Abstract
Between February 2011 and January 2014, 75 ground skinks, Scincella lateralis (Say, 1823) were collected from 13 counties of Arkansas and McCurtain County, Oklahoma, USA, and examined for coccidia. Two (3%) and 11 (15%) S. lateralis were found to be passing oocysts of a new choleoeimerian and isosporan, respectively. Oöcysts of Choleoeimeria ouachitensis n. sp. are ellipsoidal to cylindroidal with a smooth, colourless, bi– layered wall and measure 27.2 × 15.6 μm, and have a length/width (L/W) ratio of 1.7; both micropyle and oöcyst residuum are absent, but 1-2 polar granule(s) are present. Sporocysts are ovoidal, 8.9 × 6.8 μm, L/W 1.3; neither Stieda, sub-Stieda and para-Stieda bodies are present; the walls have two valves joined by longitudinal sutures; a sporocyst residuum consisted of dispersed granules between sporozoites. Oöcysts of Isospora koberi n. sp. are ovoidal with a smooth, colourless, bi-layered wall and measure 25.1 × 20.5 μm, L/W 1.2; both micropyle and oöcyst residuum are absent, but a polar granule is rarely present. Sporocysts are ovoidal, 11.4 × 8.6 μm, L/W 1.3; a nipple-like Stieda body and a subStieda body are present without a paraStieda body; a sporocyst residuum consisted of condensed granules dispersed between sporozoites. This is the second choleoeimerian and third isosporan reported from S. lateralis.
Introduction
The ground skink, Scincella lateralis (Say, 1823) is a small brownish lizard that ranges from southern New Jersey, south to the Florida Keys, and westward to eastern Kansas, and westcentral Texas, USA; isolated geographic distribution records are from central Illinois, northeastern Missouri, USA, and Coahuila, Mexico (Conant & Collins, 1998). Scincella lateralis occurs statewide in Arkansas, USA (Trauth et al., 2004). This skink is commonly found in leaf litter on the forest floor but can also be found in trash piles and dumps where it searches for arthropods.
A great deal of information is available on the natural history and ecology of this common skink (Brooks, 1975) as well as its helminth parasites (see McAllister et al., 2014a); however, less is known about its coccidian parasites. Atkinson and Ayala (1987) provided a description of an isosporan from S. lateralis in Louisiana, and Telford (1997) and Telford and Bursey (2003) described an eimerian and isosporan from Florida specimens, respectively. However, nothing, to our knowledge, has been published on coccidia of S. lateralis from Arkansas. Here, we describe a new species of Choleoeimeria and Isospora from ground skinks of the state.
Materials and methods
Between February 2011 and January 2014, 75 juvenile and adult S. lateralis (mean ± 1SD snout– vent length [SVL] = 40.3 ± 7.7, range 20–59 mm) were collected by hand from 13 counties of Arkansas, Baxter (n = 1), Calhoun (n = 5), Crawford (n = 1), Faulkner (n = 1), Lincoln (n = 2), Madison (n = 2), Marion (n = 2), Miller (n = 1), Nevada (n = 2), Ouachita (n = 6), Saline (n = 2), Searcy (n = 1) and Union (n = 29), and McCurtain County, Oklahoma (n = 20). Skinks were placed in individual bags on ice and returned to the laboratory within 24 hr for necropsy. Skinks were overdosed with an injection of concentrated Chloretone (chlorobutanol) solution and fresh faecal samples were collected from the rectum of each individual for examination of coccidia. Faeces were placed in individual vials containing 2.5% (w/v) aqueous potassium dichromate (K2Cr2O7) and screened for coccidia by light microscopy after flotation in Sheather’s sugar solution (specific gravity = 1.30). Positive samples containing both unsporulated and partially sporulated oöcysts and were placed in Petri dishes containing 2.5 % K2Cr2O7 and allowed to complete sporulation for five days at room temperature (~23°C). Samples were again examined after flotation and measurements were taken on 20 oöcysts using a calibrated ocular micrometer or Olympus© cellSens 1.7 digital imaging software and reported in micrometers (μm) with means followed by the ranges in parentheses; photographs were taken using Nomarski interference-contrast optics. Oocysts were c.385 days old (Choleoeimeria) and c.393 days old (Isospora) when measured and photographed. Descriptions of oocysts and sporocysts follow guidelines of Wilber et al. (1998) as follows: oöcyst length (L) and width (W), their ranges and ratios (L/W), micropyle (M), oöcyst residuum (OR), polar granules (PG), sporocyst length (L) and width (W), their ranges and ratio (L/W), sporocysts (SP), Stieda body (SB), sub–Stieda body (SSB), para–Stieda body (PSB), sporocyst residuum (SR), sporozoites (SZ) anterior (ARB) and posterior (PRB) refractile bodies, and nucleus (N). Photosyntypes of sporulated oöcysts were accessioned into the United States National Parasite Collection (USNPC), Beltsville, Maryland. Host voucher specimens were deposited in the Arkansas State University Herpetological Collection (ASUMZ), State University, Arkansas, U.S.A., as ASUMZ 32164–88, 32297, 32463, 32596, 32603, 32612–14, 32618, 32639–41. Scientific names of reptiles follow the TIGR Reptile Database (Uetz & Hošek, 2013).
Results
Two (3%) and 11 (15%) S. lateralis was found to be passing oöcysts of two new species of coccidia, which are described below.
Choleoeimeria ouachitensis n. sp
Type-host
Ground skink, Scincella lateralis (Say, 182)3 (Sauria: Scincidae), adult male, 43 mm SVL, symbiotype ASUMZ 32297 collected 13 April 2012.
Type-locality
Poison Spring Battlefield Historic Monument off St. Hwy 76, Ouachita County, Arkansas, USA (33.63759°N, 92.987573°W).
Type-material
Photosyntype deposited as USNPC 107852.
Prevalence
2 of 75 (3%) overall; 2 of 6 (33%) Ouachita County.
Sporulation
time
Exogenous. All oöcysts were passed in feces unsporulated or partially sporulated and fully sporulated within 5 days at c.23°C.
Site of infection
Unknown. Oöcysts were passed in faeces and host tissues were not collected or preserved for histological sectioning.
Etymology
The specific epithet is given for Ouachita County, Arkansas, USA (from –ensis) where the host was collected. The county was formed on 29 November 1842, and named for the Ouachita River.
Description (Figs. 1-2, 5)
Fig. 1.
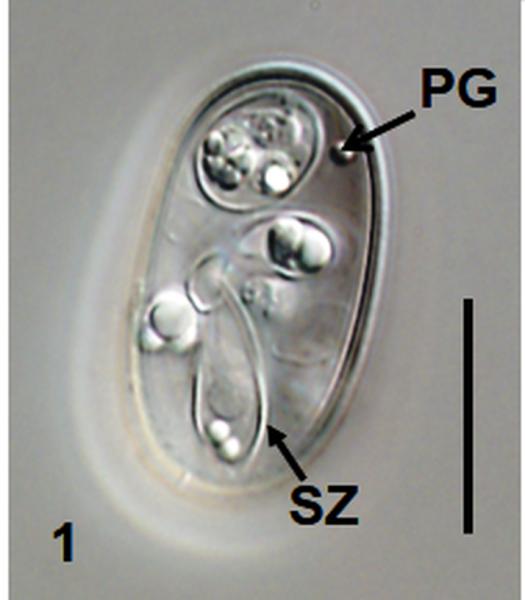
Nomarski interference-contrast photomicrographs of sporulated oöcysts of Choleoeimeria ouachitensis n. sp. and Isospora koberi n. sp. 1 Oöcyst of C. ouachitensis showing polar granule (PG) and sporozoite (SZ); 2 Oöcyst of C. ouachitensis showing sporocyst residuum (SR) in sporozoite and collapsed sporocyst wall at suture (SU); 3 Oöcyst of Isospora koberi showing Stieda body (SB) and substieda body (SSB); 4 Oöcyst of Isospora koberi showing bi-layered oocyst wall (OW). Scale bars 15 μm.
Fig. 2.
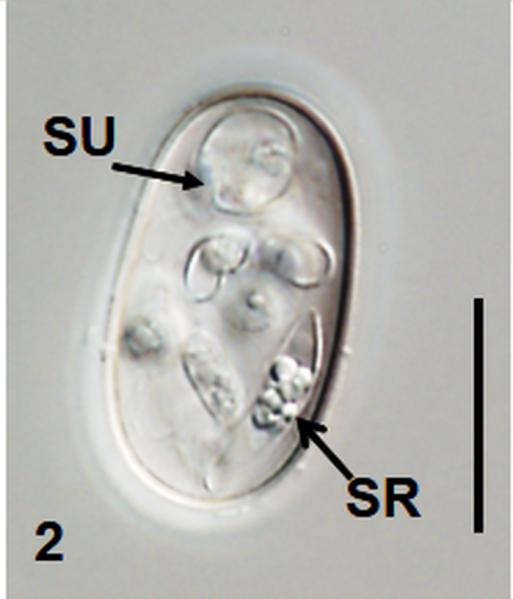
Nomarski interference-contrast photomicrographs of sporulated oöcysts of Choleoeimeria ouachitensis n. sp. and Isospora koberi n. sp. 1 Oöcyst of C. ouachitensis showing polar granule (PG) and sporozoite (SZ); 2 Oöcyst of C. ouachitensis showing sporocyst residuum (SR) in sporozoite and collapsed sporocyst wall at suture (SU); 3 Oöcyst of Isospora koberi showing Stieda body (SB) and substieda body (SSB); 4 Oöcyst of Isospora koberi showing bi-layered oocyst wall (OW). Scale bars 15 μm.
Fig. 5.

Composite line drawings of oöcysts of 5 Choleoeimeria ouachitensis n. sp.; 6 Isospora koberi n. sp.
Sporulated oöcyst
Oöcyst (n = 20) colourless, smooth, ellipsoidal to cylindroidal; 27.2 × 15.6 (26–28 × 15–17), length/width (L/W) ratio 1.7 (1.6–1.8). Wall bi–layered, c.1.0, inner c.0.4, outer c.0.6. Micropyle absent, oocyst residuum absent, 1-2 polar granule(s) present.
Sporocyst
Sporocysts (n = 20) four, colourless, smooth, ovoidal, 8.9 × 6.8 (8–10 × 6–8); L/W ratio 1.3 (1.2–1.5); wall single-layered c.0.5 with two valves joined by longitudinal sutures. Stieda body, sub-Stieda body, and para-Stieda body absent; sporocyst residuum consists of dispersed granules between sporozoite.
Sporozoite
Sporozoites two, banana–shaped, pointed at 1 end, c.10–13 long in situ; with single ellipsoidal posterior refractile body and spheroidal anterior refractile body, with nucleus slightly posterior to midpoint.
Remarks
Because we observed ellipsoidal to cylindroidal oöcysts with L/W ratios > 1.6 and sporocysts composed of two valves joined by longitudinal sutures lacking a Stieda body, we cautiously place the new species in the genus Choleoeimeria (sensu Paperna & Landsberg, 1989), rather than Eimeria. Obviously, it would be more definitive to have tissue samples showing endogenous stages in hypertrophied, displaced cells of the gall bladder or biliary epithelium and/or distinct DNA sequences (see Jirků et al., 2002) that separate Eimeria spp. from this genus. However, we feel comfortable with this placement in their absence. In addition, Modrý and Jirků (2006), in their taxonomic revision of Eimeria spp. from skinks, transferred nine cylindroidal Eimeria spp. to the genus Choleoeimeria, including one (E. scincellae, see below) from S. lateralis.
Choleoeimeria ouachitensis is most similar to two other coccidians described from North American skinks as follows: Choleoeimeria (=Eimeria) egregia Telford, 1997 from the Peninsula mole skink, Plestiodon egregius onocrepis (Cope, 1871) from Florida possesses wider oöcysts (17 μm) and larger sporocysts (10 × 8 μm) without a polar granule (Telford, 1997); Choleoeimeria (=Eimeria) scincellae Telford, 1997 described from S. lateralis from Florida possesses larger oöcysts (30 × 16 μm) and sporocysts (11 × 8 μm) without a polar granule (Telford, 1997). In addition, the only eimerian previously described from Arkansas skinks (Pleistiodon fasciatus) is Choleoeimeria (=Eimeria) fasciatus Upton, McAllister, and Trauth, 1991 (Upton et al., 1991). However, oöcysts of C. fasciatus are considerably larger (34.9 × 16.2 μm vs. 27.2 × 15.6 μm) than the new species.
Isospora koberi n. sp
Type-host
Scincella lateralis (Say, 1823) (Sauria: Scincidae), male, 41 mm SVL, symbiotype ASUMZ 32463 collected 5 August 2012.
Type-locality
Mull, Marion County, Arkansas, USA (36°04’20.1”N, 92°35’59.6”W).
Type-material
Photosyntype deposited as USNPC 107853.
Other hosts and other Arkansas, USA localities
10 S. lateralis; 1 female, 35 mm SVL, collected 5 October 2012 from 3 km N of Calion, Calhoun County (33°22’37.1”N, 92°30’23.7”W); 1 male, 33 mm SVL, collected 11 January 2013 from Harrell, Calhoun County (33°30’36.4”N, 92°30’55.5”W) and 1 male, 47 mm SVL collected 3 August 2012 from Mull, Marion County (36°04’20.1”N, 92°35’59.6”W); 1 male, 44 mm SVL, collected 26 October 2012 from 6.5 km SE of Bluff City at Holeman Cemetery, Ouachita County (33°40’10.9”N, 93°05’46.4”W); 5 males, 30, 32, 39, 43, 46 mm SVL and 1 female 27 mm SVL collected September 2012-January 2013 from Grady Bell Road, El Dorado, Union County (33°12’48.7”N, 92°35’9.5”W).
Prevalence
11 of 75 (15%) S. lateralis overall; 2 of 5 (20%) Calhoun County; 2 of 2 (100%) Marion County; 1 of 6 (17%) Ouachita County; 6 of 29 (21%) Union County.
Sporulation time
Exogenous. All oocysts were passed in faeces unsporulated or partially sporulated and fully sporulated within 5 days at c.23°C.
Site of infection
Unknown. Oöcysts were passed in feces and host tissues were not collected or preserved for histological sectioning.
Etymology
The specific epithet is given in honor of Christian Albert Kober (1901–1968), who accompanied his maternal grandson (CTM) on many memorable occasions in the field and who first taught him to appreciate the fauna of Arkansas.
Description (Figs. 3-4, 6)
Fig. 3.

Nomarski interference-contrast photomicrographs of sporulated oöcysts of Choleoeimeria ouachitensis n. sp. and Isospora koberi n. sp. 1 Oöcyst of C. ouachitensis showing polar granule (PG) and sporozoite (SZ); 2 Oöcyst of C. ouachitensis showing sporocyst residuum (SR) in sporozoite and collapsed sporocyst wall at suture (SU); 3 Oöcyst of Isospora koberi showing Stieda body (SB) and substieda body (SSB); 4 Oöcyst of Isospora koberi showing bi-layered oocyst wall (OW). Scale bars 15 μm.
Fig. 4.

Nomarski interference-contrast photomicrographs of sporulated oöcysts of Choleoeimeria ouachitensis n. sp. and Isospora koberi n. sp. 1 Oöcyst of C. ouachitensis showing polar granule (PG) and sporozoite (SZ); 2 Oöcyst of C. ouachitensis showing sporocyst residuum (SR) in sporozoite and collapsed sporocyst wall at suture (SU); 3 Oöcyst of Isospora koberi showing Stieda body (SB) and substieda body (SSB); 4 Oöcyst of Isospora koberi showing bi-layered oocyst wall (OW). Scale bars 15 μm.
Fig. 6.

Composite line drawings of oöcysts of 5 Choleoeimeria ouachitensis n. sp.; 6 Isospora koberi n. sp.
Sporulated oöcyst
Oöcyst (n = 20) colourless, smooth, ovoidal; 25.1 × 20.5 (20–26 × 19–21); L/W ratio 1.2 (1.1– 1.2). Wall bilayered, c.1.2, outer c.0.7, inner c.0.5; Micropyle absent, oöcyst residuum absent; single polar granule present (rarely).
Sporocyst
Sporocysts (n = 20) two, colourless, smooth, ovoidal, 11.4 × 8.6 (10–12 × 8–9); L/W ratio 1.3 (1.2–1.4); wall single-layered wall c.0.4. Prominent nipple–like Stieda body present, sub-Stieda body present and para-Stieda body absent; sporocyst residuum consists of condensed granules dispersed between sporozoite.
Sporozoites
Sporozoites (not measured) four, sausage–shaped; with single with spheroidal anterior refractile body and single posterior refractile body with nucleus slightly posterior to midpoint .
Remarks
Oöcysts of Isospora koberi are most similar to those of Isospora manchacensis Atkinson and Ayala, 1987 from S. lateralis collected from six sites near New Orleans, Louisiana, USA (Atkinson & Ayala, 1987) and Isospora neosepsorum Telford and Bursey, 2003 from Florida sand skinks, Plestiodon (=Neoseps) reynoldsi (Stejneger, 1910) from Florida, USA (Telford & Bursey, 2003). However, sporocysts of I. koberi are smaller than both species, particularly in width (8.6 vs.10.2 μm and 10.0 μm, respectively) and the prominent Stieda and sub-Stieda bodies are clearly visible compared to I. neosepsorum, neither of which are prominent (see Telford & Bursey, 2003, their Fig. 3). We also observed a polar granule rarely in our samples whereas none were ever reported in >100 oocysts measured by Atkinson and Ayala (1987). Most importantly, Atkinson and Ayala (1987) reported a single–layered oöcyst wall for I. manchacensis (measurements were not given by the authors) and I. koberi clearly has a bi– layered wall (see Fig. 4). In addition, the only isosporan described previously from Arkansas skinks (Plestiodon fasciatus) is Isospora scinci Upton, McAllister, and Trauth, 1991 (Upton et al., 1991). However, oöcysts and sporocysts of I. scinci are larger (26.5 × 24.3 μm and 14.9 × 10.4 μm) than the new species. Given these differences when compared to three other described isosporans from North American skinks, we feel confident that we are describing a new species.
Discussion
The lizard family Scincidae contains over 1,500 species worldwide with only 13 species in North America north of Mexico (Uetz & Hošek, 2013). Although several skinks of the South Pacific (particularly Papua New Guinea) have been recently surveyed for coccidia (see McAllister et al., 2014b), as far as we can determine and including our descriptions herein, there are only nine species of coccidians (four isosporans, five eimerians) reported from skinks from North America, including those in three southern states (Arkansas, Louisiana and Florida) (see Table I). Of the 13 species of North American skinks, coccidians have now been reported from five (38%) species.
Table 1.
Summary of coccidian parasites from North American Scincidae.
| Coccidian | Host | Locality | Prevalence* | Reference |
|---|---|---|---|---|
| Choleoeimeria egregia † | Plestiodon egregius | Florida | 2/34 (6%) | Telford (1997) |
| Plestiodon reynoldsi | Florida | 1/21 (5%) | Telford & Bursey (2003) | |
| Choleoeimeria fasciatus † | Plestiodon fasciatus | Arkansas | 3/13 (23%) | Upton et al. (1991) |
| 3/14 (21%) | McAllister et al. (1994) | |||
| Choleoeimeria ouachitensis | Scincella lateralis | Arkansas | 2/75 (3%) | This study |
| Choleoeimeria scincellae † | S. lateralis | Florida | 1/14 (7%) | Telford (1997) |
| 2/14 (14%) | Telford & Bursey (2003) | |||
| Eimeria sp. ‡ | S. lateralis | Louisiana | 14/137 (10%) | Atkinson & Ayala (1987) |
| Isospora koberi | S. lateralis | Arkansas | 10/75 (13%) | This study |
| Isospora manchacensis | S. lateralis | Louisiana | 59/137 (43%) | Atkinson & Ayala (1987) |
| Isospora neosepsorum | P. reynoldsi | Florida | 3/21 (14%) | Telford & Bursey (2003) |
| Isospora scinci | P. fasciatus | Arkansas | 3/13 (23%) | Upton et al. (1991) |
| 3/14 (21%) | McAllister et al. (1994) | |||
| Plestiodon laticeps | Arkansas | 1/4 (25%) | McAllister et al. (1994) |
Prevalence = number infected/number examined (%).
Originally described as Eimeria spp. (see Modrý and Jirků, 2006, for taxonomic changes of new combinations).
An undescribed eimerian reported from gall bladder epithelium and most likely a Choleoeimeria sp.
McAllister et al. (1994) previously examined a sample of 26 S. lateralis from Arkansas, Oklahoma, and Texas and did not find any to be passing coccidia. In addition, their survey included 20 other non-infected skinks, including two southern coal skinks, Plestiodon anthracinus pluvialis (Cope, 1880) from Arkansas, and two Great Plains skinks, Plestiodon obsoletus Baird & Girard, 1852, eight southern prairie skinks, Plestiodon septentrionalis obtusirostris (Bocourt, 1879) and eight four-lined skinks, Plestiodon tetragrammus Baird, 1859 from Texas. In addition, none of the 20 S. lateralis we examined in the present survey from Oklahoma was found to be passing coccidia. It appears that prevalence of coccidia among North American skinks is low and finding infected hosts outside of species already reported herein (particularly those which range into the more arid western US states) may be challenging.
Acknowledgements
We thank Patricia A. Pilitt (USNPC) for her expert curatorial assistance. The Arkansas Game & Fish Commission issued Scientific Collecting Permits to CTM and MBC. This study was funded, in part, by grants from the National Center for Research Resources (P20RR016474) and the National Institute of General Medical Sciences (P20GM103432) from the National Institutes of Health to RSS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Chris T. McAllister, Science and Mathematics Division Eastern Oklahoma State College Idabel, Oklahoma 74745, USA cmcallister@se.edu
R. Scott Seville, Department of Zoology and Physiology University of Wyoming Casper, Wyoming 82601, USA.
Matthew B. Connior, Health and Natural Sciences South Arkansas Community College El Dorado, Arkansas 71730, USA
Stanley E. Trauth, Department of Biological Sciences Arkansas State University State University, Arkansas 72467, USA
Henry W. Robison, Department of Biology Southern Arkansas University Magnolia, Arkansas 71754, USA
References
- Atkinson CT, Ayala SC. Isospora manchacensis n. sp., an intranuclear coccidian from the Louisiana ground skink, Scincella lateralis (Say, 1823) (Lacertilia: Scincidae). Journal of Parasitology. 1987;73:817–823. [PubMed] [Google Scholar]
- Brooks GR., Jr. Scincella lateralis. Catalogue of American Amphibians and Reptiles. 1975;169:1–4. [Google Scholar]
- Conant R, Collins JT. A field guide to reptiles and amphibians of eastern and central North America. 3rd ed. (expanded). Houghton Mifflin; Boston: 1998. p. 616. [Google Scholar]
- Jirků M, Modrý D, Šlapeta JR, Koudela B, Lukes J. The phylogeny of Goussia and Choleoeimeria (Apicomplexa: Eimeriorina) and the evolution of excystation structures in coccidian. Protist. 2002;153:379–390. doi: 10.1078/14344610260450118. [DOI] [PubMed] [Google Scholar]
- McAllister CT, Bursey CR, Connior MB, Durden LA, Robison HW. Helminth and arthropod parasites of the ground skink, Scincella lateralis (Sauria: Scincidae), from Arkansas and Oklahoma, U.S.A. Comparative Parasitology. 2014a;81 in press. [Google Scholar]
- McAllister CT, Duszynski DW, Fisher RN, Austin CC. A new species of Eimeria Schneider, 1875 (Apicomplexa: Eimeriidae) from the Solomon ground skink, Sphenomorphus solomonis (Sauria: Scincidae) from Papua New Guinea. Systematic Parasitology. 2014b;87:83–86. doi: 10.1007/s11230-013-9455-2. [DOI] [PubMed] [Google Scholar]
- McAllister CT, Upton SJ, Trauth SE. New host and distributional records for coccidia (Apicomplexa: Eimeriidae) from North American lizards (Reptilia: Sauria). Journal of the Helminthological Society of Washington. 1994;61:221–224. [Google Scholar]
- Modrý D, Jirků M. Three new species of coccidia (Apicomplexa: Eimeriorina) from the marble-throated skink, Marmorosphax tricolor Bavay, 1869 (Reptilia: Scincidae), endemic to New Caledonia with a taxonomic revision of Eimeria spp. from scincid hosts. Parasitology Research. 2006;99:416–428. doi: 10.1007/s00436-005-0106-7. [DOI] [PubMed] [Google Scholar]
- Paperna I, Landsberg JH. Description and taxonomic discussion of eimerian coccidia from African and Levantine geckoes. South African Journal of Zoology. 1989;24:245–355. [Google Scholar]
- Telford SR., Jr. Two new species of Eimeria Schneider, 1875 (Apicomplexa: Eimeriidae) from skinks in Florida. Systematic Parasitology. 1997;36:27–30. [Google Scholar]
- Telford SR, Jr., Bursey CR. Comparative parasitology of squamate reptiles endemic to scrub and sandhills communities of north-central Florida, U.S.A. Comparative Parasitology. 2003;70:172–181. [Google Scholar]
- Trauth SE, Robison HW, Plummer MV. The amphibians and reptiles of Arkansas. University of Arkansas Press; Fayetteville: 2004. p. 421. [Google Scholar]
- Uetz P, Hošek J. [26 December 2013];The TIGR Reptile Database. World Wide Web electronic publication. 2013 http://www.reptile-database.org/.
- Upton SJ, McAllister CT, Trauth SE. Two new species of coccidia (Apicomplexa: Eimeriidae) from Eumeces fasciatus (Sauria: Scincidae) in Arkansas. Canadian Journal of Zoology. 1991;69:2028–2030. [Google Scholar]
- Wilber PG, Duszynski DW, Upton SJ, Seville RS, Corliss JO. A revision of the taxonomy and nomenclature of the Eimeria spp. (Apicomplexa: Eimeriidae) from rodents in the Tribe Marmotini (Sciuridae). Systematic Parasitology. 1998;39:113–135. [Google Scholar]


