Abstract
A triply bridged fused diporphyrin appended with six thioglucose units is reported. This new, chemically and photochemically stable amphiphilic compound is taken up by breast cancer cells and causes cell death upon light exposure. Photophysical studies reveal absorption bands in the near IR region, and photosensitized formation of singlet oxygen in high quantum yields.
Keywords: Porphyrin, Thioglucose, Photodynamic Therapy, Fluorescence, Click Chemistry
Introduction
There are many applications of dyes in biochemistry, imaging and therapy that require appended, robust biotargeting motifs. Since there is wide range of biotargeting motifs available for all of these applications, core dye platforms with appropriate photophysical properties that can be rapidly and efficiently appended with the targeting motif avoids the complex redesign of synthetic strategies. Photodynamic therapy (PDT), for example, is a non-invasive treatment for cancer involving the interaction of light, a photosensitizer and oxygen to result in generation of singlet oxygen and other reactive oxygen species that cause necrosis or apoptosis.1,2 There are many cancer targeting motifs, including sugars and peptides.
The photophysics of porphyrin and phthalocyanine derivatives can be tuned for many of the above applications. For example, those with good triplet quantum yields can serve as photosensitizers for PDT, while those with good fluorescence quantum yields can serve as imaging agents and biochemical trackers. Thus there is a need for a palate of these core dye platforms with different photophysical properties. Porphyrinoids are generally non-toxic under dark conditions.3,4 Imaging and PDT of various forms of cancers are more efficient if the dye absorbs light in the therapeutic window (ca. 700–1100 nm) especially for deep cancers,5–7 so bio-targeted dye systems with longer wavelength absorptions are needed for next-generation imaging and PDT agents.8,9
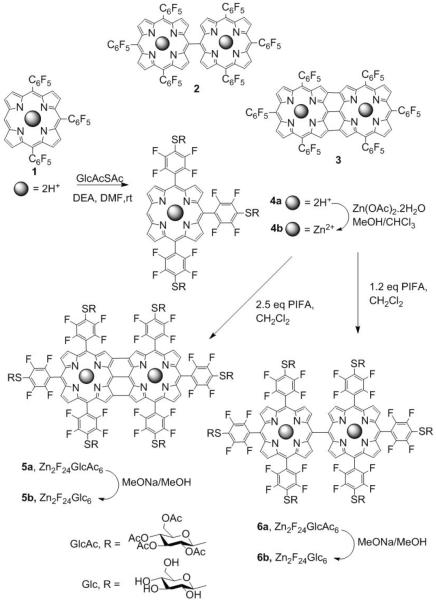
Direct, covalent linking of porphyrins (e.g. 2 and 3 in Fig. 1) yields systems with photophysical properties such as high polarizability and high nonlinear optical character10 that arise from strong electronic coupling of the macrocycles. Compounds such as 1 and 2 are fluorescent. Fused (β-β, meso-meso, β-β) triply bridged porphyrins have low energy absorption bands in the infrared region because of extended conjugation and coplanar geometries.11 Compounds such as 3 are minimally fluorescent but exhibit large two-photon absorption (2PA) cross sections (σ) where two low energy 1400 – 2000 nm photons are simultaneously absorbed and have good triplet quantum yields.12,13 Sugar moieties appended to porphyrinoids increase the selective uptake by cancer cells.14 The number, type, and position of the sugar moieties effect cell uptake,15,16 but the hydrolysis of O-glyco linkages of many reported conjugates can diminish in vivo effectiveness compared to non-hydrolysable derivatives.14
Figure 1.
Oxidative coupling of 1 with DDQ/Sc(OTf)3yields 2 and 3,25 but substitution of the para fluoro groups 2 or 3 is inefficient. Adding the sugars to compound 1 proceeds in high yields, followed by oxidative coupling of 4b with PIFA efficiently yields 5a and 6a, which are readily deprotected 5b and 6b.
Our work on perfluorophenylporphyrins suggests that the triply linked fused diporphyrin bearing six perfluorophenyl groups 5, and singly bridged derivative 6 (Fig. 1) could serve as platforms to form bioconjugates and biocompatible compounds thereby exploiting the photophysical properties of 2 and 3 reported by Kim and coworkers.8,13,17–20 Herein we report the synthesis, optical properties, cell uptake, and photodynamic induced necrosis activity of hexa-glycosylated diporphyrin, 5b, and the fluorescence imaging properties of 6b. Since the substitution of all six para fluoro groups on 3 and isolation of this product proved difficult, we used the trisperfluorophenyl porphyrin precursor (1, Fig. 1) as the platform to append the thiosugars, and then formed the fused porphyrin by a mild oxidative coupling reaction. The generality of the substitution chemistry of 1 affords a platform to append a diversity of conjugates, thereby broadening the applications of the two dimers.
Results and Discussion
5,10,15-tris(pentafluorophenyl)porphyrinatozinc(II) (1) was synthesized using the procedure reported21 (Scheme ESI 1), and is the platform molecule for the synthesis of singly and triply linked diporphyrins with diverse biotargeting motifs. The oxidative coupling reported by Osuka used DDQ/Sc(OTf)3 to form 3.13 Since the electron withdrawing groups on 1 inhibit the oxidative coupling and DDQ/Sc(OTf)3 reacts with the sugars, we employed the hypervalent iodine(III) reagent, phenyliodine bis(trifluoroacetate) (PIFA) for oxidative coupling.22 The oxidation of 1 bearing an open meso position with different equivalents of PIFA results in the formation of meso-meso linked diporphyrin 2 and the β-β, meso-meso, β-β triply linked diporphyrins 3 (Scheme, ESI 2).23–27 The metal ion chelated by the macrocycle improves the yields.
Porphyrin 1 was treated with 3.5 equivalents of thioglucose and then Zn(II) was inserted to efficiently yield 4b. The glycosylated triply linked compound 5b is obtained in 40% yield after reacting with 2.5 equivalents of PIFA, and 1.2 equivalents yield predominately 6a. The structures of all porphyrinoids were confirmed by 1H, 19F and 13C NMR spectroscopy, UV-visible, and MALDI-TOF spectra (ESI).
The UV-visible and emission spectra of the glycosylated derivative 5b and 6b are similar to that reported for 3 and 2, respectively.13 Because the porphyrins are orthogonal in 6b, the photophysical properties are similar to meso aryl porphyrins and aggregates somewhat less than the fused derivative. However, the UV-visible spectra 5b exhibits a broad Soret band at 413 nm and Q-bands between 557 nm and 1068 nm; in different solvents because the compound can partition into different cellular environments (Table 1 and ES1). No apparent aggregation of 5b was observed in DMSO, but in other solvents the compound aggregates as indicated by shoulders on both blue (H-aggregate) and red (J-aggregate) edges of the main absorption peaks.
Table 1.
UV-visible spectral data for 5b
| Solvent | UV-visible λmax (nm)a |
|---|---|
| DMSO | 429, 462, 569, 610, 636, 749, 876, 943, 1090 |
| toluene | 427, 462, 525, 565, 606, 667, 829, 966, 1079 |
| ethylacetate | 428, 462, 524, 565, 606, 671, 848, 952, 1070 |
| PBS | 426, 461, 526, 565, 607, 671, 849, 950, 1071 |
| ethanol | 421, 455, 514, 561, 602, 664, 837, 935, 1029, 1078 |
1 cm path length. See ESI. In CHC13 the lifetime is 3.3 ps, but no quantum yield is reported (ref. 13).
The aggregation of 5b was confirmed by dynamic light scattering (DLS) measurements in these solvents (ESI Table 1). The size and structural organization of nanoaggregates of porphyrinoids depends on a variety of factors such as concentration, type of solvent used, temperature and nature of peripheral groups attached to the chromophore.28 After shaking in phosphate buffered saline (PBS), DLS shows two populations with diameters of 30±4 nm and 284±15 nm, but after sonication for about 15 min, only 82±7 nm particles are observed. The size of the nanoaggregates and strength of intermolecular interactions significantly affects the endocytosis of the photosensitizer by cancer cells, and the propensity of the dyes to disaggregate while adsorbed on the cell membrane or within the cell. Because fewer dyes are excited and energy transfer processes in aggregates, the effective triplet quantum yield is reduced, thereby reducing the PDT effectiveness.
However, fluorescence microscopy shows that nanoaggregates of 6b are taken up by MDA-MB-231 human breast cancer cells and slowly disaggregates inside the cells as indicated by the increased fluorescence intensity (Fig. 2). Since 5b does not fluoresce, the disaggregation is indicated by the PDT effects, wherein the light may contribute to the disaggregation via internal conversion.
Figure 2.
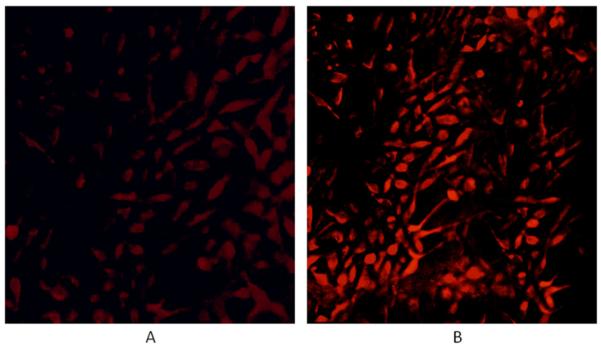
MDA-MB-231 cells were incubated with ca. 50 nM of the singly linked dimer 6b for 24 hours, rinsed four times with PBS buffer to remove any unbound dye and fixed with 4% paraformaldehyde solution in NaOH. Fluorescence images were captured using 540–580 nm band pass excitation and 600–650 nm band pass emission, magnification 20× under identical instrumental conditions. (A) Just after preparation of the fixed cells, and (B) the same slide after 3 days. The contrast in each case was enhanced by 40% for publication.
The singlet oxygen quantum yield (1O2) production (ΦΔ) for 5b in DMSO was determined to be 0.78±0.03 in which the 1O2 phosphorescence at 1270 nm was monitored29–31 and meso-tetra(4-sulfonatophenyl)porphyrin dihydrochloride (TSPP) was employed as a reference sensitizer (ΦΔ TSPP = 0.63 in D2O).31 Both 5b and 6b were examined for PDT effect on the MDA-MB-231 human breast cancer cells (Fig. 3) where the percent of viable cells is significantly reduced upon light exposure. Irradiation with white light causes necrosis with an IC50 for 5b of ca. 13 γM and for 6b ca. 19 μM measured just after photodynamic treatment, and 24 hours later, ca. 75 % of the cell are necrotic for compound 5b and ca. 66% necrotic for compound 6b, indicating induction of apoptosis.14
Figure 3.
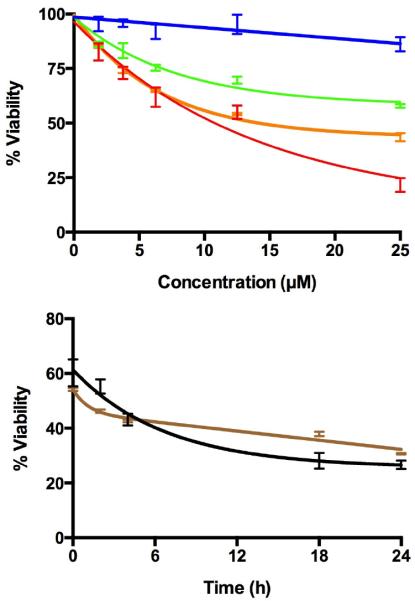
Top: after treating MDA-MB-231 human breast cancer cells with 5b and 6b for 24 hours and removing the unbound dye, dark toxicity of 5b (blue), 6b (green) and phototoxicity of 5b (red) and 6b (orange) upon irradiation of cells with white light (0.92 mW cm−2; 11.04 kJ m−2 for 20 min) were determined using a trypan blue assay. Bottom: the cells continue to become necrotic for the next 18 hours after similar treatment with 10 μM of 5b (black) and 6b (brown).
Conclusions
The mechanism of uptake of non-hydrolysable thioglycosylated porphyrinoids by cancer cells is not well understood. The overexpression of glucose receptors on the cell mediate adsorption to the membrane, but the compounds must diffuse or the nanoaggregates be internalized by endocytosis.32 These two porphyrin dimers add the palate of dyes for platforms for biomedical applications.
Supplementary Material
Highlights.
A fused diporphyrin with six thioglucose is made by click-type chemistry.
The glycoporphyrin is taken up by breast cancer cells.
Upon light exposure the glycoporphyirn causes cell death.
The glycoporphyrin photosensitizes formation of singlet oxygen in high quantum yields.
Acknowledgments
Supported by the U.S. National Science Foundation (NSF) CHE-0847997 to C.M.D. and (DMR-0611539) to R.G. Hunter College science infrastructure is supported by the NSF, the National Institute on Minority Health and Health Disparities (8G12 MD007599) and the City University of New York.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.MacDonald IJ, Dougherty TJ. J. Porphyrins Phthalocyanines. 2001;5:105–129. [Google Scholar]
- 2.Wilson BC, Patterson MS. Phys. Med. Biol. 2008;53:R61–109. doi: 10.1088/0031-9155/53/9/R01. [DOI] [PubMed] [Google Scholar]
- 3.Davia K, King D, Hong Y, Swavey S. Inorg. Chem. Commun. 2008;11:584–586. [Google Scholar]
- 4.Ko Y-J, Yun K-J, Kang M-S, Park J, Lee K-T, Park SB, Shin J-H. Bioorg. Med. Chem. Lett. 2007;17:2789–2794. doi: 10.1016/j.bmcl.2007.02.083. [DOI] [PubMed] [Google Scholar]
- 5.Achelle S, Couleaud P, Baldeck P, Teulade-Fichou M-P, Maillard P. Eur. J. Org. Chem. 2011;2011:1271–1279. [Google Scholar]
- 6.Waynant RW, Ediger MN, editors. Electrooptics Handbook. McGraw-Hill; New York: 1993. [Google Scholar]
- 7.Sharman WM, Allen CM, van Lier JE. Drug Discov. Today. 1999;4:507–517. doi: 10.1016/s1359-6446(99)01412-9. [DOI] [PubMed] [Google Scholar]
- 8.Singh S, Aggarwal A, Thompson S, Tomé JPC, Zhu X, Samaroo D, Vinodu M, Gao R, Drain CM. Bioconjugate Chem. 2010;21:2136–2146. doi: 10.1021/bc100356z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aggarwal A, Singh S, Zhang Y, Anthes M, Samaroo D, Gao R, Drain CM. Tetrahedron Lett. 2011;52:5456–5459. doi: 10.1016/j.tetlet.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka T, Lee BS, Aratani N, Yoon M-C, Kim D, Osuka A. Chem. Eur. J. 2011;17:14400–14412. doi: 10.1002/chem.201102889. [DOI] [PubMed] [Google Scholar]
- 11.Roberts DA, Fückel B, Clady RGCR, Cheng YY, Crossley MJ, Schmidt TW. J. Phys. Chem. A. 2012;116:7898–7905. doi: 10.1021/jp3043895. [DOI] [PubMed] [Google Scholar]
- 12.Tsuda A, Osuka A. Science. 2001;293:79–82. doi: 10.1126/science.1059552. [DOI] [PubMed] [Google Scholar]
- 13.Mori H, Tanaka T, Aratani N, Lee BS, Kim P, Kim D, Osuka A. Chem. Asian J. 2012;7:1811–1816. doi: 10.1002/asia.201200247. [DOI] [PubMed] [Google Scholar]
- 14.Chen X, Hui L, Foster DA, Drain CM. Biochem. 2004;43:10918–10929. doi: 10.1021/bi049272v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Drain CM. Drug Des. Rev. - Online. 2004;1:215–234. [Google Scholar]
- 16.Hirohara S, Nishida M, Sharyo K, Obata M, Ando T, Tanihara M. Bioorg. Med. Chem. 2010;18:1526–1535. doi: 10.1016/j.bmc.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Shaw SJ, Edwards C, Boyle RW. Tetrahedron Lett. 1999;40:7585–7586. [Google Scholar]
- 18.Samaroo D, Soll CE, Todaro LJ, Drain CM. Org. Lett. 2006;8:4985–4988. doi: 10.1021/ol060946z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costa JIT, Tomé AC, Neves MGPMS, Cavaleiro JAS. J. Porphyrins Phthalocyanines. 2011;15:1116–1133. [Google Scholar]
- 20.Drain CM, Singh S. In: The Handbook of Porphyrin Science with Applications to Chemistry, Physics, Materials Science, Engineering, Biology and Medicine. Kadish KM, Smith KM, Guilard R, editors. World Scientific Publishers; Singapore: 2010. pp. 485–540. [Google Scholar]
- 21.Dogutan DK, Bediako DK, Teets TS, Schwalbe M, Nocera DG. Org. Lett. 2010;12:1036–1039. doi: 10.1021/ol902947h. [DOI] [PubMed] [Google Scholar]
- 22.Ouyang Q, Zhu Y-Z, Zhang C-H, Yan K-Q, Li Y-C, Zheng J-Y. Org. Lett. 2009;11:5266–5269. doi: 10.1021/ol902198w. [DOI] [PubMed] [Google Scholar]
- 23.Jin L-M, Yin J-J, Chen L, Guo C-C, Chen Q-Y. Synlett. 2005;2005:2893, 2898. [Google Scholar]
- 24.Jin L-M, Chen L, Yin J-J, Guo C-C, Chen Q-Y. Eur. J. Org. Chem. 2005;2005:3994–4001. [Google Scholar]
- 25.Dohi T, Morimoto K, Kiyono Y, Maruyama A, Tohma H, Kita Y. Chem. Commun. 2005:2930–2932. doi: 10.1039/b503058g. [DOI] [PubMed] [Google Scholar]
- 26.Dohi T, Morimoto K, Maruyama A, Kita Y. Org. Lett. 2006;8:2007–2010. doi: 10.1021/ol060333m. [DOI] [PubMed] [Google Scholar]
- 27.Zhdankin VV, Stang PJ. Chem. Rev. 2002;102:2523–2584. doi: 10.1021/cr010003+. [DOI] [PubMed] [Google Scholar]
- 28.Drain CM, Smeureanu G, Patel S, Gong XC, Garno J, Arijeloye J. New J. Chem. 2006;30:1834–1843. [Google Scholar]
- 29.Aebisher D, Azar NS, Zamadar M, Gandra N, Gafney HD, Gao R, Greer A. J. Phys. Chem. B. 2008;112:1913–1917. doi: 10.1021/jp709829z. [DOI] [PubMed] [Google Scholar]
- 30.Gandra N, Frank AT, Le Gendre O, Sawwan N, Aebisher D, Liebman JF, Houk KN, Greer A, Gao R. Tetrahedron. 2006;62:10771–10776. [Google Scholar]
- 31.Tanielian C, Wolff C, Esch M. J. Phys. Chem. 1996;100:6555–6560. [Google Scholar]
- 32.Bechet D, Couleaud P, Frochot C, Viriot M-L, Guillemin F, Barberi-Heyob M. Trends in Biotechnology. 2008;26:612–621. doi: 10.1016/j.tibtech.2008.07.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.