Abstract
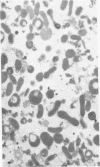
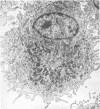
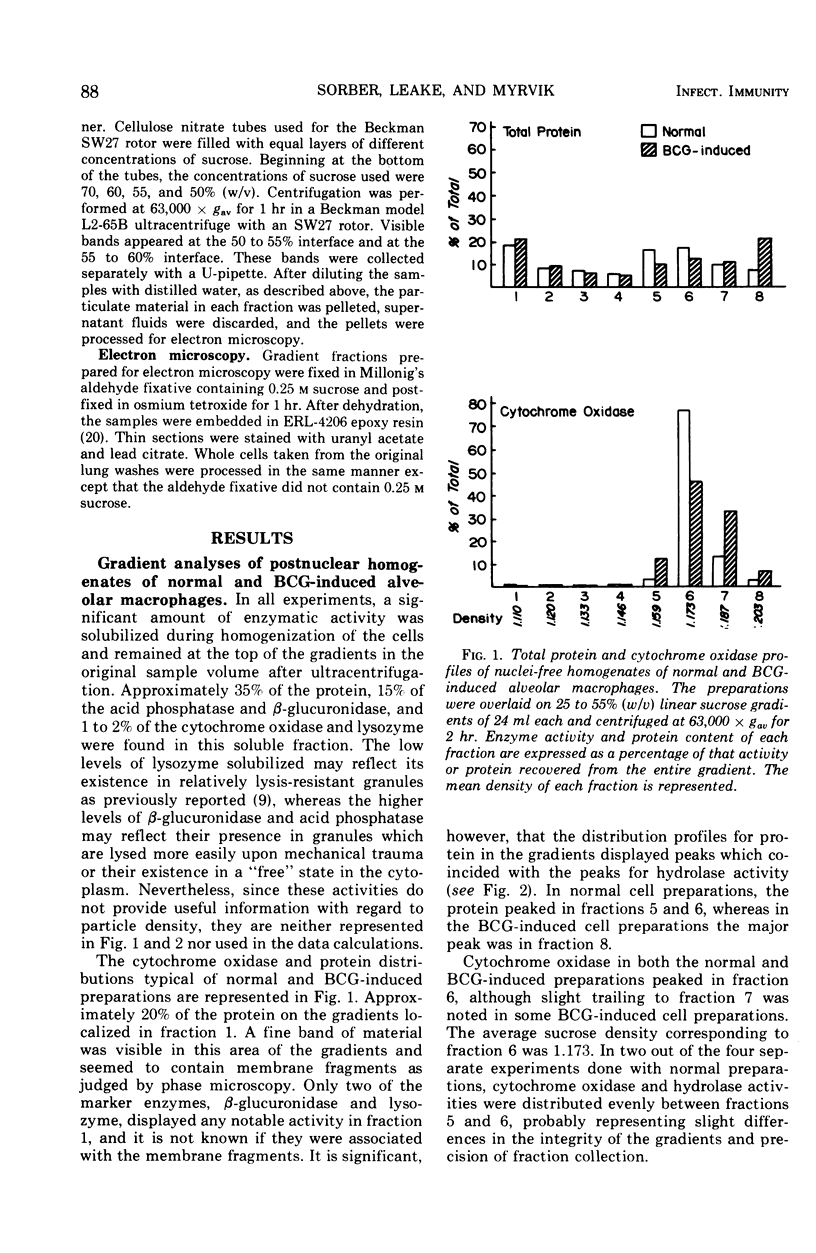
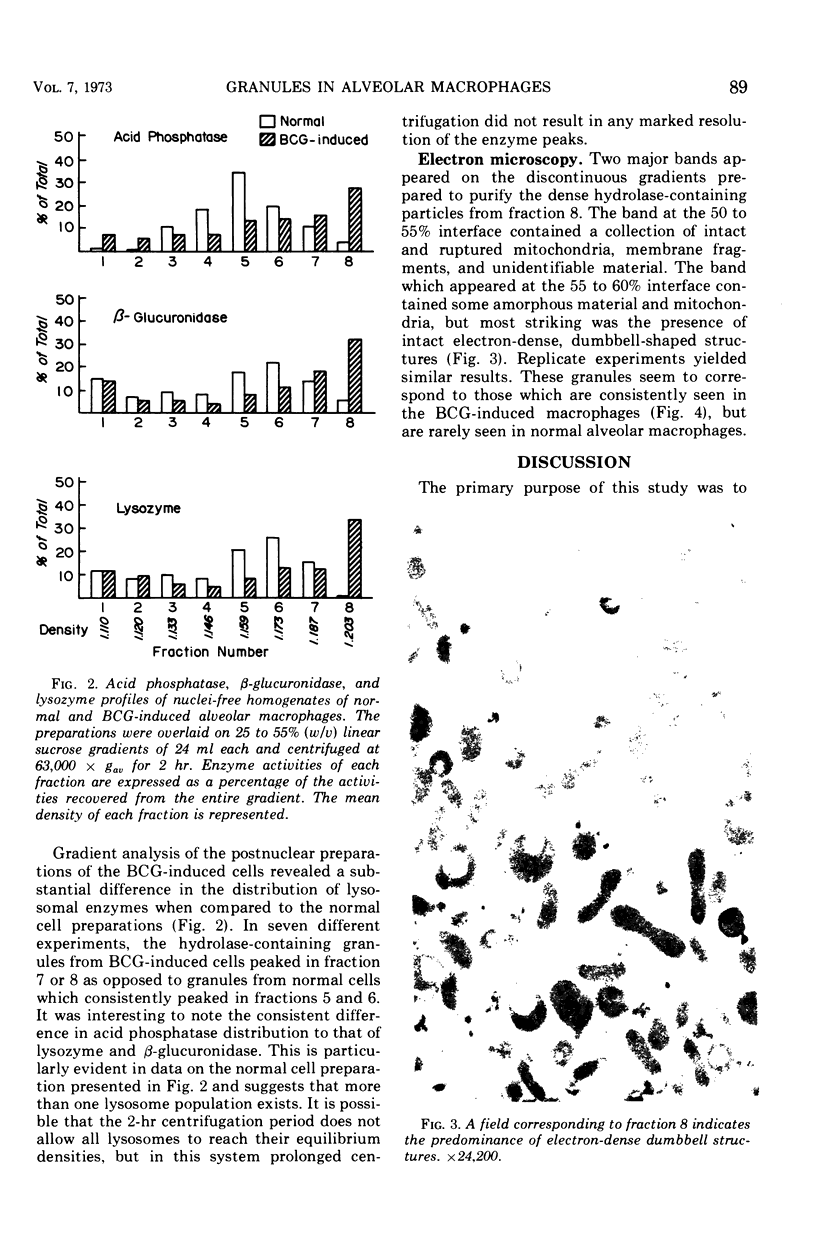
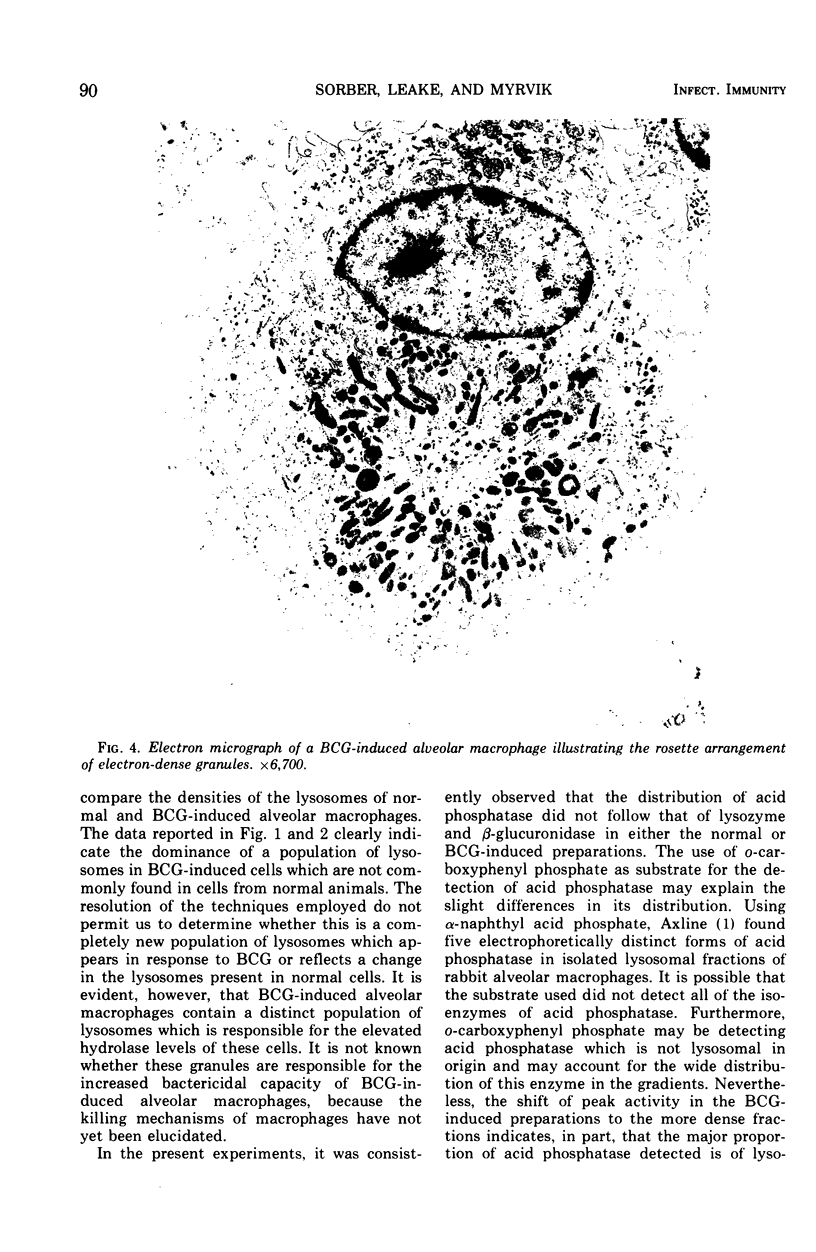
Compared to cells from normal rabbit lungs, BCG-induced alveolar macrophages have a marked increase in hydrolase levels and the number of large electron-dense subcellular structures. This study was performed to investigate the possibility that these electron-dense structures were responsible for the increased hydrolase levels of these cells. Using sucrose gradient centrifugation, nuclei-free homogenates of normal and BCG-induced macrophages were analyzed with respect to the subcellular particles they contain. Gradient fractions were assayed for enzymes commonly associated with lysosomes as well as the mitochondrial cytochrome oxidase. Fractions of peak hydrolase activity from the BCG-induced preparations were consistently more dense than those from the normal cell preparations. Ultrastructural studies of the particulate material found in fractions of peak hydrolase activity from BCG-induced preparations revealed the presence of electron-dense, often dumbbell-shaped, granules. The data suggest that these peculiar granules are responsible for the elevated hydrolase levels of BCG-induced alveolar macrophages.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axline S. G. Isozymes of acid phosphatase in normal and Calmette-Guérin bacillus-induced rabbit alveolar macrophages. J Exp Med. 1968 Nov 1;128(5):1031–1048. doi: 10.1084/jem.128.5.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggiolini M., Hirsch J. G., De Duve C. Resolution of granules from rabbit heterophil leukocytes into distinct populations by zonal sedimentation. J Cell Biol. 1969 Feb;40(2):529–541. doi: 10.1083/jcb.40.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., WIENER E. THE PARTICULATE HYDROLASES OF MACROPHAGES. I. COMPARATIVE ENZYMOLOGY, ISOLATION, AND PROPERTIES. J Exp Med. 1963 Dec 1;118:991–1008. doi: 10.1084/jem.118.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont A., Sheldon H. Changes in the fine structure of macrophages in experimentally produced tuberculous granulomas in hamsters. Lab Invest. 1965 Nov;14(11):2034–2055. [PubMed] [Google Scholar]
- Groves W. E., Davis F. C., Jr, Sells B. H. Spectrophotometric determination of microgram quantities of protein without nucleic acid interference. Anal Biochem. 1968 Feb;22(2):195–210. doi: 10.1016/0003-2697(68)90307-2. [DOI] [PubMed] [Google Scholar]
- HEISE E. R., MYRVIK Q. N., LEAKE E. S. EFFECT OF BACILLUS CALMETTE-GU'ERIN ON THE LEVELS OF ACID PHOSPHATASE, LYSOZYME AND CATHEPSIN IN RABBIT ALVEOLAR MACROPHAGES. J Immunol. 1965 Jul;95:125–130. [PubMed] [Google Scholar]
- HOFSTEE B. H. Direct and continuous spectrophotometric assay of phosphomonoesterases. Arch Biochem Biophys. 1954 Jul;51(1):139–146. doi: 10.1016/0003-9861(54)90461-0. [DOI] [PubMed] [Google Scholar]
- LEAKE E. S., MYRVIK Q. N. DIFFERENTIAL RELEASE OF LYSOZYME AND ACID PHOSPHATASE FROM SUB-CELLULAR GRANULES OF NORMAL RABBIT ALVEOLAR MACROPHAGES. Br J Exp Pathol. 1964 Aug;45:384–392. [PMC free article] [PubMed] [Google Scholar]
- Leake E. S., Myrvik Q. N. Changes in morphology and in lysozyme content of free alveolar cells after the intravenous injection of killed BCG in oil. J Reticuloendothel Soc. 1968 Feb;5(1):33–53. [PubMed] [Google Scholar]
- Leake E. S., Myrvik Q. N. Rosette arrangement of electron-dense structures of granulomatous alveolar macrophges. J Reticuloendothel Soc. 1972 Sep;12(3):305–313. [PubMed] [Google Scholar]
- MYRVIK Q. N., LEAKE E. S., OSHIMA S. A study of macrophages and epitheloid-like cells from granulomatous (BCG-induced) lungs of rabbits. J Immunol. 1962 Nov;89:745–751. [PubMed] [Google Scholar]
- MYRVIK Q., LEAKE E. S., FARISS B. Studies on pulmonary alveolar macrophages from the normal rabbit: a technique to procure them in a high state of purity. J Immunol. 1961 Feb;86:128–132. [PubMed] [Google Scholar]
- Moore V. L., Myrvik Q. N. Effect of antimacrophage serum on dermal tuberculin sensitivity and allergic pulmonary granuloma formation in rabbits. Infect Immun. 1970 Dec;2(6):810–814. doi: 10.1128/iai.2.6.810-814.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrvik Q. N., Evans D. G. Metabolic and immunologic activities of alveolar macrophages. Arch Environ Health. 1967 Jan;14(1):92–96. doi: 10.1080/00039896.1967.10664700. [DOI] [PubMed] [Google Scholar]
- Myrvik Q. N. Function of the alveolar macrophage in immunity. J Reticuloendothel Soc. 1972 May;11(5):459–468. [PubMed] [Google Scholar]
- Nichols B. A., Bainton D. F., Farquhar M. G. Differentiation of monocytes. Origin, nature, and fate of their azurophil granules. J Cell Biol. 1971 Aug;50(2):498–515. doi: 10.1083/jcb.50.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMOLELIS A. N., HARTSELL S. E. The determination of lysozyme. J Bacteriol. 1949 Dec;58(6):731–736. doi: 10.1128/jb.58.6.731-736.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Truitt G. L., Mackaness G. B. Cell-mediated resistance to aerogenic infection of the lung. Am Rev Respir Dis. 1971 Dec;104(6):829–843. doi: 10.1164/arrd.1971.104.6.829. [DOI] [PubMed] [Google Scholar]
- van Furth R. Origin and kinetics of monocytes and macrophages. Semin Hematol. 1970 Apr;7(2):125–141. [PubMed] [Google Scholar]