx2 induces a GABAergic identity (Heinrich et al., 2010, 2011). Interestingly, newer in vitro data were shown that lineage reprogramming could also occur in the human brain, with the added twist that some of the cells capable of reprogramming might be pericytes. All together, the data provides evidence that in the neocortex differentiated cell types may be instructed via cell intrinsic and extrinsic signals to acquire neuronal fates. The results pave the way for the investigation of the limits and boundaries of these events not only in different mammalian species but also in the aged and diseased brain.
Will Stem Cells Fulfill their Promise? Cell Replacement Strategies for Parkinson’s Disease
Small open-lab clinical trials of fetal-cell transplantation in Parkinson’s disease (PD) have supported the viability of transplantation as a therapy for PD (Brundin et al., 1986; Lindvall and Hagell, 2000; Dunnett et al., 2001; Hagell and Brundin, 2001). These early trials showed that human fetal dopaminergic neurons are able to survive and function for more than 10 years in the striatum of patients with PD. These studies have also provided a clear indication that transplanted fetal dopaminergic neurons can be effective. While later trials showed variable and at times disappointing (albeit perhaps explainable) results, there are great ethical barriers that preclude the use of human fetal material for transplantation. In addition, large amounts of grafted material are needed, which would require the use of many embryos per patient. This and extensive evidence from work in rodents, support the idea that transplanted DA neurons may be able to compensate for nigrostriatal neurons that are lost due to the disease. This suggests that if alternative sources of human DA neurons were available, they could have a therapeutically beneficial impact. Anders Björklund (Lund University, Sweden) presented data at the meeting on the use of ES-derived DA neurons for transplantation in mouse models of PD. Building on a protocol originally developed by Ericson and colleagues (Andersson et al., 2006), the Björklund’s group used cell-autonomous expression of the transcription factor Lmx1a to generate ES-derived murine DA neurons and demonstrated that in vivo, Lmx1a-expressig ES cells develop into fully mature DA neurons with extensive axonal terminal network throughout the host striatum. The results suggest that in the future ES-derived midbrain DA neurons might be an alternative source of cells for therapeutic transplantation in patients with PD. Along these same lines, Veronica Martinez Cerdeño (University of California Davis, Sacramento, USA) showed data on the use of embryonic, MGE-derived cells for transplantations into the striatum of a rat model of PD. They performed MGE cell transplantation into the basal ganglia of control and 6-OHDA-lesioned rats. Transplanted MGE cells survived, differentiated into GABA+ neurons, integrated into host circuitry, and modified motor behavior in both lesioned and control rats. Ethical barriers may limit the use of embryonic MGE tissue for transplantation; however, it is reasonable to imagine that in the future a similar source of cells might be generated from pluripotent iPS cells of patients. Recent work on the generation of GABAergic cortical interneurons from mouse ES cells (Maroof et al., 2010), certainly support these considerations.
As progress in uncovering the signals that control the differentiation of distinct types of neurons continues, it is plausible to speculate that in a not so distant future protocols will be available to generate therapeutically relevant neuron types for transplantation, high-throughput chemical and drug screenings and disease modeling.
Acknowledgments
The authors thank Benedikt Berninger, Alejandro Schinder, and Jose Manuel Verdugo for a truly stimulating meeting and for facilitating lively discussions across field of study. They also thank Joaquín Torreblanca, the Universidad Internacional de Andalucía (UNIA) and the Sede Antonio Machado for providing a truly unique venue and support for this meeting. They apologize to the colleagues whose work could not be cover here due to space limitations, and do thank all the speakers at the meeting for providing input on this article.
REFERENCES
- Aimone JB, Deng W, Gage FH. Adult neurogenesis: Integrating theories and separating functions. Trends Cogn Sci. 2010;14:325–337. doi: 10.1016/j.tics.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson E, Tryggvason U, Deng Q, Friling S, Alek-seenko Z, Robert B, Perlmann T, et al. Identification of intrinsic determinants of midbrain dopamine neurons. Cell. 2006;124:393–405. doi: 10.1016/j.cell.2005.10.037. [DOI] [PubMed] [Google Scholar]
- Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neurosci. 2002;3:517–530. doi: 10.1038/nrn874. [DOI] [PubMed] [Google Scholar]
- Brundin P, Nilsson OG, Strecker RE, Lindvall O, Astedt B, Bjorklund A. Behavioural effects of human fetal dopamine neurons grafted in a rat model of Parkinson’s disease. Exp Brain Res. 1986;65:235–240. doi: 10.1007/BF00243848. [DOI] [PubMed] [Google Scholar]
- Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36:827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- Colak D, Mori T, Brill MS, Pfeifer A, Falk S, Deng C, Monteiro R, et al. Adult neurogenesis requires Smad4-mediated bone morphogenic protein signaling in stem cells. J Neurosci. 2008;28:434–446. doi: 10.1523/JNEUROSCI.4374-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, REFAU>Mitsumoto H, Chung W, Croft GF, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Hen R. Young and excitable: The function of new neurons in the adult mammalian brain. Curr Opin Neurobiol. 2005;15:121–128. doi: 10.1016/j.conb.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, Liu XB, et al. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130:1146–1158. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnett SB, Bjorklund A, Lindvall O. Cell therapy in Parkinson’s disease—Stop or go? Nat Rev Neurosci. 2001;2:365–369. doi: 10.1038/35072572. [DOI] [PubMed] [Google Scholar]
- Ehm O, Goritz C, Covic M, Schaffner I, Schwarz TJ, Karaca E, Kempkes B, et al. RBPJkappa-dependent signaling is essential for long-term maintenance of neural stem cells in the adult hippocampus. J Neurosci. 2010;30:13794–13807. doi: 10.1523/JNEUROSCI.1567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH, Coates PW, Palmer TD, Kuhn HG, Fisher LJ, Suhonen JO, Peterson DA, et al. Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc Natl Acad Sci USA. 1995;92:11879–11883. doi: 10.1073/pnas.92.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspard N, Bouschet T, Hourez R, Dimidschstein J, Naeije G, van den Ameele J, Espuny-Camacho I, et al. An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature. 2008;455:351–357. doi: 10.1038/nature07287. [DOI] [PubMed] [Google Scholar]
- Ge S, Pradhan DA, Ming GL, Song H. GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci. 2007;30:1–8. doi: 10.1016/j.tins.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Gohlke JM, Armant O, Parham FM, Smith MV, Zimmer C, Castro DS, Nguyen L, et al. Characterization of the proneural gene regulatory network during mouse telencephalon development. BMC Biol. 2008;6:15. doi: 10.1186/1741-7007-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagell P, Brundin P. Cell survival and clinical outcome following intrastriatal transplantation in Parkinson disease. J Neuropathol Exp Neurol. 2001;60:741–752. doi: 10.1093/jnen/60.8.741. [DOI] [PubMed] [Google Scholar]
- Heinrich C, Blum R, Gascon S, Masserdotti G, Tripathi P, Sanchez R, Tiedt S, et al. Directing astroglia from the cerebral cortex into subtype specific functional neurons. PLoS Biol. 2010;8:e1000373. doi: 10.1371/journal.pbio.1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich C, Gascon S, Masserdotti G, Lepier A, Sanchez R, Simon-Ebert T, Schroeder T, et al. Generation of subtype-specific neurons from postnatal astroglia of the mouse cerebral cortex. Nat Protoc. 2011;6:214–228. doi: 10.1038/nprot.2010.188. [DOI] [PubMed] [Google Scholar]
- Kim JC, Cook MN, Carey MR, Shen C, Regehr WG, Dymecki SM. Linking genetically defined neurons to behavior through a broadly applicable silencing allele. Neuron. 2009;63:305–315. doi: 10.1016/j.neuron.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara T, Hsieh J, Muotri A, Yeo G, Warashina M, Lie DC, Moore L, et al. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat Neurosci. 2009;12:1097–1105. doi: 10.1038/nn.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DA, Tramontin AD, Trevejo JM, Herrera DG, Garcia-Verdugo JM, Alvarez-Buylla A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Hagell P. Clinical observations after neural transplantation in Parkinson’s disease. Prog Brain Res. 2000;127:299–320. doi: 10.1016/s0079-6123(00)27014-3. [DOI] [PubMed] [Google Scholar]
- Lopez-Barneo J, Pardal R, Ortega-Saenz P, Duran R, Villadiego J, Toledo-Aral JJ. The neurogenic niche in the carotid body and its applicability to antiparkinsonian cell therapy. J Neural Transm. 2009;116:975–982. doi: 10.1007/s00702-009-0201-5. [DOI] [PubMed] [Google Scholar]
- Maroof AM, Brown K, Shi SH, Studer L, Anderson SA. Prospective isolation of cortical interneuron precursors from mouse embryonic stem cells. J Neurosci. 2010;30:4667–4675. doi: 10.1523/JNEUROSCI.4255-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- Miles GB, Yohn DC, Wichterle H, Jessell TM, Rafuse VF, Brownstone RM. Functional properties of motoneurons derived from mouse embryonic stem cells. J Neurosci. 2004;24:7848–7858. doi: 10.1523/JNEUROSCI.1972-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongiat LA, Schinder AF. Adult neurogenesis and the plasticity of the dentate gyrus network. Eur J Neurosci. 2011;33:1055–1061. doi: 10.1111/j.1460-9568.2011.07603.x. [DOI] [PubMed] [Google Scholar]
- Pacary E, Heng J, Azzarelli R, Riou P, Castro D, Lebel-Potter M, Parras C, et al. Proneural transcription factors regulate different steps of cortical neuron migration through Rnd-mediated inhibition of RhoA signaling. Neuron. 2011;69:1069–1084. doi: 10.1016/j.neuron.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer TD, Ray J, Gage FH. FGF-2-responsive neuronal progenitors reside in proliferative and quiescent regions of the adult rodent brain. Mol Cell Neurosci. 1995;6:474–486. doi: 10.1006/mcne.1995.1035. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Markakis EA, Willhoite AR, Safar F, Gage FH. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J Neurosci. 1999;19:8487–8497. doi: 10.1523/JNEUROSCI.19-19-08487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Peljto M, Dasen JS, Mazzoni EO, Jessell TM, Wichterle H. Functional diversity of ESC-derived motor neuron subtypes revealed through intraspinal transplantation. Cell Stem Cell. 2010;7:355–366. doi: 10.1016/j.stem.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatti VC, Esposito MS, Schinder AF. The timing of neuronal development in adult hippocampal neurogenesis. Neuroscientist. 2006;12:463–468. doi: 10.1177/1073858406293538. [DOI] [PubMed] [Google Scholar]
- Platel JC, Dave KA, Bordey A. Control of neuroblast production and migration by converging GABA and glutamate signals in the postnatal forebrain. J Physiol. 2008;586:3739–3743. doi: 10.1113/jphysiol.2008.155325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platel JC, Stamboulian S, Nguyen I, Bordey A. Neurotransmitter signaling in postnatal neurogenesis: The first leg. Brain Res Rev. 2010;63:60–71. doi: 10.1016/j.brainresrev.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plath K, Lowry WE. Progress in understanding reprogramming to the induced pluripotent state. Nat Rev Genet. 2011;12:253–265. doi: 10.1038/nrg2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Castillejo C, Sanchez-Sanchez F, Andreu-Agullo C, Ferron SR, Aroca-Aguilar JD, Sanchez P, Mira H, et al. Pigment epithelium-derived factor is a niche signal for neural stem cell renewal. Nat Neurosci. 2006;9:331–339. doi: 10.1038/nn1657. [DOI] [PubMed] [Google Scholar]
- Rouaux C, Arlotta P. Fezf2 directs the differentiation of corticofugal neurons from striatal progenitors in vivo. Nat Neurosci. 2010;13:1345–1347. doi: 10.1038/nn.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamoto K, Wichterle H, Gonzalez-Perez O, Cholfin JA, Yamada M, Spassky N, Murcia NS, et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311:629–632. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- Schuurmans C, Guillemot F. Molecular mechanisms underlying cell fate specification in the developing telencephalon. Curr Opin Neurobiol. 2002;12:26–34. doi: 10.1016/s0959-4388(02)00286-6. [DOI] [PubMed] [Google Scholar]
- Shen J, Xie L, Mao X, Zhou Y, Zhan R, Greenberg DA, Jin K. Neurogenesis after primary intracerebral hemorrhage in adult human brain. J Cereb Blood Flow Metab. 2008;28:1460–1468. doi: 10.1038/jcbfm.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhonen JO, Peterson DA, Ray J, Gage FH. Differentiation of adult hippocampus-derived progenitors into olfactory neurons in vivo. Nature. 1996;383:624–627. doi: 10.1038/383624a0. [DOI] [PubMed] [Google Scholar]
- Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, Schinder AF. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008;11:901–907. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]