Abstract
Major long-term complications in patients with diabetes are related to oxidative stress, caused by the hyperglycaemia characteristic for diabetes mellitus. The anti-oxidant coenzyme Q10 (CoQ10) has therefore been proposed as a beneficial supplement to diabetes treatment. Apart from its anti-oxidative function, CoQ10 appears to modulate immune functions by largely unknown mechanisms. The aim of this study was therefore to investigate the effect of CoQ10 on antimicrobial peptides and natural killer (NK) cells, both innate immune components implicated in the pathogenesis of diabetes and diabetes-associated long-term complications such as cardiovascular disease. We determined serum levels of antimicrobial peptides and the phenotype of NK cells isolated from peripheral blood of patients with type 1 (T1DM) or type 2 diabetes mellitus (T2DM) and from healthy controls. In addition, the same parameters were determined in diabetic patients after a 12-week period of CoQ10 supplementation. Two antimicrobial peptides, the human cathelicidin antimicrobial peptide (CAMP) and the human beta defensin 1 (hBD1), were reduced in serum from patients with T1DM. This defect was not reversible by CoQ10 supplementation. In contrast, CoQ10 reduced the levels of circulating hBD2 in these patients and induced changes in subset distribution and activation markers in peripheral NK cells. The results of the present study open up novel approaches in the prevention of long-term complications associated to T1DM, although further investigations are needed.
Keywords: diabetes, human, natural killer cells, reactive oxygen species
Introduction
Autoimmune type 1 diabetes mellitus (T1DM) is the result of defects in immune regulation. However, type 2 diabetes mellitus (T2DM) is also associated with immune defects and inflammation [1,2], mainly as a consequence of hyperglycaemia-induced oxidative stress due to increased production of reactive oxygen species (ROS). These processes are suggested to underlie the pathogenesis for late diabetes complications, including arteriosclerosis and cardiovascular disease (CVD) [3]. The development of CVD is complex, and involves innate immune mediators such as antimicrobial peptides (AMPs) and natural killer (NK) cells [4–8].
As well as their antimicrobial activity, AMPs exhibit a number of further functions related to inflammation, tissue protection and repair mechanisms [9]. Most recently, the participation of pancreatic, neutrophil-derived cathelicidin-related antimicrobial peptide CRAMP was described to contribute to the pathogenesis of T1DM in mice [10]. In addition, diabetes-associated defects in AMP production have been observed [11–15]. NK cells are innate lymphocytes involved in the early protection against certain infections and tumours [16,17]. Their implication in the pathogenesis of autoimmune disease including T1DM is recognized, although their exact role in this context is complex and still not fully known [18–20].
Coenzyme Q10 (CoQ10) has been suggested to decrease CVD risk by largely uncharacterized mechanisms [21,22]. As an anti-oxidant, CoQ10 has neutralizing effects on ROS, but appears to affect different aspects of the innate immune system independently of its radical scavenging properties [22–24], e.g. by impacting upon gene expression [25,26] and influencing the cytokine response to bacterial stimuli [27,28].
The aim of this study was to investigate AMPs and NK cells in diabetes patients and the influence of CoQ10 on these immune components in the diabetic milieu.
Materials and methods
Ethics statement
This study was approved by the regional ethics committee in Stockholm. The participants included in the study gave their written informed consent.
Patients and healthy controls
Patients (n = 58) and healthy controls (n = 19) were recruited from the Department of Endocrinology, Metabolism and Diabetes, Karolinska University Hospital, Stockholm, Sweden. Patients with diabetes (n = 23) received CoQ10 orally for 12 weeks (100 mg twice daily). The CoQ10 capsules (Bio-Quinon) were a generous gift from Pharma Nord (Vejle, Denmark). Clinical data and samples were collected directly before and after CoQ10 supplementation. The lipid profile, glycaemic status and C-reactive protein (CRP) were determined according to standard procedures at the Karolinska University Laboratory. The lower detection limit of the CRP assay was 1 mg/l and lower levels were assigned a value of 0·9 mg/l for statistical analysis. Plasma cytokine and chemokine levels were measured using a Bioplex assay kit (Bio-Rad Laboratories, Hercules, CA, USA), according to the manufacturer's recommendations. In some of the patients (n = 19), serum levels of CoQ10 were measured by high performance liquid chromatography (HPLC) [29] before and after the 12-week supplementation period.
Detection of AMPs
Serum levels of the human cathelicidin LL-37/hCAP18 (CAMP) and human beta defensins (hBDs) 1–3 were determined by enzyme-linked immunosorbent assay (ELISA) according to the manufacturers' recommendations (Hycult Biotech, Uden, the Netherlands and Alpha Diagnostics, San Antonio, TX, USA, respectively).
Preparation of peripheral blood mononuclear cells (PBMCs)
PBMCs were separated by density gradient centrifugation using the BD Vacutainer CPT Cell Preparation Tubes (Becton Dickinson, Stockholm, Sweden) containing sodium citrate, according to the manufacturer's instructions.
Flow cytometry
PBMCs were stained with monoclonal antibodies to CD56, CD3, CD11c, CD16, CD69 and NKG2D for 30 min at 4°C; mouse IgG1 was used as negative control (all BD Biosciences, CA, USA). Data were acquired on a fluorescence activated cell sorter (FACS) FACSortrrrr™ flow cytometer using CellQuest™ software (BD Biosciences Immunocytometry Systems, San Jose, CA, USA).
Statistical analysis
Normally distributed data are presented as mean ± standard error (s.e.m.) and were compared by one-way analysis of variance (anova) with Bonferroni's post-hoc test or unpaired t-test, as appropriate; otherwise data are presented with median and range, and non-parametric tests were used. Changes induced by CoQ10 treatment were evaluated using a paired t-test or Wilcoxon's signed-rank test for data without normal distribution. Differences with P < 0·05 were considered statistically significant.
Results
Clinical characteristics
A total of 58 patients with diabetes, 27 with T1DM and 31 with T2DM, and 19 healthy controls were included. The groups did not differ significantly in age (T1DM, 53·2 ± 15·5 years; T2DM, 58·7 ± 8 years; controls, 50·3 ± 14·8 years) or gender distribution (59·3, 51·6 and 68·4% females among T1DM, T2DM and controls, respectively). A subset of 23 diabetic patients was subjected to a 12-week supplementation with CoQ10. Diabetes duration was significantly higher (P < 0·001) in T1DM (median 22 years) than in T2DM patients (median 9 years). Waist circumference was significantly higher (P < 0·05) in patients with T2DM (103·7 ± 2·2 cm) compared to T1DM patients (89·6 ± 2·3 cm) and controls (86·9 ± 2·6 cm). CRP was not significantly different between controls, T1DM and T2DM patients (median 2·7, 0·9 and 3·0 mg/l, respectively). Haemoglobin A1c (HbA1c) levels were significantly higher in diabetic patients compared to controls (57·5 ± 3·8 mmol/mol and 50·43 ± 3·3 mmol/mol in T1DM and T2DM, respectively, versus 30·5 ± 2·3 mmol/mol in controls; P < 0·01 and P < 0·001, respectively). Baseline serum levels of CoQ10 (1·73 nmol/ml, 0·97–3·44 nmol/ml) did not differ between T1DM and T2DM patients. Supplementation with CoQ10 resulted in a 3·27-fold increase (range 1·45–6·94-fold; P = 0·0001), and this effect was similar in the two patient groups.
Serum levels of the AMPs CAMP and hBD1 were reduced in patients with T1DM
The serum levels of AMPs in diabetic patients and controls were determined. CAMP and hBD1 levels were significantly lower in patients with T1DM compared to patients with T2DM (Fig. 1a,b), while no difference could be detected for hBD2 and hBD3 (data not shown). Interestingly, levels of CAMP and hBD1 were correlated positively with CRP (Spearman's r = 0·31, P = 0·02 for CAMP; r = 0·29, P = 0·03 for hBD1) but not hBD2 and hBD3 in the diabetic patients studied. A similar relationship was determined for interleukin (IL)-6; Spearman's r = 0·45, P = 0·03 for CAMP; r = 0·33, P = 0·12 for hBD1; r = 0·47, P = 0·02 for hBD3; n = 23). Interestingly, both serum CAMP and hBD1 correlated with plasma triglycerides (TG) (Spearman's r = 0·51, P < 0·0001 for CAMP; r = 0·45, P = 0·0004 for hBD1); moreover, CAMP correlated negatively with high-density lipoprotein (HDL)-cholesterol (Spearman's r = −0·32, P = 0·02).
Fig. 1.
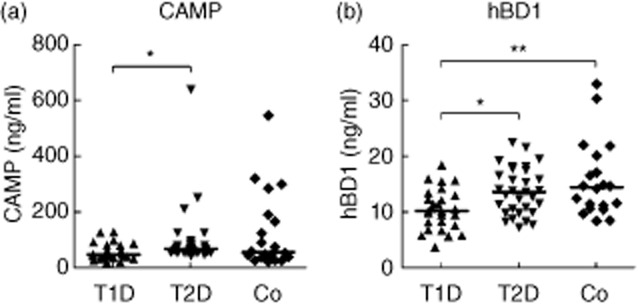
Serum levels of antimicrobial peptides in diabetic patients and in healthy controls. The levels of human cathelicidin antimicrobial peptide CAMP (a) and human beta defensin hBD1 (b) were measured in the serum from patients with type 1 diabetes mellitus (T1DM), type 2 diabetes mellitus (T2DM) and from healthy controls (Co) by enzyme-linked immunosorbent assay (ELISA). Individual values with median are shown. *P < 0·05; **P < 0·01.
Many studies during past years have highlighted the importance of NK cells in the pathogenesis of autoimmunity and T1DM. However, we found no differences in the frequency of NK cells in PBMCs between T1DM and T2DM (data not shown).
Changes induced by CoQ10 supplementation
Because the impact of CoQ10 on the immune system has been suggested previously, we sought to investigate whether oral CoQ10 supplementation could be beneficial for the diabetes-associated changes. In the 23 patients undergoing CoQ10 supplementation for 12 weeks, metabolic control was improved and oxidative stress was reduced [47·7 ± 2·7 mmol/mol at week 0 to 44·7 ± 2·6 mmol/mol at week 12, P = 0·048 for HbA1c; 44·3 ± 3·0 U/l at week 0 to 36·1 ± 1·6 U/l at week 12, P = 0·001 for oxidized LDL (ox-LDL)]. There was no significant difference between T1DM and T2DM patients, even though HbA1c appeared to be affected more strongly in T2DM, while ox-LDL seemed to be affected more strongly in T1DM patients. In contrast, TG and HDL-cholesterol levels were unaffected, and the inflammatory parameters CRP and IL-6 and the AMPs CAMP, hBD1 and hBD3 remained unchanged. However, we noticed a strong decrease of serum hBD2 levels in the T1DM patients after CoQ10 supplementation (Fig. 2a).
Fig. 2.
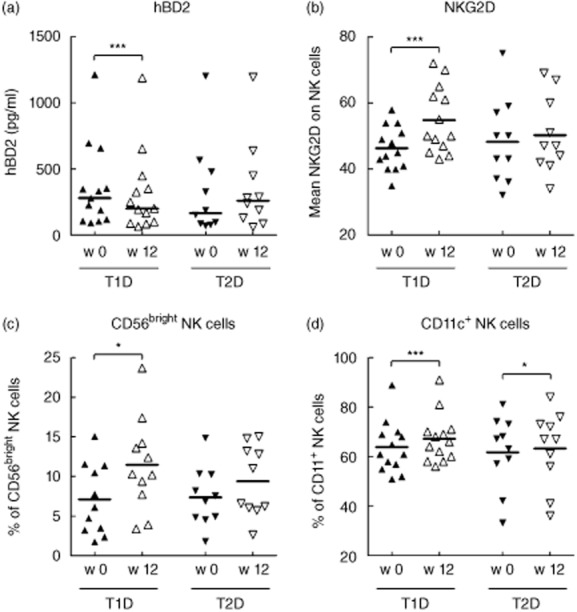
The influence of CoQ10 on antimicrobial peptide hBD2 and on natural killer cells. The serum levels of the human beta defensin 2 (hBD2) (a) and the phenotype of natural killer (NK) cells, defined as CD3-CD56+ lymphocytes, in peripheral blood mononuclear cells (b–d) was determined in patients with type 1 diabetes mellitus (T1DM) or type 2 diabetes mellitus (T2DM) before (filled symbols) and after (open symbols) a 12-week period of CoQ10 supplementation. Individual values with median (a) or mean (b–d) are shown. *P < 0·05; **P < 0·01; ***P < 0·001.
Interestingly, while there were no differences in NK cell frequency in patients before supplementation, CoQ10 induced several phenotypical alterations, primarily in T1DM patients (Fig. 2b–d). The activating receptor NKG2D on NK cells from T1DM patients was up-regulated (Fig. 2b) and the proportion of CD56bright NK cells increased (Fig. 2c). In patients with both T1DM and T2DM, CoQ10 induced a slight increase of CD11c-expressing NK cells (Fig. 2d). Expression of the early activation marker CD69 or the differentiation marker CD16 was, however, not influenced by CoQ10 (data not shown).
Discussion
The positive impact of CoQ10 on diabetes-associated conditions has been demonstrated repeatedly. The data presented in this study indicate that CoQ10 treatment can also support the innate immunity in T1DM and may help to prevent late complications.
Decreased serum hBD2 indicates reduced inflammation in T1DM patients
We observed a deficiency of CAMP and hBD1, primarily in T1DM patients. This is in line with a previously reported negative impact of low glucose and insulin levels on the production of beta defensins [12,30]. Moreover, T2DM patients suffering from diabetic foot ulcers showed defects in CAMP expression [14], indicating that AMP production might be generally impaired in the diabetic milieu. In contrast, hBD2, which is generally induced by proinflammatory stimuli, was decreased after CoQ10 supplementation in T1DM patients. This suggests that diabetes-associated inflammatory processes were reduced by CoQ10. Moreover, as hBD2 itself exhibits proinflammatory activity, a further reduction of the proinflammatory burden by CoQ10 may be assumed in these patients. Overall, our findings indicate that expression of various AMPs is affected differentially by the diabetic condition. We cannot rule out, however, that the underlying pathogenesis of the AMP-related defects differs between T1DM and T2DM patients.
Altered NK cell features in T1DM patients after CoQ10 supplementation implies increased cytokine production capacity
The activating NK cell receptor NKG2D has been shown previously to be down-regulated in NK cells from T1DM patients [20]. Our findings of increased NKG2D on NK cells after supplementation with CoQ10 in this patient group could thus indicate a normalization of NKG2D levels. NKG2D down-regulation has also been related to reactive oxygen species [31]. Using HbA1c and ox-LDL levels as indicators for oxidative imbalance, CoQ10 had a slight but statistically significant effect on the oxidative status of diabetic patients. In contrast to the changes of NKG2D expression, where up-regulated NKG2D levels were associated with Q10 supplementation only in T1DM patients, the anti-oxidative effect was more pronounced in T2DM patients, and therefore cannot explain conclusively the increase of NKG2D-positive NK cells. The enrichment of CD56bright and CD11c+ NK cells implies a shift towards NK cells, with a higher cytokine production capacity in PBMCs after CoQ10 treatment, especially in T1DM patients [32]. This is particularly interesting as the loss of NK cell activity has been reported previously for T1DM and other autoimmune diseases [18,20].
In summary, we show distinct differences between T1DM and T2DM patients regarding serum antimicrobial peptide levels and NK cell subsets. Supplementation with CoQ10 improved NK cell activity and reduced hBD2 expression in T1DM patients. This suggests that the anti-oxidant CoQ10 also targets the immune system and especially corrects T1DM-induced disorders. This novel mechanism, by which CoQ10 could affect late complications in T1DM patients, merits further investigation.
Acknowledgments
We would like to thank Agneta Hilding, Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden for help with statistical analysis. The project was supported by the Family Erling-Persson Foundation (K. B.), The von Kantzow Foundation (J. G.), Storstockholms Diabetes Association (J. G.) and the Swedish Medical Research Council (K.B.). H. B. was supported by grants from Stiftelsen Sigurd och Elsa Goljes Minne, the Swedish Diabetes Foundation and Magnus Bergvalls stiftelse.
Disclosures
The authors have no financial conflicts of interest.
Author contributions
H. B., P. L., K. B., G. D. and A. B. designed the study. H. B., P. L. and J. G. performed the experiments and analysed the data. N. R. E. collected patient material. H. B., P. L. and A. B. wrote the paper. J. G., N. R. E., G. D. and K. B. commented on the writing of the paper.
References
- 1.Garcia C, Feve B, Ferre P, et al. Diabetes and inflammation: fundamental aspects and clinical implications. Diabetes Metab. 2010;36:327–338. doi: 10.1016/j.diabet.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Kalupahana NS, Moustaid-Moussa N, Claycombe KJ. Immunity as a link between obesity and insulin resistance. Mol Aspects Med. 2012;33:26–34. doi: 10.1016/j.mam.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 4.Ciornei CD, Tapper H, Bjartell A, Sternby NH, Bodelsson M. Human antimicrobial peptide LL-37 is present in atherosclerotic plaques and induces death of vascular smooth muscle cells: a laboratory study. BMC Cardiovasc Disord. 2006;6:49. doi: 10.1186/1471-2261-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bobryshev YV, Lord RS. Identification of natural killer cells in human atherosclerotic plaque. Atherosclerosis. 2005;180:423–427. doi: 10.1016/j.atherosclerosis.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 6.Edfeldt K, Agerberth B, Rottenberg ME, et al. Involvement of the antimicrobial peptide LL-37 in human atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:1551–1557. doi: 10.1161/01.ATV.0000223901.08459.57. [DOI] [PubMed] [Google Scholar]
- 7.Barnathan ES, Raghunath PN, Tomaszewski JE, Ganz T, Cines DB, Higazi Aa-R. Immunohistochemical localization of defensin in human coronary vessels. Am J Pathol. 1997;150:1009–1020. [PMC free article] [PubMed] [Google Scholar]
- 8.Higazi AA, Nassar T, Ganz T, et al. The alpha-defensins stimulate proteoglycan-dependent catabolism of low-density lipoprotein by vascular cells: a new class of inflammatory apolipoprotein and a possible contributor to atherogenesis. Blood. 2000;96:1393–1398. [PubMed] [Google Scholar]
- 9.Niyonsaba F, Nagaoka I, Ogawa H. Human defensins and cathelicidins in the skin: beyond direct antimicrobial properties. Crit Rev Immunol. 2006;26:545–576. doi: 10.1615/critrevimmunol.v26.i6.60. [DOI] [PubMed] [Google Scholar]
- 10.Diana J, Simoni Y, Furio L, et al. Crosstalk between neutrophils, B-1a cells and plasmacytoid dendritic cells initiates autoimmune diabetes. Nat Med. 2013;19:65–73. doi: 10.1038/nm.3042. [DOI] [PubMed] [Google Scholar]
- 11.Lan CC, Wu CS, Huang SM, et al. High-glucose environment inhibits p38MAPK signaling and reduces human beta-defensin-3 expression in keratinocytes. Mol Med. 2011;17:771–779. doi: 10.2119/molmed.2010.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Froy O, Hananel A, Chapnik N, Madar Z. Differential effect of insulin treatment on decreased levels of beta-defensins and Toll-like receptors in diabetic rats. Mol Immunol. 2007;44:796–802. doi: 10.1016/j.molimm.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Malik AN, Al-Kafaji G. Glucose regulation of beta-defensin-1 mRNA in human renal cells. Biochem Biophys Res Commun. 2007;353:318–323. doi: 10.1016/j.bbrc.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 14.Rivas-Santiago B, Trujillo V, Montoya A, et al. Expression of antimicrobial peptides in diabetic foot ulcer. J Dermatol Sci. 2012;65:19–26. doi: 10.1016/j.jdermsci.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Lan CC, Wu CS, Huang SM, et al. High-glucose environment reduces human beta-defensin-2 expression in human keratinocytes: implications for poor diabetic wound healing. Br J Dermatol. 2012;166:1221–1229. doi: 10.1111/j.1365-2133.2012.10847.x. [DOI] [PubMed] [Google Scholar]
- 16.Vidal SM, Khakoo SI, Biron CA. Natural killer cell responses during viral infections: flexibility and conditioning of innate immunity by experience. Curr Opin Virol. 2011;1:497–512. doi: 10.1016/j.coviro.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becknell B, Caligiuri MA. Natural killer cells in innate immunity and cancer. J Immunother. 2008;31:685–692. doi: 10.1097/CJI.0b013e318182de23. [DOI] [PubMed] [Google Scholar]
- 18.Brauner H, Elemans M, Lemos S, et al. Distinct phenotype and function of NK cells in the pancreas of nonobese diabetic mice. J Immunol. 2010;184:2272–2280. doi: 10.4049/jimmunol.0804358. [DOI] [PubMed] [Google Scholar]
- 19.Gur C, Porgador A, Elboim M, et al. The activating receptor NKp46 is essential for the development of type 1 diabetes. Nat Immunol. 2010;11:121–128. doi: 10.1038/ni.1834. [DOI] [PubMed] [Google Scholar]
- 20.Rodacki M, Svoren B, Butty V, et al. Altered natural killer cells in type 1 diabetic patients. Diabetes. 2007;56:177–185. doi: 10.2337/db06-0493. [DOI] [PubMed] [Google Scholar]
- 21.Thomas SR, Witting PK, Stocker R. A role for reduced coenzyme Q in atherosclerosis? Biofactors. 1999;9:207–224. doi: 10.1002/biof.5520090216. [DOI] [PubMed] [Google Scholar]
- 22.Turunen M, Wehlin L, Sjoberg M, et al. Beta2-integrin and lipid modifications indicate a non-antioxidant mechanism for the anti-atherogenic effect of dietary coenzyme Q10. Biochem Biophys Res Commun. 2002;296:255–260. doi: 10.1016/s0006-291x(02)00871-9. [DOI] [PubMed] [Google Scholar]
- 23.Sohet FM, Neyrinck AM, Pachikian BD, et al. Coenzyme Q10 supplementation lowers hepatic oxidative stress and inflammation associated with diet-induced obesity in mice. Biochem Pharmacol. 2009;78:1391–1400. doi: 10.1016/j.bcp.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Dallner G, Stocker R. Coenzyme Q10. In: Coates PM, Betz JM, Blackman MR, et al., editors. Encyclopedia of dietary supplements. Boca Raton, FL: CRC Press; 2010. pp. 157–165. [Google Scholar]
- 25.Schmelzer C, Döring F. Identification of LPS-inducible genes downregulated by ubiquinone in human THP-1 monocytes. Biofactors. 2010;36:222–228. doi: 10.1002/biof.93. [DOI] [PubMed] [Google Scholar]
- 26.Schmelzer C, Kubo H, Mori M, et al. Supplementation with the reduced form of Coenzyme Q10 decelerates phenotypic characteristics of senescence and induces a peroxisome proliferator-activated receptor-alpha gene expression signature in SAMP1 mice. Mol Nutr Food Res. 2010;54:805–815. doi: 10.1002/mnfr.200900155. [DOI] [PubMed] [Google Scholar]
- 27.Schmelzer C, Lorenz G, Rimbach G, Döring F. In vitro effects of the reduced form of coenzyme Q(10) on secretion levels of TNF-alpha and chemokines in response to LPS in the human monocytic cell line THP-1. J Clin Biochem Nutr. 2009;44:62–66. doi: 10.3164/jcbn.08-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmelzer C, Lorenz G, Rimbach G, Döring F. Influence of coenzyme Q10 on release of pro-inflammatory chemokines in the human monocytic cell line THP-1. Biofactors. 2007;31:211–217. doi: 10.1002/biof.5520310308. [DOI] [PubMed] [Google Scholar]
- 29.Theuri G, Dallner G, Brismar K, Tekle M. Effects of lifestyle on plasma levels of the IGF system and the antioxidants coenzyme Q10 and vitamin E in Kenyan rural and urban populations. Growth Horm IGF Res. 2013;23:68–75. doi: 10.1016/j.ghir.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Barnea M, Madar Z, Froy O. Glucose and insulin are needed for optimal defensin expression in human cell lines. Biochem Biophys Res Commun. 2008;367:452–456. doi: 10.1016/j.bbrc.2007.12.158. [DOI] [PubMed] [Google Scholar]
- 31.Peraldi MN, Berrou J, Dulphy N, et al. Oxidative stress mediates a reduced expression of the activating receptor NKG2D in NK cells from end-stage renal disease patients. J Immunol. 2009;182:1696–1705. doi: 10.4049/jimmunol.182.3.1696. [DOI] [PubMed] [Google Scholar]
- 32.Blasius AL, Barchet W, Cella M, Colonna M. Development and function of murine B220+CD11c+NK1.1+ cells identify them as a subset of NK cells. J Exp Med. 2007;204:2561–2568. doi: 10.1084/jem.20070991. [DOI] [PMC free article] [PubMed] [Google Scholar]


