Abstract
Research investigating biological motion perception in children with ASD has revealed conflicting findings concerning whether impairments in biological motion perception exist. The current study investigated how children with high-functioning ASD (HF-ASD) performed on two tasks of biological motion identification: a novel schematic motion identification task and a point-light biological motion identification task. Twenty-two HFASD children were matched with 21 TD children on gender, non-verbal mental, and chronological, age (M years = 6.72). On both tasks, HF-ASD children performed with similar accuracy as TD children. Across groups, children performed better on animate than on inanimate trials of both tasks. These findings suggest that HF-ASD children's identification of both realistic and schematic biological motion identification is unimpaired.
Keywords: Autism spectrum disorder, Animacy, Biological motion
1. Introduction
One of the most robust and replicable findings in psychology is that the visual system is exquisitely tuned to detect point-light biological motion (Blake & Shiffrar, 2007). In typical development, newborn infants and toddlers have been shown to prefer biological motion over non-biological motion (Simion, Regolin, & Bulf, 2008) or inverted motion (Klin, Lin, Gorrindo, Ramsay, & Jones, 2009). The predisposition to attend to biological motion has been shown in typical development using multiple methods including, point-light display (Klin et al., 2009; Morita et al., 2012; Simion et al., 2008), schematic motion such as the Michotte “caterpillar” stimulus (Michotte, 1963; Schlottmann & Ray, 2010), or the motion of a single animated dot (Rutherford, Pennington, & Rogers, 2006; Schultz & Bulthoff, 2013). This early sensitivity and preference for animate motion has been difficult to reconcile with some research suggesting that individuals with autism spectrum disorder (ASD) have deficits in biological motion perception (Annaz et al., 2010; Annaz, Campbell, Coleman, Milne, & Swettenham, 2012; Blake, Turner, Smoski, Pozdol, & Stone, 2003; Centelles, Assaiante, Etchegoyhen, Bouvard, & Schmitz, 2013; Congiu, Schlottmann, & Ray, 2010; David et al., 2013; Herrington et al., 2007; Kaiser, Delmolino, Tanaka, & Shiffrar, 2010; Klin et al., 2009; Koldewyn, Whitney, & Rivera, 2010; Koldewyn, Whitney, & Rivera, 2011). However, a number of other studies do not support the contention that biological motion processing deficits are characteristic of ASD, particularly among high-functioning, older individuals with ASD (Cleary, Looney, Brady, & Fitzgerald, 2013; Freitag et al., 2008; Hubert et al., 2007; Moore, Hobson, & Lee, 1997; Murphy, Brady, Fitzgerald, & Troje, 2009; Parron et al., 2008; Rutherford & Troje, 2012; Saygin, Cook, & Blakemore, 2010).
The processing of biological motion has been hypothesized to relate to the development of core cognitive abilities such as the ability to differentiate animate and inanimate categories (Gelman & Opfer, 2002; Mandler, 1992; Opfer & Gelman, 2010; Rakison & Poulin-Dubois, 2001), as well as social-cognitive abilities, commonly affected in individuals with ASD. The aims of the current research are to (a) investigate whether children with high-functioning ASD (HF-ASD) identify animate (biological) and inanimate (mechanical) motion and (b) explore whether children's performance on biological motion identification tasks relate to parental report of ASD symptoms.
Animate motion cues such as an entity's ability to cause motion at a distance, change direction or speed, and engage in self-propulsion are important for the development of the concept of living and non-living things in infancy (Csibra, 2008; Csibra, Gergely, Bíró, Koós, & Brockbank, 1999; Mandler, 1992; Premack, 1990; Rakison & Poulin-Dubois, 2001; Schlottmann, Surian, & Ray, 2009; Tremoulet & Feldman, 2000). Similarly, typically-developing (TD) preschool and school-aged children use motion information to guide their animacy judgements (Gelman & Coley, 1990; Mak & Vera, 1999). However, this research has not specifically evaluated whether biological motion, as a single animacy cue, is sufficient for typically-developing children and children with HF-ASD to accurately differentiate animate–inanimate categories. The current research aims to investigate this question using a novel schematic motion categorization task.
Research regarding the perception of biological motion has generally used point-light displays (Johansson, 1973), or schematic, non-rigid “caterpillar” motion (Michotte, 1963). Different aspects of biological motion are emphasized with each method and existing research has yet to compare performance across methodologies. Whereas point-light biological motion emphasizes the “gravity-defined trajectory” of the limbs of living organisms (Troje, 2013), schematic motion depicts non-rigid, expansion–contraction movement, which only animate beings are capable of. One advantage of using schematic biological motion over point-light displays is that schematic motion stimuli do not provide information about the general form of the organism (e.g. limbs), and therefore the attribution of animacy is based on the object's motion alone. While schematic biological motion is an advantageous method to study the perception of animacy, the majority of studies testing biological motion understanding in children with ASD have primarily used point-light display (PLD).
1.1. Point-light biological motion
Point-light displays depict the movement of an animate being by placing point-light dots on all the major joints of the body and rendering the rest of the body invisible. Although the resulting motion is considerably degraded, the point-light markers have been shown to convey important information about both the structure of the body and the dynamic movements of each of the parts (Chang & Troje, 2008; Troje, 2002). In one of the few studies to examine the development of children's ability to identify biological motion, Pavlova, Kraägeloh-Mann, Sokolov, and Birbaumer (2001) found that 5-year-old children could accurately identify animate point-light displays of humans, dogs, and birds with the presentation of a single motion trial (Pavlova et al., 2001). However, children were not tested on their ability to identify non-biological, or mechanical motion, as a comparison. Other research has investigated how well human and mechanical motion is visually detected in typically-developing children, adults, and young adults with ASD. Kaiser et al. (2010) presented coherent and scrambled motion point-light displays of a human and a tractor, which were either masked (among noise) or unmasked. Scrambled motion displays contain the same dots as coherent motion, but are displaced to remove the form cues in coherent motion displays. While both TD groups showed greater visual sensitivity to human motion (both masked and unmasked), compared to the motion of a tractor, young adults with ASD (M age = 20) showed equal sensitivity to the motion of a human or a tractor.
The large majority of investigations concerning how children with ASD perceive biological and mechanical motion have been confined to studies of visual preference and discrimination, while few studies provide a direct test of how children identify the form represented in point-light displays. Of the studies that have asked children with ASD to verbally identify biological motion displays, most have involved identifying complex physical actions (Swettenham et al., 2013), subjective states, or emotions (Hubert et al., 2007; Moore et al., 1997; Parron et al., 2008). While children's ability to identify an agent's emotions and internal states when presented using point-light display has been investigated, how accurately children with ASD identify animate vs. inanimate motion exemplars has yet to be examined. In the current study, we compare children with HF-ASD with typically-developing (TD) children on their ability to identify biological and mechanical motion point-light displays. Additionally, we wanted to explore whether children are equally able to identify schematic, compared to point-light, biological motion.
1.2. Schematic biological motion
Classic studies by Michotte (1963), Heider and Simmel (1944), Premack (1990), and Tremoulet and Feldman (2000) demonstrate how the motion of simple geometric forms is sufficient to give rise to the impression of animacy. This perception of animacy has been described as a rapid, automatic, and largely stimulus-driven process (Heider & Simmel, 1944; Schlottmann & Ray, 2010). Schematic presentations of biological motion such as the Michotte “caterpillar” depict a rectangular-shaped stimulus that moves by elongating from one side, then contracting on the opposite side. These stimuli have been shown to elicit the perception of goal-directedness in infants as young as 6 months of age (Schlottmann & Ray, 2010) and are judged as ‘animal-like’ in typically-developing children as young as 3 years of age (Schlottmann, Allen, Linderoth, & Hesketh, 2002).
Although schematic biological motion has been widely used to research animacy perception in typically-developing children, this methodology has seldom been used to study animacy understanding in children with ASD. To our knowledge, only two studies have been conducted using schematic biological motion with children with ASD (Congiu et al., 2010; Ray & Schlottmann, 2007). Ray and Schlottmann (2007) investigated ASD children's perception of launch and reaction events presented as schematic animations that moved either biologically (non-rigidly) or inanimately (rigidly). Children with ASD (M age = 8.4 years) were shown to have difficulty perceiving launch events. In particular, when launch events were presented using rigid (inanimate) motion children with ASD did not prefer to attribute physical causality. In a similar study, Congiu et al. (2010) presented older high-functioning children with ASD with schematic stimuli that either moved rigidly, or non-rigidly. Children were tested on their understanding of physical and psychological causality using different variations of the launch event. Although 13-year-old children with HF-ASD did not show difficulty understanding the principles of physical and psychological causality, they had difficulty identifying and describing the non-rigid, biological motion stimulus as animate (Congiu et al., 2010). Only 37% of children with HF-ASD described the stimulus as “caterpillars,” “snakes,” or “slugs” compared to 77% of typically-developing children. Interestingly, 42% of children with ASD provided inanimate responses such as “rectangles,” while only 23% of control children provided inanimate responses. It is, however, possible that asking children to identify the schematic stimulus as animate using an open-response format may have underestimated children's animacy understanding as it allows children to provide literal descriptions (e.g. rectangles) instead of more abstract descriptions of what the rectangle looks like (e.g., a caterpillar).
1.3. Biological motion and social functioning
The hypothesis that the development of social functioning is dependent upon, or at least, related to, the perception of biological motion has received recent support in a number of studies (see Kaiser & Shiffrar, 2013 for a review). Among children with ASD, a relationship between biological motion perception and measures of social competence has been demonstrated, whereby children's visual fixation patterns while viewing social and non-social stimuli were found to relate to standardized measures of social competence (Klin, Jones, Schultz, Volkmar, & Cohen, 2002). Additionally, ASD severity on the ADOS has also been shown to correlate with children's ability to detect human point-light biological motion (Blake et al., 2003). Using an analog population of adult undergraduates, traits of ASD as measured by the Autism Quotient (AQ: Baron-Cohen, Wheelwright, Skinner, Martin, & Clubley, 2001) were found to relate to sensitivity in detecting human biological motion, but not object motion (Kaiser & Shiffrar, 2013). Thus, deficits in biological motion perception, or visual orienting to social stimuli, may be critical in explaining the myriad of social-cognitive symptoms characteristic of individuals with ASD (Dakin & Frith, 2005; Kaiser & Shiffrar, 2013; Klin et al., 2002; Pavlova, 2011; Swettenham et al., 1998). To date, these investigations have correlated visual preference, and ability to discriminate, biological motion, but have not evaluated whether a relation exists between the ability to identify biological motion stimuli and aspects of social functioning. In the present study it was expected that better performance on animate trials of the biological motion identification tasks would be associated with lower scores on the Social Communication Questionnaire (Rutter, Bailey, & Lord, 2003), a screening instrument for ASD, and symptoms of the broader autism phenotype as measured by the Social Responsiveness Scale (SRS; Constantino & Gruber, 2005). In contrast, we would not expect identification of inanimate, mechanical, motion to be related to scores on the SCQ or SRS.
1.4. Study aims
The main goal of the present research was to compare the performance of children with high-functioning ASD to TD children on tasks requiring the identification of biological motion across two methodologies: point-light motion and schematic motion. Our knowledge of how children with ASD perceive biological and mechanical motion has been largely confined to studies assessing visual preference and discrimination, while less is known about what young children with ASD recognize when viewing the degraded movements of humans, animals, and vehicles shown in point-light displays. A novel task was developed to test whether TD children and children with HF-ASD identify schematic biological motion as consistent with animate entities. Additionally, given that this is the first study to present children with both point-light and schematic biological motion displays, performance across tasks was compared to shed light on the empirical question of whether the same over-arching animacy concept is assessed for both types of biological motion. Given that the perception of biological motion has been hypothesized to be important for the development of cognitive and social abilities (Kaiser & Shiffrar, 2013; Pavlova, 2011, 2013), an additional exploratory aim of the present study was to investigate whether performance of children with HF-ASD across biological motion identification tasks correlates with cognitive ability and scores on two measures of ASD symptomatology.
2. Method
2.1. Participants
The ASD group consisted of 22 children (16 males) diagnosed with a diagnosis on the autism spectrum and a control group of 21 Typically-Developing (TD) children (13 males) matched on gender, chronological age, and nonverbal mental age. Three participants in each group were tested but not included in the final analysis for the point-light motion identification task due to missing data (technical difficulties). Thus, analyses for the schematic motion identification task included 22 children with HF-ASD, while 19 children with HF-ASD were included in the analysis of point-light biological motion identification. Participants in the ASD group were recruited from a University database, a hospital with specialized autism diagnostic services, as well as referrals from specialized centers treating children with ASD. Participants were not included if their full scale IQ on a standardized IQ test was below 70. All participants in the ASD group had previously received a clinical diagnosis of Autistic Disorder or Asperger's Syndrome by satisfying diagnostic criteria on the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2000). Children recruited from specialized diagnostic and treatment centers also satisfied diagnostic thresholds on the Autism Diagnostic Interview (ADI-R; Lord, Rutter, & Le Couteur, 1994). The Social Communication Questionnaire (SCQ; Rutter et al., 2003)1 and Social Responsiveness Scale (SRS; Constantino & Gruber, 2005) were used to provide additional information about symptomatology and autism severity. Participants with HF-ASD all had a primary diagnosis of Autistic Disorder (n = 11), Pervasive Developmental Disorder-Not Otherwise Specified (PDD-NOS) (n = 4), or Asperger's Syndrome (n = 7). Five participants with HF-ASD also had a secondary diagnosis of Attention Deficit Hyperactivity Disorder (ADHD), two participants had epilepsy, and three participants were born prematurely (less than 37 weeks gestation). Participants from the control group were recruited from a University database. TD participants had no other neuropsychological or developmental disorders (e.g. epilepsy, language delay) and did not have any (known) first-degree relative with an autism spectrum disorder. All TD and HF-ASD participants had normal, or corrected to normal vision. All participants completed the Differential Abilities Scale, Second Edition (DAS-II; Elliott, 2007). ASD and TD groups were subsequently matched on nonverbal mental age and chronological age. Table 1 shows the mean nonverbal mental age and chronological age for each group.
Table 1.
Participant characteristics for HF-ASD and TD groups.
| Gender (m:f) | Chronological age (SD) | Nonverbal mental age (SD) | Nonverbal IQ (SD) | SRS total score | SCQ total | |
|---|---|---|---|---|---|---|
| HF-ASD (n = 22) | 19:3 | 6.96 (1.50) | 7.31 (2.00) | 103.14 (14.59) | 79.04 (10.87) | 16.73 (6.56) |
| TD (n = 21) | 16:5 | 6.49 (1.77) | 7.30 (2.48) | 107.05 (10.00) | - |
Notes: Test of equality of means for gender χ2 (1, N = 43) = 0.73, p = 0.39, chronological age, t(41) = 0.94, p = 0.35, and nonverbal mental age, t(41) = 0.01, p = 0.99. SRS = Social Responsiveness Scale; scores on the SRS are reported as t-scores (M = 50, SD = 15). SCQ= Social Communication Questionnaire; scores of 15 or above are considered to meet cut-off for ASD on this screening measure. However, since the initial validation study it has been suggested that a lower cut-off score (e.g., 13 or 11) improves the sensitivity and specificity of the SCQ (Corsello et al., 2007; Snow & Lecavalier, 2008).
2.2. Materials and procedure
The point-light motion identification task and schematic motion categorization task were administered using a 30 in. computer monitor. The schematic motion categorization task was always administered before the point-light motion identification task. Since children also completed other cognitive tasks not related to biological motion, only four trials of each task were administered to ensure attentiveness and avoid fatigue. As such, measures were taken to ensure the reliability of data, namely, the presentation of inter-trial fixation points with an attractive sound to orient children's attention to the screen. Children's performance was recorded using a camcorder. Parents either sat behind the child as they completed the tasks, or in an adjacent room.
2.2.1. Schematic motion categorization task
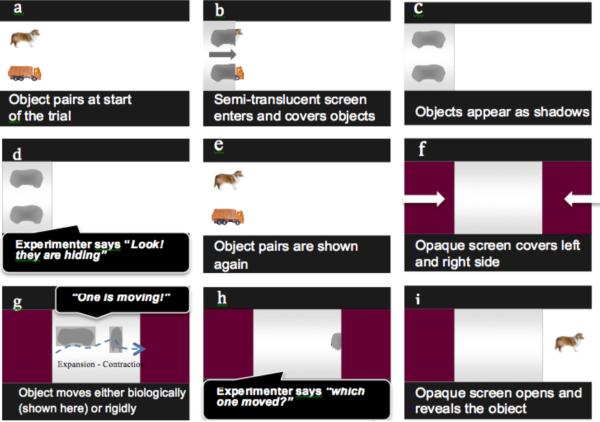
A novel forced-choice biological motion identification task was developed to test whether schematic biological motion (e.g. non-rigid expansion–contraction) is sufficient for children to infer the animate–inanimate category membership of an ambiguous geometric entity (Trauble, Poulin-Dubois, & Pauen, 2014). In this task, all other static and dynamic animacy cues were removed in order to determine whether biological motion is understood as an animacy cue among children with HFASD. The biological motion stimulus was adapted from the Michotte (1963) “caterpillar” stimuli, which consisted of a rectangle that moved non-rigidly by elongating and contracting. The novel stimulus had an organic-like, curvilinear form that was consistent with the overall shape of either an animate, or an animate, category exemplar. The general idea of this paradigm was to present children with an animate and an inanimate category exemplar (e.g. realistic images of a dog and truck), which became masked by a semi-translucent curtain, producing two identical “shadows.” Children observed one shadow moving across the screen (either biologically or non-biologically) and were subsequently asked to identify which of the two objects had moved as the shadow. The animation was divided into three phases: a familiarization phase, a motion phase, and a test phase. The main elements of the animation are shown in Fig. 1.
Fig. 1.
Diagram of the schematic biological motion categorization task. Notes: Frames (a)–(f) show the familiarization phase, frame (g) shows the motion phase, frame (h) shows the test phase, and frame (i) provides children with feedback on their response. During the familiarization phase children are introduced to two identical shadows representing each response option. The shadows of each object (shown) were designed to be identical, yet resemble either response option as closely as possible. Children viewed one shadow move across the screen either animately by expanding and contracting (i.e., dog), or inanimately by sliding across the screen (i.e., truck) during the motion phase. At test, children are asked which they thought had moved.
During the familiarization phase, children viewed static images of an animal and a vehicle on the left-hand portion of the screen. Children were asked to label the objects, then a semi-translucent curtain covered both objects so that they appeared as identical “shadows.” Children's attention was drawn to the objects hiding behind the curtain, “look they’re hiding.” The curtain was lifted and both objects were revealed again, then a completely opaque curtain covered both objects so they were no longer visible. During the motion phase, children were told, “one of them is going to move.” One shadow moved across the middle portion of the screen and disappeared behind a second opaque curtain. At test, children were asked, “which one moved?” If children did not provide a verbal response, a prompt (“was it the [dog] or the [truck]?”) was provided. Following the child's verbal response the opaque curtain was lifted and the identity of the moving shadow was revealed. Identification of the moving stimulus as either animate (e.g., dog) or inanimate (e.g., truck) could be made only by attending to the movement of the shadow, as no other animacy cues (e.g., change in speed or direction, self-propulsion) were provided. Children were not told that the manner of motion was the relevant feature they should attend to when making this decision. Two motion conditions were presented: biological motion (non-rigid expansion and contraction), or non-biological (rigid sliding across the screen).
Prior to completing test trials children were familiarized with the task by completing two warm-up trials. These trials used the same basic set-up and the same verbal explanations as test trials. However, warm-up trials differed in that the familiarization images were a caterpillar and a bicycle, and more importantly, the task could be successfully completed without attending to the motion, but to the shape of the shadow. In the warm-up phase, each familiarization image cast an appropriately shaped “shadow,” thus, children could respond correctly by simply matching the moving shadow that looked like a bicycle to the image of the bicycle. In contrast, on test trials an ambiguously shaped blob was used to represent both animate and inanimate familiarization images. In order to continue on to the test trials, children were required to demonstrate an understanding of the task by responding correctly on two consecutive trials. Thus, if an error was made on one of the warm-up trials, both warm-up trials were repeated.
Children completed a total of four test trials, whereby two sets of familiarization objects (cow–motorcycle pairing, dog– truck pairing) were presented with two types of moving shadows (biological and non-biological). The presentation of trials was counterbalanced for the type of motion presented on the first trial (biological, non-biological) and trials were pseudo-randomized so that the first two trials contained one of each set of familiarization stimuli and presented one biological and one non-biological motion scene.
Coding. Children's verbal responses were coded as either correct or incorrect on each trial; therefore, children could receive a maximum score of 4. Performance on animate (biological motion) and inanimate (non-biological motion) trials was scored separately and a percentage correct (out of 2) was calculated. Performance on the first and second presentation of each type of motion was also scored as a percentage correct (out of 2), to investigate whether the provision of feedback would produce improvement across trials.
2.2.2. Point-light motion identification task
To test whether children with high-functioning ASD identify biological and non-biological motion patterns, children were presented with point-light videos of a human, a cat, a truck, and a bicycle (Troje, 2002, provided the human point-light video stimuli; all other stimuli were adapted from Arterberry & Bornstein, 2001). The human point-light display was shown in front view, while all other stimuli were shown at a visual angle of 60 degrees to allow all four limbs of the cat and two wheels of each vehicle to be visible. Each video contained 11 point-light dots, which were placed on major parts of the human and cat (e.g. head, neck, shoulders [2], elbows [2], pelvis, knees [2], feet [2]) and major areas of the truck (e.g., wheels [3 per wheel], front bumper [2], back bumper [2], roof [1]) and bicycle's frame (e.g., wheels [3 per wheel], seat [2], frame [67_TD$DIFF][1], handle bars [2]. In each video, the stimulus was shown to be moving rightward, but remained stationary with no horizontal translation. Each trial was 6 s in duration and depicted either 3 complete gait cycles (animates) or 3 complete wheel rotations (vehicles) [0.5 s per cycle]. Videos were presented in random order. Prior to the presentation of each trial, a central fixation cross appeared accompanied with an attractive sound in order to orient children's attention to the screen.
Children were instructed to “watch” each video and were subsequently asked, “What is that?” If children did not provide a verbal response within 30 s, the experimenter encouraged them by saying, “What does it look like?” If children still did not provide a response the experimenter proceeded to the next trial “Let's try another one.” Children's verbal responses on each trial were recorded.
Coding. Verbal responses for each trial were recorded verbatim and scored as either correct or incorrect. Acceptable responses were coded as follows: human point-light display [man/woman, someone walking, person, or human], cat point-light display [cat, dog], bicycle point-light display [bicycle, motorcycle, truck], truck point-light display [truck, car, sedan, bicycle]. Since the point-light display of the bicycle and truck were both presented in side view, each depicting only two wheels, children who labeled the truck as a bicycle, and vice versa, were still considered correct. However, labeling the truck or bicycle as a morphologically different vehicle (e.g. train, airplane) was not considered a correct response. Thus, on each of four trials children's responses were scored as correct or incorrect and a total score was calculated as a percentage correct (out of 4). Children also received a score for their identification of animate, biological motion trials (out of 2), and a score for their identification of inanimate, mechanical motion trials (out of 2).
3. Results
3.1. Schematic motion categorization task
Preliminary analyses revealed no significant main effect of Order, or Group × Order interaction. Additionally, no significant main effect of Trial (first or second), or Group × Trial interaction found. was Thus, all subsequent analyses collapse across these factors. To examine whether performance of children with HF-ASD differed from TD children on animate and inanimate motion trials a 2 (Group) × 2 (Motion) mixed-model ANOVA was computed with Bonferroni corrections for multiple comparisons. Results revealed no main effect of Group, F(1, 41) = 0.0, p = 0.99, partial η2 = 0.00, that is, overall, children with HF-ASD performed as well as TD children matched on gender, age, and non-verbal cognitive ability (M of HFASD = 77.28%, M of TD = 77.38%). No significant main effect for Motion was found, F(1, 41) = 2.34, p = 0.13, partial η2 = 0.05, indicating that children generally performed equally well on animate (M = 81.40%) as opposed to inanimate (M = 73.26%) motion trials. The Group Motion interaction was also not significant, F(1, 41) = 0.03, p = 0.86, partial η2 = 0.00 (see Table 2 for scores).
Table 2.
Percentage of correct responses on the schematic and point-light biological motion identification tasks for each group.
| Point-light motion identification |
Schematic motion identification |
||||
|---|---|---|---|---|---|
| Animate | Inanimate | Animate | Inanimate | ||
| HF-ASD | 86.84% | 65.79% | 81.82% | 72.73% | |
| TD | 77.78% | 61.11% | 80.95% | 73.81% | |
A group of 21 adults were also tested to provide validation of the task. Adults’ overall performance on animate trials was 95.24% correct, while performance on inanimate trials was 83.33% correct.
3.2. Point-light motion identification task
To examine whether the accuracy of children's identification of point-light motion differed as a function of Motion Type (animate vs. inanimate) or Group (HF-ASD vs. TD) a 2 (Group) × 2 (Motion Type) mixed-model ANOVA was computed. A main effect of Motion Type was found, F(1,35) = 5.64, p = 0.02, partial η2 = 0.14, wherein children from both groups were better able to identify animate (M = 82.43%), compared to inanimate (M = 63.51%), point-light displays. No difference in performance between children with HF-ASD (M = 76.32%) and TD controls (M = 69.45%) was found, F(1,35) = 0.72, p = 0.40, partial η2 = 0.02. The Group Motion Type interaction was also not significant, F(1,35) = 0.08, p = 0.78, partial η2 = 0.00.
Given that no group differences in accuracy of point-light identification were found, we also provide a qualitative description of the types of errors children made. Of particular interest is whether children in either group incorrectly labeled an animate as an inanimate, and vice versa. When asked to identify animate point-light displays, all children in the TD group either labeled the animate stimuli as another animate (e.g., seal), or did not provide a response. On the inanimate point-light displays, one TD child labeled an inanimate (e.g., bicycle) as an animate (e.g. human), while all other children either labeled stimuli incorrectly, but as inanimates (e.g., airplane), or provided no response. Children with HF-ASD also largely followed this pattern, whereby errors on animate point-light trials consisted of incorrect, animate responses (e.g., cow), or no response. However, one child with HF-ASD incorrectly labeled an animate (e.g., cat) as an inanimate (e.g., car). Errors HF-ASD children made on inanimate point-light trials consisted of other inanimate responses (e.g. train), no response, or, in the case of two children, providing an incorrect animate label (e.g. kids, turtle).
A group of 21 adults were also tested to provide validation of the stimuli. Adults’ accuracy in identifying the agent in each animate display was 97%, while adults’ accuracy in identifying each object in the inanimate displays was 91%.
3.3. Inter-task and correlational analyses
Children's scores on both tasks were compared to determine whether children were equally successful at identifying schematic and realistic point-light biological motion. A 2 (Group) χ 2 (Task) χ 2 (Motion Type) mixed-design ANOVA with Bonferroni for multiple comparisons was computed. This analysis revealed a significant main effect of Motion Type, F(1,35) = 7.99, p < 0.01, partial η2 = 0.19, indicating that across tasks, HF-ASD and TD children performed better when identifying animate (M = 82.30%), as opposed to inanimate (M = 68.90%), motion. The main effect of Task was not significant, F(1,35) = 1.70, p = 0.20, partial η2 = 0.05, indicating that children performed similarly on the schematic (M = 78.30%) and point-light (M = 72.90%) biological motion identification tasks. No significant main effect of Group, F(1,35) = 0.60, p = 0.45, partial η2 = 0.02 (M HF-ASD = 7830%, M TD = 72.9%), Group χ Task interaction, F(1,35) = 0.13, p = 0.72, partial η2 = 0.00, or Group Motion Type interaction, F(1,35) = 0.04, p = 0.84, partial η2 = 0.00, was found. Non-parametric correlations (Kendall Tau rank coefficients) between tasks were also performed, whereby scores on each task (out of 4) were compared. Kendall's Tau was selected since scores on the motion identification tasks were not continuous and performance tended to cluster toward the upper range of values. Interestingly, among children with HF-ASD performance on the point-light motion identification and schematic motion identification tasks was significantly correlated, τ(17) = 0.55, p < 0.01, however, this was not the case for TD children, τ(16) = 0.16, p = 0.45.
Correlational analyses (Kendall Tau rank coefficients) were also conducted to investigate whether developmental variables such as cognitive ability or chronological age were related to biological motion identification performance. For the HF-ASD group, it was expected that developmental age, rather than chronological age, would relate to performance on motion identification measures, whereas among TD children chronological age was hypothesized to relate to performance. Among children with HF-ASD, performance on the schematic motion identification task was correlated with non-verbal cognitive ability, τ(20) = 0.39, p = 0.02, but not with chronological age, τ(20) = 0.24, p = 0.16. For the TD group, performance on the schematic motion identification task only tended to relate to chronological age, t(19) = 0.33, p = 0.07, and non-verbal cognitive ability, τ(19) = 0.31, p = 0.09. On the point-light motion identification task, performance in the HF-ASD group was significantly correlated with non-verbal cognitive ability, τ(17) = 0.37, p = 0.05, but not chronological age, τ(17) = 0.22, p = 0.23. While in the TD group, both non-verbal cognitive ability, τ(16) = 0.60, p < 0.01, and chronological age, τ(16) = 0.40, p = 0.05, was correlated with performance on the point-light motion identification task.
3.4. Biological motion and ASD symptoms
Non-parametric correlations were computed to examine the relation between task performance and social-cognitive difficulties as measured by the Social Communication Questionnaire (SCQ) and the Social Responsiveness Scale (SRS). To test the hypothesis that identification of biological motion relates to children's social-cognitive abilities, children's performance on biological motion trials of each procedure were collapsed to create an animate motion identification score (out of 4). Similarly, children's performance across inanimate motion trials of the point-light motion identification task and schematic motion trials to create an inanimate motion identification score (out of 4). Based on previous research, one would expect that performance on animate motion identification trials would negatively correlate with parental report of ASD symptoms on the SRS, and screening scores on the SCQ, whereas performance on inanimate motion identification trials would not be expected to relate to either parent report measure. As expected, performance of children with HF-ASD on animate motion identification trials tended to negatively relate to parental reports on the Social Communication Questionnaire, τ(17) =–0.36, p = 0.07, while performance on inanimate motion trials was not related to SCQ scores, τ(17) =– 0.21, p = 0.28. However, performance on neither animate, nor inanimate, motion identification trials were significantly related to ASD symptomatology on the Social Responsiveness Scale, τ(17) =–0.27, p = 0.16, τ(17) = 0.11, p = 0.57, respectively.
4. Discussion
Whether deficits in biological motion perception are one of the hallmarks of autism spectrum disorder has been a topic of much debate. The primary goal of the current research was to compare the performance of children with HF-ASD on two tasks of biological motion identification: a point-light motion identification task and a novel schematic motion identification task. Although schematic biological motion is frequently used to study animacy perception in typical development, few studies have used this methodology to study motion perception in ASD. The observed performance across tasks suggests that insofar as biological motion is used as a cue for animacy judgments, school-aged children with high-functioning autism perform as well as TD controls, matched on chronological age, nonverbal cognitive ability, and gender. Consistent with the results of Rutherford et al. (2006), no reliable autism-specific deficit in animacy perception was found. Furthermore, children with HF-ASD were able to process biological motion cues to make accurate animacy judgments even when presented with a single trial.
While much of the literature on biological motion perception has focused on the psychophysical aspects of point-light stimuli (e.g., visual preference, discrimination of masked stimuli) the current study sought to determine whether children with high-functioning ASD identify simple point-light presentations of animate and inanimate stimuli. Children were asked to identify point-light displays of a person, a cat, a truck, and a bicycle. Overall, children with high-functioning ASD performed similarly to TD children. Across HF-ASD and TD groups, children were better able to identify animate point-light displays (human and cat), compared to inanimate displays (truck and bicycle). Although it is not surprising that TD children were better able to identify animate, compared to inanimate motion, that children with high-functioning ASD also showed the same pattern was somewhat unexpected. Given the body of evidence suggesting that, unlike TD children, children with ASD do not preferentially attend to social stimuli (Dawson, Meltzoff, Osterling, Rinaldi, & Brown, 1998; Fletcher-Watson, Leekam, Benson, Frank, & Findlay, 2009; Kaiser et al., 2010; Klin et al., 2002, 2009; Maestro et al., 2002), it might be hypothesized that this lack of attentional preference might lead to a reduced ability to identify point-light displays of people or animals.
A novel schematic biological motion categorization task (the “shadow” paradigm) was designed to examine whether children identify an ambiguous “shadow” as animate or inanimate based its movement. Consistent with the biological motion point-light display identification task, children with HF-ASD performed similarly to TD controls. That children with HF-ASD readily associated non-rigid, expansion–contraction movement with other living things that do not move in this specific way (e.g. dog, cow) suggests that children with HF-ASD are able to make abstract inferences across the animate domain. The results of this study can be contrasted with Congiu et al. (2010) who asked 13-year-old children with HF-ASD to describe the ‘Michotte’ stimulus using an open-response format. While 77% of TD children described the stimulus as animate (e.g., “caterpillar,” “slug,” “snake”) only 37% of children with ASD provided animate descriptions. While the results of Congiu et al. (2010) study suggest that children with ASD experience difficulty with the perception of animacy, it is possible that lower cognitive ability (Mean FSIQ = 75, range 40 to 110), or children's preference to provide literal responses (e.g., rectangles, squares) could account for these differences. In the present study, younger children (M = 6.49 years) with HF-ASD were shown to associate the ‘Michotte’ expansion–contraction motion with mammals when presented as a forced-choice task.
The present study is the first to compare children with HF-ASD's performance on schematic and point-light biological motion identification tasks. Among children with HF-ASD, performance was significantly correlated across the two tasks. That is, those children who performed well on the point-light motion task generally performed well on the schematic biological motion task, and those who performed poorly on the point-light motion task generally performed poorly on the schematic motion task. Interestingly, performance of TD children was not correlated across point-light and schematic biological motion tasks. This absence of relationship may suggest that the TD children and children with ASD approached the two tasks differently or that different cognitive resources were required to successfully complete each task. However, it is possible to rule out the hypothesis that children could have been selecting their responses based on idiosyncratic features of the stimuli, such as a general preference for animate or inanimate stimuli, as this strategy would have resulted in chance level performance.
Results of the present study replicate and extend previous studies that reported a significant relation between cognitive ability and biological motion processing in individuals with ASD (Atkinson, 2009; Koldewyn et al., 2010; Rutherford & Troje, 2012). As expected across both biological motion identification tasks, nonverbal mental age was found to correlate with performance among children with HF-ASD. A number of different explanations are possible for these results. The stronger relation between cognitive ability and performance in HF-ASD, but not in TD children, may be explained by the fact that children with HF-ASD generally had a larger range of scores on the standardized measure of nonverbal mental abilities. Within the ASD group, relatively poorer performance on the biological motion tasks may be related to below-average cognitive ability whereas above-average cognitive ability may not necessarily provide children an additional advantage. That performance among children with HF-ASD was not impaired overall, but was significantly related to nonverbal cognitive ability suggest that deficits in biological motion processing may not be specific to a high-functioning ASD population, but instead may be a correlate of cognitive delay. Since the current study only included children with HF-ASD it is possible that performance on biological motion identification tasks is impaired among younger, or lower-functioning, children.
A secondary aim of this study was to investigate the relation between children's ability to identify biological motion and measures of social-cognitive functioning, namely those used to screen for ASD (e.g., SCQ) and assess the severity of symptomatology (e.g., SRS). Consistent with our hypothesis HF-ASD children who had higher scores on the SCQ tended to perform more poorly when identifying animate point-light and schematic motion, while scores on the SCQ were unrelated to performance on inanimate motion identification trials. However, symptoms of ASD measured by the SRS were not found to relate to children's ability to identify animate or inanimate point-light or schematic motion. One possible explanation for these results is that the SRS was originally designed to assess the broader autism phenotype (Constantino & Gruber, 2005), therefore, SRS scores of children who already had a diagnosis on the autism spectrum were clustered in the severe range of symptom scores and were not variable enough to correlate with our performance measures. Had we also administered the SRS to the TD group a relationship between broader autism spectrum traits and performance on animate biological motion identification trials may have emerged. That the SCQ and SRS were not administered to the TD group is one limitation to the current study.
Results of the current study are not in conflict with the view that early deficits in biological motion perception may be a diagnostic predictor of the development of ASD (Kaiser & Pelphrey, 2012). It is possible that deficits in biological motion processing are present in infancy (Klin et al., 2009), and over the course of development, compensatory strategies are increasingly relied on in order to successfully complete biological motion tasks. Mixed empirical results concerning whether deficits in biological motion processing are enduring into childhood (Annaz et al., 2010; Blake et al., 2003; Centelles et al., 2013; Congiu et al., 2010; Milne et al., 2002; Parron et al., 2008; Rutherford et al., 2006; Swettenham et al., 2013) provide evidence for neuroplasticity in the mechanisms responsible for biological motion perception. Grossman, Blake, and Kim (2004) demonstrated that not only can biological motion performance be improved with training, but improvement among TD adults was correlated with changes in activation in brain regions implicated in the processing of biological motion (Grossman et al., 2004). In another study, adults with Asperger's Syndrome were shown to perform similarly to controls on a task requiring individuals to identify the direction of biological motion, however, the Asperger's Syndrome group demonstrated decreased brain activation in areas typically recruited in biological motion processing (Herrington et al., 2007).
The ability to extract information from biological motion displays has far-reaching implications for children's concept formation and understanding of the social world (Klin & Jones, 2008; Pavlova, 2011; Yoon & Johnson, 2009). In the current study, the perception of animacy in children with high-functioning ASD was assessed with the “shadow” paradigm, a forced-choice task that required children to associate animate stimuli with the ability to move biologically by expanding and contracting. However, modifying the current procedure to test children's ability to discriminate biological motion by manipulating the salience of biological motion cues (e.g., varying the degree of expansion and contraction) may potentially be a more sensitive measure to detect whether differences in performance across groups exist. Additionally, aside from biological motion, a number of other animacy cues exist and remain to be explored in and ASD population. Future research may wish to examine animacy understanding in children with ASD using a wide range of cues, such as the ability to self-start, change speed, or change direction. A developmental adaptation of the schematic biological motion identification task has been used to test nonverbal infants as young as 7 months of age using a violation-of-expectation paradigm (Trauble et al., 2014). This non-verbal adaptation would not only allow for the assessment of animacy understanding in younger, nonverbal children with ASD, but also makes possible the investigation of animacy understanding in a high-risk infant study (i.e. infant siblings of children with ASD). In order to evaluate the hypothesis that biological motion processing impairments are a hallmark of ASD, and affect the development of social-cognitive abilities, an examination of biological motion understanding in infant siblings at risk for autism spectrum disorder would be extremely valuable.
Acknowledgements
This research was supported by a grant from the Natural Science and Engineering Research Council of Canada (2003– 2013) to Dr. Poulin-Dubois. This research was also supported by NICHD under award #R01HD468058 to the last author and does not necessarily represent the views of the National Institutes of Health. We also wish to acknowledge the support provided by the Autism Research Training (ART) program, a Canadian Institute for Health Research (CIHR) Strategic Training initiative in autism research. Portions of this research were presented at Development 2014: A Canadian Conference on Developmental Psychology, Ottawa, Ontario, Canada. The authors would like to thank Dr. Martha Arterberry for her contribution to the development of the point-light stimuli, Dr. Birgit Trauble and Dr. Sabina Pauen for their collaboration in developing the schematic biological motion animations, as well as Jenny Coutu who assisted with coding.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Although all children in our sample of HF-ASD carried a valid clinical diagnosis on the autism spectrum, a number of children did not meet the accepted cut-off of 15 on the Social Communication Questionnaire. However, recent research suggests that lowering the cut-off to 13 or 11 improves sensitivity and specificity, especially among high-functioning children with ASD (Corsello et al., 2007; Oosterling, Swinkels, Jan van der Gaag, Visser, Dietz, & Buitelaar, 2009). Of the 22 children with HF-ASD in our sample, 9 failed to meet the cut-off of 15 or above. However, it was not the case that children who failed to meet SCQ thresholds were less impaired, or more likely to be identified by a diagnosis on the broader Autism Spectrum (e.g. PDD-NOS, Asperger's Syndrome), compared with children who did meet cut-off, χ2 (n = 22) = 0.24, p = 0.89.
References
- Annaz D, Campbell R, Coleman M, Milne E, Swettenham J. Young children with autism spectrum disorder do not preferentially attend to biological motion. Journal of Autism and Developmental Disorders. 2012;42(3):401–408. doi: 10.1007/s10803-011-1256-3. http://dx.doi.org/10.1007/s10803-011-1256-3. [DOI] [PubMed] [Google Scholar]
- Annaz D, Remington A, Milne E, Coleman M, Campbell R, Thomas MSC, et al. Development of motion processing in children with autism. Developmental Science. 2010;13(6):826–838. doi: 10.1111/j.1467-7687.2009.00939.x. http://dx.doi.org/10.1111/j.1467-7687.2009.00939.x. [DOI] [PubMed] [Google Scholar]
- Arterberry ME, Bornstein MH. Three-month-old infants’ categorization of animals and vehicles based on static and dynamic attributes. Journal of Experimental Child Psychology. 2001;80(4):333–346. doi: 10.1006/jecp.2001.2637. http://dx.doi.org/10.1006/jecp.2001.2637. [DOI] [PubMed] [Google Scholar]
- Atkinson AP. Impaired recognition of emotions from body movements is associated with elevated motion coherence thresholds in autism spectrum disorders. Neuropsychologia. 2009;47(13):3023–3029. doi: 10.1016/j.neuropsychologia.2009.05.019. [DOI] [PubMed] [Google Scholar]
- Blake R, Shiffrar M. Perception of human motion. Annual Review of Psychology. 2007;58:47–73. doi: 10.1146/annurev.psych.57.102904.190152. http://dx.doi.org/10.1146/annurev.psych.57.102904.190152. [DOI] [PubMed] [Google Scholar]
- Blake R, Turner LM, Smoski MJ, Pozdol SL, Stone WL. Visual recognition of biological motion is impaired in children with autism. Psychological Science. 2003;14(2):151–157. doi: 10.1111/1467-9280.01434. [DOI] [PubMed] [Google Scholar]
- Centelles L, Assaiante C, Etchegoyhen K, Bouvard M, Schmitz C. From action to interaction: Exploring the contribution of body motion cues to social understanding in typical development and in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2013;43(5):1140–1150. doi: 10.1007/s10803-012-1655-0. http://dx.doi.org/10.1007/s10803-012-1655-0. [DOI] [PubMed] [Google Scholar]
- Chang DHF, Troje NF. Perception of animacy and direction from local biological motion signals. Journal of Vision. 2008;8:1–10. doi: 10.1167/8.5.3. http://dx.doi.org/10.1167/8.5.3.Introduction. [DOI] [PubMed] [Google Scholar]
- Cleary L, Looney K, Brady N, Fitzgerald M. Inversion effects in the perception of the moving human form: A comparison of adolescents with autism spectrum disorder and typically developing adolescents. Autism: The International Journal of Research and Practice. 2013 doi: 10.1177/1362361313499455. http://dx.doi.org/10.1177/136236131349945. [DOI] [PubMed]
- Congiu S, Schlottmann A, Ray E. Unimpaired perception of social and physical causality, but impaired perception of animacy in high functioning children with autism. Journal of Autism and Developmental Disorders. 2010;40(1):39–53. doi: 10.1007/s10803-009-0824-2. http://dx.doi.org/10.1007/s10803-009-0824-2. [DOI] [PubMed] [Google Scholar]
- Constantino J, Gruber C. The Social Responsiveness Scale. Western Psychological Services; Los Angeles: 2005. [Google Scholar]
- Corsello C, Hus V, Pickles A, Risi S, Cook EH, Leventhal BL, et al. Between a ROC and a hard place: Decision making and making decisions about using the SCQ. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2007;48(9):932–940. doi: 10.1111/j.1469-7610.2007.01762.x. http://dx.doi.org/10.1111/j.1469-7610.2007.01762.x. [DOI] [PubMed] [Google Scholar]
- David N, Schultz J, Milne E, Schunke O, Schottle D, Munchau A, et al. Right temporoparietal gray matter predicts accuracy of social perception in the autism spectrum. Journal of Autism and Developmental Disorders. 2013:1–14. doi: 10.1007/s10803-013-2008-3. [DOI] [PubMed] [Google Scholar]
- Dawson G, Meltzoff A, Osterling J, Rinaldi J, Brown E. Children with autism fail to orient to naturally occurring social stimuli. Journal of Autism and Developmental Disorders. 1998;28(6):479–485. doi: 10.1023/a:1026043926488. [DOI] [PubMed] [Google Scholar]
- Elliott C. Introductory and technical handbook. 2nd ed. The Psychological Corporation; San Antonio, TX: 2007. Differential Ability Scale. [Google Scholar]
- Fletcher-Watson S, Leekam SR, Benson V, Frank MC, Findlay JM. Eye-movements reveal attention to social information in autism spectrum disorder. Neuropsychologia. 2009;47(1):248–257. doi: 10.1016/j.neuropsychologia.2008.07.016. http://dx.doi.org/10.1016/j.neuropsychologia.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Freitag CM, Konrad C, Haäberlen M, Kleser C, von Gontard A, Reith W, et al. Perception of biological motion in autism spectrum disorders. Neuropsychologia. 2008;46(5):1480–1494. doi: 10.1016/j.neuropsychologia.2007.12.025. http://dx.doi.org/10.1016/j.neuropsychologia.2007.12.025. [DOI] [PubMed] [Google Scholar]
- Gelman SA, Coley JD. The importance of knowing a dodo is a bird: Categories and inferences in 2-year-old children. Developmental Psychology. 1990;26(5):796–804. http://dx.doi.org/10.1037/0012-1649.26.5.796. [Google Scholar]
- Gelman SA, Opfer JE. Development of the animate–inanimate distinction. In: Goswami U, editor. Blackwell handbook of childhood cognitive development. Blackwell Publishers Ltd.; 2002. pp. 151–166. [Google Scholar]
- Grossman ED, Blake R, Kim C-Y. Learning to see biological motion: Brain activity parallels behavior. Journal of Cognitive Neuroscience. 2004;16(9):1669–1679. doi: 10.1162/0898929042568569. http://dx.doi.org/10.1162/0898929042568569. [DOI] [PubMed] [Google Scholar]
- Heider F, Simmel M. An experimental study of apparent behavior. The American Journal of Psychology. 1944;57(2):243–259. [Google Scholar]
- Herrington JD, Baron-Cohen S, Wheelwright SJ, Singh KD, Bullmore ET, Brammer M, et al. The role of MT+/V5 during biological motion perception in Asperger Syndrome: An fMRI study. Research in Autism Spectrum Disorders. 2007;1(1):14–27. http://dx.doi.org/10.1016/j.rasd.2006.07.002. [Google Scholar]
- Hubert B, Wicker B, Moore DG, Monfardini E, Duverger H, Da Fonséca D, et al. Brief report: Recognition of emotional and non-emotional biological motion in individuals with autistic spectrum disorders. Journal of Autism and Developmental Disorders. 2007;37(7):1386–1392. doi: 10.1007/s10803-006-0275-y. http://dx.doi.org/10.1007/s10803-006-0275-y. [DOI] [PubMed] [Google Scholar]
- Johansson G. Visual perception of biological motion and a model for its analysis. Perception and Psychophysics. 1973;14(2):201–211. [Google Scholar]
- Kaiser MD, Delmolino L, Tanaka JW, Shiffrar M. Comparison of visual sensitivity to human and object motion in autism spectrum disorder. Autism Research. 2010;3(4):191–195. doi: 10.1002/aur.137. http://dx.doi.org/10.1002/aur.137. [DOI] [PubMed] [Google Scholar]
- Kaiser MD, Pelphrey K. Disrupted action perception in autism: Behavioral evidence, neuroendophenotypes, and diagnostic utility. Developmental Cognitive Neuroscience. 2012;2(1):25–35. doi: 10.1016/j.dcn.2011.05.005. http://dx.doi.org/10.1016/j.dcn.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser MD, Shiffrar M. Variability in visual perception of human motion as a function of observers autistic traits. In: Johnson K, Shiffrar M, editors. People watching: Social, perceptual and neurophysiological studies of body perception. Oxford University Press; New York, NY: 2013. [Google Scholar]
- Klin A, Jones W. Altered face scanning and impaired recognition of biological motion in a 15-month-old infant with autism. Developmental Science. 2008;11(1):40–46. doi: 10.1111/j.1467-7687.2007.00608.x. http://dx.doi.org/10.1111/j.1467-7687.2007.00608.x. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Archives of General Psychiatry. 2002;59(9):809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Klin A, Lin DJ, Gorrindo P, Ramsay G, Jones W. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459(7244):257–261. doi: 10.1038/nature07868. http://dx.doi.org/10.1038/nature07868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koldewyn K, Whitney D, Rivera SM. The psychophysics of visual motion and global form processing in autism. Brain. 2010;(133):599–610. doi: 10.1093/brain/awp272. http://dx.doi.org/10.1093/brain/awp272. [DOI] [PMC free article] [PubMed]
- Koldewyn K, Whitney D, Rivera SM. Neural correlates of coherent and biological motion perception in autism. Developmental Science. 2011;14(5):1075–1088. doi: 10.1111/j.1467-7687.2011.01058.x. http://dx.doi.org/10.1111/j.1467-7687.2011.01058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. Journal of Autism and Developmental Disorders. Vol. 30. Western Psychological Services; 2000. Autism diagnostic observation schedule (ADOS). pp. 205–223. http://dx.doi.org/10.1007/BF02211841. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disabilities. 1994;24(5) doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Maestro S, Muratori F, Cavallaro MC, Pei F, Stern D, Golse B, et al. Attentional skills during the first 6 months of age in autism spectrum disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(10):1239–1245. doi: 10.1097/00004583-200210000-00014. http://dx.doi.org/10.1097/01.CHI.0000020277.43550.02. [DOI] [PubMed] [Google Scholar]
- Mak B, Vera AH. The role of motion in children's categorization of objects. Cognition. 1999;71(1):B11–B21. doi: 10.1016/s0010-0277(99)00019-0. http://dx.doi.org/10.1016/S0010-0277(99)00019-0. [DOI] [PubMed] [Google Scholar]
- Mandler J. How to build a baby: II. Conceptual primitives. Psychological Review. 1992;99(4):587–604. doi: 10.1037/0033-295x.99.4.587. [DOI] [PubMed] [Google Scholar]
- Michotte A. The perception of causality. Basic Books; New York: 1963. [Google Scholar]
- Milne E, Swettenham J, Hansen P, Campbell R, Jeffries H, Plaisted K. High motion coherence thresholds in children with autism. Journal of Child Psychology and Psychiatry. 2002;43(2):255–263. doi: 10.1111/1469-7610.00018. [DOI] [PubMed] [Google Scholar]
- Moore DG, Hobson RP, Lee A. Components of person perception: An investigation with autistic, non-autistic retarded and typically developing children and adolescents. British Journal of Developmental Psychology. 1997;15(4):401–423. http://dx.doi.org/10.1111/j.2044-835X.1997.tb00738.x. [Google Scholar]
- Morita T, Slaughter V, Katayama N, Kitazaki M, Kakigi R, Itakura S. Infant and adult perceptions of possible and impossible body movements: An eye-tracking study. Journal of Experimental Child Psychology. 2012;113(3):401–414. doi: 10.1016/j.jecp.2012.07.003. http://dx.doi.org/10.1016/j.jecp.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Murphy P, Brady N, Fitzgerald M, Troje NF. No evidence for impaired perception of biological motion in adults with autistic spectrum disorders. Neuropsychologia. 2009;47(14):3225–3235. doi: 10.1016/j.neuropsychologia.2009.07.026. http://dx.doi.org/10.1016/j.neuropsychologia.2009.07.026. [DOI] [PubMed] [Google Scholar]
- Opfer JE, Gelman SA. Development of the animate–inanimate distinction. In: Goswami U, editor. The Wiley-Blackwell handbook of childhood cognitive development. Wiley-Blackwell; 2010. pp. 213–238. http://dx.doi.org/10.1002/9780470996652.ch7. [Google Scholar]
- Parron C, Da Fonseca D, Santos A, Moore DG, Monfardini E, Deruelle C. Recognition of biological motion in children with autistic spectrum disorders. Autism. 2008;12(3):261–274. doi: 10.1177/1362361307089520. http://dx.doi.org/10.1177/1362361307089520. [DOI] [PubMed] [Google Scholar]
- Pavlova M. Biological motion processing as a hallmark of social cognition. Cerebral Cortex. 2011;22(5):981–995. doi: 10.1093/cercor/bhr156. http://dx.doi.org/10.1093/cercor/bhr156. [DOI] [PubMed] [Google Scholar]
- Pavlova M. The development of biological motion processing in normalcy and pathology. In: Johnson K, Shiffrar M, editors. People watching: Social, perceptual and neurophysiological studies of body perception. Oxford University Press; New York, NY: 2013. [Google Scholar]
- Pavlova M, Kraägeloh-Mann I, Sokolov A, Birbaumer N. Recognition of point-light biological motion displays by young children. Perception. 2001;30(8):925–933. doi: 10.1068/p3157. http://dx.doi.org/10.1068/p3157. [DOI] [PubMed] [Google Scholar]
- Premack D. The infant's theory of self-propelled objects. Cognition. 1990;36(1):1–16. doi: 10.1016/0010-0277(90)90051-k. [DOI] [PubMed] [Google Scholar]
- Rakison DH, Poulin-Dubois D. Developmental origin of the animate–inanimate distinction. Psychological Bulletin. 2001;127(2):209–228. doi: 10.1037/0033-2909.127.2.209. [DOI] [PubMed] [Google Scholar]
- Rutherford MD, Pennington BF, Rogers SJ. The perception of animacy in young children with autism. Journal of Autism and Developmental Disorders. 2006;36(8):983–992. doi: 10.1007/s10803-006-0136-8. http://dx.doi.org/10.1007/s10803-006-0136-8. [DOI] [PubMed] [Google Scholar]
- Rutherford MD, Troje NF. IQ predicts biological motion perception in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2012;42(4):557–565. doi: 10.1007/s10803-011-1267-0. http://dx.doi.org/10.1007/s10803-011-1267-0. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. Social communication questionnaire. Western Psychological Services; Los Angeles, CA: 2003. [Google Scholar]
- Saygin AP, Cook J, Blakemore S-J. Unaffected perceptual thresholds for biological and non-biological form-from-motion perception in autism spectrum conditions. PLoS ONE. 2010;5(10):e13491. doi: 10.1371/journal.pone.0013491. http://dx.doi.org/10.1371/journal.pone.0013491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlottmann A, Allen D, Linderoth C, Hesketh S. Perceptual causality in children. Child Development. 2002;73(6):1656–1677. doi: 10.1111/1467-8624.00497. [DOI] [PubMed] [Google Scholar]
- Schlottmann A, Ray E. Goal attribution to schematic animals: Do 6-month-olds perceive biological motion as animate? Developmental Science. 2010;13(1):1–10. doi: 10.1111/j.1467-7687.2009.00854.x. http://dx.doi.org/10.1111/j.1467-7687.2009.00854.x. [DOI] [PubMed] [Google Scholar]
- Schultz J, Bulthoff H. Parametric animacy percept evoked by a single moving dot mimicking natural stimuli. Vision. 2013;13(4):1–19. doi: 10.1167/13.4.15. http://dx.doi.org/ 10.1167/13.4.15. [DOI] [PubMed] [Google Scholar]
- Simion F, Regolin L, Bulf H. A predisposition for biological motion in the newborn baby. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(2):809–813. doi: 10.1073/pnas.0707021105. http://dx.doi.org/10.1073/pnas.0707021105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow AV, Lecavalier L. Sensitivity and specificity of the Modified Checklist for Autism in Toddlers and the Social Communication Questionnaire in preschoolers suspected of having pervasive developmental disorders. Autism: The International Journal of Research and Practice. 2008;12(6):627–644. doi: 10.1177/1362361308097116. http://dx.doi.org/10.1177/1362361308097116. [DOI] [PubMed] [Google Scholar]
- Swettenham J, Baron-Cohen S, Charman T, Cox A, Baird G, Drew A, et al. The frequency and distribution of spontaneous attention shifts between social and nonsocial stimuli in autistic, typically developing, and nonautistic developmentally delayed infants. Journal of Child Psychology and Psychiatry. 1998;39(5):747–753. [PubMed] [Google Scholar]
- Swettenham J, Remington A, Laing K, Fletcher R, Coleman M, Gomez J-C. Perception of pointing from biological motion point-light displays in typically developing children and children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2013;43(6):1437–1446. doi: 10.1007/s10803-012-1699-1. http://dx.doi.org/10.1007/s10803-012-1699-1. [DOI] [PubMed] [Google Scholar]
- Trauble B, Poulin-Dubois D, Pauen S. Infants can detect the animacy status of moving shadows. 2014 Manuscript submitted for publication. [Google Scholar]
- Tremoulet PD, Feldman J. Perception of animacy from the motion of a single object. Perception. 2000;29(8):943–951. doi: 10.1068/p3101. http://dx.doi.org/10.1068/p3101. [DOI] [PubMed] [Google Scholar]
- Troje NF. Decomposing biological motion: A framework for analysis and synthesis of human gait patterns. Journal of Vision. 2002;2(5):371–387. doi: 10.1167/2.5.2. http://dx.doi.org/10.1167/2.5.2. [DOI] [PubMed] [Google Scholar]
- Troje NF. What is biological motion? Definition, stimuli, and paradigms. In: Rutherford MD, Kuhlmeier VA, editors. Social perception: Detection and interpretation of animacy, agency, and intention. MIT Press; Cambridge, MA: 2013. pp. 13–36. [Google Scholar]
- Yoon JMD, Johnson SC. Biological motion displays elicit social behavior in 12-month-olds. Child Development. 2009;80(4):1069–1075. doi: 10.1111/j.1467-8624.2009.01317.x. http://dx.doi.org/10.1111/j.1467-8624.2009.01317.x. [DOI] [PubMed] [Google Scholar]