Synopsis
Sodium current in the heart flows principally through the pore protein NaV1.5, which is part of a complex of interacting proteins that serve both to target and localize the complex in the membrane, and to modulate function by such post-translational modifications as phosphorylation and nitrosylation. Multiple mutations in seven different NaV1.5 interacting proteins have been associated with dysfunctional sodium current and inherited cardiac diseases, including long QT syndrome, Brugada syndrome, atrial fibrillation, and cardiomyopathy, as well as sudden infant death syndrome (SIDS). Mutations in as yet unidentified interacting proteins may account for cardiac disease for which a genetic basis has not yet been established. Characterizing the mechanisms by which these mutations cause disease may give insight into etiologies and treatments of more common acquired cardiac disease, such as ischemia and heart failure.
Keywords: Arrhythmia, Macromolecular complex, Sodium channel, Long QT syndrome, Brugada syndrome, Sudden Infant Death Syndrome, Cardiomyopathy, Sodium current, Late Sodium Current, SCN5a
Clinical Importance
In this review we summarize current understanding of cardiac diseases caused by mutations in proteins that interact with the cardiac sodium channel NaV1.5. NaV1.5 is encoded by the gene SCN5A and forms the pore through which flows the majority of sodium current (INa). More than 30 sodium channel interacting proteins (SCIPs) have been identified1, 2 (Table 1). Excluding the four β-subunits, which are covered in Chapter 13, mutations in seven additional SCIPs have been associated with cardiac diseases (Table 2). These diseases include inherited arrhythmia syndromes such as long QT syndrome (LQTS), Brugada syndrome (BrS) and atrial fibrillation (AF), as well as inherited cardiomyopathy. We also include sudden infant death syndrome (SIDS) as a disease where deaths may be presumed to have a cardiac cause and where ~10% have been associated with channelopathies3. Mutations in SCIPs are a rare cause of inherited arrhythmia (<1% of LQTS and BrS), but they account for ~5% of SIDS overall3 or half of the SIDS cases associated with channelopathies. Mutations in SNTA1 alone represent ~25% of SIDS associated channelopathies (~2.5% of SIDS overall). Despite the overall rarity, the study of these mutations has provided insight into the regulation of INa and the pathophysiology of arrhythmia in common acquired cardiac diseases, such as ischemia and heart failure. In addition, an important percentage of BrS and SIDS remain without a linked genotype and SCIPs, both known (Table 1) or as yet unidentified, could account for the etiology of an important percentage of these syndromes.
Table 1.
Nav1.5 interacting proteins (SCIPs)
| Protein | Gene | Size (aa) | Evidence for NaV1.5 Association | Effect on INa |
|---|---|---|---|---|
| 14-3-3η | Ywhah | 246 | Co-IP64 | (↓)RecR, (−) SSI64 |
| α-Actinin 2 | Actn2 | 894 | Co-IP65 | (↑) INa Peak65 |
| Ankyrin G | Ank3 | 4377 | Co-IP66, 67 | (↑)Surface Density, (↑)INa Peak66, 67 |
| α1-Syntrophin | Snta1 | 505 | GST-PD, Co-IP18, 19, 23 | Regulate INa Late 18, 19, 23 |
| β1, β1b | Scn1b | β1 218 β1b 268 |
Co-IP68 | Kinetics conflicting (↓) INa Late69, 70 (↑)RecR71 (↑)INa Peak72, 73 |
| β2 | Scn2b | 215 | Co-IP74 | Sialylation Status75, (↑)Late Current76 |
| β3 | Scn3b | 215 | Co-IP77 | (↑)INa, (↑)RecR78(+) SSI, (↑)RefractPer.,79 |
| β4 | Scn4b | 228 | Co-IP80 | (↑) AP Upstroke Velocity, (+) SSI81 |
| β4 Spectrin | Sptbn4 | 2,564 | Co-IP82 | (↑)INa Peak, (+) SSI via Phos.@ S57182 |
| Calmodulin | Calm1 | 152 | GST-PD83 NMR84 |
(+)SSI85, 86 |
| CaMKinase IIδ isoform 3 | Camk2d | 510 | Co-IP82 | (−) SSI, Phos. @ S571S, 516, T594 (↑) INa Late87 |
| Caveolin-3 | Cav3 | 151 | Co-IP32 | (↓) INa Late29, 30, 32 |
| Connexin 40 | Gja5 | 358 | Co-loc88 | ? |
| Connexin 43 | Gja1 | 382 | Co-IP89 | (↑) INa Peak90 |
| Desmoglein 2 | Dsg2 | 1118 | Co-IP91 | (↑) INa Peak91 |
| Dystrophin | Dmd | 3,685 | Co-IP92 | (↑)INa Peak92 |
| Fibroblast growth factor FGF12B, 13 |
Fgf12 Fgf13 |
181 | Co-IP93 | (+) SSI, (↑) RecR93 |
| λ2 syntrophin | Sntg2 | 539 | GST-PD94 | (+) Activ Ou, 2003 175/id} |
| GPD1L | Gpd1l | 351 | GST-PD37 | (↑)INa by phosphorylation37 80 or ROS39 |
| MOG1 | Rangrf | 218 | GST-PD, co-IP44 | (↑)Surface Density, (↑)INa Peak44 |
| Nedd4-2 | Nedd4l | 975 | GST-PD8 | (↑) INa Peak8 |
| nNOS | Nos1 | 1434 | GST-PD, Co-IP19 | (↑)INa Late19 (↑) INa Peak22, 23 |
| Plakophilin-2 | Pkp2 | 881 | Co-IP54 | (↑) INa Peak, (+) SSI56 |
| PMCA4b | Atp2b4 | 1241 | GST-PD, Co-IP19, 20 | (↓) INa Late19 |
| SAP97 | Dlg1 | 904 | Co-IP10 | (↑) INa Peak10 |
| SLMAP | Slmap | 828 | *59 | (↑) INa Peak59 |
| Telethonin | Tcap | 167 | Co-IP13 | (−) Activ13 |
| PTPH1 | Ptpn3 | 913 | GST-PD95 | (−) SSI95 |
| Utrophin | Utrn | 3433 | Co-IP92 | (↓) INa Peak92 |
| Zasp | Ldb3 | 727 | GST-PD63 | (↑) INa Peak 63 |
Adapted from Adsit GS, Vaidyanathan R, Galler CM, Kyle JW, Makielski JC. Channelopathies from mutations in the cardiac sodium channel protein complex. J Mol Cell Cardiol 2013.
SLMAP has not yet been shown to associate with NaV1.5.
Abbreviations: Co-IP = co-immunoprecipitation; GST-PD = pull-down with a GST fusion protein; (↓) = decrease in; (↑) = increase in; (+) = depolarizing shift; (−) = hyperpolarizing shift; Activ.= activation; RecR = recovery rate; RefractPer.=refractory period; SSI=steady state inactivation; INa Peak= peak sodium current; INa Late =late sodium current or persistent current
Table 2.
Sodium channel interacting protein (SCIP) with disease-causing mutations that have been shown to affect INa
| SCIP | Gene name | Common Name | Chromosome | MW kDa | Splice Variants | Disease | Disease mutations |
|---|---|---|---|---|---|---|---|
| SNTA1 | SNTA1 | α1-Syntrophin | 20q11.2 | 54 | 1 | LQT1219, 25 SIDS23 |
A257G, A261V, 390V T262P, T372M, G460S |
| CAV3 | CAV3 | Caveolin-3 | 3p25 | 17 | 1 | LQT929 SIDS30 |
A85T, F97C, S141R V14L, T78M, L79R |
| GPD11 | GPD1L | Glycerol -3- Phoshphate deydrogenase 1-Like | 3p22.3 | 38 | 1 | BrS12, 38 SIDS37, 96 |
A280V E83K, I124V, R273C |
| MOG1 | RANGRF | MOG1 | 17p13.1 | 20 | 4 | BrS46 AF47 |
E83D E61X |
| PKP2 | PKP2 | Plakophillin-2 | 12p11 | 97 | 2 | BrS56 | S183N, M365V, R635Q T526A |
| ZASP | LDB3 | Z-band alternatively spliced PDZ motif protein | 10q23.2 | 77 | 7 | LVNC61, 63 | D117N (isoform2) |
| SLMAP | SLMAP | Sarcolemma Membrane- Associated Protein | 3p21.2 | 95 | 8 | BrS59 | V269I, E710A |
| NaV1.5 | SCN5A | Cardiac Sodium Channel | 3p21 | 227 | 6 | LQT3, BrS, SIDS, CM, and others | >400 |
LQT, long QT; SIDS, sudden infant death syndrome; BrS, Brugada syndrome; AF, atrial fibrillation; LVNC, left ventricular non-compaction; CM, cardiomyopathy. Underlined mutations reported to be associated with more than one syndrome. Information on splice variants can be found at the Uniprot web site, specifically for SNTA1 http://www.uniprot.org/uniprot/Q13424, CAV3 http://www.uniprot.org/uniprot/P56539, GPD1L http://www.uniprot.org/uniprot/Q8N335 MOG1 http://www.uniprot.org/uniprot/Q9HD47 PKP2 http://www.uniprot.org/uniprot/Q13835 SLMAP http://www.uniprot.org/uniprot/Q14BN4 ZASP http://www.uniprot.org/uniprot/O75112
Definition of SCIPs and the Sodium Channel Complex (SSC)
SCIPs are defined as proteins that co-localize with NaV1.5 as part of a Sodium Channel Complex (SCC) and where a physical association has been shown by co-immunoprecipitation, GST-fusion protein pull-down, or other binding assay, such as yeast-two hybrid assay. SCIPs have been variously called and classified as accessory proteins, auxiliary proteins, associated proteins, anchoring proteins, β-subunits, scaffolding proteins, adaptor proteins, and regulatory proteins2, 4–6. Each term has intuitive implications for structure or function, but the terms are not usually rigorously defined and classifications may overlap. Co-localization may occur through direct interaction with NaV1.5 or indirectly through other SCIPs. For example both α1-syntrophin and ankyrin-G serve to connect SCIPs to NaV1.5 and are called adapter or scaffolding proteins. In many cases the sites of physical interaction are known, and most SCIPs have also been shown to affect INa function. On the other hand some proteins affect INa or “interact functionally” but have not been shown to be physically associated with the SCC and do not meet this formal definition of a SCIP. For example, PKC affects NaV1.5 density7 but has not been shown to be associated with the SCC. A broader definition of SCIPs might include proteins such as chaperones that associate during trafficking to the membrane or tags for recycling/degradation, such as ubiquitin8, but for this review we use the narrower definition. Another issue with the definition is that through the cytoskeleton and scaffolding proteins the SCC may be attached more distantly to many other complexes in the cell. As the field evolves, more precise definitions of “interacting” based on proximity, time, location, and function will emerge.
Although more than 30 SCIPs have been identified (Table 1), they are not present in every complex at all times. Indeed two distinct “pools” of SSCs have been identified in cardiac myocytes, one at the lateral membrane associated with SNTA1 and a second at the intercalated disc with SAP97/plakophilin-2 (PKP2)9, 10. Also, the dynamic nature of the SCC is not known; are the components “permanent” for the ~35 hour half-life11 of the channel at the cell surface, or are they transient over some time period?
Mutations, disease, and causality
When a mutation is identified in a patient with a particular disease, perhaps the strongest evidence of causality is the strength of genetic linkage analysis. As an example, GPD1L was discovered through a strong genetic linkage to the BrS phenotype12, however, most mutations in SCIPs do not have this level of genetic linkage evidence for causality. Absence of a mutation in “control” populations and high conservation of the residue across species provides additional genetic evidence for causality. Plausible pathogenicity of a candidate disease mutation is often built on the functional importance of the mutation by studies at the molecular and cellular level. The mechanisms by which INa dysfunction causes clinical syndromes is covered in detail in other articles in this issue, but can be briefly conceptualized as a gain of function where INa, particularly late INa corresponding to phase 2 and 3 of the action potential is increased, or as “loss of function” where INa, particularly peak or early INa corresponding to phases 0 and 1 of the action potential, is decreased.
Scope of this review
We feature five SCIPs that meet the definition above and also include SLMAP and ZASP as two more putative SCIPs (Table 2 and Fig. 1) that have evidence of causation of a cardiac disease through changes in INa. SCIPs that are not considered include mutations in the SCIP telethonin associated with irritable bowel syndrome13, but not associated with cardiac disease, which is the scope of this review. A mutation within NaV1.5 disrupted association with the SCIP ankyrin-G in BrS patients14, but this is not included because no disease causing mutations in ankyrin-G itself have been yet identified. Mutations (D130G and F142L) in the SCIP calmodulin (CALM1) (Table 1) were discovered in infants with long QT arrhythmia15; both showed decreased sensitivity to calcium, but effects on INa are not yet established.
Figure 1.
Diagrams of SCIPs with locations of mutations causing NaV1.5-related cardiac disease and select functional domains. The N- and C-termini are indicated as N and C with the amino acid number beneath. The amino acids corresponding to each domain are shown below (amino acid range for domains are assigned based on http://www.uniprot.org/). Disease-causing mutations are indicated at the top of each panel with the box color coded for diseases long QT syndrome (LQTS), Brugada syndrome (BrS), sudden infant death syndrome (SIDS), and atrial fibrillation (AF) as noted at the figure bottom, with references for these mutations given in Table 2. A. SNTA1: Two parts of the split plextrin homology domain are shown as PH1N and PH1C with the PDZ domain located between them. PH2 is the second plextrin homology domain and SU is the syntrophin unique domain. http://www.uniprot.org/uniprot/Q13424 B. CAV3: SD is the CAV3 scaffold domain and IMD is the intramembrane domain. http://www.uniprot.org/uniprot/P56539 C. GPD1L: NAD binding sites are indicated as diamonds, substrate binding regions are red boxes and the active site is indicated as *. http://www.uniprot.org/uniprot/Q8N335 D. MOG1: RanBD/NR is the Ran binding/GTP release domain42 http://www.uniprot.org/uniprot/Q9HD47 E. PKP2: The N-terminal domain is marked NT and the eight armadillo repeats are indicated as red boxes. http://www.uniprot.org/uniprot/Q13835 F. SLMAP: The long cytoplasmic N-terminus with a forkhead-associated domain is marked FHA, coiled-coiled leucine zipper domains labeled CC are shown as coils, and a transmembrane domain is labeled TMD. http://www.uniprot.org/uniprot/Q14BN4 G. ZASP: The PDZ domain at the N-terminal end is marked PDZ and the 3 Lim zinc binding domains labeled Lim1, Lim2 and Lim3. http://www.uniprot.org/uniprot/O75112
SNTA1 and LQTS/SIDS
SNTA1 or α1-syntrophin is a 54 kDa cytoplasmic membrane-associated adaptor protein that is a member of the multigene syntrophin family and is coded for by the SNTA1 gene16. Syntrophins contain three protein interacting domains: 1) a PDZ domain (postsynaptic density protein-95/disc large/zona occludens-1), 2) a plextrin homology (PH) domain, one of which is split by the PDZ domain in SNTA1, and 3) a syntrophin unique domain (SU) (Fig. 1A). SNTA1 also contains a second intact PH domain following the split PH domain. SNTA1 serves as an adaptor to link proteins with signaling molecules, such as nNOS or calmodulin, or even other adaptor proteins, such as dystrophin.16 An adaptor protein itself does not possess intrinsic activity, but localization of bound signaling molecules to the microenvironment provides specificity17. The PDZ domain on SNTA1 interacts with a PDZ binding domain on the last 3 amino acids of the C-terminus of NaV1.5 as shown by pull-down of SNTA1 by a GST-fusion protein containing 66 amino acids of the NaV1.5 C-terminus (Fig. 2).18 When the last 3 amino acids (SIV) of the NaV1.5 C-terminus were deleted syntrophins and dystrophins were no longer pulled down.
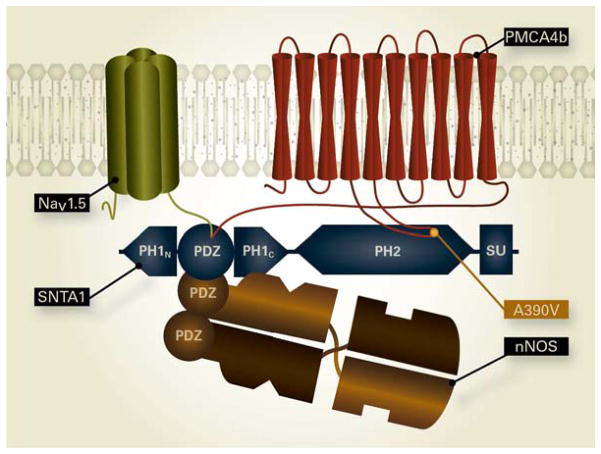
Figure 2.
Cartoon of the NaV1.5/SNTA1/nNOS/PMCA4b complex. The PDZ binding domain at the C-terminus of NaV1.5 binds to the PDZ domain of SNTA1. One of the PDZ domains from the nNOS dimer associates with the PDZ domain of SNTA1. PMCA4b interacts with SNTA1 at two locations, the 4–5 cytoplasmic loop binds to the PH2/SU region of SNTA1 and the PDZ binding domain in the C-terminus of PMCA4b can also associate with PDZ in SNTA1. The A390V-SNTA1 BrS mutation that disrupts binding of PMCA4b to SNTA1 is shown. The release of PMCA4b from the complex results in upregulation of nNOS and increased nitrosylation of NaV1.5 and increased late INa.
A screen for mutations in SNTA1 in a cohort of 50 unrelated LQTS patients negative for 11 established LQTS genes yielded a missense mutation (A390V-SNTA) in an 18 year old male with syncope19. Previous work on neuronal nitric oxide synthase (nNOS) in brain established a physical association between SNTA1, nNOS, and the plasma membrane calcium ATPase subtype 4b calcium pump (PMCA4b)20. Other studies in heart established that PMCA4b inhibited nNOS21. SNTA1 also associated with NaV1.5 in heart18, and together these studies raised the possibility that SNTA1 was part of an SCC in heart with SNTA1 connecting nNOS and PMCA4b to NaV1.5 (Fig. 2). Confirmation of NaV1.5/SNTA1/nNOS/PMCA4b complex was demonstrated in transfected HEK cells where GST- NaV1.5 C-terminal fusion constructs pulled-down WT-SNTA1, nNOS and PMCA4b. When A390V-SNTA1 was co-expressed instead of WT-SNTA1, the association with PMCA4b was lost19. The A390V mutation is within the second PH2 domain in SNTA1 (Fig. 1A & Fig. 2) and is thought to be the stronger of two association sites between PMCA4b and SNTA1, the weaker association being between a PDZ domain on the C-terminus tail of PMCA4b and the PDZ domain within the split PH1 domain on SNTA116. The loss of association of PMCA4b with A390V-SNTA1 resulted in increased S-nitrosylation of NaV1.5 and increased late INa and both increases were prevented by the addition of L-NMMA, an arginine analogue and specific inhibitor of nNOS19. These results support the idea that late INa was increased secondary to S-nitrosylation of NaV1.5 caused by A390V-SNTA1 disruption of association of the nNOS inhibitor PMCA4b (Fig. 2).
A screen of 39 LQTS patients negative for mutations in known LQTS susceptibility genes identified a single missense mutation in SNTA1 (A257G-SNTA1) in 3 unrelated patients22. A257G is in a highly conserved region of SNTA1 and it was not found in controls. Expression studies with A257G-SNTA1 showed increased peak INa but no change in late INa. Kinetic effects with co-expression of A257G-SNTA1 included a negative shift of 7–10 mV in steady-state activation. Computer modelling supported the role of A257G-SNTA1 in triggering arrhythmia22. It should be noted that the expression studies did not include nNOS or PMCA4b, which were shown19 to be required for increased late INa with A390V-SNTA1, and this could explain why no disproportionate increase in late INa was observed. Together these studies19, 22 established SNTA1 as a cause of LQTS and was designated LQT12.
SNTA1 was screened for mutations in 282 SIDS cases and 6 rare missense mutations (G54R, P56S, T262P, S287R, T372M and G460S) were found in 8 cases that were absent in 800 controls23. When these were co-expressed with NaV1.5, PMCA4b, and nNOS the results for S287R, T372M and G460S were similar to A390V-SNTA showing increased late INa that was blocked by nNOS inhibitors, thereby establishing mutations in SNTA1 as a plausible cause of SIDS. Missense mutations in both SNTA1 and NaV1.5 (A261V-SNTA and R800L-NaV1.5) were found in a three generation family with LQTS24. Co-expression of both mutations showed increased late INa that was the sum of each mutation expressed alone, suggesting additive effects to produce the phenotype. In another example of interaction, the P74L-SNTA1 polymorphism mitigated the deleterious effect of the LQT12 A257G-SNTA1 mutation25.
CAV3 and LQTS/SIDS
Caveolin-3 (CAV3) is a small ~17 kDa integral membrane protein highly expressed in skeletal muscle and heart26–28. CAV3 is a member of a family of caveolins which are the major proteins of caveolae, cholesterol-enriched invaginations of the sarcolemma (Fig 3). CAV3 mutations (Fig. 1B) were identified in LQTS (classified as LQT9)29 and SIDS30 and showed increased late INa when co-expressed with NaV1.5. CAV3 has also been shown to associate with and inhibit nNOS31 (Fig. 3), raising the possibility that LQT9, analogous to LQT12, is caused by a loss of function by CAV3 to inhibit nNOS leading to increased S-nitrosylation of NaV1.5 and increased late INa.. Expression of the LQT9 mutation F97C-CAV3 in both HEK cells and rat myocytes showed increased late INa and rat myocytes showed increased action potential duration that were abrogated by the NOS inhibitor L-NMMA32. Evidence for increased S-nitrosylation of NaV1.5 was provided by experiments in HEK cells where S-nitrosylation of NaV1.5 determined by biotin switch assay was increased by F97C-CAV3 compared to WT-CAV3 32. Interestingly F97C-CAV3 remained associated with the SCC but lost the nNOS inhibition activity as determined by enzymatic assay32. This suggested that CAV3 and SNTA1 mutations share a common mechanism to increase late INa by releasing inhibition of nNOS leading to an increase in S-nitrosylation of NaV1.5 (or other SCC) and an increased late INa. It is important to note that CAV3 interacts with multiple ion channels and transporters, and ion currents other than INa may also be affected by these mutations. Indeed these mutations decrease the inward rectifier current by decreasing KIR2.1 channel protein expression at the surface33, and this likely contributes to the pathogenesis of LQT9.
Figure 3.
The CAV3/nNOS/NaV1.5 complex in caveolae. The triangles on CAV3 represents amino acids 62–72 and are homologous to the CAV1 region that has been shown to bind to and inhibit eNOS activity97. The binding site on nNOS shown is located at W681 and W683 and is based on the homologous site on eNOS97.
GPD1L and BrS
GPD1L (Glycerol Phosphate Dehydrogenase 1 Like) is a 38 kDa protein (Fig. 1C) with very high homology to human cytoplasmic GPD1(cGPD1)34, an enzyme that catalyzes the reversible reaction converting dihydroxyacetone phosphate to glycerol 3-phospate with the subsequent oxidation of NADH to NAD+. cGDP1 is an NAD-dependent cytosolic enzyme that is an important link between the glycolytic pathway and triglyceride synthesis. GPD1L was unknown as a human protein until the mutation A280V-GPD1L was implicated in BrS by linkage analysis and gene walking in a large family35. This BrS mutation12 and three novel SIDS-associated GPDL1 mutations (E83K, I124V and R273C)36 decreased INa by >50% when the mutant GPD1L cDNAs were transfected into HEK cells expressing NaV1.512, 36. The SIDS mutations transfected in mouse myocytes36 also increased late INa. In heterologous expression systems GPD1L co-localized with NaV1.5 and was shown to be associated with NaV1.5 by co-immunoprecipitation, and NaV1.5 was pulled down by GST-GPD1L (both WT and GPD1L mutants)37, establishing GPD1L as a SCIP. GPD1L was localized at the cell surface12 and the A280V mutant decreased cell surface expression ~30%12, but the mechanism for the decrease was unknown. How does a defect in an enzyme that is presumably involved in metabolic pathways specifically target NaV1.5? Two different but not mutually exclusive mechanisms have been proposed. One hypothesis37 has the A280V-GPD1L mutation leading to an increased concentration of glycerol-3-phospate which would increase other substrates in the pathway to diacyl-glycerol (DAG). DAG concentration would increase primarily in the area near the NaV1.5/GPD1L SCC and upregulate PKC, which is known to decrease INa by direct channel phosphorylation at S15037. In support of this hypothesis E83K-SNTA1 and A280V-SNTA1 decreased both INa and cell surface expression of NaV1.5 and these decreases were abrogated both by pharmacological blockers of the PKC-related pathway and also by co-expression with the PKC phosphorylation deficient NaV1.5 mutant S1503A37. This mechanism could account for specificity by co-localization of GPD1L and intermediates with NaV1.5 substrate and direct phosphorylation of NaV1.5. Another proposed mechanism has the mutant increase NADH, and through PKC effects on mitochondria, to increase ROS, which then decreases INa by unspecified mechanisms38, 39. It is not clear however, how this mechanism would provide specificity for NaV1.5 as a general increase in ROS would have wide effects. Either or both mechanisms could be operant depending upon the direction of the reversible reaction that GPD1L regulates. GPD1L causing BrS is very rare40 and is only slightly more common in SIDS3, but of wider interest is linkage of a SNP upstream of GPD1L that has been associated with sudden cardiac death in patients with coronary artery disease41. Whether or not this association occurs through NaV1.5 is unknown, but the localization to NaV1.5, the functional interaction, and the genetic associations suggest an important role for GPD1L in regulating cardiac excitability.
MOG1 and BrS/AF
MOG1 is a 20 kDa protein (Fig. 1D) coded for by the RANGRF gene (Ran GTP release factor). The yeast homolog, scMOG1, is primarily located in the nucleus and acts as a Ran GTP release factor regulating nuclear import and export of proteins. Human MOG1 binds to both yeast and human Ran, has a GTP release activity that has been mapped to the first 45 amino acids42 (Fig 1D), and can partially rescue the growth defect in yeast cells lacking scMOG1, showing that it retains some of the activity of the yeast homologue43. MOG1 was identified as a SCIP in human heart by a yeast two-hybrid screen using a human heart cDNA library as prey44 and the second intracellular loop of Nav1.5 between repeats II-III as bait44. Although MOG1 is mostly found in the nucleus it also co-localizes with NaV1.5 at the intercalated disc44. NaV1.5 and MOG1 co-immunoprecipitation and GST pull-down assays confirmed the association between the NaV1.5 cytoplasmic loop II and in vitro translated MOG1. The GST pull-down assay using in vitro translated proteins is notable because it demonstrates a direct interaction without the need for intermediates to link MOG1 to NaV1.5, although the precise interaction sites are not known. Co-expression of NaV1.5 and MOG1 in HEK cells increased INa 2-fold without affecting steady-state activation, steady-state inactivation or recovery from inactivation. Similarly, overexpression of MOG1 in mouse neonatal cardiomyocytes increased INa with no change in single channel conductance, suggesting that the increased INa was caused by an increase in channel number at the cell surface. Knock-down of MOG1 by siRNA in neonatal mouse cardiomyocytes decreased INa45, 45.
A screen of 246 BrS patients46 yielded a missense mutation (E83D-MOG1) in a female patient with BrS that was absent in controls. Co-expression of E83D-MOG1 with NaV1.5 in HEK cells caused ~50% reduced INa without a change in kinetics, and it exerted a dominant-negative effect on WT-MOG1 in co-expression experiments. The E61X nonsense mutation causing a premature stop (E61X) was reported in 4 patients in a screen of 197 patients with lone AF and in one of 23 patients with BrS47, but this mutation was also detected in two control subjects. Expression of E61X-MOG1 with NaV1.5 in CHO-K1 cells showed ~50% decreased INa but when E61X-MOG1 was co-expressed with WT-MOG1 there was no dominant-negative effect and the levels of INa were comparable to control. The pathogenicity of E61X was further questioned when it was detected in an asymptomatic patient with a BrS ECG and in five other asymptomatic family members48.
PKP2 and BrS and Cardiomyopathy
Plakophilin (PKP2) is a 98 kDa protein and a member of a family of desmosomal proteins localized primarily at the intercalated disc in cardiomyocytes. PKP2 has a 335 amino acid N-terminus that is the site of interaction with binding partners (Fig. 1E)49, 50. There are 8–9 armadillo repeats, a signature feature of the family, and a characteristic bend occurs between armadillo repeats 5 and 6 followed by a short C-terminus49, 50. PKP2 is a scaffolding protein that with other desmosomal proteins forms a bridge between cadherens and intermediate filaments. Mutations in PKP2 have been linked to familial arrhythmogenic cardiomyopathy (AC), also called arrhythmogenic right ventricular cardiomyopathy51. As with heart failure52 and other cardiac diseases, NaV1.5 is often reduced in AC53. In autopsy samples from 5 AC patients and 5 normal controls, immunohistochemistry at the intercalated discs showed that levels of NaV1.5, Cx43 and plakoglobin were reduced in most patients (65–74%) while PKP2 was not affected unless there was a PKP2 mutation53. These results show that levels of expression of NaV1.5 at the intercalated disc can be reduced independently of PKP2. Evidence that PKP2 is a SCIP was first provided in siRNA knock down experiments of PKP2 in rat cardiomyocytes54 where PKP2 protein was reduced and INa reduced ~50% with no apparent loss of total NaV1.5 protein. This suggested a redistribution of NaV1.5 from the cell surface to intracellular locations. Evidence for association between PKP2 and NaV1.5 was provided by pull-down of NaV1.5 by a GST-fusion construct containing the N-terminus of PKP254. The kinetics of NaV1.5 were altered after siRNA knock down of PKP2 with a negative shift in steady-state inactivation and slower recovery from inactivation54. Optical mapping showed a slowing of conduction55 suggesting PKP2 as a potential causative agent in BrS. Subsequently a screen of 200 BrS patients without evidence of AC and negative for mutations in other genes linked to BrS yielded 5 PKP2 missense mutations (Q62K, S183N, M365V, T526A and R635Q)56 (Fig. 1E). INa and the number of NaV1.5 channels found at the intercalated disc were decreased when PKP2 mutants were expressed in a rat atrial cell line (HL1 cells), human embryonic stem cell derived cardiomyocytes, or in induced pluripotent stem cell derived cardiomyocytes from an AC patient. A dominant-negative effect was absent when WT and mutant PKP2 were co-expressed. In contrast to PKP2 siRNA knock down studies in cardiomyocytes, sodium channel kinetics were not affected56. Single channel properties were also unaffected and taken together with immunohistochemical data are consistent with a reduction of NaV1.5 protein at the intercalated discs56. These data support a role for PKP2 in targeting and transport of NaV1.5 to the intercalated disc56 as recently reviewed57.
SLMAP and BrS
Sarcolemma membrane-associated protein (SLMAP or SLAP) is a 95 kDa protein that is localized to the sarcolemma and t-tubules near the sarcoplasmic reticulum. SLAMP3, the longest of three alternatively splice forms (37, 46, 74 kDa respectively) is predominant in heart (Fig. 1F)58. SLMAP and ZASP (see below) are recently identified disease causing SCIPs included in this review despite falling outside the strict definition of a disease causing SCIP put forth earlier. A screen of 190 unrelated BrS patients identified two missense mutations (V269I and E710A) in SLMAP59. Both mutations decreased INa and also cell surface expression of both SLMAP and NaV1.5 in HEK cells, with no change in INa gating kinetics. SLMAP and NaV1.5, failed to co-immunoprecipitate suggesting that SLMAP may not be a tightly associated part of the SCC 59. SLMAP may play a role in targeting of NaV1.5 and is a candidate for further study as a BrS linked gene, but it has not yet been firmly established as a SCIP.
ZASP and Dilated Cardiomyopathy/Left Ventricular Noncompaction Syndrome
ZASP (Z-band alternatively spliced PDZ motif protein) is encoded by the gene LDB3Z4 (Lim Domain-Binding Protein 3) and is a member of a group of 10 genes that code for proteins that contain both PDZ and multiple LIM domains (Fig. 1G)60. Multiple alternative spliced forms of ZASP exist and the protein coded by longest transcript is 77 kDa (Table 2). All alternatively spliced forms of ZASP have an N-terminal PDZ domain but some forms lack the C-terminal Lim domains (Table 2). LIM domains contain a cysteine-rich consensus Zinc-finger sequence and are protein interaction domains60. Mutations in ZASP have been identified in patients with dilated cardiomyopathy and left ventricular non-compaction61 and cardiac specific loss of the murine ZASP homologue results in a severe dilated cardiomyopathy and premature death62. In addition to a role in stabilizing the sarcomere structure, ZASP can act as an adaptor protein as, for example, to bridge alpha-actinin-2 through its N-terminal PDZ domain with PKC or PKA through its C-terminal LIM domains5 or to bridge the L-type calcium channel through its C-terminal PDZ binding motif with PKA through the LIM binding domain5. Mutations in ZASP have been associated with myofibrillar myopathy, and dilated cardiomyopathy61. D117N-ZASP, a mutation reported previously61, was found in a patient with Left Ventricular Noncompaction Syndrome63 and when co-expressed with NaV1.5 in HEK293 cells and in rat neonatal myocytes it caused a ~30% reduction in INa. Steady-state activation was shifted +9 mV in HEK cells and +15 mV in myoctes and inactivation was shifted by + 4 mV in HEK cells and +10 mV in myocytes, and recovery from inactivation was slowed63. Computer modeling supported the hypothesis that the reduction in INa mediated by ZASP-D117N would generate arrhythmias. Association with Nav1.5 was demonstrated by co-immunoprecipitation using purified ZASP (or D117N- ZASP) produced in E. coli. mixed with homogenates from NaV1.5 transfected HEK cells or myocytes63. The reported association of ZASP with NaV1.5 63 and subsequently with L-type calcium channels 5 acts as a reminder that SCIPs can be promiscuous (see also Cav3) and the pathogenesis may be complex.
Summary
SCIPs serve multiple functions including targeting of the SCC to the sarcolemma and regulating function through such mechanisms as post-translational modification (phosphorylation and nitrosylation). Specificity to NaV1.5 and INa can be achieved by directly interacting with the NaV1.5 channel protein but also by localizing signaling pathway components to the local milieu 17. When this regulation is disturbed by mutations in SCIPs the resulting dysregulation of INa can be a mechanism for diseases such as inherited arrhythmia syndromes and SIDS. Although at present mutations in SCIPs are a relatively rare cause of cardiac disease, they are prime candidates to account for BrS syndrome and other inherited arrhythmia syndromes, as well as SIDS and cardiomyopathies, where genetic causes are suspected but not yet demonstrated. At a more basic level, understanding the mechanisms of how mutations in SCIPs cause disease may give insight into the etiology and treatment options of the more common acquired cardiac diseases, including the contribution of subtle genetic variations as susceptibility variants to cardiac disease.
Key points.
Sodium current, which underlies cardiac excitability, flows through a pore protein NaV1.5 which is part of a larger complex of interacting proteins
Mutations in 7 NaV1.5 interacting proteins have been associated with dysfunctional sodium current and inherited cardiac diseases.
The mechanisms by which mutations in interacting proteins cause specific dysfunction involve targeting/trafficking and phosphorylation/nitrosylation of the NaV1.5 complex.
Mutations in as yet unidentified interacting proteins may account for cardiac disease for which a genetic basis is not yet known
Acknowledgments
The authors thank Maeve Makielski for Figure artwork.
Footnotes
Disclosure Statement: No conflicts to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Adsit GS, Vaidyanathan R, Galler CM, Kyle JW, Makielski JC. Channelopathies from mutations in the cardiac sodium channel protein complex. J Mol Cell Cardiol. 2013 doi: 10.1016/j.yjmcc.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abriel H. Cardiac sodium channel Na(v)1. 5 and interacting proteins: Physiology and pathophysiology. J Mol Cell Cardiol. 2010;48(1):2–11. doi: 10.1016/j.yjmcc.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 3.Makielski JC. SuddenInfant Death Syndrome. In: Zipes DP, Jalife J, editors. Cardiac Electrophysiology: From Cell to Bedside. 6. Philadelphia: Elsevier; 2014. pp. 975–980. [Google Scholar]
- 4.Isom LL, De Jongh KH, Catterall WA. Auxiliary subunits of voltage-gated ion channels. Neuron. 1994;12:1183–1194. doi: 10.1016/0896-6273(94)90436-7. [DOI] [PubMed] [Google Scholar]
- 5.Lin C, Guo X, Lange S, et al. Cypher/ZASP is a novel A-kinase anchoring protein. J Biol Chem. 2013;288(41):29403–29413. doi: 10.1074/jbc.M113.470708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaidyanathan R, Makielski JC. Scaffolding proteins and ion channel diseases. In: Zipes DP, Jalife J, editors. Cardiac Electrophysiology: From Cell to Bedside. 6. Philadelphia: Elsevier; 2014. pp. 229–234. [Google Scholar]
- 7.Murray KT, Hu NN, Daw JR, et al. Functional effects of protein kinase C activation on the human cardiac Na+ channel. Circ Res. 1997;80(3):370–376. doi: 10.1161/01.res.80.3.370. [DOI] [PubMed] [Google Scholar]
- 8.van Bemmelen MX, Rougier JS, Gavillet B, et al. Cardiac voltage-gated sodium channel Nav1. 5 is regulated by Nedd4-2 mediated ubiquitination. Circ Res. 2004;95(3):284–291. doi: 10.1161/01.RES.0000136816.05109.89. [DOI] [PubMed] [Google Scholar]
- 9.Shy D, Gillet L, Abriel H. Cardiac sodium channel NaV1. 5 distribution in myocytes via interacting proteins: the multiple pool model. Biochim Biophys Acta. 2013;1833(4):886–894. doi: 10.1016/j.bbamcr.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Petitprez S, Zmoos AF, Ogrodnik J, et al. SAP97 and dystrophin macromolecular complexes determine two pools of cardiac sodium channels Nav1. 5 in cardiomyocytes. Circ Res. 2011;108(3):294–304. doi: 10.1161/CIRCRESAHA.110.228312. [DOI] [PubMed] [Google Scholar]
- 11.Maltsev VA, Kyle JW, Mishra S, Undrovinas A. Molecular identity of the late sodium current in adult dog cardiomyocytes identified by Nav1. 5 antisense inhibition. Am J Physiol Heart Circ Physiol. 2008;295(2):H667–H676. doi: 10.1152/ajpheart.00111.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.London B, Michalec M, Mehdi H, et al. Mutation in glycerol-3-phosphate dehydrogenase 1 like gene (GPD1-L) decreases cardiac Na+ current and causes inherited arrhythmias. Circulation. 2007;116(20):2260–2268. doi: 10.1161/CIRCULATIONAHA.107.703330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazzone A, Strege PR, Tester DJ, et al. A mutation in telethonin alters nav1. 5 function. J Biol Chem. 2008;283(24):16537–16544. doi: 10.1074/jbc.M801744200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohler PJ, Rivolta I, Napolitano C, et al. Nav1.5 E1053K mutation causing Brugada syndrome blocks binding to ankyrin-G and expression of Nav1. 5 on the surface of cardiomyocytes. Proc Natl Acad Sci U S A. 2004;101(50):17533–17538. doi: 10.1073/pnas.0403711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crotti L, Johnson CN, Graf E, et al. Calmodulin mutations associated with recurrent cardiac arrest in infants. Circulation. 2013;127(9):1009–1017. doi: 10.1161/CIRCULATIONAHA.112.001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhat HF, Adams ME, Khanday FA. Syntrophin proteins as Santa Claus: role(s) in cell signal transduction. Cell Mol Life Sci. 2013;70(14):2533–2554. doi: 10.1007/s00018-012-1233-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barouch LA, Harrison RW, Skaf MW, et al. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature. 2002;416(6878):337–339. doi: 10.1038/416337a. [DOI] [PubMed] [Google Scholar]
- 18.Gavillet B, Rougier JS, Domenighetti AA, et al. Cardiac sodium channel Nav1. 5 is regulated by a multiprotein complex composed of syntrophins and dystrophin. Circ Res. 2006;99(4):407–414. doi: 10.1161/01.RES.0000237466.13252.5e. [DOI] [PubMed] [Google Scholar]
- 19.Ueda K, Valdivia C, Medeiros-Domingo A, et al. Syntrophin mutation associated with long QT syndrome through activation of the nNOS-SCN5A macromolecular complex. Proc Natl Acad Sci U S A. 2008;105(27):9355–9360. doi: 10.1073/pnas.0801294105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams JC, Armesilla AL, Mohamed TM, et al. The sarcolemmal calcium pump, alpha-1 syntrophin, and neuronal nitric-oxide synthase are parts of a macromolecular protein complex. J Biol Chem. 2006;281(33):23341–23348. doi: 10.1074/jbc.M513341200. [DOI] [PubMed] [Google Scholar]
- 21.Oceandy D, Cartwright EJ, Emerson M, et al. Neuronal nitric oxide synthase signaling in the heart is regulated by the sarcolemmal calcium pump 4b. Circulation. 2007;115(4):483–492. doi: 10.1161/CIRCULATIONAHA.106.643791. [DOI] [PubMed] [Google Scholar]
- 22.Wu G, Ai T, Kim JJ, et al. alpha-1-syntrophin mutation and the long-QT syndrome: a disease of sodium channel disruption. Circ Arrhythm Electrophysiol. 2008;1(3):193–201. doi: 10.1161/CIRCEP.108.769224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng J, Van Norstrand DW, Medeiros-Domingo A, et al. Alpha1-syntrophin mutations identified in sudden infant death syndrome cause an increase in late cardiac sodium current. Circ Arrhythm Electrophysiol. 2009;2(6):667–676. doi: 10.1161/CIRCEP.109.891440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu RM, Tan BH, Orland KM, et al. Digenic inheritance novel mutations in SCN5a and SNTA1 increase late I(Na) contributing to LQT syndrome. Am J Physiol Heart Circ Physiol. 2013;304(7):H994–H1001. doi: 10.1152/ajpheart.00705.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng J, Norstrand DW, Medeiros-Domingo A, et al. LQTS-associated mutation A257G in alpha1-syntrophin interacts with the intragenic variant P74L to modify its biophysical phenotype. Cardiogenetics. 2011;1(1) doi: 10.4081/cardiogenetics.2011.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balijepalli RC, Kamp TJ. Caveolae, ion channels and cardiac arrhythmias. Prog Biophys Mol Biol. 2008;98(2–3):149–160. doi: 10.1016/j.pbiomolbio.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li S, Galbiati F, Volonte D, et al. Mutational analysis of caveolin-induced vesicle formation. Expression of caveolin-1 recruits caveolin-2 to caveolae membranes. FEBS Lett. 1998;434(1–2):127–134. doi: 10.1016/s0014-5793(98)00945-4. [DOI] [PubMed] [Google Scholar]
- 28.McNally EM, de Sa ME, Duggan DJ, et al. Caveolin-3 in muscular dystrophy. Hum Mol Genet. 1998;7(5):871–877. doi: 10.1093/hmg/7.5.871. [DOI] [PubMed] [Google Scholar]
- 29.Vatta M, Ackerman MJ, Ye B, et al. Mutant caveolin-3 induces persistent late sodium current and is associated with long-QT syndrome. Circulation. 2006;114(20):2104–2112. doi: 10.1161/CIRCULATIONAHA.106.635268. [DOI] [PubMed] [Google Scholar]
- 30.Cronk LB, Ye B, Kaku T, et al. Novel mechanism for sudden infant death syndrome: Persistent late sodium current secondary to mutations in caveolin-3. Heart Rhythm. 2007;4(2):161–166. doi: 10.1016/j.hrthm.2006.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Venema VJ, Ju H, Zou R, Venema RC. Interaction of neuronal nitric-oxide synthase with caveolin-3 in skeletal muscle. Identification of a novel caveolin scaffolding/inhibitory domain. J Biol Chem. 1997;272(45):28187–28190. doi: 10.1074/jbc.272.45.28187. [DOI] [PubMed] [Google Scholar]
- 32.Cheng J, Valdivia CR, Vaidyanathan R, Balijepalli RC, Ackerman MJ, Makielski JC. Caveolin-3 suppresses late sodium current by inhibiting nNOS-dependent S-nitrosylation of SCN5A. J Mol Cell Cardiol. 2013;61:102–110. doi: 10.1016/j.yjmcc.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaidyanathan R, Vega AL, Song C, et al. The Interaction of Caveolin 3 with the Inward Rectifier Channel Kir2.1; Physiology and Pathology related to LQT9. J Biol Chem. 2013 doi: 10.1074/jbc.M112.435370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ou X, Ji C, Han X, et al. Crystal structures of human glycerol 3-phosphate dehydrogenase 1 (GPD1) J Mol Biol. 2006;357(3):858–869. doi: 10.1016/j.jmb.2005.12.074. [DOI] [PubMed] [Google Scholar]
- 35.Weiss R, Barmada MM, Nguyen T, et al. Clinical and molecular heterogeneity in the Brugada syndrome: a novel gene locus on chromosome 3. Circulation. 2002;105(6):707–713. doi: 10.1161/hc0602.103618. [DOI] [PubMed] [Google Scholar]
- 36.Van Norstrand DW, Valdivia CR, Tester DJ, et al. Molecular and Functional Characterization of Novel Glycerol-3-Phosphate Dehydrogenase 1 Like Gene (GPD1-L) Mutations in Sudden Infant Death Syndrome. Circulation. 2007 doi: 10.1161/CIRCULATIONAHA.107.704627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valdivia CR, Ueda K, Ackerman MJ, Makielski JC. GPD1L links redox state to cardiac excitability by PKC-dependent phosphorylation of the sodium channel SCN5A. Am J Physiol Heart Circ Physiol. 2009;297(4):H1446–H1452. doi: 10.1152/ajpheart.00513.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu M, Sanyal S, Gao G, et al. Cardiac Na+ current regulation by pyridine nucleotides. Circ Res. 2009;105(8):737–745. doi: 10.1161/CIRCRESAHA.109.197277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu M, Liu H, Dudley SC., Jr Reactive oxygen species originating from mitochondria regulate the cardiac sodium channel. Circ Res. 2010;107(8):967–974. doi: 10.1161/CIRCRESAHA.110.220673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Makiyama T, Akao M, Haruna Y, et al. Mutation analysis of the glycerol-3 phosphate dehydrogenase-1 like (GPD1L) gene in Japanese patients with Brugada syndrome. Circ J. 2008;72(10):1705–1706. doi: 10.1253/circj.cj-08-0508. [DOI] [PubMed] [Google Scholar]
- 41.Westaway SK, Reinier K, Huertas-Vazquez A, et al. Common variants in CASQ2, GPD1L, and NOS1AP are significantly associated with risk of sudden death in patients with coronary artery disease. Circ Cardiovasc Genet. 2011;4(4):397–402. doi: 10.1161/CIRCGENETICS.111.959916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steggerda SM, Paschal BM. Identification of a conserved loop in Mog1 that releases GTP from Ran. Traffic. 2001;2(11):804–811. doi: 10.1034/j.1600-0854.2001.21109.x. [DOI] [PubMed] [Google Scholar]
- 43.Marfatia KA, Harreman MT, Fanara P, Vertino PM, Corbett AH. Identification and characterization of the human MOG1 gene. Gene. 2001;266(1–2):45–56. doi: 10.1016/s0378-1119(01)00364-x. [DOI] [PubMed] [Google Scholar]
- 44.Wu L, Yong SL, Fan C, et al. Identification of a new co-factor, MOG1, required for the full function of cardiac sodium channel Nav 1. 5. J Biol Chem. 2008;283(11):6968–6978. doi: 10.1074/jbc.M709721200. [DOI] [PubMed] [Google Scholar]
- 45.Chakrabarti S, Wu X, Yang Z, et al. MOG1 rescues defective trafficking of Na(v)1. 5 mutations in Brugada syndrome and sick sinus syndrome. Circ Arrhythm Electrophysiol. 2013;6(2):392–401. doi: 10.1161/CIRCEP.111.000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kattygnarath D, Maugenre S, Neyroud N, et al. MOG1: a new susceptibility gene for Brugada syndrome. Circ Cardiovasc Genet. 2011;4(3):261–268. doi: 10.1161/CIRCGENETICS.110.959130. [DOI] [PubMed] [Google Scholar]
- 47.Olesen MS, Jensen NF, Holst AG, et al. A novel nonsense variant in Nav1. 5 cofactor MOG1 eliminates its sodium current increasing effect and may increase the risk of arrhythmias. Can J Cardiol. 2011;27(4):523. doi: 10.1016/j.cjca.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 48.Campuzano O, Berne P, Selga E, et al. Brugada syndrome and p.E61X_RANGRF. Cardiol J. 2014 doi: 10.5603/CJ.a2013.0125. [DOI] [PubMed] [Google Scholar]
- 49.Bass-Zubek AE, Godsel LM, Delmar M, Green KJ. Plakophilins: multifunctional scaffolds for adhesion and signaling. Curr Opin Cell Biol. 2009;21(5):708–716. doi: 10.1016/j.ceb.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kowalczyk AP, Green KJ. Structure, function, and regulation of desmosomes. Prog Mol Biol Transl Sci. 2013;116:95–118. doi: 10.1016/B978-0-12-394311-8.00005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Tintelen JP, Entius MM, Bhuiyan ZA, et al. Plakophilin-2 mutations are the major determinant of familial arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circulation. 2006;113(13):1650–1658. doi: 10.1161/CIRCULATIONAHA.105.609719. [DOI] [PubMed] [Google Scholar]
- 52.Valdivia CR, Chu WW, Pu JL, et al. Increased late sodium current in myocytes from a canine heart failure model and from failing human heart. J Mol Cell Cardiol. 2005;38(3):475–483. doi: 10.1016/j.yjmcc.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 53.Noorman M, Hakim S, Kessler E, et al. Remodeling of the cardiac sodium channel, connexin43, and plakoglobin at the intercalated disk in patients with arrhythmogenic cardiomyopathy. Heart Rhythm. 2013;10(3):412–419. doi: 10.1016/j.hrthm.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sato PY, Musa H, Coombs W, et al. Loss of plakophilin-2 expression leads to decreased sodium current and slower conduction velocity in cultured cardiac myocytes. Circ Res. 2009;105(6):523–526. doi: 10.1161/CIRCRESAHA.109.201418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cerrone M, Noorman M, Lin X, et al. Sodium current deficit and arrhythmogenesis in a murine model of plakophilin-2 haploinsufficiency. Cardiovasc Res. 2012;95(4):460–468. doi: 10.1093/cvr/cvs218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cerrone M, Lin X, Zhang M, et al. Missense mutations in plakophilin-2 cause sodium current deficit and associate with a brugada syndrome phenotype. Circulation. 2014;129(10):1092–1103. doi: 10.1161/CIRCULATIONAHA.113.003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cerrone M, Delmar M. Desmosomes and the sodium channel complex: Implications for arrhythmogenic cardiomyopathy and Brugada syndrome. Trends Cardiovasc Med. 2014 doi: 10.1016/j.tcm.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wielowieyski PA, Sevinc S, Guzzo R, Salih M, Wigle JT, Tuana BS. Alternative splicing, expression, and genomic structure of the 3′ region of the gene encoding the sarcolemmal-associated proteins (SLAPs) defines a novel class of coiled-coil tail-anchored membrane proteins. J Biol Chem. 2000;275(49):38474–38481. doi: 10.1074/jbc.M007682200. [DOI] [PubMed] [Google Scholar]
- 59.Ishikawa T, Sato A, Marcou CA, et al. A novel disease gene for Brugada syndrome: sarcolemmal membrane-associated protein gene mutations impair intracellular trafficking of hNav1. 5. Circ Arrhythm Electrophysiol. 2012;5(6):1098–1107. doi: 10.1161/CIRCEP.111.969972. [DOI] [PubMed] [Google Scholar]
- 60.te Velthuis AJ, Isogai T, Gerrits L, Bagowski CP. Insights into the molecular evolution of the PDZ/LIM family and identification of a novel conserved protein motif. PLoS One. 2007;2(2):e189. doi: 10.1371/journal.pone.0000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vatta M, Mohapatra B, Jimenez S, et al. Mutations in Cypher/ZASP in patients with dilated cardiomyopathy and left ventricular non-compaction. J Am Coll Cardiol. 2003;42(11):2014–2027. doi: 10.1016/j.jacc.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 62.Zheng M, Cheng H, Banerjee I, Chen J. ALP/Enigma PDZ-LIM domain proteins in the heart. J Mol Cell Biol. 2010;2(2):96–102. doi: 10.1093/jmcb/mjp038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xi Y, Ai T, De Lange E, et al. Loss of function of hNav1. 5 by a ZASP1 mutation associated with intraventricular conduction disturbances in left ventricular noncompaction. Circ Arrhythm Electrophysiol. 2012;5(5):1017–1026. doi: 10.1161/CIRCEP.111.969220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Allouis M, Le Bouffant F, Wilders R, et al. 14-3-3 is a regulator of the cardiac voltage-gated sodium channel Nav1. 5. Circ Res. 2006;98(12):1538–1546. doi: 10.1161/01.RES.0000229244.97497.2c. [DOI] [PubMed] [Google Scholar]
- 65.Ziane R, Huang H, Moghadaszadeh B, Beggs AH, Levesque G, Chahine M. Cell membrane expression of cardiac sodium channel Na(v)1. 5 is modulated by alpha-actinin-2 interaction. Biochemistry. 2010;49(1):166–178. doi: 10.1021/bi901086v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mohler PJ, Wehrens XH. Mechanisms of human arrhythmia syndromes: abnormal cardiac macromolecular interactions. Physiology (Bethesda) 2007;22:342–350. doi: 10.1152/physiol.00018.2007. [DOI] [PubMed] [Google Scholar]
- 67.Lowe JS, Palygin O, Bhasin N, et al. Voltage-gated Nav channel targeting in the heart requires an ankyrin-G dependent cellular pathway. J Cell Biol. 2008;180(1):173–186. doi: 10.1083/jcb.200710107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dhar MJ, Chen C, Rivolta I, et al. Characterization of sodium channel alpha- and beta-subunits in rat and mouse cardiac myocytes. Circulation. 2001;103(9):1303–1310. doi: 10.1161/01.cir.103.9.1303. [DOI] [PubMed] [Google Scholar]
- 69.Valdivia CR, Nagatomo T, Makielski JC. Late currents affect kinetics for heart and skeletal Na channel α and β1 subunits expressed in HEK293 cells. Journal of Molecular & Cellular Cardiology. 2002 doi: 10.1006/jmcc.2002.2040. In press. [DOI] [PubMed] [Google Scholar]
- 70.Maltsev VA, Kyle JW, Undrovinas A. Late Na+ current produced by human cardiac Na+ channel isoform Nav1. 5 is modulated by its beta1 subunit. J Physiol Sci. 2009;59(3):217–225. doi: 10.1007/s12576-009-0029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Herfst LJ, Potet F, Bezzina CR, et al. Na+ channel mutation leading to loss of function and non-progressive cardiac conduction defects. J Mol Cell Cardiol. 2003;35(5):549–557. doi: 10.1016/s0022-2828(03)00078-6. [DOI] [PubMed] [Google Scholar]
- 72.Nuss HB, Chiamvimonvat N, Perez-Garcia MT, Tomaselli GF, Marban E. Functional association of the β1 subunit with human cardiac (hH1) and rat skeletal muscle (μ1) sodium channel α subunits expressed in Xenopus oocytes. J Gen Physiol. 1995;106(6):1171–1191. doi: 10.1085/jgp.106.6.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lopez-Santiago LF, Meadows LS, Ernst SJ, et al. Sodium channel Scn1b null mice exhibit prolonged QT and RR intervals. J Mol Cell Cardiol. 2007 doi: 10.1016/j.yjmcc.2007.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Malhotra JD, Chen C, Rivolta I, et al. Characterization of sodium channel α- and β-Subunits in rat and mouse cardiac myocytes. Circulation. 2001;103(9):1303–1310. doi: 10.1161/01.cir.103.9.1303. [DOI] [PubMed] [Google Scholar]
- 75.Johnson D, Bennett ES. Isoform-specific effects of the beta2 subunit on voltage-gated sodium channel gating. J Biol Chem. 2006;281(36):25875–25881. doi: 10.1074/jbc.M605060200. [DOI] [PubMed] [Google Scholar]
- 76.Mishra S, Undrovinas NA, Maltsev VA, Reznikov V, Sabbah HN, Undrovinas A. Post-transcriptional silencing of SCN1B and SCN2B genes modulates late sodium current in cardiac myocytes from normal dogs and dogs with chronic heart failure. Am J Physiol Heart Circ Physiol. 2011;301(4):H1596–H1605. doi: 10.1152/ajpheart.00948.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hakim P, Brice N, Thresher R, et al. Scn3b knockout mice exhibit abnormal sino-atrial and cardiac conduction properties. Acta Physiol (Oxf) 2010;198(1):47–59. doi: 10.1111/j.1748-1716.2009.02048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fahmi AI, Patel M, Stevens EB, et al. The sodium channel beta-subunit SCN3b modulates the kinetics of SCN5a and is expressed heterogeneously in sheep heart. J Physiol. 2001;537(Pt 3):693–700. doi: 10.1111/j.1469-7793.2001.00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hakim P, Gurung IS, Pedersen TH, et al. Scn3b knockout mice exhibit abnormal ventricular electrophysiological properties. Prog Biophys Mol Biol. 2008;98(2–3):251–266. doi: 10.1016/j.pbiomolbio.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Medeiros-Domingo A, Kaku T, Tester DJ, et al. SCN4B-Encoded Sodium Channel {beta}4 Subunit in Congenital Long-QT Syndrome. Circulation. 2007;116:136–142. doi: 10.1161/CIRCULATIONAHA.106.659086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Remme CA, Scicluna BP, Verkerk AO, et al. Genetically determined differences in sodium current characteristics modulate conduction disease severity in mice with cardiac sodium channelopathy. Circ Res. 2009;104(11):1283–1292. doi: 10.1161/CIRCRESAHA.109.194423. [DOI] [PubMed] [Google Scholar]
- 82.Hund TJ, Koval OM, Li J, et al. A beta(IV)-spectrin/CaMKII signaling complex is essential for membrane excitability in mice. J Clin Invest. 2010;120(10):3508–3519. doi: 10.1172/JCI43621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim J, Ghosh S, Liu H, Tateyama M, Kass RS, Pitt GS. Calmodulin mediates Ca2+ sensitivity of sodium channels. J Biol Chem. 2004;279(43):45004–45012. doi: 10.1074/jbc.M407286200. [DOI] [PubMed] [Google Scholar]
- 84.Chagot B, Chazin WJ. Solution NMR structure of Apo-calmodulin in complex with the IQ motif of human cardiac sodium channel NaV1. 5. J Mol Biol. 2011;406(1):106–119. doi: 10.1016/j.jmb.2010.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tan HL, Kupershmidt S, Zhang R, et al. A calcium sensor in the sodium channel modulates cardiac excitability. Nature. 2002;415(6870):442–447. doi: 10.1038/415442a. [DOI] [PubMed] [Google Scholar]
- 86.Aiba T, Hesketh GG, Liu T, et al. Na+ channel regulation by Ca2+/calmodulin and Ca2+/calmodulin-dependent protein kinase II in guinea-pig ventricular myocytes. Cardiovasc Res. 2010;85(3):454–463. doi: 10.1093/cvr/cvp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Koval OM, Snyder JS, Wolf RM, et al. Ca2+/Calmodulin-Dependent Protein Kinase II-Based Regulation of Voltage-Gated Na+ Channel in Cardiac Disease. Circulation. 2012;126(17):2084–2094. doi: 10.1161/CIRCULATIONAHA.112.105320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van der Velden HM, Jongsma HJ. Cardiac gap junctions and connexins: their role in atrial fibrillation and potential as therapeutic targets. Cardiovasc Res. 2002;54(2):270–279. doi: 10.1016/s0008-6363(01)00557-0. [DOI] [PubMed] [Google Scholar]
- 89.Malhotra JD, Thyagarajan V, Chen C, Isom LL. Tyrosine-phosphorylated and nonphosphorylated sodium channel beta1 subunits are differentially localized in cardiac myocytes. J Biol Chem. 2004;279(39):40748–40754. doi: 10.1074/jbc.M407243200. [DOI] [PubMed] [Google Scholar]
- 90.Jansen JA, Noorman M, Musa H, et al. Reduced heterogeneous expression of Cx43 results in decreased Nav1. 5 expression and reduced sodium current that accounts for arrhythmia vulnerability in conditional Cx43 knockout mice. Heart Rhythm. 2012;9(4):600–607. doi: 10.1016/j.hrthm.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rizzo S, Lodder EM, Verkerk AO, et al. Intercalated disc abnormalities, reduced Na(+) current density, and conduction slowing in desmoglein-2 mutant mice prior to cardiomyopathic changes. Cardiovasc Res. 2012;95(4):409–418. doi: 10.1093/cvr/cvs219. [DOI] [PubMed] [Google Scholar]
- 92.Albesa M, Ogrodnik J, Rougier JS, Abriel H. Regulation of the cardiac sodium channel Nav1. 5 by utrophin in dystrophin-deficient mice. Cardiovasc Res. 2011;89(2):320–328. doi: 10.1093/cvr/cvq326. [DOI] [PubMed] [Google Scholar]
- 93.Wang C, Hennessey JA, Kirkton RD, et al. Fibroblast growth factor homologous factor 13 regulates Na+ channels and conduction velocity in murine hearts. Circ Res. 2011;109(7):775–782. doi: 10.1161/CIRCRESAHA.111.247957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ou YJ, Strege P, Miller SM, et al. Syntrophin gamma 2 regulates SCN5A Gating by a PDZ domain-mediated interaction. J Biol Chem. 2003;278(3):1915–1923. doi: 10.1074/jbc.M209938200. [DOI] [PubMed] [Google Scholar]
- 95.Jespersen T, Gavillet B, van Bemmelen MX, et al. Cardiac sodium channel Na(v)1. 5 interacts with and is regulated by the protein tyrosine phosphatase PTPH1. Biochem Biophys Res Commun. 2006;348(4):1455–1462. doi: 10.1016/j.bbrc.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 96.Van Norstrand DW, Valdivia CR, Tester DJ, et al. Molecular and functional characterization of novel glycerol-3-phosphate dehydrogenase 1 like gene (GPD1-L) mutations in sudden infant death syndrome. Circulation. 2007;116(20):2253–2259. doi: 10.1161/CIRCULATIONAHA.107.704627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Trane AE, Pavlov D, Sharma A, et al. Deciphering the binding of caveolin-1 to client protein endothelial nitric oxide synthase (eNOS): scaffolding sub-domain identification, interaction modeling, and biological significance. J Biol Chem. 2014 doi: 10.1074/jbc.M113.528695. [DOI] [PMC free article] [PubMed] [Google Scholar]