Abstract
B memory cells remain in circulation and secrete alloantibodies without antigen exposure > 20 years after alloimmunization postpartum or by transplantation. These long-lived B cells are resistant to cytostatic drugs. Therapeutically, intravenous immunoglobulin (IVIg) is administered to reduce allo-human leucocyte antigen (HLA) antibodies pre- and post-transplantation, but the mechanism of reduction remains unclear. Recently, we reported that IVIg reacts with several HLA-I alleles and the HLA reactivity of IVIg is lost after its HLA-E reactivity is adsorbed out. Therefore, we have generated an anti-HLA-E monoclonal antibody that mimics the HLA-reactivity of IVIg to investigate whether this antibody suppresses IgG secretion, as does IVIg. B cells were purified from the blood of a woman in whose blood the B memory cells remained without antigen exposure > 20 years after postpartum alloimmunization. The B cells were stimulated with cytokines using a well-defined culture system. The anti-HLA-E monoclonal antibody (mAb) significantly suppressed the allo-HLA class-II IgG produced by the B cells, and that this suppression was far superior to that by IVIg. These findings were confirmed with HLA-I antibody secreted by the immortalized B cell line, developed from the blood of another alloimmunized woman. The binding affinity of the anti-HLA-E mAb for peptide sequences shared (i.e. shared epitopes) between HLA-E and other β2-microglobulin-free HLA heavy chains (open conformers) on the cell surface of B cells may act as a ligand and signal suppression of IgG production of activated B memory cells. We propose that anti-HLA-E monoclonal antibody may also be useful to suppress allo-HLA IgG production in vivo.
Keywords: allo-HLA IgG, B memory cells, HLA-reactivity, immunosuppression, intravenous immunoglobulin (IVIg)
Introduction
Classical B memory (Bmem) cells, such as those generated after infection, are antigen-experienced, disappearing from circulation in the absence of the immunizing agent and reappearing upon antigen re-exposure [1]. In contrast, the Bmem/plasma cells that produce immunoglobulin (Ig)G antibodies against alloantigens secrete antibodies continuously without antigen exposure for > 20 years [2,3]. These long-lived Bmem cells are resistant to cytostatic drugs while classical Bmem cells are not [4,5]. Furthermore, the antigen specificities of the antibodies produced by the Bmem cells remain unaltered for many years [4]. The existence of long-lived Bmem cells has become an established concept, but until now had not been established in connection with human leucocyte antigens (HLA) – a matter of particular importance with the HLA-alloimmunization that occurs during pregnancy. Similar HLA-directed alloimmunization may occur during organ transplantation, generating long-lived Bmem cells. The primary HLA alloantibodies secreted by these cells after organ transplantation are de-novo donor-specific antibodies (DSA), and are detrimental to the survival of allografts in transplant recipients [6,7]. In addition to these primary alloantibodies, the blood of an allograft recipient may contain other detrimental secondary alloantibodies (non-donor-specific alloantibodies) [6,7].
The characteristics of long-lived Bmem cells geared to allo-HLA exposure and the factors controlling their antibody secretion remain to be elucidated in a well-defined culture system without other cells (such as feeder cells and T cells) or their undefined cellular products. The long-lived Bmem or plasma cell population from the blood of a postpartum-alloimmunized woman would provide an ideal in-vitro model to evaluate and define the effects of therapeutic agents aimed at suppressing allo-HLA antibody secretion.
The need to suppress HLA antibody formation in pretransplant patients has increased as the population of such sensitized patients has increased [8,9]. Inhibition of alloantibody secretion in allograft recipients has also become a more urgent matter, as chronic rejection has been shown to be caused by post-transplant HLA alloantibodies [10]. The use of intravenous immunoglobulin (IVIg) for these patients was approved by the US Food and Drug Administration (FDA) to reduce anti-HLA antibody levels prior to transplantation and to reverse humoral rejection [11–15]. Therapeutic preparations of IVIg contain IgG purified from plasma pooled from > 10 000 donors, and were first used against infections, either as prophylactic therapy or following exposure to pathogens. Later, IVIg became a substitution therapy for patients with immunodeficiencies [16] and has been used increasingly as treatment for autoimmune and systemic inflammatory diseases [17], as well as in organ and bone marrow transplantation [18–20]. The mechanism of antibody suppression by IVIg is far from clear and a problem with the use of IVIg is the inability to standardize IVIg preparations, as they come from different sources, the sera obtained from thousands of individuals in different regions of the world, treated with a variety of stabilizing agents and albumin levels. In addition, there are no in-vitro measurements to determine those preparations' potency or even to distinguish from one lot to another.
We have shown previously that different preparations of IVIg react with an array of HLA alleles, both class Ia (HLA-A/-B/-Cw) and class Ib (HLA-E/-F/-G); that when anti-HLA-E antibodies are depleted specifically from IVIg, its HLA-Ia reactivity is abolished, suggesting that IVIg's HLA-Ia reactivity is due primarily to the presence of anti-HLA-E antibodies; and that there are anti-HLA-E monoclonal antibodies (mAbs) that simulate the HLA-reactivity of IVIg [21]. These observations led us to hypothesize that anti-HLA-E mAbs that simulate the HLA-reactivity of IVIg may also mimic the suppressive activities of IVIg. This hypothesis was tested by comparing the efficacy of IVIg versus that of the anti-HLA-E mAb Terasaki Foundation Laboratory (TFL)-007 (formerly called ‘PTER-007’ [21]), in suppressing the secretion of allo-HLA antibodies by activated long-lived Bmem cells. These cells came from the peripheral blood of a woman alloimmunized postpartum 23 years ago, and were grown in a well-defined culture system. A separate, confirmatory comparison used a hybridoma cell line (HML16) from a different postpartum-alloimmunized woman. In all, our study provides a better understanding of the nature of the exogenous agents that can suppress the antibody secretion of activated long-lived Bmem cells.
Material and methods
Intravenous immunoglobulin (IVIg) and mAb TFL-007
IVIg-GamaSTAN™ (Talecris Biotherapeutics, Inc., Research Triangle Park, NC, USA) was used in the experiments on human B cell cultures and human hybridoma cell lines; IVIgGamunex®-C (Talecris) was used only with the human hybridoma cells. The GamaSTAN was lot 26NJ651, formulated as a 15–18% protein solution at a pH of 6·4–7·2 in 0·21–0·32 M glycine; the Gamunx was lot 26NKLG1, a solution at a pH of 6·4–7·2 in 0·16–0·24 M glycine, albumin < 20 μg/ml. There were two preparations of TFL-007 (an anti-HLA-E mAb): TFL-007s (culture supernatant) and TFL-007a (ascites).
The mouse hybridoma clones producing TFL-007 were generated and cultured as described elsewhere [21]. They were injected into the peritoneal cavity of BALB/c mice; the TFL-007a ascites obtained after 2 weeks were used for immortalized human B cells. The IgG isotype of the mAb TFL-007 (IgG2a) and the absence of IgM in the culture supernatant and ascites were confirmed.
The IgG in the culture supernatant (400 μl) and ascites (400 μl) were purified by passing each aliquot of supernatant or ascites through Protein G columns (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The ascites, purified with in-house buffers free of NaN2, were tested for anti-HLA reactivity. For TFL-007s, we used a Millipore concentrator (100 K membrane) for condensation (500 μl to approximately 20 μl). The purified TFL-007s culture supernatants were tested for HLA-reactivity. The protein concentrations of the mAbs were measured with a BioPhotometer (Eppendorf, Hauppauge, NY, USA). The therapeutic preparations of IVIg and the two preparations of mAb TFL-007 were diluted in the respective media used for cell culture.
Human peripheral B cells isolation and culture
For this investigation, B lymphocytes were isolated from the fresh peripheral blood of a woman known to have anti-HLA-DRB IgG alloantibodies, after obtaining her informed consent and Institutional Review Committee approval. HLA typing of husband, wife, first child (age 23 years) and second child (age 18 years) were performed at TFL. Ficoll-Paque Plus (GE Healthcare, Pittsburgh, PA, USA) was used to isolate peripheral blood mononuclear cells (PBMC). The B cells (resting) were isolated from the PBMC by positive selection using CD19 Pan B Dynabeads® magnetic beads (Invitrogen, Life Technologies Corporation, Carlsbad, CA, USA) and B cells were detached by DETACHaBEAD® CD19 (Invitrogen). Purified human B cells were > 95% CD19+, determined by flow cytometry analysis.
Purified B cells were plated at 0·2 × 106/200 μl/well in a sterile 96-well, round-bottomed plate (Thermo Fisher Scientific, Inc.). B cells were cultured in Iscove's modified Dulbecco's medium, containing HEPES, L-glutamine and sodium pyruvate (Gibco-Invitrogen) supplemented with 10% AB-human serum (Gibco), 5 μg/ml recombinant human (rh) insulin (Sigma-Aldrich, St Louis, MO, USA), 50 μg/ml rh-transferring (Sigma), 25 μg/ml gentamycin (Sigma) and 50 μM 2-mercaptoethanol (2-ME) (Sigma). The resting B cells were activated with 25 ng/ml rh interleukin (rhIL)-2 (R&D Systems, Minneapolis, MN, USA) [43–46], 100 ng/ml rhIL-4 (R&D Systems) [43,46,47], 100 ng/ml rhIL-6 (R&D Systems) [45,46], 50 ng/ml rhIL-10 (R&D Systems) [43,45–47], 50 ng/ml rhIL-2) (PeproTech, Rocky Hill, NJ, USA) [44,47] and 1 μg/ml human CD40 antibody (R&D Systems) [44]. On day 7 of the culture, 10 μl of culture supernatant from each well were analysed for the presence of anti-HLA class II IgG alloantibodies. Cells from the wells that contained the HLA antibodies were further harvested, washed three times, seeded into four wells and activated as above. On days 8 and 9, the culture supernatants were tested for the secretion of allo-HLA antibodies.
After ascertaining the consistency of the mean fluorescent intensity (MFI) of HLA antibodies in each well on days 8 and 9, the cells were pooled, washed three times and aliquoted into three different wells: with medium alone; with GamaSTAN IVIg at 1:100 dilution, 1·5 mg/ml; and with mAb TFL-007s at 1:100 dilution containing 5 μg/ml. The cells were maintained in culture without any cytokine activators or anti-CD40 antibody for an additional 3 days, and 10 μl of culture supernatants from each well were analysed for HLA-alloantibodies at 0, 12, 24, 48 and 72 h.
Human hybridoma cell line secreting anti-HLA class I antibody
The human hybridoma cell line HML16 was generated from the resting B cells of a multiparous woman. The primary B cell clone was generated by Epstein–Barr virus (EBV) transformation, then the clone was fused with the murine, non-producing myeloma cell line P3X63-Ag8·653 (American Type Culture Collection, ATCC® CRL 1580™; ATCC, Rockville, MD, USA). The hybridoma cell line produced anti-HLA class I alloantibodies with differing MFIs: high against B*0702, B*8101, B*6701 and B*4201; and low against B*2708, B*2705, B*5501, B*5601 and B*8201. The cell line was cultured in RPMI-1640, supplemented with 20% heat-inactivated fetal bovine serum (JR Scientific, Inc., Woodland, CA, USA), 1 mM sodium pyruvate (Sigma), L-Glutamine-Pen-Strep Solution (Gemini BioProducts, West Sacramento, VA, USA) and 50 μM 2-ME. The cells were seeded at 1000/100 μl/well in a Falcon 96-well, flat-bottomed plate, and divided into three different treatment groups: medium control, mouse IgG control (MP Biomedicals, Santa Ana, CA, USA) and TFL-007a. In a separate experiment, medium control was compared with treatment by IVIg preparations from GamaSTAN and Gamunex-C. Two subgroups (100 and 50 μg/ml) for mouse IgG control, three subgroups for each of the IVIg preparations or four subgroups for TFL-007a were established for different doses except medium control. Six or more repetitions were performed with each subgroup. The three subgroups of GamaSTAN-IVIg were at dilutions 1:10 (15 mg/ml), 1:20 (7·5 mg/ml) and 1:40 (3·75 mg/ml), the subgroups of Gamunex-C were at 1:10 (10 mg/ml), 1:20 (5 mg/ml) and 1:40 (2·5 mg/ml), and those of mAb TFL-007a were at 1:10 (100 μg/ml), 1:20 (50 μg/ml), 1:40 (25 mg/ml) and 1:80 (12·5 μg/ml). Twenty μl of culture supernatant from each well were analysed for allo-HLA antibodies at 0 and 72 h.
Flow cytometry for human B cell differentiation
To ascertain the purity of the isolated resting B cells, expression of CD19 was monitored using the fluorescein isothiocyanate (FITC)-labelled anti-human CD19 (HIB19; BioLegend, San Diego, CA, USA) [48] in a fluorescence activated cell sorter (FACS)Calibur (BD Biosciences, San Jose, CA, USA) using CellQuest™ software (BD Biosciences). On days 0 and 7, both resting and activated B cells were stained with phycoerythrin (PE) anti-human CD20 (2H7, BioLegend) [49], peridinin chlorophyll (PerCP) anti-human CD27 (0323; BioLegend) [50] and FITC anti-human CD38 (HIT2; BioLegend) [51] to examine the differentiation of activated B cells. Prior to staining with antibodies, Human TruStain FcX™ (BioLegend) was used to block FcR-involved unwanted staining.
Anti-HLA IgG antibody detection
The Multiplex Luminex-based immunoassay was used [32–34] to measure the MFI of anti-HLA alloantibodies in the serum, culture supernatants, IVIg and anti-HLA-E mAbs (the latter two at different dilutions). The assays were carried out for data acquisition and analysis using either a HLA-II single-beads combo or single–single beads (B*0702 and B*8101) (One Lambda, Inc., Canoga Park, CA, USA). The array of HLA alleles on the beads is listed in the product information at http://www.onelambda.com. The HLA-II microbeads have built-in control beads: positive control beads are coated with human IgG and negative control beads are coated with serum albumin. Separate positive and negative control beads were also added to single–single bead assays. Mean and standard deviation of MFI for each allele were recorded as .csv files.
HLA molecular typing
DNA from the blood cells of the family of a woman alloimmunized postpartum was isolated by using the QIAamp DNA Mini Kit (Qiagen, Inc., Valencia, CA, USA). Extracted DNA was then polymerase chain reaction (PCR)-amplified and typed using the LABType® SSO Typing Test Kit (One Lambda). HLA fusion 2·0 (One Lambda) was used to analyse the typing profile.
Statistics
This study used two different statistical analyses. The first set of experiments evaluated the comparative effectiveness of IVIg and anti-HLA-E antibody (TFL-007s) in suppressing the antibody production of isolated and cultured Bmem cells. Both IVIg and TFL-007s were used at a single concentration. The antibody production was observed and recorded at 0, 12, 24, 48 and 72 h for the wells containing the Bmem cells and either IVIg or TFL-007s, and for control wells whose cells were treated with medium only. The paired-sample two-tailed t-test was used to compare the results obtained at the stated hours with IVIg and TFL-007s against those for the control wells. The second set of experiments involved a hybridoma cell line, again with treatment by IVIG or TFL-007a, but with medium-only or mouse isotype control cells. In these experiments, which were conducted in culture wells each for cells treated with IVIg with TFL-007a, and for the control cells, the IVIg and TFL-007a were used at different concentrations. At each concentration, the equal-variance two-tailed t-test was used to compare the differences between the treatment and control groups.
Results
Primary alloantibodies persist in serum 23 years after alloimmunization
HLA typing of the family showed that the first child shared the father's unique alleles, DRB1*0101 and DQA1*0101/DQB1*0501 (Table 1a), which may have been responsible for eliciting alloantibodies in the woman against both DRB and DQ. (Table 1b) These alloantibodies (anti-DRB1*0101 IgG and anti-DQA1*0101/DQB1*0501 IgG) with high MFI persisted in her blood 23 years after alloimmunization, indicating the presence of Bmem cells. Because these alloantibodies in the woman showed affinity for her husband's HLA class-II alleles (primary alleles), they are designated ‘primary alloantibodies’. Periodic screening indicated that the high MFI of the primary allo-HLA antibodies continued during the last 2 years. In addition, the sera contained several other allo-HLA antibodies (secondary alloantibodies) reacting to DRB1*0102, DRB1*0404, DRB1*0405, DRB1*1402 and DRB1*0401 (the secondary alleles), these secondary alloantibodies due possibly to the cross-reactivity of the primary alloantibodies.
Table 1.
Human leucocyte antigen (HLA) types (a) and HLA immunoglobulin (Ig)G alloantibodies (b) in the serum of a woman alloimmunized postpartum 23 years ago. (a) HLA genotype of an alloimmunized woman and her family. (b) HLA class II IgG alloantibodies in the serum of the alloimmunized woman. The HLA class II allele found in the husband and first child was DRB1*0101, against which IgG antibody was found both in the serum and culture supernatant. The serum antibody levels are expressed as mean fluorescent intensity (MFI) after correcting against negative control (MFI: < 80). The antibodies found in wife's serum against the specific alleles (DRB1*0101 and DQA1*0101/DQB1*0501) of the husband (shown in bold type) are referred to as primary alloantibodies. The primary alloantibodies may cross-react with closely related alleles, resulting in secondary alloantibodies. Asterisks indicate the antibodies found in the activated B cell culture. Only one primary antibody is found (shown as ***) and five different secondary antibodies with varying MFI (* or **) are present
| (a) Alleles shared by husband, wife and children with their ages | |||||
|---|---|---|---|---|---|
| HLA classes | Haplotype | Husband (53) and 1st child (23) | Husband (53) and 2nd child (18) | Wife (54) and 1st child | Wife (54) and 2nd child |
| I | A | *1101 | *3002 | *2402 | *3301 |
| B | *3501 | *2703 | *4801 | *5801 | |
| Cw | *0410 | *0327 | *0302 | *0803 | |
| II | DRB1 | 1*0101 | 1*1501 | 1*1302 | 1*1501 |
| DRB5 | 5*0101 | 5*0101 | 5*0101 | 3*0301 | |
| DQA1 | 1*0101 | 1*0102 | 1*0505 | 1*0102 | |
| DQB1 | 1*0501 | 1*0602 | 1*0609 | 1*0301 | |
| DPA1 | 1*0202 | 1*0103 | 1*0103 | 1*0202 | |
| DPB1 | 1*0402 | 1*0501 | 1*0501 | 1*0301 | |
| (b) Anti-HLA-IgG antibodies (expressed in MFI) found in the serum of a woman 23 years after postpartum alloimmunization | ||||||
|---|---|---|---|---|---|---|
| Alleles present in the husband and 1st child | Alleles recognized by the IgG antibodies | Serum dilutions | IgG detected in vitro | |||
| (1:10) | (1:20) | (1:40) | (1:80) | |||
| (+) | DRB1*0101 | 4 912 | 2785 | 1415 | 709 | *** |
| DRB1*0102 | 4 320 | 2231 | 1179 | 612 | ** | |
| DRB1*1001 | 2 276 | 1161 | 530 | < 500 | ||
| DRB1*0404 | 1 860 | 971 | 513 | < 500 | * | |
| DRB1*0103 | 1 290 | 615 | < 500 | < 500 | ||
| DRB4*0101 | 1 179 | 747 | 515 | < 500 | ||
| DRB1*0401 | 971 | 519 | < 500 | < 500 | * | |
| DRB1*0405 | 970 | 500 | < 500 | < 500 | * | |
| DRB1*1402 | 933 | 500 | < 500 | < 500 | * | |
| DRB1*1401 | 859 | < 500 | < 500 | < 500 | ||
| DRB1*0403 | 608 | < 500 | < 500 | < 500 | ||
| (+) | DQA1*0101\DQB1*0501 | 12 153 | 7814 | 4507 | 2483 | |
| DQA1*0102\DQB1*0502 | 7 106 | 4335 | 2443 | 1313 | ||
| DQA1*0501\DQB1*0201 | 5 677 | 4145 | 2395 | 1265 | ||
| DQA1*0401\DQB1*0201 | 538 | < 500 | < 500 | < 500 | ||
Activation of Bmem cells
To evaluate the effects of IVIg and mAb TFL-007s on suppression of the secretion of allo-HLA antibodies, the B cell subpopulation in the woman's blood was examined (Fig. 1). The B cell population (CD19+), isolated from PBMC using positive selection on day 0, consisted of a major fraction (R1) including naive B cells (CD20+/CD27−/CD38+/−) (74·47%), Bmem cells (CD20+/CD27+/CD38−) (8·47%) and plasma cells (CD20−/CD27++/CD38++) (0·26%) (Fig. 1). These cells, upon activation by a selected battery of cytokines IL-2/IL-4/IL-6/IL-10/IL-21 (at a 1:4:4:2:2 ratio) and 1 μg/ml human CD40 antibody for 7 days resulted in an increase in plasma cells from 0·26% on day 0 to 36·25% on day 7. All wells of B cells were monitored for secretion of alloantibodies. No primary alloantibodies directed against DQA1*0101/DQB1*0501 were observed in any wells, but several wells contained high-MFI alloantibodies against DRB1*0101 (Table 1b). Therefore, all observations were restricted to antibodies reacting to DRB alleles. The positive wells secreting the primary allo-DRB IgG were isolated and recultured in four wells on day 7. The consistency of MFI of the antibodies among these four wells was confirmed on day 8 and 9 (data not shown). On day 9, the cells were pooled and aliquoted into three wells and maintained without the cytokine combo or anti-CD40 mAb. These wells were exposed to medium or IVIg (1/100, 1·5 mg/ml) or mAb TFL-007s (1/100, 5 μg/ml) for 72 h. To study the effect of IVIg on secretion of allo-HLA IgG antibodies, we used IVIg at a protein concentration 300-fold higher than that of purified culture supernatant of TFL-007s (5 μg/ml) used in treatment of B cells in culture. The supernatants recovered from the respective wells were screened for the HLA-alloantibodies.
Fig. 1.
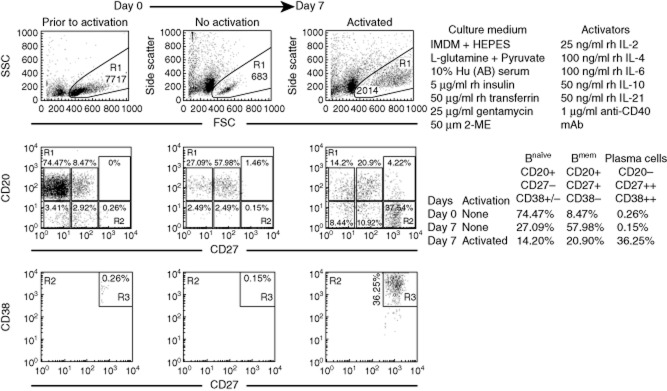
Flow cytometric profiles of B lymphocytes of the alloimmunized woman on days 0 and 7 after in-vitro culture. R1 illustrates gating strategy and the side- and forward-scatter profiles. R1 is examined for the expression of CD20 and CD27, which includes CD20+ and CD20− populations; CD20+ population comprises CD27− (Bnaive cells) and CD27+ (Bmem cells) subpopulations. R2 and R3 illustrate the CD20−/CD27++/CD38++ (plasma cells). Note, in the upper chart, the increase in the number of plasma cells on day 7 after activation with the activators used. B cell populations are defined in the lower chart. Flow cytometric analyses of B cells were performed every time blood was drawn from the donor: in this case, three times.
Profiles of anti-HLA-DRB alloantibodies in serum and of those secreted by activated B cells
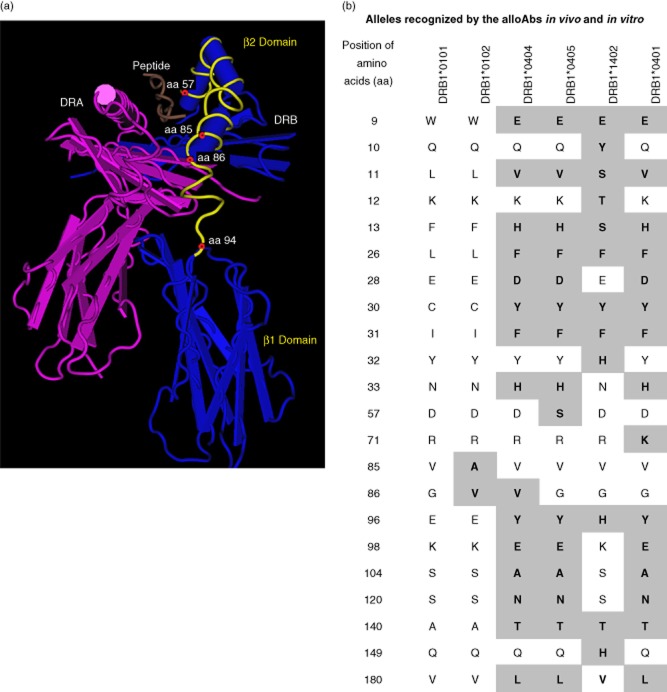
Both serum and culture supernatants showed the presence of several alloantibodies in addition to the primary alloantibody, anti-DRB1*0101 IgG (Table 1b). The secondary alloantibodies were not directed against the husband's alleles. The reason they may represent cross-reactive alloantibodies is that they occurred in cultures containing the primary alloantibody, anti-DRB1*0101. These alleles (DRB1*0102, DRB1*0404, DRB1*0405, DRB1*1402 and DRB1*0401) shared amino acids or amino acid sequences with the primary allele, DRB1*0101, whose structure is illustrated in Fig. 2a. Figure 2b shows the distinguishing amino acid sequences of the primary and secondary alleles and the possible antibody binding site in the vicinity of the peptide grove in the β2-domain. The antibody binding site is inferred from two features: first, the amino acid sequence at positions 85 and 86 of DRB1*0102 (alanine and valine) differ from those of DRB1*0101 (valine and glycine) (Fig. 2b); secondly, the correlation between the ranking of secondary antibodies (based on MFI) and the evolving differences in the amino acids at the β2-domain (Table 1b and Fig. 2b). It is such differences in amino acids between DRB1*0101 and other reactive DRB alleles that suggest that the secondary alloantibodies are cross-reactive alloantibodies, hence their designation as secondary alloantibodies.
Fig. 2.
The amino acid sequences of the primary and secondary human leucocyte antigen (HLA)-DRB alleles recognized by the immunoglobulin (Ig)G antibodies in the serum and in culture supernatants. The secondary alleles differ from the primary allele (DRB1*0101) in their amino acid sequence of DRB at both the β1 and β2 domains of DRB. (a) The structure of DRB1*0101 is illustrated to document the possible site (amino acid positions 57–94, marked yellow) at which the alloantibodies may bind. The position of the exogenous peptide is shown in brown. DRA heavy chain is indicated in reddish purple. (b) The amino acids in the alleles, recognized by the secondary antibodies, differ from those of DRB1*0101 (primary allele); they are in the shaded areas and in bold type. HLA-DRB1*0102 is placed next to DRB1*0101 because the mean fluorescent intensity (MFI) of the antibody recognizing this allele is less than that of the primary alloantibody (formed against DRB1*0101). The amino acid features that distinguish these two alleles are at amino acid positions 85 and 86. Other DRB alleles are arranged in order, based on their MFI relative to the primary allele. The amino acids that differ at the β2 domain of other secondary alleles may be recognized by the secondary antibodies.
TFL-007, but not IVIg, suppressed secretion of allo-HLA-DRB antibodies by activated B cells
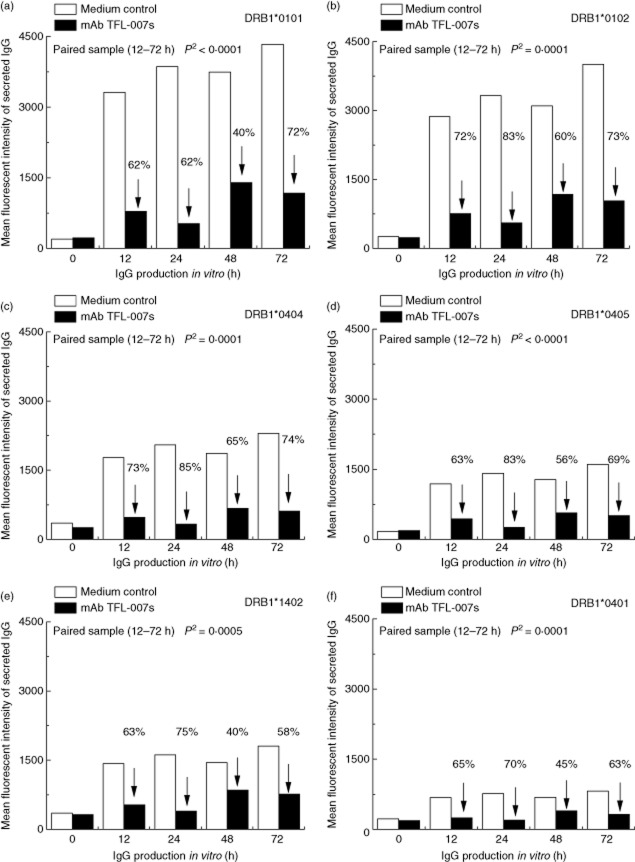
Results presented in Fig. 3 document the effect of IVIg-GamaSTAN on secretion of both primary (Fig. 3a) and secondary (Fig. 3b–f) allo-HLA antibodies in vitro. IVIg-GamaSTAN suppressed the secretion of the primary alloantibody against DRB1*0101 only marginally at different time-points (P < 0·04), and did not significantly affect the secondary alloantibodies against DRB1*0102. Importantly, the MFI of other secondary alloantibodies actually increased after IVIg treatment, the increase ranking from low to high: DRB1*0404 (NS), DRB1*0405 (P < 0·01), DRB1*1402 (P < 0·01) and DRB1*0401 (P < 0·002).
Fig. 3.
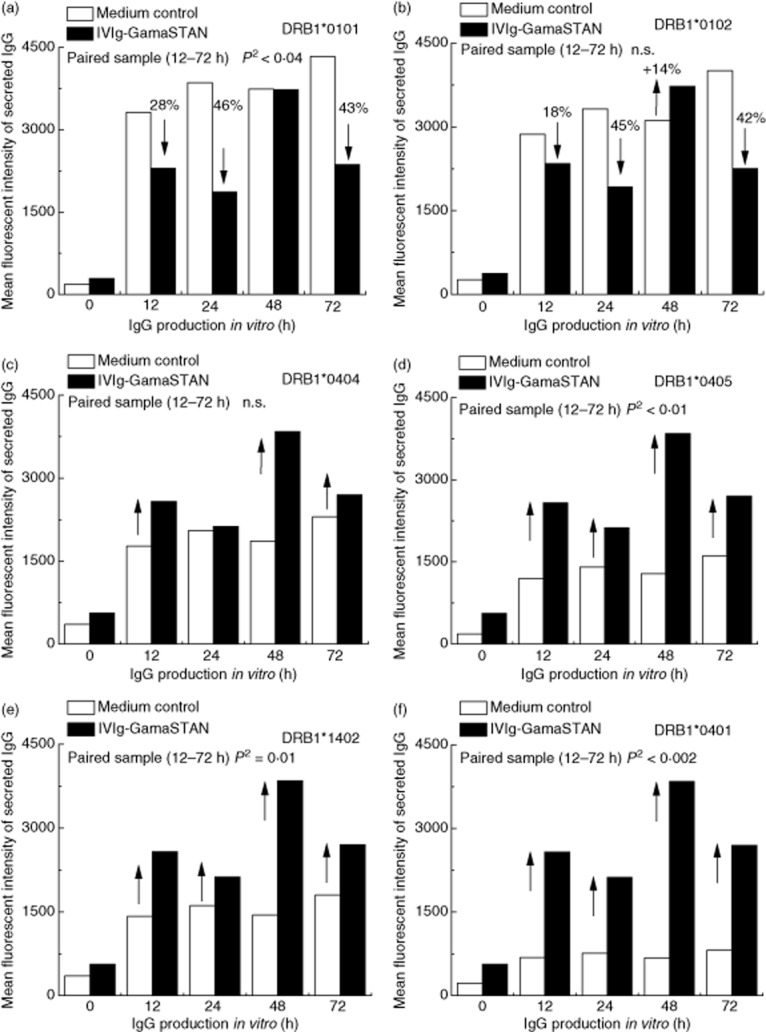
The effects of IVIg on the secretion of primary and secondary allo-human leucocyte antigen (HLA) class II immunoglobulin (Ig)G antibodies by activated B lymphocytes obtained from the alloimmunized woman. GamaSTAN S/D IVIg was used at 1:100 dilution; 1·5 mg protein/ml. At the time when IVIg was added, cytokine combo and anti-CD40 antibody were not added. In all panels, the mean fluorescent intensity (MFI) of alloantibody secretion is compared between medium control and treatment with IVIg. (a) Effects of IVIg on the secretion of the primary alloantibody directed against DRB1*0101. (b–f) Effects of IVIg on the secretion of the secondary alloantibodies (b). DRB1*0102; (c) DRB1*0404; (d) DRB1*0405; (e) DRB1*1402; (f) DRB1*0401). IVIg inhibited the secretion of the primary alloantibody (P2 < 0·04) but no such inhibition is evident with other alloantibodies. Indeed, IVIg increased the secretion of several alloantibodies (DRB1*0405, DRB1*1402, DRB1*0401) (see arrows) at significant levels.
Suppression by TFL-007s was markedly different from that by IVIg. Figure 4 shows that TFL-007s significantly reduced the secretion of both primary (Fig. 4a) and secondary alloantibodies (Fig. 4b–f) (P < 0·001–0·0001). TFL-007s suppressed the secretion of the antibodies reacting to DRB1*0101 and DRB1*0102 more strongly than did IVIg. The percentage difference at each different hour of secretion of the primary alloantibody against DRB1*0101 in Fig. 4a, and of the secondary alloantibody against DRB1*0102 in Fig. 4b, confirm the suppressive efficacy of TFL-007s. Taken together, these results indicate that mAb TFL-007s, not IVIg, prominently arrested the secretion of both primary and secondary allo-HLA-DRB IgG antibodies secreted by activated normal healthy human Bmem cells.
Fig. 4.
The effects of monoclonal antibody (mAb) Terasaki Foundation Laboratory (TFL)-007s on the secretion of primary and secondary allo-human leucocyte antigen (HLA) class II immunoglobulin (Ig)G antibodies by activated B lymphocytes obtained from an alloimmunized woman. TFL-007s was used at 1:100 dilution; 5 μg of protein/ml. At the time that the TFL-007s was added, cytokine combo and anti-CD40 antibody were not added. In all panels, the mean fluorescent intensity (MFI) of the alloantibody secretion is compared between medium control and treatment with monoclonal antibody (mAb) TFL-007s. (a) Effects of mAb TFL-007s on the secretion of the primary alloantibody directed against DRB1*0101. (b–f) Effects of mAb TFL-007s on the secretion of the secondary alloantibodies (b) DRB1*0102; (c) DRB1*0404; (d) DRB1*0405; (e) DRB1*1402; (f) DRB1*0401). The mAb TFL-007s inhibited most significantly the primary alloantibody (P2 < 0·0001), and also significantly inhibited the secretion of secondary alloantibodies. It is important to note that the ability of TFL-007s to inhibit secretion of primary antibody is highly significant and much greater than that of intravenous (IV)Ig. The mAb TFL-007s had a similar high ability to inhibit all the secondary alloantibodies, a feature that puts TFL-007s in stark contrast to the effect of IVIg on antibody secretion.
TFL-007, but not IVIg, suppressed secretion of anti-HLA-B alloantibodies secreted by immortalized B cells
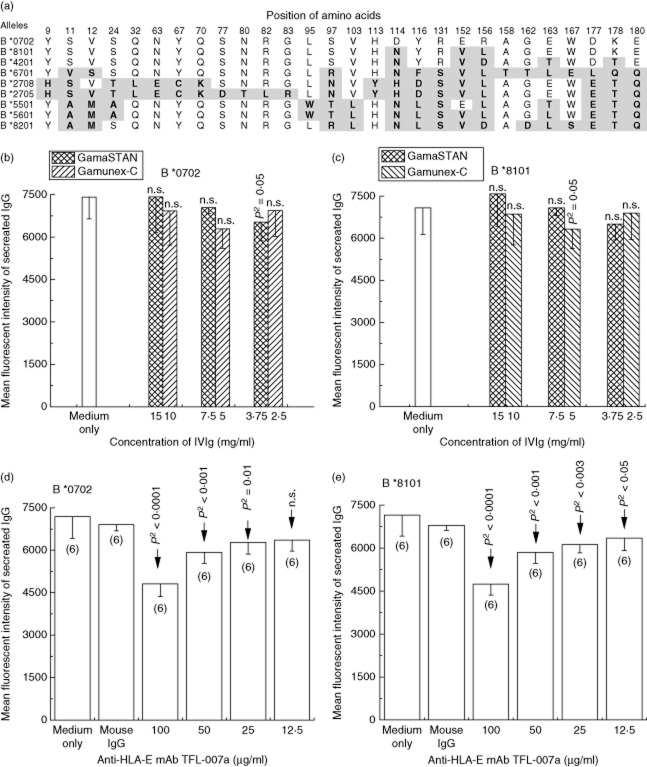
The observation that the overall extent of IVIg-mediated apoptosis of resting and activated human B cells was significantly lower than that observed with B cell hybridoma [22] prompted us to examine and compare the effects of IVIg and TFL-007 on alloantibodies secreted by immortalized B cells. The B cell hybridoma (HML16) was generated from the B cells of a postpartum-alloimmunized woman, the cells immortalized with EBV. The hybridoma cell line produced anti-HLA class I alloantibodies with differing MFIs: high against B*0702, B*8101, B*4201 and B*6701 and low against B*2708, B*2705, B*5501, B*5601 and B*8201. Figure 5a shows the differences in amino acid sequences between the allelic structures recognized by alloantibodies. As no information on HLA typing of the woman and her family was available, the primary alloantibody could not be identified, although the antibodies to HLA- B*0702 and B*8101 showed the highest MFI.
Fig. 5.
Human leucocyte antigen (HLA) class I recognition of the antibodies secreted by the hybridoma cell line HML16. (a) Illustration of the amino acid composition of HLA class I alleles recognized by the antibodies secreted by the hybridoma cell line. Alleles are ranked according to the decreasing mean fluorescent intensity (MFI) of the alloantibody. The MFI of the major alleles correlate with the differences in the amino acids among different alleles: MFI of antibodies is high against B*8101, B*0702, B*4201 and B*6701, low against B*2708, B*2705, B*5501, B*5601 and B*8201. (b–e) The effects of IVIg (b,c) and anti-HLA-E monoclonal antibody (mAb) Terasaki Foundation Laboratory (TFL)-007a (d,e) on the secretion of anti-HLA-B IgG antibodies by the hybridoma are compared with medium control and/or with mouse IgG control. The therapeutic preparations of IVIg from GamaSTAN and Gamunex-C had least impact on the secretion both antibodies (b,c). In contrast, anti-HLA-E mAb TFL-007a significantly (P2 < 0·0001) suppressed the IgG secretion against HLA-B*0702 (d) and B*8101 (e).
The treatment effects of two different therapeutic preparations of IVIg (GamaSTAN and Gamunex) were tested on HML16 at different dilutions and protein concentrations and compared with the media control (Fig. 5b,c). In contrast to the concentration of GamaSTAN used for freshly obtained B cells from the alloimmunized woman, the concentration used for the hybridoma reflects the concentrations of high-dose IVIg used in transplant patients. IVIg-GamaSTAN was tested at dilutions 1:10, 1:20 and 1:40, with dosages of 15 mg/ml, 7·5 mg/ml and 3·75 mg/ml, respectively, whereas the IVIg-Gamunex was tested at dilutions 1:10, 1:20 and 1:40, with dosages of 10 mg/ml, 5 mg/ml and 2·5 mg/ml, respectively. Neither IVIg preparation evidenced any suppression of the secretion of allo-HLA-B IgG by the hybridoma cells (Fig. 5b,c). In contrast, the mAb TFL-007a had a strikingly significant suppressive effect on the secretion of both HLA-B*0702 and B*8101 (Fig. 5d,e). The treatment effect of TFL-007a on alloantibody secretion by the hybridoma HML16 was compared with that of media control, mouse IgG control and the mAb at different dilutions: 1:10, 1:20, 1:40 and 1:80, with dosages of 100 μg/ml, 50 μg/ml, 25 μg/ml and 12·5 μg/ml, respectively. More importantly, mAb TFL-007a showed dosimetric suppression of allo-HLA antibodies. When HML16 was treated with the highest concentration of TFL-007a (100 μg/ml), suppression was 33% for B*0702 and 34% for B*8101 compared with the medium control group. When the dosage of TFL-007a was decreased, the suppression effect declined. In summary, in marked contrast to IVIg preparations, anti-HLA-E mAb TFL-007a significantly suppressed the secretion of both allo-HLA-B antibodies.
Discussion
Our results are consistent with reports that were unable to show inhibition of in-vitro antibody formation by IVIg [22–25]; conversely, our results do not support the implication by another report [26] that IgG production can be suppressed by IVIg. These investigators reported IVIg's dose-dependent suppression of IgG secretion by PBML (which include T cells) activated by pokeweed mitogen, so secretion was not by isolated B cells. By contrast, the Bmem cells activated in our experiments were purified CD19+ B cells (> 95%) maintained in a well-defined culture system, excluding any of the indirect effect from feeder or T cells or their secretary products discussed by others [26,27]. Equally important, the group reporting suppression of IgG did not use therapeutic IVIg or even IVIg as is; they used sulphonated IVIg, polyethylene glycol-treated IVIg and pH 4-treated IVIg. When they tried human serum albumin or pepsin-treated IVIg, those preparations did not suppress the IgG secretion. Our observations, therefore, suggest that the findings of dose-dependent suppression by IVIg may not have been due to direct interaction with B cells. As other groups [28,29] have reported, the observed suppression of allo-HLA antibody production in vivo by modified IVIg [26] could be due to suppression of T cell proliferation, activation or antigen presentation.
Reports consistent with ours seem quite persuasive. For instance, Heidt et al. documented IVIg's lack of effect on IgG-mRNA synthesis by B cells [23]. While, in our study, IVIg marginally decreased secretion of the primary alloantibody anti-DRB1*0101, the most striking (and paradoxical) effect was that IVIg increased secretion of HLA-II secondary alloantibodies in vitro, as others also documented in vitro [22,30] and in mice [24,27]. The report by de Grandmont et al. [22] is the most conclusive, as they radiolabelled the secreted antibodies to measure the augmentation of IgG secretion. They showed that IVIg could not induce a significant level of apoptosis in the presence of CD154 (used instead of anti-CD40 antibody) and cytokines and, indeed, in our study the augmentation of secondary allo-DRB antibodies following the addition of IVIg occurred after anti-CD40 antibody and cytokines were removed from the culture wells. de Grandmont's finding is especially important considering the report of Prasad et al. [31], who suggested that IVIg may bind to negative FcγIIb receptors on B cells to cause the cells' apoptosis or anergy but not activation. In this study, as in ours, IVIg was added after removing the anti-CD40 antibody. The augmentation of secondary allo-HLA antibodies supports de Grandmont's contention, not Prasad's.
The impact of anti-HLA-E mAb TFL-007 on secretion of anti-HLA alloantibodies is remarkably significant. The mAb TFL-007 was selected because it mimics the HLA-Ia and Ib reactivity of IVIg in addition to its very high reactivity with HLA-E [21]. Indeed, the HLA-Ia reactivity of anti-HLA-E mAbs can be inhibited with peptides shared by HLA-E and HLA-Ia alleles [32–34]. Furthermore, when anti-HLA-E antibodies were depleted, the entire HLA-Ia reactivity of IVIg was abolished [21]. This supports the contention that TFL-007 not only mimics IVIg but is also a better agent than therapeutic IVIg for arresting secretion of allo-HLA antibodies by activated Bmem cells. TFL-007 most strikingly (P2 < 0·0001) arrested the secretion of both HLA-DRB primary and secondary alloantibodies.
We studied the effects of IVIg and anti-HLA-E mAb TFL-007 on the secretion of HLA-alloantibodies by circulatory B cells – freshly obtained from a woman alloimmunized postpartum 23 years – and also studied their effects on the secretion of HLA-B alloantibodies by immortalized B cells obtained from another woman's alloimmunized postpartum. The mAb TFL-007, but not IVIg, strongly and significantly (P2 < 0·0001) suppressed the secretion of HLA-B alloantibodies by hybridoma. There was scant difference in the MFI of alloantibodies secreted by hybridoma between those treated with IVIg and the control cells, a finding that does not support the contention [31] that IVIg can induce apoptosis and anergy of hybridoma higher than the apoptosis of the resting and activated B cell population in a normal healthy individual. In short, IVIg failed to suppress both HLA-I and HLA-II alloantibodies secreted by both hybridoma and long-lasting Bmem cells and plasma cells from, respectively, two different healthy women alloimmunized postpartum many years before. In this respect, mAb TFL-007 does not mimic IVIg, but may serve better to suppress the allo-HLA antibody secretion by allo-HLA-exposed/immunized B cells.
The mechanism of suppression of allo-HLA antibody secretion by TFL-007 can be inferred from the high affinity of TFL-007 for HLA-ER. This unique affinity, in spite of cross-reactivity with other HLA-class I alleles, underscores the fact that the heavy chain of HLA-E is the target antigen which could function as an inhibitory ligand on B cells, as does FcγRIIb. The inhibitory ligand is the β2-microglobulin-free heavy chain of HLA-E, but it may not be intact; HLA-E is inferred from the unique property of anti-HLA-E mAb TFL-007, which is that it binds to peptide sequences in the heavy chain that is shared with other HLA class I alleles and is not available for binding because it is masked by β2-microglobulin. Indeed, Coupel et al. [35] confirmed the expression of HLA-ER on B cells [35]. Innumerable reports document that in activated or stimulated B cells, β2-microglobulin-free heavy chains (also known as ‘open conformers’) are over-expressed (for review, see [36]).
The possible mechanisms underlying IVIg-mediated suppression in patients with DSA are speculative. Anti-idiotype antibodies in IVIg are implicated in suppression of allo-HLA antibody production. While it was reported that ‘an antibody against another antibody could bind to the antibody on the surface of a B cell and either stimulate or inhibit further antibody formation’ [emphasis added] ([37], p. 65), another study found no evidence for the presence anti-HLA anti-idiotypic antibodies in IVIg [38]. IVIg may suppress proliferation and induce apoptosis by binding through the Fc portion of IgG to the inhibitory FcγRIIb expressed on B cells [39]. Another group reported that ‘the overall extent of apoptosis (mediated by IVIg) of resting and activated (human B) cells was significantly lower than that observed with transformed cells (B cell hybridoma)’ [text in parentheses added] ([31], p. 3786). In spite of these speculations, several recent reports [40–42] question the IVIg-mediated suppression of allo-HLA antibody levels in patients. The results of our investigation do not support the contention that IVIg may interact directly with B cells to suppress allo-HLA antibody secretion. However, the present investigation does not rule out that IVIg may be involved indirectly in suppressing allo-HLA antibody secretion by B cells, which may include suppression of T cell help.
Finally, if the objective of IVIg therapy is to suppress IgG production of allo- and autoantigens, as required for autoimmune diseases or for lowering anti-HLA non-donor-specific or pre-existing DSA in patients waiting for allograft or for lowering DSA in allograft recipients to prevent humoral rejection, then anti-HLA-E mAb TFL-007 may be a simple and better agent, if humanized or chimerized.
In conclusion, mAb TFL-007, but not IVIg, suppressed the anti-HLA alloantibodies produced by activated Bmem cells/plasma cells and immortalized B cells. During monitoring, the effect of TFL-007, the cytokine combo and anti-CD40 antibody were both removed to specifically evaluate the impact of the antibody. Possibly, mAb TFL-007 may also be useful in suppressing other antibodies produced by B cells, and alloantibodies as well.
Acknowledgments
The authors wish to express their sincere thanks to Dr Stanley Jordan, Cedars-Sinai, Los Angeles, for providing the Gamunex-C, Dr Junchao Cai, TFL, for his valuable suggestions on human B cell culture, Dr Etsuko Maruya, former member of TFL, for her guidance in hybridoma cell culture and all other active members of TFL for their support throughout this investigation. The entire research project is supported by grants from Terasaki Family Foundation mediated through Terasaki Foundation Laboratory.
Author contributions
D. Z. designed the study, performed the experiments, collected and validated the data and assisted in writing the paper. M. H. R. developed and characterized the anti-HLA-E mAb TFL-007, analysed the HLA-reactivity of the sera of the subject involved; formulated the hypothesis, periodically monitored the experimental outcome; analysed the data and performed statistical analysis; prepared the figures; discussed and wrote the paper. P. I. T. was instrumental in undertaking this investigation and contributed to designing the study, examined the results and provided interpretations. T. M. developed the immortalized anti-HLA antibody-secreting B cell line and characterized the antibody secretion by the B cells. T. P. assisted in carrying out the experiments and provided continuous support for data analyses and discussion. V. J. assisted in carrying out the experiments and was involved in the data analysis and in discussion of results.
Disclosures
None of the authors have any conflicts of interest.
References
- 1.Fulcher DA, Basten A. B cell life span: a review. Immunol Cell Biol. 1997;75:446–455. doi: 10.1038/icb.1997.69. [DOI] [PubMed] [Google Scholar]
- 2.Manz RA, Löhning M, Cassese G, Thiel A, Radbruch A. Survival of plasma cells is independent of Ag. Int Immunol. 1998;10:1703–1711. doi: 10.1093/intimm/10.11.1703. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed R, Gray D. Immunological memory and protective immunity: understand their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 4.Radbruch A, Muehlinghaus G, Luger EO, et al. Competence and competition: the challenge of becoming a long-live plasma cell. Nat Rev Immunol. 2006;6:741–750. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- 5.Luger EO, Fokuhl V, Wegmann M, et al. Induction of allergen-specific plasma cells by mucosal allergen challenge. J Allergy Clin Immunol. 2009;124:819–826. doi: 10.1016/j.jaci.2009.06.047. [DOI] [PubMed] [Google Scholar]
- 6.Terasaki PI, Cai J. Human leukocyte Ag Abs and chronic rejection: from association to causation. Transplantation. 2008;86:377–383. doi: 10.1097/TP.0b013e31817c4cb8. [DOI] [PubMed] [Google Scholar]
- 7.Terasaki PI, Cai J. Humoral theory of transplantation: further evidence. Curr Opin Immunol. 2005;17:541–545. doi: 10.1016/j.coi.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 8.Marfo K, Lu A, Ling M, Akalin R. Desensitization protocols and their outcome. Clin J Am Soc Nephrol. 2011;6:922–936. doi: 10.2215/CJN.08140910. [DOI] [PubMed] [Google Scholar]
- 9.Montgomery RA, Lonze BE, King KE, et al. Desensitization in HLA-incompatible kidney recipients and survival. N Engl J Med. 2011;365:318–326. doi: 10.1056/NEJMoa1012376. [DOI] [PubMed] [Google Scholar]
- 10.Mao Q, Terasaki PI, Cai J, et al. Extremely high association between appearance of HLA Abs and failure of kidney grafts in a five-year longitudinal study. Am J Transplant. 2007;7:864–871. doi: 10.1111/j.1600-6143.2006.01711.x. [DOI] [PubMed] [Google Scholar]
- 11.Glotz D, Antoine C, Julia P, et al. Desensitization and subsequent kidney transplantation of patients using intravenous immunoglobulins (IVIg) Am J Transplant. 2002;2:758–760. doi: 10.1034/j.1600-6143.2002.20809.x. [DOI] [PubMed] [Google Scholar]
- 12.Jordan SC, Vo A, Bunnapradist S, et al. Intravenous immune globulin treatment inhibits crossmatch positivity and allows for successful transplantation of incompatible organs in living-donor and cadaver recipients. Transplantation. 2003;76:631–636. doi: 10.1097/01.TP.0000080685.31697.FC. [DOI] [PubMed] [Google Scholar]
- 13.Vo AA, Lukovsky M, Toyoda M, et al. Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med. 2008;359:242–251. doi: 10.1056/NEJMoa0707894. [DOI] [PubMed] [Google Scholar]
- 14.Rocha PN, Butterly DW, Greenberg A, et al. Beneficial effect of plasmapheresis and intravenous immunoglobulin on renal allograft survival of patients with acute humoral rejection. Transplantation. 2003;75:1490–1495. doi: 10.1097/01.TP.0000060252.57111.AC. [DOI] [PubMed] [Google Scholar]
- 15.Jordan SC, Vo AA, Tyan D, Nast CC, Toyoda M. Current approaches to treatment of Ab-mediated rejection. Pediatr Transplant. 2005;9:408–415. doi: 10.1111/j.1399-3046.2005.00363.x. [DOI] [PubMed] [Google Scholar]
- 16.Buckley RH, Schiff RI. The use of intravenous immune globulin in immuno-deficiency diseases. N Engl J Med. 1991;325:110–117. doi: 10.1056/NEJM199107113250207. [DOI] [PubMed] [Google Scholar]
- 17.Kaveri SV, Dietrich G, Hurez V, Kazatchkine MD. Intravenous immunoglobulins (IVIg) in the treatment of autoimmune diseases. Clin Exp Immunol. 1991;86:192–198. doi: 10.1111/j.1365-2249.1991.tb05794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McIntyre JA, Higgins N, Britton R, et al. Utilization of intravenous immunoglobulin to ameliorate all-Abs in a highly sensitized patient with a cardiac assist device awaiting heart transplantation. Fluorescence-activated cell sorter analysis. Transplantation. 1996;62:691–693. doi: 10.1097/00007890-199609150-00027. [DOI] [PubMed] [Google Scholar]
- 19.Glotz D, Haymann JP, Sansonetti N, et al. Suppression of HLA-specific alloAbs by high-dose intravenous immunoglobulins (IVIg). A potential tool for transplantation of immunized patients. Transplantation. 1993;56:335–337. doi: 10.1097/00007890-199308000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Tyan DB, Li VA, Czer L, Trento A, Jordan SC. Intravenous immunoglobulin suppression of HLA alloantibody in highly sensitized transplant candidates and transplantation with a histoincompatible organ. Transplantation. 1994;57:553–562. [PubMed] [Google Scholar]
- 21.Ravindranath MH, Terasaki PI, Pham T, Jucaud V, Kawakita S. Therapeutic preparations of IVIg contain naturally occurring anti-HLA-E Abs that react with HLA-Ia (HLA-A/-B/-Cw) alleles. Blood. 2013;121:2013–2028. doi: 10.1182/blood-2012-08-447771. [DOI] [PubMed] [Google Scholar]
- 22.de Grandmont MJ, Racine C, Roy A, Lemieux R, Néron S. Intravenous immunoglobulins induce the in vitro differentiation of human B lymphocytes and the secretion of IgG. Blood. 2003;101:3065–3073. doi: 10.1182/blood-2002-06-1684. [DOI] [PubMed] [Google Scholar]
- 23.Heidt S, Roelen DL, Eijsink C, Eikmans M, Claas FH, Mulder A. Intravenous immunoglobulin preparations have no direct effect on B cell proliferation and immunoglobulin production. Clin Exp Immunol. 2009;158:99–105. doi: 10.1111/j.1365-2249.2009.03996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sundblad A, Huetz F, Portnoi D, Coutinho A. Stimulation of B and T cells by in vivo high dose immunoglobulin administration in normal mice. J Autoimmun. 1991;4:325–339. doi: 10.1016/0896-8411(91)90028-b. [DOI] [PubMed] [Google Scholar]
- 25.Sundblad A, Marcos MA, Malanchere E, et al. Observations on the mode of action of normal immunoglobulin at high doses. Immunol Rev. 1994;139:125–158. doi: 10.1111/j.1600-065x.1994.tb00860.x. [DOI] [PubMed] [Google Scholar]
- 26.Kondo N, Ozawa T, Mushiake K, et al. Suppression of immunoglobulin production of lymphocytes by intravenous immunoglobulin. J Clin Immunol. 1991;11:152–158. doi: 10.1007/BF00918683. [DOI] [PubMed] [Google Scholar]
- 27.Han M, Rogers JA, Lavingia B, Stastny P. Peripheral blood B cells producing donor-specific HLA Abs in vitro. Hum Immunol. 2009;70:29–34. doi: 10.1016/j.humimm.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 28.Amran D, Renz H, Lack G, Bradley K, Gelfand EW. Suppression of cytokine-dependent human T-cell proliferation by intravenous immunoglobulin. Clin Immunol Immunopathol. 1994;73:180–186. doi: 10.1006/clin.1994.1186. [DOI] [PubMed] [Google Scholar]
- 29.Sbrana S, Ruocco L, Vanacore R, Azzarà A, Ambrogi F. In vitro effects of an immunoglobulin preparation for intravenous use (IVIG) on T-cells activation. Allerg Immunol (Paris) 1993;25:35–37. [PubMed] [Google Scholar]
- 30.Bayry J, Fournier EM, Maddur MS, et al. Intravenous immunoglobulin induces proliferation and immunoglobulin synthesis from B cells of patients with common variable immunodeficiency: a mechanism underlying the beneficial effect of IVIg in primary immunodeficiencies. J Autoimmun. 2011;36:9–15. doi: 10.1016/j.jaut.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Prasad NK, Papoff G, Zeuner A, et al. Therapeutic preparations of normal polyspecific IgG (IVIg) induce apoptosis in human lymphocytes and monocytes: a novel mechanism of action of IVIg involving the Fas apoptotic pathway. J Immunol. 1998;161:3781–3790. [PubMed] [Google Scholar]
- 32.Ravindranath MH, Taniguichi M, Chen CW, et al. HLA-E monoclonal Abs recognize shared peptide sequences on classical HLA class Ia: relevance to human natural HLA Abs. Mol Immunol. 2010;47:1121–1131. doi: 10.1016/j.molimm.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 33.Ravindranath MH, Pham T, El-Awar N, Kaneku H, Terasaki PI. Anti-HLA-E mAntibody 3D12 mimics MEM-E/02 in binding to HLA-B and HLA-C alleles: web-tools validate the immunogenic epitopes of HLA-E recognized by the Abs. Mol Immunol. 2011;48:423–430. doi: 10.1016/j.molimm.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 34.Ravindranath MH, Kaneku H, El-Awar N, Morales-Buenrostro LE, Terasaki PI. Abs to HLA-E in nonalloimmunized males: pattern of HLA-Ia reactivity of anti-HLA-E-positive sera. J Immunol. 2010;185:1935–1948. doi: 10.4049/jimmunol.1000424. [DOI] [PubMed] [Google Scholar]
- 35.Coupel S, Moreau A, Hamidou M, Horejsi V, Soulillou JP, Charreau B. Expression and release of soluble HLA-E is an immunoregulatory feature of endothelial cell activation. Blood. 2007;109:2806–2814. doi: 10.1182/blood-2006-06-030213. [DOI] [PubMed] [Google Scholar]
- 36.Arosa FA, Santos SG, Powis SJ. Open conformers: the hidden face of MHC-I molecules. Trends Immunol. 2007;28:115–123. doi: 10.1016/j.it.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Schiff RI. Intravenous gammaglobulin: pharmacology, clinical uses and mechanisms of action. Pediatr Allergy Immunol. 1994;5:63–87. doi: 10.1111/j.1399-3038.1994.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 38.Barocci S, Fenoglio D, Leprini A, Nocera A. Anti-HLA Abs(Ab1) and anti-idiotypic Abs (Ab2) directed against Anti-HLA Ab1 in various preparations of polyspecific immunoglobulins for intravenous use. Boll Soc Ital Biol Sper. 1990;66:1193–1200. [PubMed] [Google Scholar]
- 39.Ott VL, Fong DC, Cambier JC. Fc gamma RIIB as a potential molecular target for intravenous gamma globulin therapy. J Allergy Clin Immunol. 2001;108(Suppl):S95–98. doi: 10.1067/mai.2001.117822. [DOI] [PubMed] [Google Scholar]
- 40.Nair V, Sawinski D, Akalin E, et al. Effect of high-dose intravenous immunoglobulin on anti-HLA Abs in sensitized kidney transplant candidates. Clin Transplant. 2012;26:E261–268. doi: 10.1111/j.1399-0012.2012.01657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alachkar N, Lonze BE, Zachary AA, et al. Infusion of high-dose intravenous immunoglobulin fails to lower the strength of human leukocyte Ag Abs in highly sensitized patients. Transplantation. 2012;94:165–171. doi: 10.1097/TP.0b013e318253f7b6. [DOI] [PubMed] [Google Scholar]
- 42.Marfo K, Ling M, Bao Y, et al. Lack of effect in desensitization with intravenous immunoglobulin and rituximantibody in highly sensitized patients. Transplantation. 2012;94:345–351. doi: 10.1097/TP.0b013e3182590d2e. [DOI] [PubMed] [Google Scholar]
- 43.Fecteau JF, Neron S. CD40 stimulation of human peripheral B lymphocytes: distinct response from naive and memory cells. J Immunol. 2005;171:4621–4629. doi: 10.4049/jimmunol.171.9.4621. [DOI] [PubMed] [Google Scholar]
- 44.Ettinger R, Sims GP, Fairhurst AM, et al. IL-21 induces differentiation of human naive and memory B cells into Ab-secreting plasma cells. J Immunol. 2005;175:7867–7879. doi: 10.4049/jimmunol.175.12.7867. [DOI] [PubMed] [Google Scholar]
- 45.Jego G, Bataille R, Pellat-Deceunynck C. Interleukin-6 is a growth factor for non-malignant human plasmablasts. Blood. 2001;97:1817–1822. doi: 10.1182/blood.v97.6.1817. [DOI] [PubMed] [Google Scholar]
- 46.Jourdan M, Caraux A, De Vos J, et al. An in vitro model of differentiation of memory B cells into plasmablasts and plasma cells including detailed phenotypic and molecular characterization. Blood. 2009;114:5173–5181. doi: 10.1182/blood-2009-07-235960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Avery DT, Deenick EK, Ma CS, et al. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing antibody responses in humans. J Exp Med. 2010;207:155–171. doi: 10.1084/jem.20091706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walker JD, Maier CL, Pober JS. Cytomegalovirus-infected human endothelial cells can stimulate allogeneic CD4+ memory T cells by releasing Agic exosomes. J Immunol. 2009;182:1548–1559. doi: 10.4049/jimmunol.182.3.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Polyak MJ, Deans JP. Alanine-170 and proline-172 are critical determinants for extracellular CD20 epitopes; heterogeneity in the fine specificity of CD20 monoclonal Abs is defined by additional requirements imposed by both amino acid sequence and quaternary structure. Blood. 2002;99:3256–3262. doi: 10.1182/blood.v99.9.3256. [DOI] [PubMed] [Google Scholar]
- 50.Correia DV, Fogli M, Hudspeth K, da Silva MG, Mavilio D, Silva-Santos B. Differentiation of human peripheral blood Vdelta1+ T cells expressing the natural cytotoxicity receptor NKp30 for recognition of lymphoid leukemia cells. Blood. 2011;118:992–1001. doi: 10.1182/blood-2011-02-339135. [DOI] [PubMed] [Google Scholar]
- 51.Roura-Mir C, Catálfamo M, Cheng TY, et al. CD1a and CD1c activate intrathyroidal T cells during Graves' disease and Hashimoto's thyroiditis. J Immunol. 2005;174:3773–3780. doi: 10.4049/jimmunol.174.6.3773. [DOI] [PubMed] [Google Scholar]