Abstract
A diagnosis of idiopathic anaphylaxis following a detailed clinical assessment remains very challenging for patients and clinicians. Risk reduction strategies such as allergen avoidance are not possible. This study investigated whether the (ISAC) allergen array with 103 allergens would add diagnostic value in patients with idiopathic anaphylaxis. We extended the specific immunoglobulin (Ig)E testing in 110 patients with a diagnosis of idiopathic anaphylaxis from five UK specialist centres using ISAC arrays. These were divided into three groups: score I identified no new allergen sensitization beyond those known by previous assessment, score II identified new sensitizations which were not thought likely to explain the anaphylaxis and score III identified new sensitizations felt to have a high likelihood of being responsible for the anaphylaxis. A proportion (50%) of score III patients underwent clinical reassessment to substantiate the link to anaphylaxis in this group. The results show that 20% of the arrays were classified as score III with a high likelihood of identifying the cause of the anaphylaxis. A wide range of major allergens were identified, the most frequent being omega-5-gliadin and shrimp, together accounting for 45% of the previously unrecognized sensitizations. The ISAC array contributed to the diagnosis in 20% of patients with idiopathic anaphylaxis. It may offer additional information where a careful allergy history and follow-on testing have not revealed the cause of the anaphylaxis.
Keywords: component resolved diagnostics (CRD), exercise induced anaphylaxis (EIA), food dependent exercise induced anaphylaxis (FDEIA), idiopathic anaphylaxis, ISAC allergen array
Introduction
Anaphylaxis is a severe hypersensitivity reaction of rapid onset, which may be fatal if untreated [1–3]. The causes of anaphylaxis have been divided into four main categories: immunologically mediated, which may be either immunoglobulin (Ig)E-dependent or IgE-independent, non-immunological and idiopathic [3] (Table 1).
Table 1.
Causes of anaphylaxis
| Immunological IgE-dependent | Immunological IgE-independent | Non-immunological | Idiopathic | Disorders which may mimic anaphylaxis |
|---|---|---|---|---|
| Foods (peanut, tree nut, shellfish, fish, milk, egg, soybean, peach, sesame, wheat) | Radio contrast media | Physical (exercise, cold, heat, sunlight) | Previously unrecognized allergen | Carcinoid syndrome |
| Stinging insects (venom) | NSAIDs | Ethanol | Mastocytosis or clonal mast cell disorders | Phaeochromcytoma |
| Medications (β lactam antibiotics, NSAIDs, biological agents, e.g. monoclonal antibodies) | Dextrans | Medications (e.g. opioids) | Hereditary angioedema | |
| Natural rubber latex | Biological agents (e.g. monoclonal antibodies) | Acquired angioedema | ||
| Occupational allergens | Panic attacks | |||
| Seminal fluid | ||||
| Aeroallergens | ||||
| Radio contrast media |
Ig = immunoglobulin; NSAID = non-steroidal anti-inflammatory drugs. [modified from Simons et al., World Allergy Organization (WAO) anaphylaxis guidelines [3]]
The most common causes of anaphylaxis are food, insect venom and drug allergies. Food-mediated anaphylaxis is more common in children and teenagers than adults and typically associated with milk, egg, nuts and seafood (fish and shellfish) [4], with peanut being the most common cause in the United States [5,6]. Anaphylaxis due to insect stings and drugs is more common in adults [7,8].
Exercise-induced anaphylaxis (EIA) [9] is thought to be the cause of 5–10% of all cases of anaphylaxis [10]. In approximately one-third to a half of patients with EIA, the ingestion of specific foods followed by physical effort within 4–6 h is necessary to induce anaphylaxis [11,12]. Commonly implicated foods in food-dependent exercise-induced anaphylaxis (FDEIA) include wheat (omega-5-gliadin), shellfish, peanuts, seeds, cow's milk, fruits and vegetables [12].
This study investigates patients with idiopathic anaphylaxis who may have anaphylaxis due to a previously unrecognized allergen. Idiopathic anaphylaxis for the purposes of this study is defined as anaphylaxis where, despite a careful allergy history in a specialist centre and a combination of skin prick testing (SPT) and laboratory testing for specific IgE, it has not been possible to determine the trigger for the anaphylaxis. In addition, every attempt has been made to exclude an underlying mast cell disorder and other conditions which may occasionally mimic anaphylaxis (Table 1).
Idiopathic anaphylaxis is a difficult and challenging diagnosis. For the patient, this means living with the uncertainty and the potential risk of future episodes of anaphylaxis from an unidentified trigger and having to carry an adrenaline auto-injector. From the perspective of the clinician, the inability to identify a trigger means that usual anaphylaxis interventions such as avoidance measures, specific education and modification of risk are not possible. Moreover, these patients usually require long-term follow-up and reinvestigation.
The prevalence of anaphylaxis is increasing [13], with the current lifetime prevalence rate between 0·5 and 2% in the Western world [14]. Although idiopathic anaphylaxis involves a small proportion of patients with anaphylaxis, the clinical implications are highly significant. While the allergy history and subsequent testing informed by the history remains the gold standard, the aim of this study was to determine whether the use of an allergen microarray (ImmunoCAP ISAC; Phadia/Thermo Fisher, Uppsala, Sweden) improved the detection of relevant trigger factors in idiopathic anaphylaxis.
Methods
Patients
Patients included in the study had been diagnosed with idiopathic anaphylaxis on the basis of clinical assessment, SPT and laboratory testing for allergen specific IgE and mast cell tryptase (MCT) with no causative allergen identified. Serum from a total of 110 adult patients (73 female : 37 male, ratio 2:1, mean age 42 years (range 20–76 years) from five UK National Health Service (NHS) specialist allergy centres (University Hospital of Wales, Cardiff, Derriford Hospital, Plymouth/Exeter, Bristol/Gloucester, St Helier Hospital, Carshalton and Guildford) was tested to extend the routine clinical evaluation of specific IgE (sIgE). Fifty per cent of patients in whom a relevant trigger was identified (11 of 22) were recalled in order to obtain further clinical history or undergo additional SPT and challenge testing to substantiate the relationship between the sIgE results from the ISAC array and the clinical symptoms. The South West 1 Research Ethics Committee Chair confirmed that the project and patient recall at Derriford (where recall took place) did not require ethical approval.
Serum analysis
Routine serum measurements of specific IgE, total IgE and MCT were performed using a fluoroenzyme immunoassay (FEIA) auto-analyser, the ImmunoCAP 250 platform (Phadia/Thermo Fisher, Uppsala, Sweden), according to the manufacturer's guidelines.
The allergen microarray assay (ImmunoCAP ISAC 103; Phadia/Thermo Fisher) was used to determine the specific IgE repertoire of each patient's serum. The clinical utility and comparability of the serum IgE measurements of the ISAC compared to the FEIA platform has been reported previously [15–17]. Serum concentrations of the specific IgEs reacting to 103 allergen components from 43 allergen sources were measured using the ImmunoCAP ISAC platform. ISAC reactions were performed according to the manufacturer's instructions [18].
Processed ISAC slides were scanned using a GenePix 4000B microarray scanner (Molecular Devices, Sunnyvale, CA, USA) and image acquisition was performed using GenePix Pro (Molecular Devices).
Image analysis was performed using the Microarray Image Analyzer (MIA: Phadia/Thermo Fisher). All new positive ISAC results were retested by ImmunoCAP and only taken to be positive if confirmed by ImmunoCAP.
Clinical scoring
A simple clinical scoring system (ISAC score) was used to determine two factors: first whether there was new information, and secondly whether this was likely to aid in diagnosis of the anaphylaxis. To ensure consistency, the evaluation was undertaken in a single centre (Cardiff) by three consultants independently assessing the clinical and laboratory information for all patients. Scores with a difference of opinion were re-evaluated to clarify the scores given, and where there was discrepancy the lower score was allocated to avoid over-estimation of higher scores.
ISAC score I
No new allergen sensitization was found using the ISAC reactions beyond those already known via routine investigations with SPT and/or ImmunoCAP testing.
ISAC score II
Score II concerned new, previously unrecognized allergen sensitizations that had not been found during routine investigations with SPT and/or ImmunoCAP testing. These new sensitizations were not thought to be associated with the anaphylaxis.
Allergens were excluded as a potential cause of anaphylaxis by the nature of the allergen reactivity detected (e.g. aeroallergens such as grass pollen, which is very unlikely to result in anaphylaxis) and by careful analysis of the clinical history and notes.
ISAC score III
Score III concerned new sensitizations that had not been found previously during routine investigations with SPT and/or ImmunoCAP testing. These new sensitizations were thought to have a strong association with the anaphylaxis.
The new sensitizations were to heat- and digestion-stable allergens in categories associated with severe reactions, including anaphylaxis (e.g. lipid transfer proteins and storage proteins); in addition, 50% of patients in the score III group were recalled for the history to be re-evaluated, further testing or challenge. Statistical analysis was performed using spss statistics version 20 (IBM, New York City, NY, USA).
Results
A total of 110 patients were studied: 73 female and 37 male, mean age 42 years (range 20–76 years). The mean serum concentration of the total IgE was 451 kU/l (n = 105, range < 2–13 946 kU/l). There was a linear relationship between total IgE and the number of positive sIgE results (data not shown). All patients analysed (107) had a baseline serum MCT concentration of < 15·0 μg/l (not measured during or within 24 h of an episode of anaphylaxis). Mean baseline serum MCT measurements were 4·3 μg/l (range 1·0–11·0 μg/l). In three patients baseline MCT measurements were recorded as < 1·0 μg/l and in 20 patients baseline MCT measurements were recorded as < 15·0 μg/l. Baseline MCT measurements were not available in three patients (one in each of the three score groups). No patients had clinical features consistent with systemic mastocytosis.
The ISAC scores for the 110 subjects in the cohort were score I, 53 (48%), score II, 35 (32%) and score III, 22 (20%) (Fig. 1). A total of 594 positive sensitizations to allergen components were found by ISAC analysis of the 110 patients, of which 183 sensitizations (31%) were not previously known.
Fig. 1.
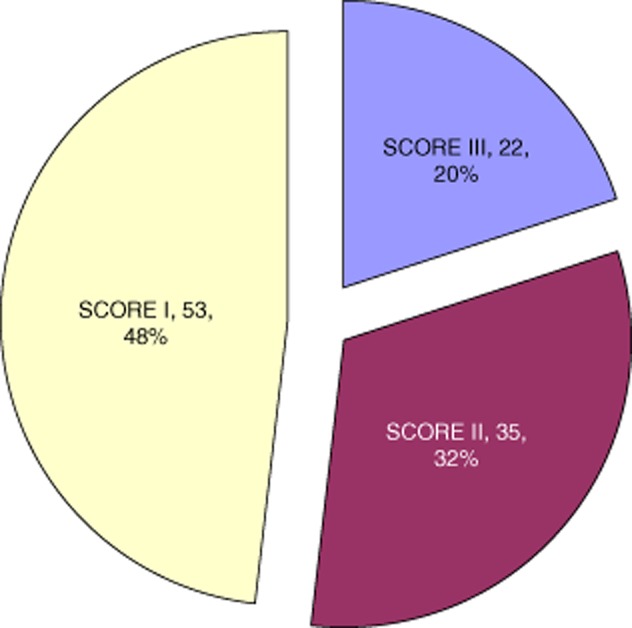
Clinical scores of ISAC arrays. The clinical scores of the ISAC arrays are shown. In score I no additional allergen sensitizations were identified; in score II new sensitizations not thought responsible for the anaphylaxis were identified; and in score III new sensitizations thought to have a strong likelihood of causing the anaphylaxis were identified.
ISAC score I
In 53 patients (48% of the cohort), no new allergen sensitizations were found by the ISAC reaction (ISAC score I). Seventy-four per cent of the score I patients (n = 39, 35% of the total cohort) had blank ISAC reactions with no allergen components revealed as positive (ISU < 0·3). The remaining 26% of the ISAC score I patients (n = 14, 12% of the cohort) had ISAC reactions which found allergic sensitizations that were already known to the investigating clinician (as a result of previous SPT and/or ImmunoCAP-specific IgE serum testing). A total of 69 positive components were detected in the 53 members of the ISAC score I group.
ISAC score II
A total of 35 subjects had an ISAC score of II (32% of the cohort) and 322 positive components were detected. New sensitizations found in the ISAC score II patients were predominantly aeroallergens, including pollens (n = 24, 69% of score II), house dust mite (HDM) (n = 22, 63%) and animal danders (n = 15, 43%).
Sensitization to multiple PR10 components (Birch Bet v1 plus multiple food and pollen PR10 components) was found in five (14%) score II patients. Of these patients, three had symptoms in addition to anaphylaxis that were compatible with OAS/pollen-food syndrome.
It should, however, be noted that potential triggers of anaphylaxis were detected in patients who were subsequently scored as ISAC score groups I and II. These included reactivity to components of honey bee venom, latex, wheat, shrimp, peanut, hazelnut, serum albumins and milk. This reflects the clinical and laboratory stringency of the scoring in that weak positives, especially if not confirmed on CAP testing, were not included in score III.
ISAC score III
Twenty-two patients from the anaphylaxis cohort (20%) had a score of III, and in this group there were 203 sensitizations. These included 35, thought on the basis of the history and the heat- and digestion-stable nature of the allergen [e.g. lipid transfer protein (LTP) or storage protein] to be highly likely to be responsible for the anaphylaxis. It was possible to recall 11 of the 22 patients in this group (50% of score III) to substantiate the relationship between the newly identified triggers and anaphylaxis by re-evaluation of the history, SPT and challenge testing, where appropriate. In all 11 cases the new trigger was confirmed as likely to be relevant to the anaphylaxis. By the time of recall, further clinical information was available to clarify the relationship in four of the patients who had challenged themselves with the allergen by accidental exposure. In a further two patients challenges were undertaken and the remainder were not challenged, as it was felt clinically inappropriate or they refused.
All recalled patients and their general practitioners (GPs) were advised to contact the Allergy Centre and return to the clinic if symptoms recurred – no patients recontacted the Centre.
Eight of this subgroup of 22 patients (36%) had more than one likely potential trigger of their anaphylactic episodes. Seven patients had two unrelated allergens and one patient had reactions to four unrelated allergic triggers, peanut, soybean, latex and fish parvalbumin (carp and cod). A summary of the allergic sensitization in score III patients is shown in Fig. 2, with a breakdown of the allergic triggers of each of the score III patients in Fig. 3. Complete ISAC results and patient demographics are shown in Supporting information, Table S1).
Fig. 2.
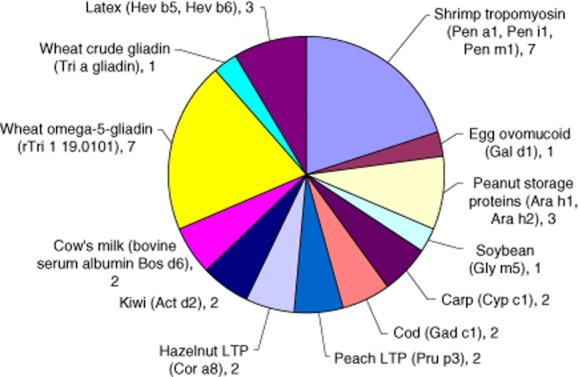
ISAC-determined positive allergen component-specific immunoglobulin (Ig)E results in the score III group. The 35 allergens and components in 22 patients from the score III (new sensitizations thought to have a strong likelihood of causing the anaphylaxis) with the number of patients positive for each are shown.
Fig. 3.
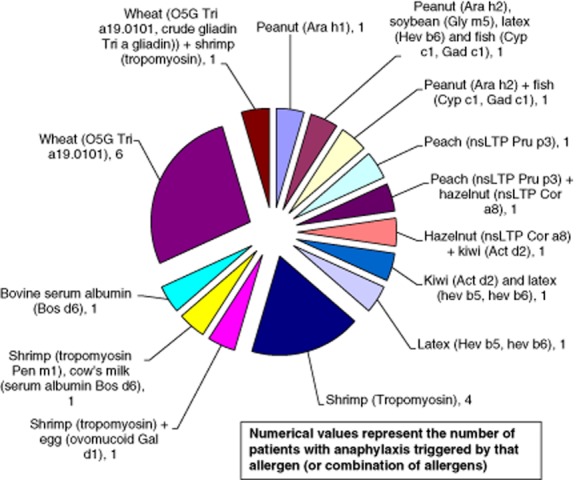
ISAC-determined allergic trigger(s) in each of the 22 score III patients. The ISAC-identified triggers of anaphylaxis in each individual patient in the score III group is shown, demonstrating the frequency of single and multiple sensitization.
Seven (32%) of the score III patients were found to have anaphylaxis as a result of exposure to wheat, specifically wheat omega-5-gliadin (O5G, Tri a 19·0101), and this was the sole trigger in six cases. In a single patient, reactivity to wheat (O5G Tri a 19 and crude gliadin Tri a gliadin) was accompanied by an allergy to shrimp tropomyosin components.
Reactivity to shrimp was found as a trigger of unexplained anaphylaxis in seven (32%) score III patients. Shrimp tropomyosin components (Pen a1, Pen i1 and Pen m1) were the sole triggers of anaphylaxis identified in four score III patients. In a further three patients, reactions to shrimp components were accompanied by reactivity to egg, beef meat/cow's milk [bovine serum albumin (BSA)] and wheat, respectively. In five of the seven shrimp allergic patients a cross-over of specific IgE reactivity was seen with other tropomyosin components from other species, including HDM (Der p10), cockroach (Bla g7) and Anisakis (Ani s3).
Peanut storage proteins (Ara h1, Arah2) as an allergic trigger of anaphylaxis were found in three of the score III patients. In a single patient, peanut Ara h1 was the sole trigger of the anaphylactic episodes.
Hazelnut non-specific LTP (nsLTP) was found as a co-trigger of anaphylaxis in two ISAC score III patients. In one of the hazelnut-allergic patients there was also reactivity to kiwi Act d2. The remaining ISAC score III individual with hazelnut-triggered episodes also had reactions to peach nsLTP (Cor a8). A single patient had anaphylaxis for which only peach nsLTP (Cor a8) was identified as a precipitating agent.
Latex was identified as an anaphylaxis-precipitating agent in three of the ISAC score III patients. In a single patient the latex components Hev b5 and Hev b6 were the only precipitating allergens identified. The remaining patients had reactions triggered by latex (Hev b5 and Hev b6) and kiwi (Act d2) or had reactivity to Hev b6 along with multiple other major allergen components of peanut (Arah2), soybean (Gly m5) and fish parvalbumins (Cyp c1, Gad c1).
Reactivity to fish parvalbumins was seen in a patient who also reacted to peanut storage proteins (Ara h2). Reactivity to egg (Gal d1) was seen only in a single patient in conjunction with reactions triggered by shrimp tropomyosin component (Pen a1). Reactions to BSA were seen in conjunction with shrimp tropomyosin (Pen m1 as well as HDM and cockroach tropomyosin) and as the only identified allergen responsible for anaphylactic episodes. In both cases reactivity to BSA was accompanied by sensitization to other serum albumins, including those from cat (Fel d2), dog (Can f3) and horse (Equ c3). Bos d6 is a minor milk allergen [19–21] and major beef allergen [22–24]. Reactivity to beef appeared to be genuine in the patient with sole BSA sensitization.
Within the ISAC score III group, additional sensitizations to allergens which were not known previously and not considered likely precipitants of the patient's anaphylaxis were also found. The majority of these sensitizations were to aeroallergens, including plant pollen components, HDM, animal danders and fungal allergens Fig. 4.
Fig. 4.
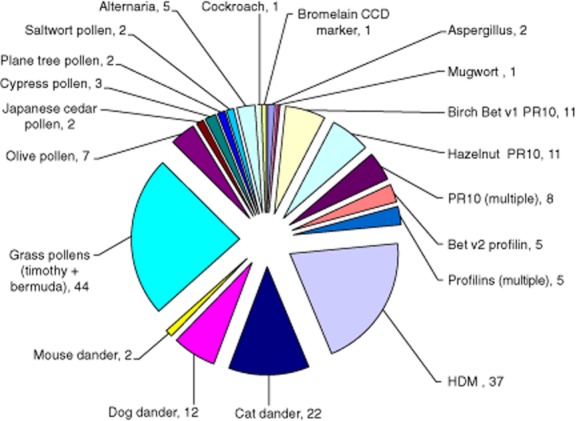
New allergen sensitizations demonstrated by the ISAC and not considered triggers of anaphylaxis. New ISAC-identified allergen sensitizations in 57 patients from score II and score III groups not thought to be triggers of the anaphylaxis which were predominantly aeroallergens. Sensitization to multiple PR10 and profilin components were detected.
Discussion
The current study addresses whether the ISAC array assists in the diagnosis of previously unrecognized sensitizations causing idiopathic anaphylaxis. In 22 of 110 cases (20%) the ISAC array showed sensitizations with score III and thus a high likelihood of a causal relationship for the anaphylaxis. This is an unexpectedly high number, given that the ISAC 103 array was not designed as a screen for idiopathic anaphylaxis and lacks a number of key allergens (including alpha-gal, lupin, walnut, horse, an extended panel of shellfish, fish and infrequently consumed nuts), some of which have been included in the more recent ISAC 113 array. In addition, tests using recombinant allergens may have a lower sensitivity than those using allergen extracts, where all isoforms of any particular allergen are expressed, this being balanced against issues of standardization and the presence of irrelevant proteins in native allergen extracts [25–27].
Potential shortcomings of this study include the effects of the stringency used to avoid over-estimating the numbers of relevant new positive results. A score III was only given when there was unanimous agreement of three independent consultant allergists, and a score II given if there was any disagreement. Therefore, it is possible that a number of potentially relevant allergens may have been given scores of II or I, resulting in a potential underestimate of score III. Infrequent descriptions of anaphylaxis to aeroallergens such as alpine slide anaphylaxis are reported [28,29], and these would also not have been included in score III. The current study also concentrated on adults, and it would be important to study the role of the ISAC array in IA in children. It was only possible to recall and reassess 50% of the patients with score III to substantiate the importance of the new findings.
The results also question the reliability of the allergy history, as it is this which most often guides subsequent testing. The history, however, is also a ‘test’ which should therefore become amenable to analysis using performance characteristics such as sensitivity and specificity. Several areas are highlighted by the study which may be important in reducing the sensitivity of the history. These include the potential effect on recall of a long interval between the event and the history being taken, the presence of sensitization to multiple relevant allergens and dissociation in time between exposure to the allergen and the reaction, as in FDEIA. The score III results include allergens in almost all of the categories for which FDEIA has been described (omega-5-gliadin, shellfish, peanuts, seeds, cow's milk, fruits and vegetables) [12]. There may also be circumstances where the patient, even with perfect recall, is not aware of an exposure. It is possible that this could arise due to mislabelling (a recent example in the United Kingdom and Europe concerned horsemeat which had been found in numerous ‘beef'-labelled products; in one case, a well-known manufacturer of beef lasagne was found to contain 100% horsemeat). In the present study reactions to horse were score II or I, given the uncertainty of the relationship to anaphylaxis.
Although the ISAC array (cost £150) is more expensive than SPT and individual CAP tests (£12–14 each) new possible causative sensitizations were found in 20% of idiopathic anaphylaxis patients that would otherwise be missed. In these patients the new information could lead to targeted risk reduction and less uncertainty for patients. This may potentially reduce recurrent episodes and the requirement for subsequent medical care, as well as the need to consider other more costly interventions such as omalizumab. Additional information from the array may also alter management in those with sensitizations not related to anaphylaxis or the 70% of score I patients with a blank array, although an analysis of this was not the purpose of the study. It is however, tempting to speculate that an array with zero positive sensitizations may be associated with a lower probability of anaphylaxis mediated through an IgE-dependent mechanism. Increasing use of multiplex testing platforms such as the ISAC array will probably lead to additional clinical challenges in the management of new unexpected positive results as well as yielding potential false positive results, and presenting a large data set to interpret.
In conclusion, while the allergy history and guided testing remains the gold standard for evaluation of patients with idiopathic anaphylaxis, the ISAC array may be of diagnostic utility in selected patients. The variability in the amount of food consumed, degree of exercise and the time interval between the two reduces the sensitivity of the history in the case of FDEIA. We suggest that consideration should be given to testing for covert allergy in unexplained anaphylaxis to omega-5-gliadin and shrimp and possibly other allergens known to be associated with FDEIA, regardless of whether there is an obvious supporting history. In this group of patients there is a case for performing an ISAC allergy microarray if diagnostic uncertainty remains. Improved understanding of the mechanism underlying FDEIA would further improve management in this group of patients.
Acknowledgments
S. J. and A. H. are supported by NISCHR Fellowships.
Disclosures
A. H. has received limited fees and travelling expenses for speaking at a symposium organized by Phadia (Thermo Scientific). ISAC reagents for this study were provided by Phadia (Thermo Scientific). E. K. received support for consumables from Phadia/Thermofisher. S. J. has received speaker/conference support and funding for consumables from Phadia/Thermofisher for this study. S. C., C. C., M. M., J. O., S. D., N. S., A. B., G. S., T. E.-S. and P. W. have no conflicts of interest.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web-site:
Table S1. Score III patient demographics, diagnoses and complete ISAC array results.
References
- 1.Sampson HA, Munoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report – Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117:391–397. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 2.Simons FE, Ardusso LR, Bilo MB, et al. World Allergy Organization anaphylaxis guidelines: summary. J Allergy Clin Immunol. 2011;127:587–93 e1-22. doi: 10.1016/j.jaci.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 3.Simons FE, Ardusso LR, Bilo MB, et al. World Allergy Organization guidelines for the assessment and management of anaphylaxis. World Allergy Organ J. 2011;4:13–37. doi: 10.1097/WOX.0b013e318211496c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol. 2010;125(Suppl. 2):S116–125. doi: 10.1016/j.jaci.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 5.Furlong TJ, DeSimone J, Sicherer SH. Peanut and tree nut allergic reactions in restaurants and other food establishments. J Allergy Clin Immunol. 2001;108:867–870. doi: 10.1067/mai.2001.119157. [DOI] [PubMed] [Google Scholar]
- 6.Sicherer SH, Furlong TJ, Munoz-Furlong A, Burks AW, Sampson HA. A voluntary registry for peanut and tree nut allergy: characteristics of the first 5149 registrants. J Allergy Clin Immunol. 2001;108:128–132. doi: 10.1067/mai.2001.115755. [DOI] [PubMed] [Google Scholar]
- 7.Golden DB. Insect sting allergy and venom immunotherapy: a model and a mystery. J Allergy Clin Immunol. 2005;115:439–447. doi: 10.1016/j.jaci.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Bilo MB, Bonifazi F. The natural history and epidemiology of insect venom allergy: clinical implications. Clin Exp Allergy. 2009;39:1467–1476. doi: 10.1111/j.1365-2222.2009.03324.x. [DOI] [PubMed] [Google Scholar]
- 9.Sheffer AL, Austen KF. Exercise-induced anaphylaxis. J Allergy Clin Immunol. 1980;66:106–111. doi: 10.1016/0091-6749(80)90056-1. [DOI] [PubMed] [Google Scholar]
- 10.Du Toit G. Food-dependent exercise-induced anaphylaxis in childhood. Pediatr Allergy Immunol. 2007;18:455–463. doi: 10.1111/j.1399-3038.2007.00599.x. [DOI] [PubMed] [Google Scholar]
- 11.Kidd JM, III, Cohen SH, Sosman AJ, Fink JN. Food-dependent exercise-induced anaphylaxis. J Allergy Clin Immunol. 1983;71:407–411. doi: 10.1016/0091-6749(83)90070-2. [DOI] [PubMed] [Google Scholar]
- 12.Barg W, Medrala W, Wolanczyk-Medrala A. Exercise-induced anaphylaxis: an update on diagnosis and treatment. Curr Allergy Asthma Rep. 2011;11:45–51. doi: 10.1007/s11882-010-0150-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Institute for Clinical Excellence (NICE) 2011. Clinical Guideline 134Anaphylaxis: Assessment to Confirm an Anaphylactic Episode and the Decision to Refer after Emergency Treatment for a Suspected Anaphylactic Episode. London: NICE.
- 14.Lieberman P, Camargo CA, Jr, Bohlke K, et al. Epidemiology of anaphylaxis: findings of the American College of Allergy, Asthma and Immunology Epidemiology of Anaphylaxis Working Group. Ann Allergy Asthma Immunol. 2006;97:596–602. doi: 10.1016/S1081-1206(10)61086-1. [DOI] [PubMed] [Google Scholar]
- 15.Hiller R, Laffer S, Harwanegg C, et al. Microarrayed allergen molecules: diagnostic gatekeepers for allergy treatment. FASEB J. 2002;16:414–416. doi: 10.1096/fj.01-0711fje. [DOI] [PubMed] [Google Scholar]
- 16.Wohrl S, Vigl K, Zehetmayer S, et al. The performance of a component-based allergen-microarray in clinical practice. Allergy. 2006;61:633–639. doi: 10.1111/j.1398-9995.2006.01078.x. [DOI] [PubMed] [Google Scholar]
- 17.Jahn-Schmid B, Harwanegg C, Hiller R, et al. Allergen microarray: comparison of microarray using recombinant allergens with conventional diagnostic methods to detect allergen-specific serum immunoglobulin E. Clin Exp Allergy. 2003;33:1443–1449. doi: 10.1046/j.1365-2222.2003.01784.x. [DOI] [PubMed] [Google Scholar]
- 18.Deinhofer K, Sevcik H, Balic N, et al. Microarrayed allergens for IgE profiling. Methods. 2004;32:249–254. doi: 10.1016/j.ymeth.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 19.Wal JM. Bovine milk allergenicity. Ann Allergy Asthma Immunol. 2004;93(Suppl. 3):S2–11. doi: 10.1016/s1081-1206(10)61726-7. [DOI] [PubMed] [Google Scholar]
- 20.Szepfalusi Z, Ebner C, Urbanek R, et al. Detection of IgE antibodies specific for allergens in cow milk and cow dander. Int Arch Allergy Immunol. 1993;102:288–294. doi: 10.1159/000236538. [DOI] [PubMed] [Google Scholar]
- 21.Neyestani TR, Djalali M, Pezeshki M. Isolation of alpha-lactalbumin, beta-lactoglobulin, and bovine serum albumin from cow's milk using gel filtration and anion-exchange chromatography including evaluation of their antigenicity. Protein Expr Purif. 2003;29:202–208. doi: 10.1016/s1046-5928(03)00015-9. [DOI] [PubMed] [Google Scholar]
- 22.Han GD, Matsuno M, Ito G, Ikeucht Y, Suzuki A. Meat allergy: investigation of potential allergenic proteins in beef. Biosci Biotechnol Biochem. 2000;64:1887–1895. doi: 10.1271/bbb.64.1887. [DOI] [PubMed] [Google Scholar]
- 23.Fiocchi A, Restani P, Riva E. Beef allergy in children. Nutrition. 2000;16:454–457. doi: 10.1016/s0899-9007(00)00285-9. [Review] [DOI] [PubMed] [Google Scholar]
- 24.Fuentes Aparicio V, Sanchez Marcen I, Perez Montero A, Baeza ML, de Barrio Fernandez M. Allergy to mammal's meat in adult life: immunologic and follow-up study. J Invest Allergol Clin Immunol. 2005;15:228–231. [Case reports] [PubMed] [Google Scholar]
- 25.Hamilton RG. Clinical laboratory assessment of immediate-type hypersensitivity. J Allergy Clin Immunol. 2010;125(Suppl. 2):S284–296. doi: 10.1016/j.jaci.2009.09.055. [Review] [DOI] [PubMed] [Google Scholar]
- 26.Chapman MD, Smith AM, Vailes LD, Arruda LK, Dhanaraj V, Pomes A. Recombinant allergens for diagnosis and therapy of allergic disease. J Allergy Clin Immunol. 2000;106:409–418. doi: 10.1067/mai.2000.109832. [DOI] [PubMed] [Google Scholar]
- 27.Steckelbroeck S, Ballmer-Weber BK, Vieths S. Potential, pitfalls, and prospects of food allergy diagnostics with recombinant allergens or synthetic sequential epitopes. J Allergy Clin Immunol. 2008;121:1323–1330. doi: 10.1016/j.jaci.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Spitalny KC, Farnham JE, Witherell LE, et al. Alpine slide anaphylaxis. N Engl J Med. 1984;310:1034–1037. doi: 10.1056/NEJM198404193101607. [DOI] [PubMed] [Google Scholar]
- 29.Tsunoda K, Ninomiya K, Hozaki F, Kaga K. Anaphylaxis in a child playing in tall grass. Allergy. 2003;58:955–956. doi: 10.1034/j.1398-9995.2003.00121.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Score III patient demographics, diagnoses and complete ISAC array results.


