Abstract
Tumor recognition by immune effector cells is mediated by antigen receptors and a variety of adhesion and costimulatory molecules. The evidence accumulated since the identification of CD155 and CD112 as ligands for DNAM-1 in humans and mice has suggested that the interactions between DNAM-1 and its ligands play an important role in T cell– and natural killer (NK) cell–mediated recognition and lysis of tumor cells. We have previously demonstrated that methylcholanthrane (MCA) accelerates tumor development in DNAM-1–deficient mice, and the Cd155 level on MCA-induced tumors is significantly higher in DNAM-1–deficient mice than in wild-type (WT) mice. By contrast, Cd112 expression on the tumors is similar in WT and DNAM-1-deficient mice, suggesting that CD155 plays a major role as a DNAM-1 ligand in activation of T cells and NK cells for tumor immune surveillance. To address this hypothesis, we examined MCA-induced tumor development in CD155-deficient mice. Unexpectedly, we observed no significant difference in tumor development between WT and CD155-deficient mice. Instead, we found that Cd112 expression was significantly higher in the MCA-induced tumors of CD155-deficient mice than in those of WT mice. We also observed higher expression of DNAM-1 and lower expression of an inhibitory receptor, TIGIT, on CD8+ T cells in CD155-deficient mice. These results suggest that modulation of the expression of receptors and CD112 compensates for CD155 deficiency in immune surveillance against MCA-induced tumors.
Introduction
Cancer immune surveillance to suppress tumor development is an important host protection process. Several immune effector cell types and secreted cytokines play a critical role in this process. Among them, cytotoxic T lymphocytes (CTL) and natural killer (NK) cells are major players in cell-mediated immunity against tumors [1], [2]. Interaction of cell surface receptors on CTL and NK cells with their respective ligands expressed on tumors activates the CTL and NK cells [2], [3], resulting in their secretion of cytokines and cytotoxicity against tumors [4], [5].
The leukocyte adhesion molecule DNAX accessory molecule-1 (DNAM-1, also known as CD226) is a member of the immunoglobulin (Ig) superfamily and is constitutively expressed on most CD4+ and CD8+ T cells, NK cells, monocytes, and macrophages [6]. The poliovirus receptor (PVR) CD155 and another member of the same family, CD112 (PVR-related family 2 [PRR-2], also called nectin-2), are the ligands of DNAM-1 in humans and mice [7]–[9]. Interactions between DNAM-1 on NK cells and CD8+ T cells and CD112 and CD155 on tumor cells augment cell-mediated cytotoxicity and cytokine production [7], [8].
CD155 and CD112 are present on various types of epithelial and endothelial cells in many tissues [10], [11]. A number of studies have demonstrated that CD155 and CD112 are overexpressed on certain hematopoietic and nonhematopoietic tumors [12]–[17], suggesting that DNAM-1 ligand expression might be induced by tumorigenesis and might stimulate CTL- and NK cell–mediated tumor immunity. Of note, CD155 and CD112 also bind TIGIT (T cell immunoreceptor with Ig and ITIM [immunoreceptor tyrosine-based inhibitory motif] domains), which is expressed on T cells and NK cells and mediates an inhibitory signal (either directly or indirectly) in these cells [18], [19]. CD155 also binds an immunoreceptor, CD96 (also called T cell-activated increased late expression [TACTILE]), that is expressed on both activated T cells and NK cells [20]–[22]. Taken together, the available data suggest that CD155 might be a double-edged sword balancing tumor growth and elimination.
We have previously demonstrated that the chemical carcinogens methylcholanthrane (MCA) and 7,12-dimethylbenz[a]anthracene (DMBA) result in significantly greater development of fibrosarcoma and papilloma, respectively, in DNAM-1–deficient mice than in wild-type (WT) mice [23]. Interestingly, we found that although Cd155 expression on MCA-induced fibrosarcomas was significantly higher in DNAM-1–deficient mice than in WT mice, Cd112 expression was similar, suggesting that CD155, rather than CD112, is the tumor ligand involved in DNAM-1–mediated immune surveillance against MCA-induced fibrosarcoma. In the present study, we used CD155-deficient mice to examine the role of CD155 on MCA-induced fibrosarcomas in tumor immune surveillance.
Materials and Methods
Mice
C57BL/6N and BALB/c mice were purchased from CLEA (Tokyo, Japan). CD155-deficient C57BL/6N and BALB/c mice were described previously [24]; they were additionally backcrossed twice with C57BL/6N and BALB/c mice, respectively (a total of 12 generations). All mice were housed and bred under specific-pathogen-free conditions at the Animal Resource Center of the University of Tsukuba. Animal experiments were carried out in a humane manner after receiving approval from the Animal Experiment Committee of the University of Tsukuba (Approval No.: 10-237, 11-231, 12-231), and in accordance with Fundamental Guideline for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions under the Jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology, and Japanese Act on Welfare and Management of Animals (No.105).
Flow cytometry
CD4+ T, CD8+ T, and NK cells from peripheral blood were analyzed using an LSRFortessa flow cytometer (BD Biosciences, San Jose, CA). The anti-DNAM-1 monoclonal antibody (mAb) TX42 (rat IgG2a) was generated in our laboratory [13]. All other antibodies for flow cytometry analyses were purchased from BD Biosciences. CD8+ T cells were purified by magnetic separation from spleen cells on a Mini-MACS system (Miltenyi Biotec, Bergisch Gladbach, Germany). Purified CD8+ T cells were cultured in the presence of 20 ng/ml exogenous IL-2 (BD Biosciences) in a 24-well plate coated with 1 µg/ml of anti-CD3e mAb (BD Biosciences). The cells were harvested at various time points, and the cell surface molecules were analyzed by flow cytometry. Antibodies against TIGIT and CD96 were from eBioscience (San Diego, CA) and R&D Systems (Minneapolis, MN), respectively. Cell staining and flow cytometry were performed according to standard procedures. The CellQuest (BD Biosciences) and FlowJo (Tree Star, Inc., Ashland, OR) programs were used for data acquisition and analysis.
Tumor growth assay and survival of mice
Groups of 10–20 WT or CD155-deficient male mice (8–12 week-old) were injected subcutaneously (s.c.) in the back with 5 or 100 µg (as specified in the figure legends) MCA (Sigma-Aldrich, St. Louis, MO) dissolved in 0.1 ml corn oil (Sigma-Aldrich) after anesthetization (7∶3 mixture of polyethylene glycol and isoflurane). Mice were examined at least once a week for tumor size with a caliper square as previously described [13]. Mouse survival was closely monitored 3 times per week during the experimental period and the humane endpoint was applied for euthanization by excess anesthetization.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from fibrosarcomas and naive tissues with the Isogen reagent (Nippon Gene, Tokyo, Japan). For reverse transcription, we used 2 µg of total RNA and a High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, Carlsbad, CA) in a final volume of 20 µl. qRT-PCR was performed on a 7500 FAST Real-Time PCR System (Applied Biosystems) with Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen, Grand Island, NY). The primers were as follows: Ifng, 5′-TCAAGTGGCATAGATGTGGAAGAA-3′ (forward) and 5′-TGGCTCTGCAGGATTTTCATG-3′ (reverse); Il1b, 5′-TGAAGCAGCTATGGCAACTG-3′ (forward) and 5′-CAGGTCAAAGGTTTGGAAGC-3′ (reverse); Il12b, 5′-GGAGACCCTGCCCATTGAACT-3′ (forward) and 5′-CAACGTTGCATCCTAGGATCG-3 (reverse); Il10, 5′-GCTGGACAACATACTGCTAACC-3′ (forward) and 5′-ATTTCCGATAAGGCTTGGCAA-3′ (reverse); Cd112d, 5′-CTCTGTGGATCGAATGGTCA-3′ (forward) and 5′-GGCAGCGATAATACCTCCAA-3′ (reverse); Cd226, 5′-TCGCTCAGAGGCCATTACAG-3′ (forward) and 5′-CCCTGGGCTCTTTAAGTGGAA-3′ (reverse); Tigit, 5′-CTGATACAGGCTGCCTTCCT-3′ (forward) and 5′-TGGGTCACTTCAGCTGTGTC-3′ (reverse); Cd96, 5′-TCCCCAATATGGCCTCTACTG-3′ (forward) and 5′-GACTGTAGTCTTGATGCCTTCTG-3 (reverse). The level of the β-actin or Cd2 transcripts were measured as internal controls. β-actin, 5′-GGCTGTATTCCCCTCCATCG-3′ (forward) and 5′-CCAGTTGGTAACAATGCCATGT-3′ (reverse); Cd2, 5′- TGGTAACTCATGTTCTTCTGG-3′ (forward) and 5′- GTAATGGTGTATGGCACAAATG-3′ (reverse). PCR conditions were as follows: an initial denaturation step at 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. Data were analyzed by the 2−ΔΔCt method. All values were determined in triplicate.
Statistics
The survival of mice was analyzed by the Kaplan–Meier survival method followed by the log-rank test. All other statistical analyses were performed using the unpaired Student’s t test. P<0.05 was considered statistically significant.
Results
MCA-induced tumor development is similar in WT and CD155-deficient mice
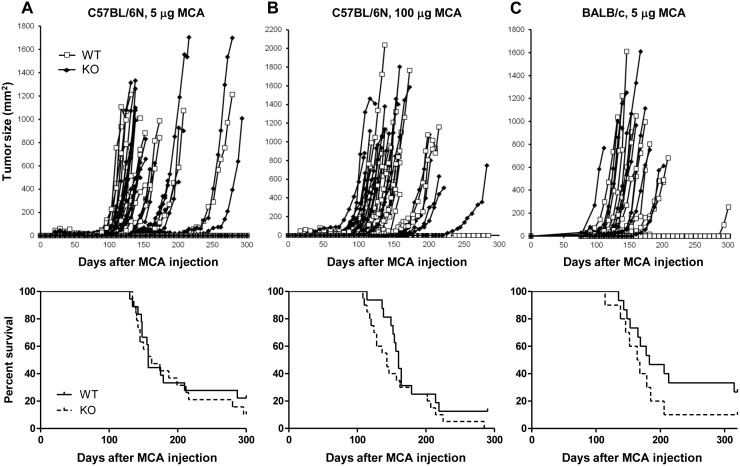
To examine the effect of CD155 on MCA-induced fibrosarcoma in tumor immune surveillance, WT and CD155-deficient mice in the C57BL/6N background were injected with MCA. Unexpectedly, both groups showed a similar course of fibrosarcoma development 80–250 days after MCA injection, and their survival rates were similar ( Fig. 1A, B ). Similar results were obtained for WT and CD155-deficient mice in the BALB/c background ( Fig. 1C ). These results suggest that, although DNAM-1 expressed on T cells and NK cells plays an important role in immune surveillance against MCA-induced fibrosarcoma, the counterpart of DNAM-1 on the fibrosarcoma may not be CD155 alone.
Figure 1. MCA-induced tumor development in WT and CD155-deficient mice.
(A, B) WT (n = 18 and 16, respectively) or CD155-deficient (KO; n = 19 and 20, respectively) C57BL/6N mice were injected s.c. with 5 µg (A) or 100 µg (B) methylcholanthrane (MCA) on day 0. (C) WT (n = 15) or KO (n = 10) BALB/c mice were injected s.c. with 5 µg MCA on day 0. Tumor size in each mouse was measured once a week. Tumor size (top) and survival data (bottom) are shown.
Expression of cytokine genes is similar in tumors from WT and CD155-deficient mice
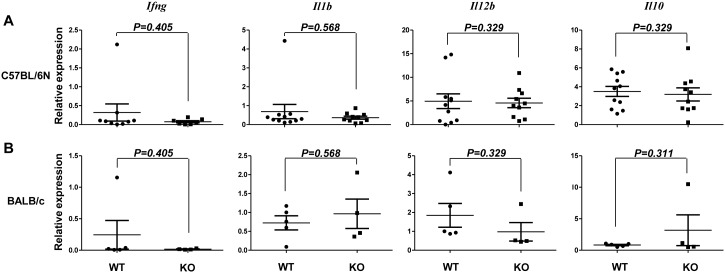
Cytokines expressed in immune cells infiltrating tumor tissues are involved in tumor immune responses. Because there was no difference in MCA-induced tumor development between WT and CD155-deficient mice, we next investigated cytokine expression in tumor tissues from the two types of mice. qRT-PCR revealed that the levels of mRNA for the proinflammatory cytokines Ifng, Il1b, and Il12b and the anti-inflammatory cytokine Il10 in fibrosarcomas induced by 5 µg MCA were similar in WT and CD155-deficient mice in both C57BL/6N and BALB/c backgrounds ( Fig. 2 ). These results suggest that cytokine expression levels in immune cells infiltrating into tumors were similar in CD155-defficient mice and WT mice.
Figure 2. Relative cytokine mRNA levels in MCA-induced tumors.
(A, B) Fibrosarcomas induced by 5 µg MCA in WT or CD155-deficient (KO) C57BL/6N (A) or BALB/c (B) mice were resected from each mouse and subjected to quantitative qRT-PCR for transcripts of the indicated cytokines. Horizontal bars represent means and error bars represent means ± SEM. P Values for Student’s t test are shown.
Cd112 expression is increased in MCA-induced fibrosarcoma in CD155-deficient mice
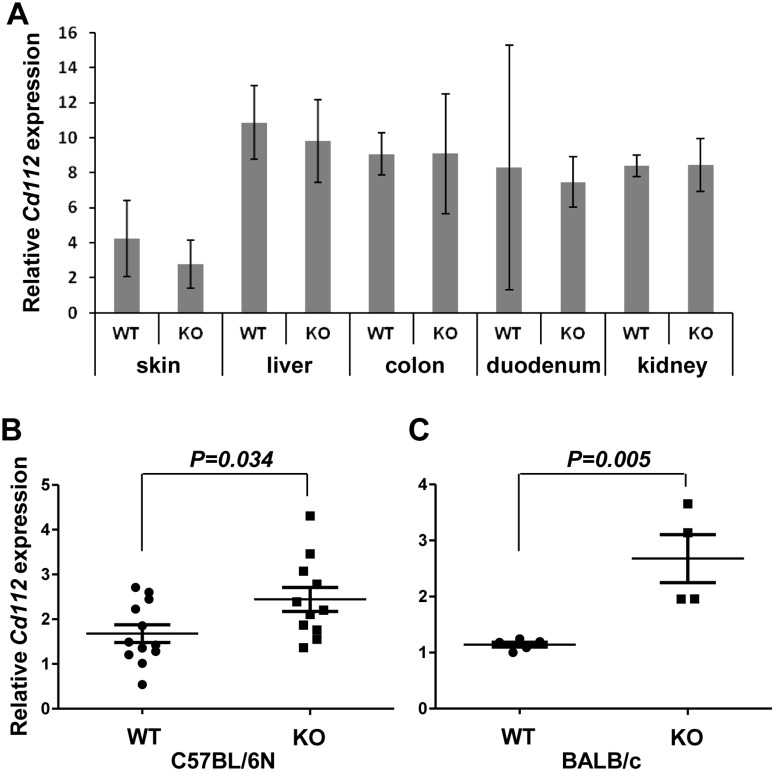
We next examined the expression of Cd112 in MCA-induced fibrosarcoma in WT and CD155-defficient mice. In naïve C57BL/6N mice, Cd112 expression was similar in several organs, including the skin, in WT and CD155-deficient mice ( Fig. 3A ). However, in MCA-induced fibrosarcoma, Cd112 expression was significantly higher in CD155-deficient mice than in WT mice ( Fig. 3B ). Similar results were obtained for CD155-deficient mice in the BALB/c background ( Fig. 3C ). These results suggest that Cd112 expression is upregulated in MCA-induced fibrosarcoma in CD155-deficient mice during the transformation.
Figure 3. Relative Cd112 mRNA levels in organs and MCA-induced tumors.
(A) Tissues from WT (n = 3) or CD155-deficient (KO; n = 3) mice were subjected to quantitative qRT-PCR for Cd112. Error bars represent means ± SD. (B, C) Fibrosarcomas induced in WT or CD155-deficient C57BL/6N (B) or BALB/c (C) mice by 5 µg MCA were resected from each mouse and subjected to qRT-PCR for Cd112. Horizontal bars represent means and error bars represent means ± SEM. P Values for Student’s t test are shown.
DNAM-1 and CD96 expressions on resting T cells are increased in CD155-deficient mice
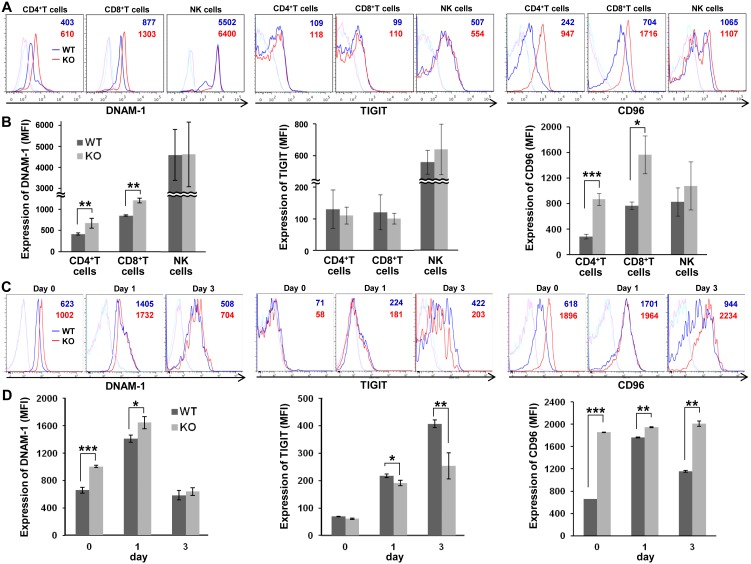
A previous report demonstrated that DNAM-1 expression on T cells was significantly higher in CD155-deficient mice than in WT mice [25]. Consistent with this, our flow cytometry analysis demonstrated significantly higher expression of DNAM-1 on peripheral blood CD4+ and CD8+ T cells ( Fig. 4A ). Fluorescence intensity analysis confirmed a significant increase in DNAM-1 expression on CD4+ and CD8+ T cells ( Fig. 4B ). At the same time, we also analyzed the expressions of TIGIT and CD96 on those cells and found that CD96 but not TIGIT expression on CD4+ and CD8+ T cells was also significantly higher in CD155-deficient mice than in WT mice. As to NK cells, however, we did not observe a difference of these molecules’ expression between in WT and in CD155-deficient mice. TIGIT was markedly expressed on NK cells compared to CD4+ and CD8+ T cells, although there was no significant difference between WT and CD155-deficient mice.
Figure 4. Expression of CD155 ligands on resting and activated T cells from WT and CD155-deficient mice.
(A) Peripheral blood lymphocytes from WT (n = 3) or CD155-deficient (KO; n = 3) C57BL/6N mice were stained with anti-DNAM-1, anti-TIGIT, or anti-CD96 antibodies (WT; blue lines, KO; red lines) or control antibodies (WT; light blue lines, KO; pink lines), and analyzed by flow cytometry. The numbers (WT; in blue, KO; in red) indicate the mean fluorescence intensity (MFI) of DNAM-1, TIGIT, and CD96 staining. Representative data are shown. (B) MFI was used to analyze DNAM-1 expression on CD4+ T, CD8+ T, and NK cells as in (A). Error bars represent means ± SD. (C) CD8+ T cells purified from spleen were activated with anti-CD3 antibody and IL-2 for the indicated number of days. Cells were stained and analyzed by flow cytometry, as described in (A). (D) The expression of DNAM-1, TIGIT, and CD96 on CD8+ T cells is shown as MFI as in (C). *, P<0.05; **, P<0.01; ***, P<0.001.
Receptor modulation by CD8+ T cell activation
As CD155 binds CD96, TIGIT, and DNAM-1, we examined the expression of these receptors on activated CD8+ T cells. CD8+ T cells from the spleens of WT and CD155-deficient mice were stimulated with anti-CD3 mAb in the presence of IL-2. DNAM-1 and CD96 expression was significantly higher on resting CD155-deficient CD8+ T cells than on resting WT CD8+ T cells, whereas TIGIT expression was not detected ( Fig. 4C, D ). Activated CD8+ T cells increased expression of TIGIT on both WT and CD155-deficient CD8+ T cells after stimulation. Upon stimulation, the levels of DNAM-1 and CD96 were still significantly higher on CD155-deficient than on WT CD8+ T cells, whereas TIGIT expression was significantly lower on CD155-deficient CD8+ T cells than on WT CD8+ T cells ( Fig. 4C, D ).
To analyze the expressions of DNAM-1, TIGIT, and CD96 on tumor-resident immune cells, fibrosarcomas induced by 5 µg MCA in WT or CD155-deficient mice were resected and subjected to quantitative RT-PCR by using Cd2 expression, which is specifically expressed on T cells and NK cells, but not on non-hematopoietic cells, for normalization. The average tumor size and time points of resection after MCA injection were comparable between two groups. However, we did not observe different expression levels of those receptors in tumors between WT and CD155-deficient mice (Fig. S1).
Discussion
CD155 and CD112 are involved in tumor immune surveillance, because ectopic expression of CD155 or CD112 on tumors can induce cell-mediated cytotoxicity [13]. Although the binding affinities of DNAM-1 to CD155 and CD112 are similar [8], CD155 rather than CD112 appears to play a predominant role in DNAM-1-dependent NK cell triggering [7]. CD112, but not CD155, mediates not only heterophilic but also homophilic binding. This ability may adversely affect DNAM-1 binding to CD112, suggesting that DNAM-1 may prefer CD155 to CD112 as a physiological ligand [8], [26].
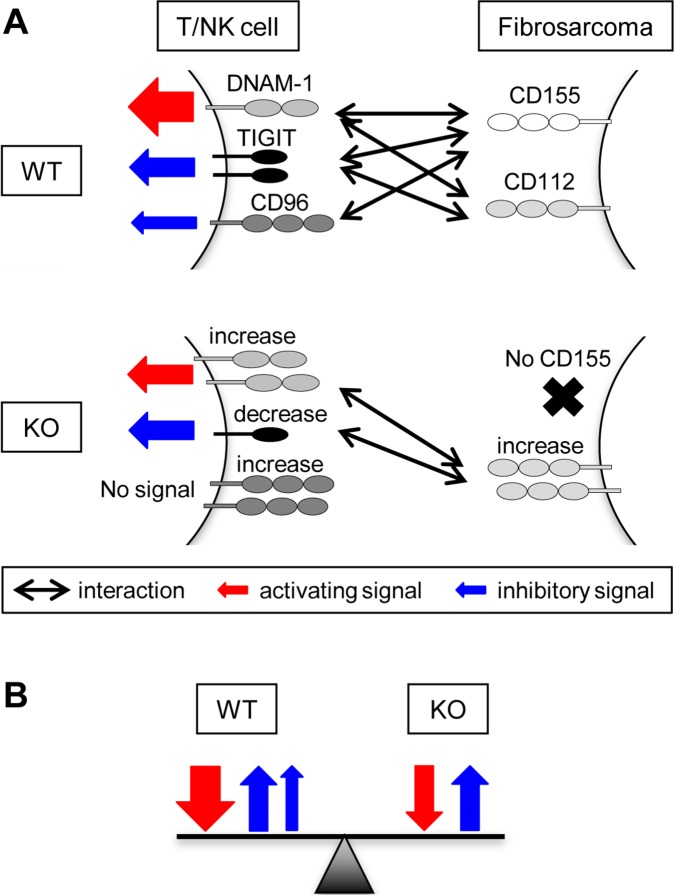
Our previous study demonstrated that the development of MCA-induced fibrosarcoma and DMBA-induced papilloma was enhanced in DNAM-1-deficient mice, suggesting that DNAM-1 on T cells, NK cells, or both plays an important role in immune surveillance against DNAM-1 ligand–expressing tumors [23]. In this study, we used CD155-deficient mice to examine whether CD155 on MCA-induced fibrosarcoma plays a reciprocal role in tumor immunity. Unexpectedly, however, we found that MCA-induced tumors developed similarly in WT and CD155-deficient mice. We suggest several possible explanations of why the absence of CD155 on tumors did not affect tumor immunity ( Fig. 5 ). First, Cd112 expression in fibrosarcomas was significantly higher in CD155-deficient mice than in WT mice, suggesting that DNAM-1 interaction with CD112 compensated for the loss of the DNAM-1–CD155 interaction. CREB and c-Jun may regulate Cd112 transcription [27] and are often activated in cancers [28], [29]. The relative amounts of Cd112 mRNA are higher in poorly differentiated gastric cancer than in normal gastric tissue [13]. Second, DNAM-1 expression was significantly upregulated on CD4+ and CD8+ T cells from CD155-deficient mice, consistent with a previous report [25]. DNAM-1 upregulation in CD8+ T cells was independent of antigen-driven activation because it was already observed in resting T cells, but it was still upregulated after stimulation. We also found that, although TIGIT expression was upregulated after stimulation with anti-CD3 mAb in both WT and CD155-deficient CD8+ T cells, it was significantly lower in CD155-deficient cells than in WT cells. TIGIT is an inhibitory receptor that binds to both CD155 and CD112 [18]. Thus, upregulated expression of the activating receptor DNAM-1 and downregulated expression of the inhibitory receptor TIGIT should be favorable for cytotoxicity mediated by T cells, NK-cells, or both against tumors with upregulated CD112, even if the affinity of CD112 for binding DNAM-1 or TIGIT is lower than that of CD155. CD96 expression was also significantly higher on CD155-deficient CD8+ T cells than WT cells. Recent evidence indicates that CD96, which binds CD155 but not CD112, negatively regulates tumor immunity and cytokine secretion by NK cells [21]. Thus, the absence of CD155 on tumors would downregulate CD96-mediated NK cell inhibition, resulting in an increase in cytotoxicity against CD155-deficient tumors. It remains unclear how the expression of DNAM-1, TIGIT, CD96, and CD112 is modulated in CD155-deficient mice. A better understanding of the mechanisms that regulate the levels of these molecules may provide new insights into possible ways to improve cancer immunotherapy.
Figure 5. A hypothetical model for interactions between activating/inhibitory receptors DNAM-1, TIGIT and CD96 on T or NK cells and CD155/CD112 ligands expressed on MCA-induced fibrosarcoma in WT or CD155-deficient mice.
(A) DNAM-1 and TIGIT bind to both of CD155 and CD112, while CD96 interacts with CD155 only. Each receptor-ligand interaction transduces either activating or inhibitory signal, as shown by the red or blue arrow, respectively. The modulation of the receptors and ligand expression on CD155-deficient (KO) fibrosarcoma are indicated. (B) The sums of the activating and inhibitory signals are similar between WT and KO.
In contrast to the peripheral blood lymphocytes, we did not observe any different expression levels of DNAM-1, TIGIT, and CD96 in tumors after normalization by Cd2 expression between WT and CD155-deficient mice. It is unclear at present how these results can be explained. However, it is possible that, during tumor development for more than 100 days, immune cell activation might be modified. For example, myeloid-derived suppressor cells (MDSC) in tumors tissues suppress both innate and adaptive immunities by secretion of various cytokines [30], which might affect the expression of the cell surface molecules.
We have previously shown that abrogating DNAM-1 activity on CD8+ T cells results in development of milder graft-versus-host disease (GVHD) [31], [32]. On the other hand, Seth et al. reported that the absence of CD155 aggravated acute GVHD, which is mainly caused by CD4+ T cells [33]. Although there are differences in the experimental settings between the two GVHD models, these observations and the results of our current study indicate that phenotypes of CD155 and DNAM-1 deficiencies are not two sides of the same coin.
Supporting Information
Relative DNAM-1, TIGIT, and CD96 mRNA levels in MCA-induced tumors. Fibrosarcomas induced by 5 µg MCA in WT or CD155-deficient (KO) C57BL/6N mice were resected and subjected to quantitative RT-PCR for the expression of transcripts of the indicated receptors as described in Materials and Methods. (A) Tumor size, days of resection after MCA injection, and relative expressions of the transcripts are shown. (B) Relative expressions of indicated receptors are shown. Horizontal bars represent means and error bars represent means ± SEM. P Values for Student’s t test are shown.
(PPT)
The ARRIVE Guidelines Checklist. We followed the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines and the ARRIVE Checklist is available as supporting information.
(PDF)
Acknowledgments
We thank S. Mitsuishi and Y. Nomura for secretarial assistance.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This research was supported by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (Grant Number 24249021 and 25114701 to AS and KS, respectively). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Dunn GP, Old LJ, Schreiber RD (2004) The three Es of cancer immunoediting. Annu Rev Immunol 22: 329–360. [DOI] [PubMed] [Google Scholar]
- 2. Swann JB, Smyth MJ (2007) Immune surveillance of tumors. J Clin Invest 117: 1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lanier LL (2005) NK cell recognition. Annu Rev Immunol 23: 225–274. [DOI] [PubMed] [Google Scholar]
- 4. Dunn GP, Koebel CM, Schreiber RD (2006) Interferons, immunity and cancer immunoediting. Nat Rev Immunol 6: 836–848. [DOI] [PubMed] [Google Scholar]
- 5. Springer TA (1990) Adhesion receptors of the immune system. Nature 346: 425–434. [DOI] [PubMed] [Google Scholar]
- 6. Shibuya A, Campbell D, Hannum C, Yssel H, Franz-Bacon K, et al. (1996) DNAM-1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes. Immunity 4: 573–581. [DOI] [PubMed] [Google Scholar]
- 7. Bottino C, Castriconi R, Pende D, Rivera P, Nanni M, et al. (2003) Identification of PVR (CD155) and nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med 198: 557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tahara-Hanaoka S, Shibuya K, Onoda Y, Zhang H, Yamazaki S, et al. (2004) Functional characterization of DNAM-1 (CD226) interaction with its ligands PVR (CD155) and nectin-2 (PRR-2/CD112). Int Immunol 16: 533–538. [DOI] [PubMed] [Google Scholar]
- 9. Tahara-Hanaoka S, Miyamoto A, Hara A, Honda S, Shibuya K, et al. (2005) Identification and characterization of murine DNAM-1 (CD226) and its poliovirus receptor family ligands. Biochem Biophys Res Commun 329: 996–1000. [DOI] [PubMed] [Google Scholar]
- 10. Lopez M, Aoubala M, Jordier F, Isnardon D, Gomez S, et al. (1998) The human poliovirus receptor related 2 protein is a new hematopoietic/endothelial homophilic adhesion molecule. Blood 92: 4602–4611. [PubMed] [Google Scholar]
- 11. Iwasaki A, Welker R, Mueller S, Linehan M, Nomoto A, et al. (2002) Immunofluorescence analysis of poliovirus receptor expression in Peyer’s patches of humans, primates, and CD155 transgenic mice: Implications for poliovirus infection. J Infect Dis 186: 585–592. [DOI] [PubMed] [Google Scholar]
- 12. Masson D, Jarry A, Baury B, Blanchardie P, Laboisse C, et al. (2001) Overexpression of the CD155 gene in human colorectal carcinoma. Gut 49: 236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tahara-Hanaoka S, Shibuya K, Kai H, Miyamoto A, Morikawa Y, et al. (2006) Tumor rejection by the poliovirus receptor family ligands of the DNAM-1 (CD226) receptor. Blood 107: 1491–1496. [DOI] [PubMed] [Google Scholar]
- 14. Carlsten M, Bjorkstrom NK, Norell H, Bryceson Y, van Hall T, et al. (2007) DNAX accessory molecule-1 mediated recognition of freshly isolated ovarian carcinoma by resting natural killer cells. Cancer Res 67: 1317–1325. [DOI] [PubMed] [Google Scholar]
- 15. Castriconi R, Dondero A, Corrias MV, Lanino E, Pende D, et al. (2004) Natural killer cell-mediated killing of freshly isolated neuroblastoma cells: Critical role of DNAX accessory molecule-1–poliovirus receptor interaction. Cancer Res 64: 9180–9184. [DOI] [PubMed] [Google Scholar]
- 16. Pende D, Spaggiari GM, Marcenaro S, Martini S, Rivera P, et al. (2005) Analysis of the receptor-ligand interactions in the natural killer-mediated lysis of freshly isolated myeloid or lymphoblastic leukemias: Evidence for the involvement of the poliovirus receptor (CD155) and nectin-2 (CD112). Blood 105: 2066–2073. [DOI] [PubMed] [Google Scholar]
- 17. El-Sherbiny YM, Meade JL, Holmes TD, McGonagle D, Mackie SL, et al. (2007) The requirement for DNAM-1, NKG2D, and NKp46 in the natural killer cell-mediated killing of myeloma cells. Cancer Res 67: 8444–8449. [DOI] [PubMed] [Google Scholar]
- 18. Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, et al. (2009) The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol 10: 48–57. [DOI] [PubMed] [Google Scholar]
- 19. Stanietsky N, Simic H, Arapovic J, Toporik A, Levy O, et al. (2009) The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Natl Acad Sci U.S.A. 106: 17858–17863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang PL, O’Farrell S, Clayberger C, Krensky AM (1992) Identification and molecular cloning of tactile. A novel human T cell activation antigen that is a member of the Ig gene superfamily. J Immunol 148: 2600–2608. [PubMed] [Google Scholar]
- 21. Chan CJ, Martinet L, Gilfillan S, Souza-Fonseca-Guimaraes F, Chow MT, et al. (2014) The receptors CD96 and CD226 oppose each other in the regulation of natural killer cell functions. Nat Immunol 15: 431–438. [DOI] [PubMed] [Google Scholar]
- 22. Fuchs A, Cella M, Giurisato E, Shaw AS, Colonna M (2004) Cutting edge: CD96 (tactile) promotes NK cell-target cell adhesion by interacting with the poliovirus receptor (CD155). J Immunol 172: 3994–3998. [DOI] [PubMed] [Google Scholar]
- 23. Iguchi-Manaka A, Kai H, Yamashita Y, Shibata K, Tahara-Hanaoka S, et al. (2008) Accelerated tumor growth in mice deficient in DNAM-1 receptor. J Exp Med 205: 2959–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maier MK, Seth S, Czeloth N, Qiu Q, Ravens I, et al. (2007) The adhesion receptor CD155 determines the magnitude of humoral immune responses against orally ingested antigens. Eur J Immunol 37: 2214–2225. [DOI] [PubMed] [Google Scholar]
- 25. Seth S, Qiu Q, Danisch S, Maier MK, Braun A, et al. (2011) Intranodal interaction with dendritic cells dynamically regulates surface expression of the co-stimulatory receptor CD226 protein on murine T cells. J Biol Chem 286: 39153–39163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu J, Qian X, Chen Z, Xu X, Gao F, et al. (2012) Crystal structure of cell adhesion molecule nectin-2/CD112 and its binding to immune receptor DNAM-1/CD226. J Immunol 188: 5511–5520. [DOI] [PubMed] [Google Scholar]
- 27. Lui WY, Sze KL, Lee WM (2006) Nectin-2 expression in testicular cells is controlled via the functional cooperation between transcription factors of the Sp1, CREB, and AP-1 families. J Cell Physiol 207: 144–157. [DOI] [PubMed] [Google Scholar]
- 28. Sakamoto KM, Frank DA (2009) CREB in the pathophysiology of cancer: Implications for targeting transcription factors for cancer therapy. Clin Cancer Res 15: 2583–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Angel P, Hattori K, Smeal T, Karin M (1988) The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell 55: 875–885. [DOI] [PubMed] [Google Scholar]
- 30. Ostrand-Rosenberg S, Sinha P (2009) Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol 182: 4499–4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nabekura T, Shibuya K, Shibuya A (2011) Reply to Seth, et al.: DNAX accessory molecule-1 (DNAM-1) plays an important role in alloreactive CD8+ T cells responsible for the exacerbation of acute graft-versus-host disease. Proc Natl Acad Sci U.S.A 108: E34. [Google Scholar]
- 32. Nabekura T, Shibuya K, Takenaka E, Kai H, Shibata K, et al. (2010) Critical role of DNAX accessory molecule-1 (DNAM-1) in the development of acute graft-versus-host disease in mice. Proc Natl Acad Sci U.S.A. 107: 18593–18598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Seth S, Ravens I, Lee CW, Glage S, Bleich A, et al. (2011) Absence of CD155 aggravates acute graft-versus-host disease. Proc Natl Acad Sci U.S.A. 108: E32–E33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Relative DNAM-1, TIGIT, and CD96 mRNA levels in MCA-induced tumors. Fibrosarcomas induced by 5 µg MCA in WT or CD155-deficient (KO) C57BL/6N mice were resected and subjected to quantitative RT-PCR for the expression of transcripts of the indicated receptors as described in Materials and Methods. (A) Tumor size, days of resection after MCA injection, and relative expressions of the transcripts are shown. (B) Relative expressions of indicated receptors are shown. Horizontal bars represent means and error bars represent means ± SEM. P Values for Student’s t test are shown.
(PPT)
The ARRIVE Guidelines Checklist. We followed the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines and the ARRIVE Checklist is available as supporting information.
(PDF)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.