Abstract
Curcumin has phototoxic effects on bacteria under <450 nm irradiation, but it is unstable in vivo and cannot exert effects on deep tissues. Near infrared light (NIR) is harmless to the body and has stronger penetration than visible light. In order to improve the effects of curcumin, upconversion nanoparticles conjugated with curcumin (UCNPs-curcumin) are designed to upconvert NIR to the excitation wavelength of curcumin. UCNPs-curcumin were synthesized using polyethyleneimine to combine curcumin and UCNPs, based on typical composition of lanthanide nitrates Re(NO)3 (Y:Yb:Er=78%:20%:2%) linked by ethylenediaminetetraacetic acid in sodium fluoride (NaF) matrix, to upconvert NIR to 432 nm light. The product was characterized by size distribution, thermogravimetric analysis, differential scanning calorimetry, and scanning electron microscopy. Growth inhibition of methicillin-resistant Staphylococcus aureus (MRSA) was not only measured in vitro but also investigated on MRSA-induced pneumonia in mice. The results showed that curcumin was covered by UCNPs to form stable nanoparticles whose average size was 179.5 nm and zeta potential was −33.7 mV in normal saline. The UCNPs-curcumin produced singlet oxygen, which reaches a stable level after 30 minutes of irradiation, and took effect on MRSA through bacterial cytoplasm leakage. They alleviated MRSA-induced pneumonia and reduced bacterial counts in lungs with 980 nm irradiation (0.5 W/cm2) on chests of mice. It is confirmed that the UCNPs-curcumin in lungs are activated under NIR irradiation and strengthen their antibacterial effects on MRSA. This research provides a new type of NIR photosensitizer, which plays an important role in phototoxic effects of curcumin in deep tissues under NIR.
Keywords: curcumin, upconversion nanoparticle, near infrared light, methicillin-resistant Staphylococcus aureus, phototoxicity
Introduction
Curcumin (diferuloylmethane) is a well-known polyphenol from Curcuma longa L., which is used as a colorant in Asian food and has anti-inflammatory, antimicrobial, and anticarcinogenic activities.1 It mainly takes effect by phototoxicity through production of singlet oxygen (1O2) upon irradiation at <450 nm to kill bacteria and tumors.2,3 Because the penetration of the laser light depends on its wavelength,4 ≤450 nm has weaker penetration than red light and near infrared light (NIR), leading to feeble action on deep lesions in vivo.
NIR falls in the region of the spectrum with the lowest absorption in tissue and therefore enables the deepest tissue penetration.5 It is also safe and causes minimal photo damage to the biological specimen involved, but it cannot activate curcumin. In order to bridge this gap, a transducer that is capable of converting NIR light to excitating light of curcumin is required. Upconversion nanoparticles (UCNPs) can convert two or more low-energy pump photons from NIR to a higher-energy output photon with a shorter wavelength.6 In recent years, UCNPs have been developed for applications in biological labeling, sensing, and imaging.7 For most UCNPs, lanthanide ion codoped sodium yttrium fluoride (NaYF4) nanoparticles possess one of the highest upconversion efficiencies, with tunable photoluminescence, controlled size, and low toxicity.8 To convert NIR to <450 nm light, we prepared NaYF4 UCNPs to conjugate with curcumin as a photosensitizer to take effect in deep tissues. The physico-chemical properties of the UCNPs, including particle morphology, size distribution, differential scanning calorimetry (DSC), and 1O2 production, were analyzed.
Staphylococcus aureus is a major pathogenic bacterium for pulmonary nosocomial infection.9 The disease is currently treated by antibiotics but easily acquires drug resistance, resulting in the deficiency of effective treatment.10 Methicillin-resistant S. aureus (MRSA), in particular, is resistant to most antibiotics and is responsible for significantly higher morbidity and mortality and increased health care costs.11 Even though curcumin shows the effect of photokilling MRSA in vitro,12 it is uncertain to be effective in deep tissue lesions such as pneumonia infected by MRSA.
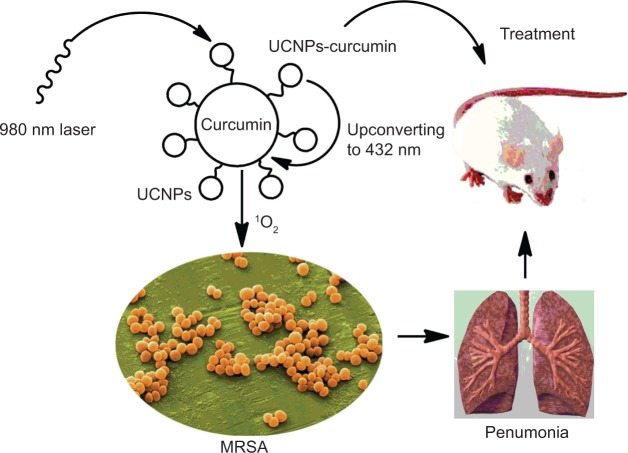
We not only evaluated the inhibitory effect of UCNPs conjugated with curcumin on bacterial growth in vitro but also investigated their effect on MRSA-induced pneumonia in mice. The scheme is illustrated in Figure 1. Bacterial count and the expression of inflammatory cytokines tumor necrosis factor (TNF)-α and interleukin (IL)-1β in lungs were analyzed after NIR radiation. Bacterial cytoplasmic leakage was measured to discuss the possible mechanism.
Figure 1.
The schematic chart of the upconversion nanoparticles conjugated with curcumin (UCNPs-curcumin) inhibiting methicillin-resistant Staphylococcus aureus (MRSA)-induced pneumonia in mice.
Materials and methods
Chemicals
Curcumin (purity 98%) was purchased from Guangzhou Qiyun Biotech Company (Guangzhou, People’s Republic of China). MRSA (ATCC 25923) was obtained from the Chinese National Institute for Food and Drug Control. Yttrium oxide (Y2O3, 99.99%), ytterbium oxide (Yb2O3, 99.99%), erbium oxide (Er2O3, 99.99%), and sodium fluoride (NaF, 98%) were purchased from Shanghai Chemical Industrial Company (Shanghai, People’s Republic of China) and used as starting materials without further purification. Amoxicillin, TNF-α, and IL-1β kits were bought from Jiancheng Biotech Company (Nanjing, People’s Republic of China). Sodium ethylenediaminetetraacetic acid (EDTA-Na), polyethyleneimine (PEI), and other chemicals were of analytical purity and were purchased from Qianhui Reagent Company (Guangzhou, People’s Republic of China).
Animals
Sixty-four Kunming mice (half male and half female) weighing 20–30 g and aged 6–8 weeks were used for the experiment. They were kept in plastic cages at 25°C±2°C with 50%±10% humidity and on a 12-hour light–dark cycle with free access to pellet food and water. The animals were acclimatized to laboratory conditions for 1 week prior to the experiments. All procedures performed were reviewed and approved by the South China University of Technology and conformed to internationally accepted principles. All efforts were made to minimize animal suffering and to reduce the number of animals to be used.
Synthesis of UCNPs conjugated with curcumin
The UCNPs were synthesized by hydrothermal reaction following literature protocol with slight modifications.13 All the lanthanide nitrates Re(NO)3 were prepared by dissolving the respective rare-earth oxides in nitric acid. Typically, 1 mmol of Re(NO)3 (Y:Yb:Er=78%:20%:2%), 20 mmol of NaF, and 2 mmol of EDTA-Na were mixed in 50 mL water and heated to 180°C in autoclave and kept at this temperature for 20 hours. After cooling down to room temperature, the products were precipitated by ethanol, washed three times with water, and dried in vacuum, and 1.2 g of product was collected. Upconversion of the UCNPs was detected by F4500 Fluorescence Spectrometer (Hitachi Instrument Co, Ltd, Japan) with excitation wavelength of 980 nm.
The conjugation of UCNPs with curcumin (UCNPs-curcumin) was by means of molecular interaction and was carried out with the following procedure. One hundred and fifty milligrams of UCNPs, 100 mg curcumin, and 50 mg PEI were dissolved in dichloromethane and stirred at room temperature for 5 hours. The product (0.3 g) was obtained by evaporation of dichloromethane and washing with ethanol.
Particle size distribution and zeta potential
Particle size distribution and zeta potential of the UCNPs-curcumin were determined by Nano-2S MDT-2 Malvern Particle Size Analyzer (Malvern Instruments Ltd, Malvern, UK). Before testing, the appropriate sample was dispersed in normal saline with a refractive index of 1.33.
Morphological observation
Morphology of the UCNPs-curcumin was investigated by scanning electron microscopy (SEM, LEO 1530 VP, Germany). Ten milligrams of sample was suspended in 1 mL water, and 2 μL of the suspension was placed on a glass surface and dried in air. The samples were gold sputtered under high vacuum before analysis at an accelerating voltage of 20 kV.
Thermogravimetric analysis and differential scanning calorimetry analysis
Thermogravimetric (TG) analysis and DSC were carried out simultaneously using a Q6000-SDT Thermogravimetric Apparatus (TA Instrument Ltd, DE, USA) with a heating rate of 20°C/min from 30°C to 800°C under nitrogen atmosphere.
Determination of 1O2
1O2 was measured by the bleaching of p-nitrosodimethylaniline (RNO) according to the reference.14 The UCNPs-curcumin (0.5 mg/mL) were mixed with 50 mmol of RNO and 100 mmol of imidazole in 20 mmol of phosphate buffer (pH 7.4) and then irradiated by a 980 nm laser (0.5 W/cm2, Threetop Optical Electric Technology Company, Dongguan, People’s Republic of China) for 10, 20, 30, 40, and 60 minutes. Power density of 0.5 W/cm2 was applied on the basis of its safety and wide use in photodynamic therapy in vivo. The RNO absorption at 440 nm was determined by UV-3010 ultraviolet (UV) spectrometer (Hitachi Instrument Co, Ltd, Japan). The reduction of optical density (OD) at 440 nm can reflect the production of 1O2. UCNPs and curcumin were treated as the controls in the same method.
Phototoxic assay against MRSA
The phototoxic activity was tested as reported.15 Aliquots of 100 μL of MRSA suspension (1×106 colony-forming units [CFU]/mL) were individually transferred to separate wells of 96-well microtiter plates. An equal volume of the UCNPs-curcumin suspension in Mueller–Hinton broth (MH) was added to each well to give final concentrations of 10, 20, 40, 80, 160, and 320 μg/mL. After dark incubation at 37°C for 20 minutes (preirradiation time), each plate was placed under 0.5 W/cm2 irradiation at 980 nm for 30 minutes. The incubation was kept in dark for 24 hours, and then OD value at 490 nm was measured with water as blank to calculate the bacterial growth inhibition rate. Minimal inhibitory concentration (MIC) was determined as the lowest concentration with clear wells. The phototoxic activities of UCNPs and curcumin were measured in the same way. UCNPs were directly dispersed in MH broth, and curcumin was dissolved in dimethyl sulfoxide to 30 mg/mL and then diluted to final concentration with MH broth.
| (1) |
OD490 OD490, OD at 490 nm, reflects bacterial concentration. The wells with bacteria and solvent were used as negative controls.
Cytoplasmic leakage of MRSA
MRSA was monitored over time for cytoplasmic leakage via absorbance at 260 nm (A260) measurements according to the reference.16 A260 can reflect the content of amino acids and nucleic acids leaked out of the cell. Δ260 is the increase in the absorbance of bacterial supernatant at 260 nm compared with untreated controls. Bacteria were treated the same as photo-toxic assay and incubated for 24 hours. Sample aliquots were centrifuged for 5 minutes at 10,000xg and room temperature to remove cellular debris. The supernatant was collected and the absorbance was detected at 260 nm by UV spectrometer to deduce cytoplasmic leakage.
Antibacterial test in pneumonia mice induced by MRSA
The pneumonia model was described by the reference with a little modification.17 Mice were divided into eight groups with eight mice in each group: normal control, negative control, amoxicillin group, and five medicated groups (UCNPs, curcumin, and UCNPs-curcumin at high, middle, and low doses) with irradiation. Mice, except the normal group, were intranasally infected with 50 μL inoculums of MRSA (1×106 CFU/mL) suspension in saline applied dropwise to the nares. The mice were allowed to aspirate the inoculums for 10 minutes before being returned to cages to recover. The mice were administered with vein injection of the UCNPs-curcumin at low dose (5 mg/kg), middle dose (15 mg/kg) and high dose (45 mg/kg) with 980 nm irradiation (0.5 W/cm2) on chest for 30 min. The control mice were administered respectively with 45 mg/kg amoxicillin, 45 mg/kg UCNPs, and 45 mg/kg curcumin instead. The drugs were suspended in normal saline, and the dosage used was based on safety and effectiveness according to our previous research and the reference.18 Three days later, the mice were euthanized and lung tissue was removed and divided into two aliquots. One aliquot was plated onto Tryptose Soya Agar plates for bacterial colony count, and the bacterial density was expressed as the number of CFU per lung. The other was used for determination of TNF-α and IL-1β by enzyme-linked immunosorbent assay according to kit description. Briefly, lung tissue was weighed and homogenized in normal saline, then centrifuged at 1,000×g for 20 minutes. TNF-α and IL-1β in the supernatant were labeled by biotin antibody and horseradish peroxidase Avidin and detected at 450 nm.
Statistical analysis
Data were expressed as mean ± standard deviation and analyzed with SPSS Version 13.0 software (IBM, Armonk, NY, USA). Significant tests among the groups were based on one-way analysis of variance and Student–Newman–Keuls test.
Results and discussion
Characters of the UCNPs-curcumin
There have been several reports on the synthesis of UCNPs.19–21 However, most of these nanoparticles are synthesized at high temperatures.22,23 In recent years, a hydrothermal synthesis method at lower temperature has been found.24 Different constitutions of nanoparticles have various upconverting effects. Yb/Er codoped NaYF4 nanoparticles have been reported as the most efficient infrared-to-visible upconversion fluorescent.25 Therefore, the NaYF4 UCNPs were synthesized by hydrothermal method in our research.
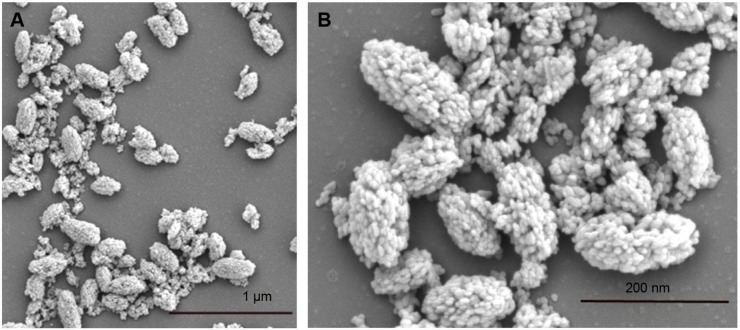
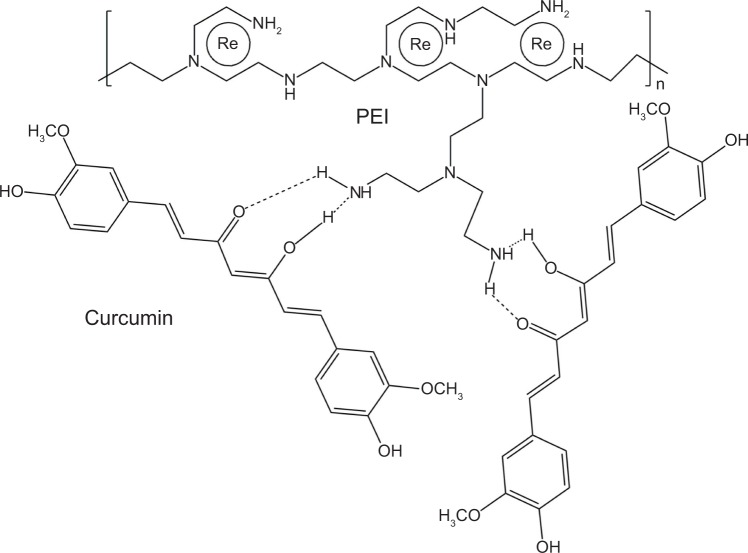
Curcumin shows many pharmacological effects in vitro, but it has disadvantages such as limited absorption, poor bio-availability, and rapid metabolism and excretion.26 Linkage of curcumin with UCNPs may improve its stability in vivo. We conjugated UCNPs with curcumin to produce the new nanoparticles (UCNPs-curcumin). The results showed that the average size of the nanoparticles was 179.5 nm and the size distributed in the range 142–263 nm. The zeta potential was −33.7 mV, suggesting that UCNPs-curcumin are stable in normal saline because of negative charge repulsion. Plain nanoparticles (UCNPs) have an average size of 6.5 nm and zeta potential of −5.4 mV, showing smaller size but easier precipitation. Morphology of the UCNPs-curcumin was observed and photographed with SEM to identify micro-structures of the nanoparticles. The SEM images of the UCNPs-curcumin displayed an oval shape with UCNPs binding together (Figure 2). Based on molecular structure, more than three UCNPs can be chelated by ion bonds with one molecule of PEI, which also binds to two molecules of curcumin by hydrogen bonds at branched chain (Figure 3). Many UCNPs are connected by PEI and curcumin and become a large particle.
Figure 2.
Scanning electron micrograph of the upconversion nanoparticles conjugated with curcumin at 5,000× (A) and 20,000× (B) amplification.
Figure 3.
Possible structure of the upconversion nanoparticles conjugated with curcumin by polyethyleneimine (PEI).
In order to further evaluate the composition of the nanoparticles, DSC and TG are applied as a simple method to verify it.27 DSC and TG curves of the UCNPs-curcumin are shown in Figure 4. It exhibits three single endothermic peaks at 59°C, 183°C, and 250°C, respectively, representing the melting points of PEI, curcumin, and EDTA. TG patterns of the nanocapsules showed gradual loss of weight because of slow decomposition of PEI, EDTA, and curcumin. Upon calculation of the weight loss by TG curves of the UCNPs and curcumin, the UCNPs-curcumin are made up of 55.5% UCNPs, 27.8% curcumin, and 16.7% PEI.
Figure 4.
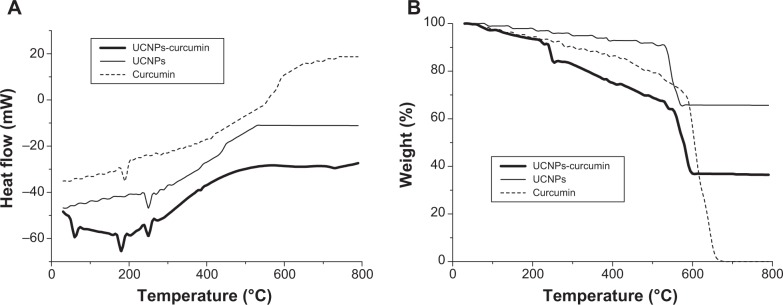
Differential scanning calorimetric (A) and thermogravimetric (B) chart of the upconversion nanoparticles conjugated with curcumin (UCNPs-curcumin).
Production of 1O2
The production of 1O2 was evaluated by the change of RNO absorbance at 440 nm. RNO is a scavenger of 1O2 and bleached by it. A440 decreased significantly (P<0.01) after the treatment of the UCNPs-curcumin with 980 nm irradiation, and reached stability at a time interval of 30 minutes (Figure 5). The UCNPs and curcumin had no obvious change in A440 alone. It suggests that the UCNPs-curcumin produce 1O2 under irradiation, and 30 minutes of illumination are appropriate for their stable effect at 0.5 mg/mL concentration with 0.5 W/cm2 irradiation.
Figure 5.
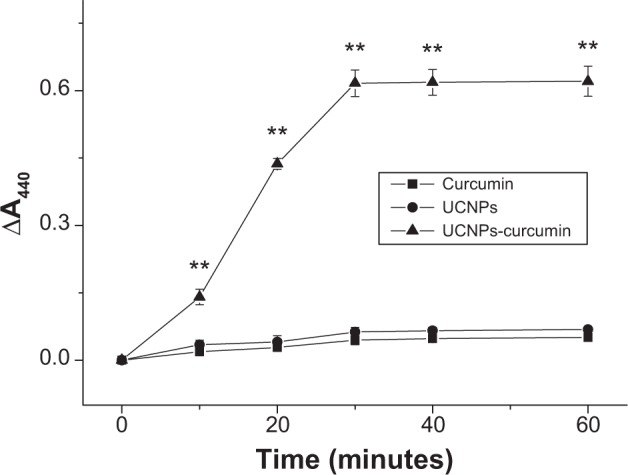
Singlet oxygen production of the samples under 980 nm irradiation (0.5 W/cm2) at different times. Singlet oxygen was measured by bleaching of p-nitrosodimethylaniline with a decrease in the absorbance of 440 nm. Data are presented as mean ± standard deviation in three repetitions.
Note: **P<0.01, compared with curcumin and upconversion nanoparticles (UCNPs).
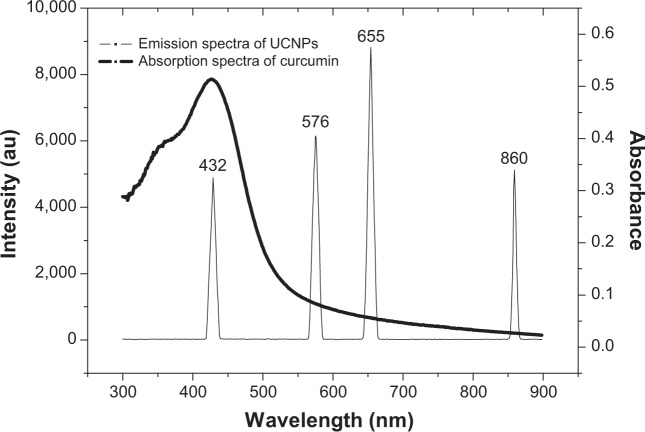
Curcumin can be excited by 450 nm laser, but it cannot be activated by 980 nm laser. The UCNPs can upconvert 980 nm light to shorter wavelength. Upconversion tests showed that the UCNPs emitted four peaks (432 nm, 576 nm, 655 nm, and 860 nm) of light in the spectrum after excitation at 980 nm (Figure 6), and the 432 nm peak completely overlaps with absorption spectra. Among the four emission peaks, only light at 432 nm can activate curcumin to produce 1O2. Others are too weak to excite curcumin. Because 432 nm light is about one-quarter of upconversion efficiency, how to increase the upconversion ratio of 980 nm to 432 nm is worth further research.
Figure 6.
Emission spectra of the upconversion nanoparticles (UCNPs) excited by 980 nm laser and absorption spectra of curcumin.
Phototoxicity of the UCNPs-curcumin on MRSA
The UCNPs-curcumin had inhibitory effects on MRSA growth at dose dependence accompanying 30 minutes of irradiation at 980 nm (Figure 7). MIC was 30 μg/mL less than the curcumin (130 μg/mL), and the MIC of curcumin was similar, as reported.28 It suggests that the UCNPs-curcumin had stronger phototoxicity on MRSA than curcumin.
Figure 7.
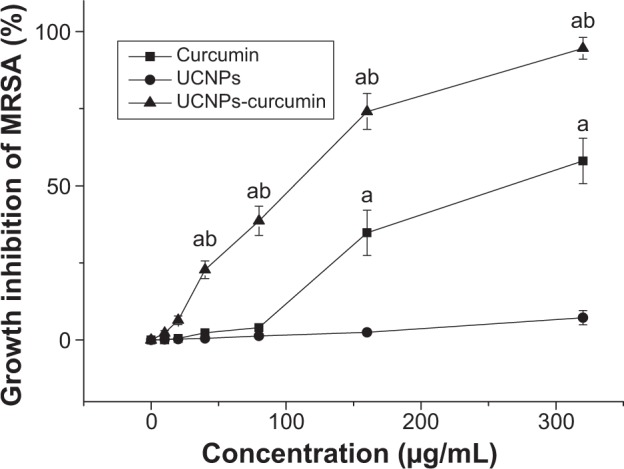
Inhibition of the samples on the growth of methicillin-resistant Staphylococcus aureus (MRSA) with 980 nm laser irradiation (0.5 W/cm2) for 30 minutes. Data are presented as mean ± standard deviation in five repetitions.
Notes: aP<0.01, compared with upconversion nanoparticles (UCNPs); bP<0.01, compared with curcumin.
Cytoplasmic leakage of MRSA was measured by the increase in the absorbance of bacterial supernatant at 260 nm compared with untreated controls. ΔA260 increased significantly (P<0.01) after treatment of the UCNPs-curcumin under 980 nm irradiation at concentration dependence (Figure 8). It suggests that the UCNPs-curcumin destroy MRSA cell membrane leading to leakage of cytoplasm. This is the possible mechanism of the UCNPs-curcumin inhibiting MRSA under irradiation.
Figure 8.
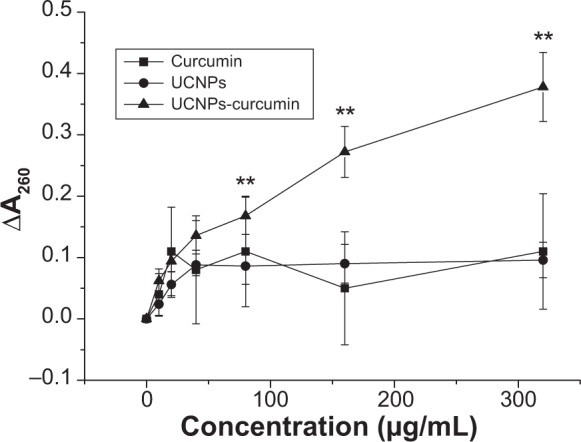
Cytoplasmic leakage of methicillin-resistant Staphylococcus aureus affected by the samples in different concentrations with 980 nm irradiation (0.5 W/cm2, 30 minutes). It was measured in the supernatant of bacterial culture with an increase in the absorbance at 260 nm. Data are presented as mean ± standard deviation .
Note: **P<0.01, compared with curcumin and upconversion nanoparticles (UCNPs).
Inhibitory effects of the UCNPs-curcumin on pneumonia in mice
Even though it is proven that curcumin can inhibit MRSA in vitro, it is difficult to play a role in pneumonia induced by MRSA in vivo because of its disadvantages, such as instability and low bioavailability. The UCNPs-curcumin improve the stability of curcumin and will have better effects in vivo. The lung, an internal organ, cannot be affected by visible light, since its penetrating depth is <10 mm;29 only NIR can reach the lung without harm30 and become an excellent light source to activate the UCNPs-curcumin in the lung.
Animal tests showed that the infectious lungs of mice had more colonies by bacterial counting. TNF-α and IL-1β in the lungs of mice increased significantly when compared with normal mice, indicating pneumonia caused by MRSA in mice. Amoxicillin had little improvement on bacterial quantity and cytokines expression in the lungs of mice, suggesting antibiotic resistance of the bacteria. The UCNPs-curcumin with irradiation significantly (P<0.01) decreased bacterial counts in lungs with dose dependence (Figure 9), proving their effectiveness on inhibition of MRSA.
Figure 9.
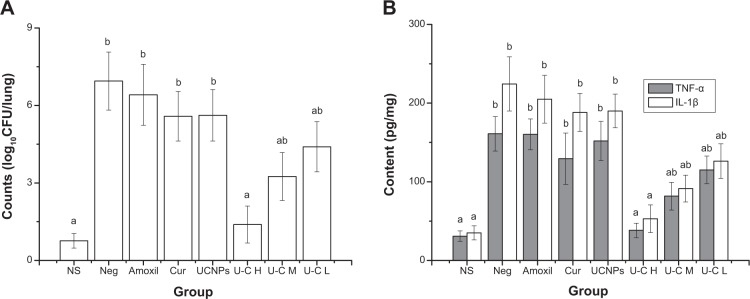
Effects of the treatments on bacterial density (A) and cytokines (B) in lungs of mice. Normal saline (NS), negative (Neg), amoxicillin (Amoxil), curcumin (Cur), upconversion nanoparticles (UCNPs) 45 mg/kg intravenous (IV); U–C H, U–C M, and U–C L are UCNPs-curcumin with IV injection at high dose (45 mg/kg), middle dose (15 mg/kg), and low dose (5 mg/kg). Mice, except the normal group, were intranasally administered with methicillin-resistant Staphylococcus aureus (1×106 colony-forming units [CFU]/mL), treated by drugs and 980 nm irradiation (0.5 W/cm2, 30 minutes) on the chest for 3 days, and then euthanized. Lungs were taken for bacterial counting and determination of tumor necrosis factor (TNF)-α and interleukin (IL)-1β by enzyme-linked immunosorbent assay. Data are presented as mean ± standard deviation .
Notes: aP<0.01, compared with negative group; bP<0.01, compared with normal group.
The concentration of UCNPs-curcumin of mouse lung was directly determined by fluorescence intensity at 860 nm after excitation at 980 nm. The results showed that there were, respectively, 3.2 μg/g, 16.5 μg/g, and 58.3 μg/g UCNPs-curcumin in lungs 2 hours after intravenous (IV) injection of UCNPs-curcumin at 5 mg/kg, 15 mg/kg, and 45 mg/kg. That is to say, about 0.3%–0.6% UCNPs-curcumin could reach the lungs and take effect after IV administration, indicating the lower effective dose.
In order to compare the effects of different treatments, the same IV administration was applied in this research. Even though inhalation is direct to deliver drugs to the lung, and may be a good way to increase drug concentration in lungs, like other drugs,31 it is difficult to measure the dose accurately in mice. This needs further investigation on different routes of administration for our next research.
TNF-α and IL-1β are key inflammatory cytokines and cause phosphorylation and degradation of inhibitor of nuclear factor kappa-B kinase, leading to translocation of nuclear factor-κB into the nucleus to promote expression of inflammatory genes.32 This experiment shows that MRSA increases the expression of TNF-α and IL-1β, triggering inflammation in the lung. Injection of the UCNPs or curcumin alone did not inhibit the bacterial growth and the expression of TNF-α and IL-1β in the lung, but they were reduced by the treatments of UCNPs-curcumin at dose dependence under 980 nm irradiation for 30 minutes. It confirms that MRSA-induced pneumonia is controlled by UCNPs-curcumin through activation by NIR to directly inhibit the bacteria in the lung.
Curcumin is a safe food additive with a maximum tolerance dose of 12 g/kg.33 UCNPs-curcumin are also safe at a dose of 5–45 mg/kg after vein injection. But irradiation excites the UCNPs-curcumin to produce 1O2, which is harmful to cell membrane. The damage is relevant to irradiation power, time, and position. No injury to lungs has been found in our research with 0.5 W/cm2 irradiation on the chest for 30 minutes. These parameters can be good references for future clinical application.
Conclusion
UCNPs-curcumin are synthesized by upconversion NaYF4 nanoparticles conjugated with curcumin by PEI. The UCNPs are based on typical composition of Re(NO)3 (Y:Yb:Er=78%:20%:2%) linked by EDTA in NaF matrix, and can upconvert NIR to 432 nm light. The product is characterized by size distribution, DSC, TG, and SEM, confirming that curcumin is covered by the UCNPs to make stable nanoparticles. UCNPs-curcumin can produce 1O2 after irradiation at 980 nm and inhibit MRSA growth by destroying cell membrane leading to bacterial cytoplasmic leakage. The antibacterial effect of UCNPs-curcumin is also proven by the control on MRSA-induced pneumonia in mice. It is mainly attributed to direct bacteria inhibition by phototoxicity through activation of the UCNPs-curcumin under NIR irradiation. The dosage, irradiation power, and time are crucial to protect normal tissue from injury. This research provides a new type of NIR photosensitizer, which improves the effects of curcumin in vivo. It is effective to inhibit MRSA-induced infections in deep organs, and has prospects in clinical application.
Acknowledgments
The authors would like to thank the staff at the South China University of Technology for data analysis and the ethical committee for approval of animal tests. The financial support (No 81173646) from the National Science Funding of China is also acknowledged.
Footnotes
Disclosure
The authors declare no conflicts of interest.
References
- 1.Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “curecumin”: from kitchen to clinic. Biochem Pharmacol. 2008;75(4):787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 2.Koon H, Leung AW, Yue KK, Mak NK. Photodynamic effect of curcumin on NPC/CNE2 cells. J Environ Pathol Toxicol Oncol. 2006;25(1–2):205–215. doi: 10.1615/jenvironpatholtoxicoloncol.v25.i1-2.120. [DOI] [PubMed] [Google Scholar]
- 3.Araújo NC, Fontana CR, Bagnato VS, Gerbi ME. Photodynamic effects of curcumin against cariogenic pathogens. Photomed Laser Surg. 2012;30(7):393–399. doi: 10.1089/pho.2011.3195. [DOI] [PubMed] [Google Scholar]
- 4.Ackermann G, Hartmann M, Scherer K, Lang EW, Hohenleutner U, Landthaler M, Bäumler W. Correlations between light penetration into skin and the therapeutic outcome following laser therapy of port-wine stains. Lasers Med Sci. 2002;17(2):70–78. doi: 10.1007/s101030200013. [DOI] [PubMed] [Google Scholar]
- 5.Pansare V, Hejazi S, Faenza W, Prud’homme RK. Review of long-wavelength optical and NIR imaging materials:contrast agents, fluorophores and multifunctional nano carriers. Chem Mater. 2012;24(5):812–827. doi: 10.1021/cm2028367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mai HX, Zhang YW, Sun LD, Yan CH. Highly efficient multicolor up-conversion emissions and their mechanisms of monodisperse NaYF4:Yb, Er core and core/shell-structured nanocrystals. J Phys Chem C. 2007;111(37):13721–13729. [Google Scholar]
- 7.Wang F, Banerjee D, Liu YS, Chen XY, Liu XG. Upconversion nanoparticles in biological labeling, imaging, and therapy. Analyst. 2010;135(8):1839–1854. doi: 10.1039/c0an00144a. [DOI] [PubMed] [Google Scholar]
- 8.Tian G, Gu ZJ, Zhou LJ, et al. Mn2+ dopant-controlled synthesis of NaYF4:Yb/Er upconversion nanoparticles for in vivo imaging and drug delivery. Adv Mater. 2012;24(9):1226–1231. doi: 10.1002/adma.201104741. [DOI] [PubMed] [Google Scholar]
- 9.Quartin AA, Scerpella EG, Puttagunta S, Kett DH. A comparison of microbiology and demographics among patients with healthcare-associated, hospital-acquired, and ventilator-associated pneumonia: a retrospective analysis of 1184 patients from a large, international study. BMC Infect. Dis. 2013;13:e561. doi: 10.1186/1471-2334-13-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haussler S, Parsek MR. Biofilms 2009: new perspectives at the heart of surface-associated microbial communities. J Bacteriol. 2010;192(12):2941–2949. doi: 10.1128/JB.00332-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maeda Y, Kenny F, Coulter WA, et al. Bactericidal activity of denture-cleaning formulations against planktonic health care-associated and community-associated methicillin-resistant Staphylococcus aureus. Am J Infect Control. 2007;35(9):619–622. doi: 10.1016/j.ajic.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Ribeiro AP, Pavarina AC, Dovigo LN, Brunetti IL, Bagnato VS, Vergani CE, Costa CA. Phototoxic effect of curcumin on methicillin-resistant Staphylococcus aureus and L929 fibroblasts. Lasers Med Sci. 2013;28(2):391–398. doi: 10.1007/s10103-012-1064-9. [DOI] [PubMed] [Google Scholar]
- 13.Wang C, Cheng L, Liu Z. Drug delivery with upconversion nanopar-ticles for multi-functional targeted cancer cell imaging and therapy. Biomaterials. 2011;32(4):1110–1120. doi: 10.1016/j.biomaterials.2010.09.069. [DOI] [PubMed] [Google Scholar]
- 14.Wang C, Tao H, Cheng L, Liu Z. Near-infrared light induced in vivo photodynamic therapy of cancer based on upconversion nanoparticles. Biomaterials. 2011;32(26):6145–6154. doi: 10.1016/j.biomaterials.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Paschoal MA, Tonon CC, Spolidório DM, Bagnato VS, Giusti JS, Santos-Pinto L. Photodynamic potential of curcumin and blue LED against Streptococcus mutans in a planktonic culture. Photodiagnosis Photodyn Ther. 2013;10(3):313–319. doi: 10.1016/j.pdpdt.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Weaver AJ, Jr, Shepard JB, Wilkinson RA, et al. Antibacterial activity of THAM trisphenylguanide against methicillin-resistant Staphylococcus aureus. PLoS One. 2014;9(5):e97742. doi: 10.1371/journal.pone.0097742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee MH, Arrecubieta C, Martin FJ, Prince A, Borczuk AC, Lowy FD. A postinfluenza model of Staphylococcus aureus pneumonia. J Infect Dis. 2010;201(4):508–515. doi: 10.1086/650204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiong L, Yang T, Yang Y, Xu C, Li F. Long-term in vivo biodistribution imaging and toxicity of polyacrylic acid-coated upconversion nanophosphors. Biomaterials. 2010;31(27):7078–7085. doi: 10.1016/j.biomaterials.2010.05.065. [DOI] [PubMed] [Google Scholar]
- 19.Yi GS, Sun BQ, Yang FZ, Chen DP, Zhou YX, Cheng J. Synthesis and characterization of high-efficiency nanocrystal up-conversion phosphors:ytterbium and erbium codoped lanthanum molybdate. Chem Mater. 2002;14(7):2910–2914. [Google Scholar]
- 20.Heer S, Lehmann O, Haase M, Gudel HU. Blue, green, and red upconversion emission from lanthanide-doped LuPO4 and YbPO4 nanocrystals in a transparent colloidal solution. Angew Chem Int Edn. 2003;42(27):3179–3182. doi: 10.1002/anie.200351091. [DOI] [PubMed] [Google Scholar]
- 21.Yi GS, Lu HC, Zhao SY, Yue G, Yang WJ, Chen DP, Guo LH. Synthesis, characterization, and biological application of size-controlled nanocrystalline NaYF4:Yb, Er infrared-to-visible up-conversion phosphors. Nano Lett. 2004;4(11):2191–2196. [Google Scholar]
- 22.Heer S, Kompe K, Gudel HU, Haase M. Highly efficient multicolour upconversion emission in transparent colloids of lanthanide-doped NaYF4 nanocrystals. Adv Mater. 2004;16(23–24):2102–2105. [Google Scholar]
- 23.Zeng JH, Su J, Li ZH, Yan RX, Li YD. Synthesis and upconversion luminescence of hexagonal-phase NaYF4:Yb, Er3+, phosphors of controlled size and morphology. Adv Mater. 2005;17(17):2119–2123. [Google Scholar]
- 24.Du HY, Zhang WH, Sun JY. Structure and upconversion luminescence properties of BaYF5:Yb, Er nanoparticles prepared by different methods. J Alloy Compd. 2011;509(7):3413–3418. [Google Scholar]
- 25.Chatterjee DK, Rufaihah AJ, Zhang Y. Upconversion fluorescence imaging of cells and small animals using lanthanide doped nanocrystals. Biomaterials. 2008;29(7):937–943. doi: 10.1016/j.biomaterials.2007.10.051. [DOI] [PubMed] [Google Scholar]
- 26.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharmaceutics. 2007;4(6):807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 27.Pignatello R, Ferro M, Puglisi G. Preparation of solid dispersions of nonsteroidal anti-inflammatory drugs with acrylic polymers and studies on mechanisms of drug-polymer interactions. AAPS Pharm Sci Tech. 2002;3(2):35–45. doi: 10.1208/pt030210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gunes H, Gulen D, Mutlu R, Gumus A, Tas T, Eren Topkaya A. Antibacterial effects of curcumin: an in vitro minimum inhibitory concentration study. Toxicol Ind Health. 2013 Oct 21; doi: 10.1177/0748233713498458. Epub. [DOI] [PubMed] [Google Scholar]
- 29.Liu B, Farrell TJ, Patterson MS. Comparison of photodynamic therapy with different excitation wavelengths using a dynamic model of aminolevulinic acid-photodynamic therapy of human skin. J Biomed Opt. 2012;17(8):e088001. doi: 10.1117/1.JBO.17.8.088001. [DOI] [PubMed] [Google Scholar]
- 30.Hemmer E, Takeshita H, Yamano T, et al. In vitro and in vivo investigations of upconversion and NIR emitting Gd2O3:Er3+, Yb3+ nanostructures for biomedical applications. J Mater Sci Mater Med. 2012;23(10):2399–2412. doi: 10.1007/s10856-012-4671-x. [DOI] [PubMed] [Google Scholar]
- 31.Jin YQ, Huang C, Chen A, He F. A comparative study on the pharmacokinetics of different delivery systems of amphotericin B after intravenous and inhalation administration to mice. Chin J Pharm. 2009;40(5):355–361. [Google Scholar]
- 32.Litteljohn D, Mangano E, Clarke M, Bobyn J, Moloney K, Hayley S. Inflammatory mechanisms of neurodegeneration in toxin-based models of Parkinson’s disease. Parkinsons Dis. 2011;2011:e713517. doi: 10.4061/2011/713517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li R, Liu XH, Kong T, Li Y, Sun CH. Evaluation of the safety and toxicology of curcumin. J Hygiene Res. 2009;40(6):747–749. [Google Scholar]