Abstract
While murine CD4+CD39+ regulatory T cells (Treg) co-express CD73 and hydrolyze exogenous (e) adenosine triphosphate (ATP) to immunosuppressive adenosine (ADO), surface co-expression of CD73 on human circulating CD4+CD39+ Treg is rare. Therefore, the ability of human Treg to produce and utilize ADO for suppression remains unclear. Using mass spectrometry, we measured nucleoside production by subsets of human CD4+CD39+ and CD4+CD39(–)CD73+ T cells or CD19+ B cells isolated from blood of 30 volunteers and 14 cancer patients. CD39 and CD73 expression was evaluated by flow cytometry, Western blots, confocal microscopy or reverse transcription–polymerase chain reaction (RT–PCR). Circulating CD4+CD39+ Treg which hydrolyzed eATP to 5′-AMP contained few intracytoplasmic granules and had low CD73 mRNA levels. Only ∼1% of these Treg were CD39+CD73+. In contrast, CD4+CD39negCD73+ T cells contained numerous CD73+ granules in the cytoplasm and strongly expressed surface CD73. In vitro-generated Treg (Tr1) and most B cells were CD39+CD73+. All these CD73+ T cell subsets and B cells hydrolyzed 5′-AMP to ADO. Exosomes isolated from plasma of normal control (NC) or cancer patients carried enzymatically active CD39 and CD73+ and, when supplied with eATP, hydrolyzed it to ADO. Only CD4+CD39+ Treg co-incubated with CD4+CD73+ T cells, B cells or CD39+CD73+ exosomes produced ADO. Thus, contact with membrane-tethered CD73 was sufficient for ADO production by CD4+CD39+ Treg. In microenvironments containing CD4+CD73+ T cells, B cells or CD39+CD73+ exosomes, CD73 is readily available to CD4+CD39+CD73neg Treg for the production of immunosuppressive ADO.
Keywords: adenosine, ectonucleotidases, exosomes, human tTreg, immune suppression, pTreg
Introduction
Adenosine (ADO) has long been known as one of major factors responsible for immune suppression in inflammatory environments [1,2]. The exogenous (e) ADO pathway is primarily responsible for hydrolysis of ATP which, if present in excess, is toxic to cells [3]. Accumulations of ATP at chronic inflammatory sites, such as tumours or microbe-infected tissues, are avoided by the engagement of ectonucleotidases, CD39 and CD73, expressed by many different cell types, including immunocytes [4,5]. In 2006, Deaglio et al. [6] and Borsellino et al. [7] reported that murine regulatory T cells (Treg) co-expressed surface CD39 and CD73 and sequentially hydrolyzed eATP to 5′-AMP and then to ADO. We and others have confirmed the ability of human Treg present in the peripheral circulation or at tissue sites of inflammation to produce 5′-AMP and ADO and utilize ADO for immune suppression [8,9]. However, unlike murine CD4+forkhead box protein 3+ (FoxP3+) Treg, most of which co-expressed surface CD39 and CD73, only ∼1% of human peripheral blood CD4+CD39+FoxP3+ Treg had CD73 on the cell surface [10,11]. Thus, the absence of CD73 from Treg surface appears to distinguish human from murine Treg.
Another enzyme known to be involved in the ADO metabolism in lymphocytes is adenosine deaminase-1 (ADA), which hydrolyzes ADO to inosine. The absence of ADA or its functional abnormalities result in severe combined immunodeficiency (SCID) [12]. On the lymphocyte cell surface, ADA is associated closely with CD26, dipeptidyl peptidase IV, which functions as a surrogate marker for this enzyme [13]. In humans, Treg lack CD26 surface expression and are, therefore, unable to metabolize ADO, while conventional effector T cells (Teff) are usually ADA-positive [8]. It has been suggested that absence of CD26 could serve to identify human Treg and distinguishes them from CD4+ Teff [14].
Recent studies of human Treg suggest a much greater functional diversity among these cells than originally suspected [15]. This diversity is especially evident in disease. Peripheral (p) Treg, referred to previously as inducible (i) Treg [16], which accumulate in tissues and peripheral blood of patients with cancer, differ phenotypically and functionally from natural or thymus-derived (t) Treg present in the blood of normal controls (NCs) [17,18]. These pTreg seem to originate by conversion from tTreg or conventional CD4+ T cells (Tconv) via differentiation induced and maintained by antigens in the presence of soluble tumour-derived factors such as transforming growth factor (TGF)-β, interleukin (IL)-10 and ADO [19]. Another subset of in-vitro-induced Treg with attributes similar to those of pTreg are now referred to as in-vitro-generated regulatory Treg (Tr1) cells [16]. Circulating iTreg in cancer patients' blood up-regulate expression of surface molecules associated with immune suppression, including CD39 and CD73, and can produce ADO and prostaglandin E2 (PGE2) [20]. Co-operation between ADO and PGE2 in inducing immune suppression of Teff via the common cyclic adenosine monophosphate (cAMP) pathway is an excellent example of biological amplification and co-operation between signals delivered to target cells by different receptors [20].
Little is known about CD39, CD73 or CD26 expression on lymphocyte subsets other than Treg in humans, although the presence of ectonucleotidases on B cells has been described previously [21–23]. We recently reported that small subsets of CD4+ and CD8+ T cells express surface CD73 [24]. In the peripheral circulation of NC, the frequency of ADO-producing CD4+CD39(–) CD73+ T cells was ∼10% of all CD4+ T cells, but in HIV-1 patients with elevated C-reactive protein (CRP) serum levels and elevated percentages of circulating CD38+/human leucocyte antigen D-related+ (HLA-DR+) (activated) T cells, their frequency decreased to 1–2% [24]. These data suggested that the depletion of this CD4+ T cell subset capable of producing ADO could be partially responsible for persistent immune activation in HIV-1-infected patients [24].
Exosomes are 30–100 nm microvesicles present in body fluids of all individuals and enriched in cancer patients' body fluids. Exosomes isolated from supernatants of cancer cell lines were reported to carry CD39 and CD73 and to be able to hydrolyze eATP to immunosuppressive ADO [25]. Similarly, exosomes produced by Treg carried CD39 and CD73, which contributed to suppression mediated by Treg [26]. Exosomes are thought to mediate cell-to-cell communication [27], and their involvement in the adenosine pathway emphasizes the concept of intercellular co-operation in ADO production among various cells.
We have taken advantage of the recent progress in cell separation technologies and studied the expression of ectonucleotidases on human lymphocyte subsets in the peripheral circulation as well as exosomes present in the plasma of NC and cancer patients. The objective of this study is to show that these enzymes responsible for ADO production are universally available in the haematopoietic system, as they can be delivered tethered to membranes of cells or exosomes to regulatory cells responsible for ADO production. We show that such co-operation endows human Treg with an unlimited capacity to mediate ADO-driven immune suppression, and thus might contribute to tumour immune escape and perhaps other biological functions involving the adenosine pathway.
Materials and methods
Peripheral blood mononuclear cells (PBMC) collection
Buffy coats obtained from healthy volunteers (70–80 ml) were purchased from the Central Blood Bank of Pittsburgh. Blood samples from 14 untreated head and neck squamous cell carcinoma (HNSCC) patients with active disease and 30 normal donors (30–40 ml) were collected under an institutional review board (IRB)-approved protocol (IRB no. 991206). All subjects signed informed consent prior to blood draws. Venous blood was drawn into heparinized tubes and centrifuged on Ficoll-Hypaque gradients (GE Healthcare Bioscience, Pittsburgh, PA, USA). PBMC were recovered, washed in AIM-V medium (Invitrogen, Carlsbad, CA, USA) and used immediately for experiments. Plasma was stored in 2-ml vials at −20°C until further use.
Cell subset isolation
For negative selection of CD4+ cells by magnetic immunobeads, a biotin-conjugated antibody cocktail specific for lineage antigens was applied. Next, CD39+ T cells were isolated from the CD4+ T cell population using biotin-conjugated anti-CD39 antibodies and magnetic beads coated with anti-biotin antibodies, as described previously [10,28]. CD39neg T cells were separated into CD73+ and CD73neg populations by anti-CD73 antibody-coated beads. To obtain CD4+CD25+ T cell subsets, CD4+ T cell-enriched populations were separated into CD25+ and CD25neg subsets using beads coated with anti-CD25. CD19+ B cells were separated by one-step isolation using anti-CD19 antibody-coated beads. Cell separations were performed using the AutoMACS system and all reagents were purchased from Miltenyi Biotec (Auburn, CA, USA), as described previously [28].
Tumour cell lines
PCI-13, a HNSCC cell line established in our laboratory [14] and Kasumi-1, a human acute myeloid leukaemia (AML) cell line purchased from the American Type Culture Collection, were cultured as described previously [28]. Supernatants were collected and used for exosome isolation.
In-vitro generation of Tr1 cells
To generate Tr1 cells, we used the culture model consisting of CD4+CD25neg Tconv, autologous dendritic cells, allogeneic irradiated tumour cells and a mix of cytokines, as described previously [29]. After 10 of days of culture, Tr1 were harvested and evaluated for the phenotype by flow cytometry, co-expression of CD39 and CD73 by fluorescence microscopy and the ability to produce adenosine by mass spectrometry. Their suppressor function was measured in proliferation assays with autologous responder T cells, as described previously [29].
Flow cytometry
The following anti-human monoclonal antibodies (mAbs) were used for staining: CD39-fluorescein isothiocyanate (FITC) (clone A1; eBioscience, San Diego, CA, USA); CD73-phycoerythrin (PE) (clone AD2; Biolegend, San Diego, CA, USA or clone 10F1; Abcam, Cambridge, MA, USA); CD26-PE (clone M-A261, eBioscience); CD19-ECD (clone J3-119; Beckman Coulter, Brea, CA, USA); CD4-PC5 (clone 13 B8·2; Beckman Coulter); and CD25-PE (clone 4E3; Miltenyi Biotec). Isotype controls were included in all experiments. B cells were treated with the FcR blocking reagent (Miltenyi Biotec). Cells were washed and incubated with mAbs specific for each surface marker in 50 μl phosphate-buffered saline (PBS) for 30 min at room temperature (RT) in the dark. Following staining, cells were washed and examined using an EPICS XL-MCL flow cytometer equipped with Expo32 software (Beckman Coulter). At least 1 × 105 events were acquired for analysis. The analysis was restricted to the lymphocyte gate based on characteristic properties of the cells in the forward- and side-scatter. Where applicable, gates were restricted to the CD4+, CD4+CD39+, CD4+CD73+ or CD19+ lymphocyte subsets.
Microscopy
Freshly isolated lymphocyte subsets were fixed with 4% (w/v) paraformaldehyde in PBS and either stained with labelled antibodies for surface expression of CD39 and CD73 or first permeabilized in 0·1% Triton X in PBS for 25 min and then stained. Cells were washed with PBS and blocked with 2% (w/v) bovine serum albumin (BSA) in PBS. They were then stained with primary anti-CD39 antibody (clone BU-61 at 1:100 dilution; Ancell, Bayport, MN, USA) and/or anti-CD73 antibody (clone 10f1, 1:50 dilution; Abcam or clone H-300, 1:500 dilution; Santa-Cruz Biotechnology, Santa Cruz, CA, USA,) and then with secondary anti-mouse antibodies conjugated with FITC (1:200; Jackson ImmunoResearch, West Grove, PA, USA) or Cy3 (1:500; Jackson ImmunoResearch), respectively. Cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA, USA). For controls, isotype control antibodies were used and also the primary antibodies were substituted by PBS in some experiments. Cells were layered onto glass slides by cytospin, covered with Gelvatol mounting medium while still wet, coverslipped and examined in the Olympus Fluo-View 500 confocal microscope, using a ×40 objective.
Isolation of exosomes
Exosomes were isolated from plasma of NC or HNSCC patients using differential centrifugation, exclusion chromatography and ultracentrifugation, as described previously [30,31]. Briefly, aliquots of plasma (5 ml) were centrifuged at 1000 g for 10 min. Supernatants were centrifuged again at 10 000 g for 10 min, passed through 0·2 μm bacterial filters (Fisher, Pittsburgh, PA, USA), applied to size-exclusion A50m columns (Bio-Rad Laboratories, Hercules, CA, USA) containing Sepharose 2B (Amersham Biosciences, Piscataway, NJ, USA) and eluted with PBS. Three 9-ml fractions were collected, and after discarding the first fraction, the second and third fractions were combined, placed in Beckman Optiseal Centrifuge Tubes and centrifuged at 100 000 g for 3h at 4°C in a Beckman Optima LE-80K Ultracentrifuge (Beckman Coulter). The pellets were resuspended in PBS (500 μl) and their protein content determined in a Lowry microassay (Bio-Rad Laboratories). In some experiments, isolated exosomes were further fractionated on continuous sucrose density gradients. Isolated exosomes were characterized by TEM, NanoSight and Western blots.
Sucrose-density gradients
To determine the density of exosomes, linear sucrose gradients (0·2 M to 2·5 M sucrose) were prepared. Upon layering of ultracentrifuged and resuspended exosomes, gradients were centrifuged as described [32,33]. Serial 1 ml fractions at an increasing sucrose density were collected and refractive index of each fraction was measured in a refractometer. The fractions were evaluated by Western blots for CD81 (an exosomal marker), CD39 and CD73 using antibodies specific for these proteins.
Co-culture of T cell subsets
Isolated CD4+CD39+ and CD4+CD39neg CD73+ T cells were incubated alone or together at the 1:1 ratio in wells of 96-well plates (25 000 cells/well) containing 200 ul Dulbecco's phosphate-buffered saline (DPBS) in the presence of eATP (20 uM) for various time-periods. Exosomes (10 μg protein) fractionated from plasma of NC were incubated under the same conditions alone or in the presence of CD4+CD39+ T cells. Control wells contained B cells or cells without eATP. All experiments were performed in duplicate. Cell supernatants were collected, centrifuged for 2 min at 6000 g, boiled for 2 min to inactivate ADO-degrading enzymes and stored at −80C° for subsequent analysis. Concentrations of 5′-AMP, ADO and inosine (INO) were then measured by mass spectrometry.
Culture of pTreg with exosomes
CD4+CD39+ and CD4+CD39neg T cells were isolated from PBMC of NC. The cells (25 000/well) were incubated with eATP (20 μM) ± exosomes (10 μg protein) obtained from plasma of NC for 16 h at 37°C. Cell supernatants were prepared for mass spectrometry as described above.
Western blots
Whole cell protein extracts were prepared from various lymphocyte subpopulations or pelleted exosomes using a lysis buffer containing 0·5% Nonidet P-40, 150 mM NaCl and 50 mM Tris base (Sigma, St Louis, MO, USA) at 4°C. Lysates were boiled and electrophoresed on 4–15% Tris-HCl gradient gels, as described previously [33]. Membranes were treated with primary antibodies to CD39 (sc-18766, clone H-85, diluted 1:400; Santa Cruz Biotechnology) or CD73 (sc-25603, clone H300, diluted 1:400), followed by goat anti-mouse secondary antibodies conjugated to horseradish peroxidase (goat, 1:40 000; Pierce/Thermo Fisher Scientific Inc., Rockford, IL, USA). Anti-CD9 or anti-CD81 antibodies were used for exosome detection (Pierce). Signal West Femto Maximum Sensitivity Substrate (Pierce) and Kodak BioMax MR Film were used to visualize the target proteins.
Real-time polymerase chain reaction (PCR)
RNA was isolated (Trizol reagent; Life Technologies, Carlsbad, CA, USA), and cDNA was synthesized using iScriptTM cDNA synthesis kit (Bio-Rad). CD39 primers were forward: 5′-TTCTCTCCCTCCTTCTGCAA-3′, reverse: 5′-ATGGCCACTGTGAAAAGGAC-3′; CD73 primers were forward: 5′-CGCAACAATGGCACAATTAC-3′, reverse: 5′-CTCGACACTTGGTGCAAAGA-3′; β-actin primers were forward: 5′-ACTCTTCCAGCCTTCCTTC-3′, reverse: 5′-ATCTCCTTCTGCATCCTGTC-3′. Real-time PCR analysis was performed using SYBR Green PCR Master Mix (Applied Biosystems/Thermo Fisher Scientific, Inc.) in the AB 7300 Real-Time PCR System (Applied Biosystems). Threshold cycle (Ct) for target was subtracted from Ct for β-actin to calculate 2ΔCt.
Mass spectrometry
Samples were evaluated using liquid chromatography-tandem mass spectrometry by selected reaction monitoring with 13C10-adenosine as internal standard. Samples were injected into the Acquity ultra-performance liquid chromatographic system (Waters, Milford, MA, USA) and were separated with a C18 column (Waters UPLC BEH C18; 1·7 μm; 2·1 × 100 mm) using the following elution conditions: mobile phase A, 1% acetic acid in H2O; mobile phase B, methanol; flow rate, 0·3 ml/min; elution gradient (A/B) was 99·5/0·5% (0–2 min), 98/2% (2–3 min), 85/15% (3–4 min) and 99·5/0·5% (4–5 min). Purines levels were analysed with a TSQ Quantum-Ultra triple–quadruple mass spectrometry equipped with a heated electrospray ionization source. The mass spectrometer was operated in the positive-ion mode and the following mass-to-charge transitions were monitored: 348→136 for 5′-AMP; 268→136 for adenosine; and 269→137 for inosine.
Statistics
The data are expressed as means. For samples with non-parametric distribution of values, Kruskal–Wallis and two-tailed exact Wilcoxon–Mann–Whitney tests were applied using spss software (IBM, version 19). Correlations were calculated by the Spearman test. P-values < 0·05 and R2 values > 0·5 were considered to be significant.
Results
Co-expression of CD39 and CD73 in PBMC of normal controls
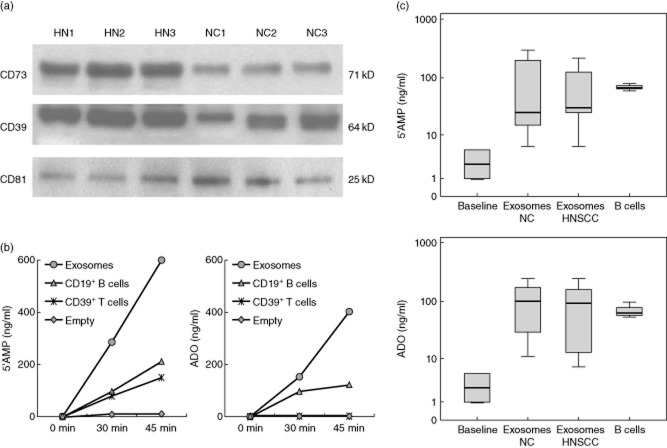
In the peripheral blood of NC, surface CD39 and CD73 were expressed on different subsets of CD4+ Tcells (Fig. 1a). Only a small percentage (∼1%) of these cells consistently co-expressed both markers. Nearly all CD19+ B cells co-expressed surface CD39 and CD73 (Fig. 1a). A subset of CD4+CD39neg T cells present in the peripheral circulation was found to express CD73, as reported previously [8,24]. The frequency of CD4+CD39+ T cells was 6·0 ± 2·9% [mean ± standard deviation (s.d.)] with a range of 1–13% and that of CD4+CD73 T cells was 6·9 ± 3·5% with a range of 3–18% (Fig. 1b). The percentages of these two T cell subsets did not correlate with one another, suggesting that they were distinct subsets of CD4+ T cells (Fig. 1c). Nearly all CD4+CD39+CD25+ Treg were FoxP3+, as we have reported previously [19,34], and no more than 4% of CD4+CD39+FoxP3+ Treg co-expressed CD39 and CD73 (Supporting information, Fig. S1). In rare NC (two of 30) higher percentages (up to 50%) of such double-positive Treg were seen.
Fig. 1.
Lymphocyte subpopulations expressing ectonucleotidases. (a) Co-expression of CD39 and CD73 on CD4+ T cells and CD19+ B cells in peripheral blood lymphocytes obtained from a representative normal donor (NC). The gates were set based on side- and forward-scatter. (b) Box-plots showing the frequency of CD4+CD73+CD39(–) T cells and CD4+CD39+CD73(–) regulatory T cells (Treg) in the peripheral circulation of 29 NC. Box-plots show medians and quartiles for 25 and 75% as boxes and values for 0% and 100% as whiskers. (c) The percentages of CD4+CD39+ Treg and CD4+CD73+ T cells in the peripheral blood of NC do not correlate with each other.
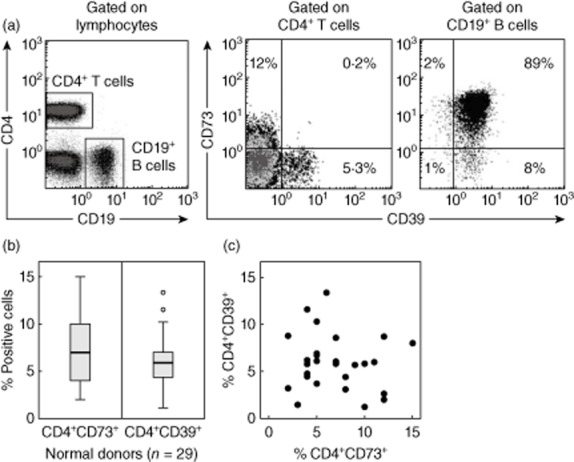
To further evaluate CD39 and CD73 co-expression in CD4+ T lymphocytes, CD4+CD25+ T cells and B cells were isolated from normal human PBMC using immunobeads. Their purity was 96 ± 0·6 and 92 ± 3%, respectively. The CD4+CD25+ T cells, consisting of CD39+, CD73+ and activated (CD4+CD25+) Tconv, were examined by confocal microscopy and Western blots. As shown in Fig. 2a, CD4+CD25+ T cells fixed with paraformaldehyde and stained for surface expression of CD39 and CD73 were either CD39+ (green) or CD73+ (red). Only rare cells co-expressed both enzymes (Fig. 2a, bottom). In contrast to CD39, which was distributed uniformly on the cell surface, CD73 formed patches or caps on the cell surface (Fig. 2a). This staining pattern suggested that CD73 may be readily stripped from the cell surface or internalized. Among permeabilized CD4+CD25+ T cells stained for CD39 (green) and CD73 (red), most were CD39+CD73neg with CD39 localized in the cytoplasmic compartment bordering the cell membrane (Fig. 2b, row 1), although some of these cells also contained intracytoplasmic red granules. We refer to these cells as CD39+CD73± Treg (Fig. 2b, row 1). In addition, we observed CD73+CD39neg T cells characterized by the presence of prominent red intracytoplasmic granules (Fig. 2b, row 2). Some of these cells also faintly expressed CD39 (green), and are referred to as CD73+CD39± Treg (Fig. 2b, row 2). Finally, Fig. 2b also illustrates intracytoplasmic staining of a CD39+CD73+ B cell and a rare CD39+CD73+ Treg cell (Fig. 2b, row 3), both containing a mass of CD73 granules (red) and a strong, uniform CD39 expression localized to the surface compartment. B cells, which are known to co-express CD39 and CD73, are used as positive controls. In aggregate, the data indicate that human Treg, which represent a major fraction of the CD4+CD25+ T cell subset [10], express both ectonucleotidases but regulate their expression differentially, presenting CD39 on the cell surface and retaining most of CD73 in the form of intracytoplasmic granules.
Fig. 2.
Microscopic examinations of isolated CD4+CD25+ T cells. The cells were separated from normal peripheral blood mononuclear cells (PBMC) by magnetic antibody-coated beads, as described in Methods. (a) Separated cells were stained for surface expression of CD39 and CD73. Only rare CD39+CD73+ cells were seen (see arrow). Note distinct surface staining for CD39 (evenly distributed) and CD73 (a cap formation). (b) CD4+CD25+ T cells were permeabilized prior to staining and examined by confocal microscopy. In row 1, a CD4+CD39+CD73neg regulatory T cells (Treg) cell shows uniform staining (green) localized to the surface compartment. Next to it is a CD4+CD39+CD73± cell which contains few CD73+ (red) cytoplasmic granules. In row 2, a CD4+CD73+CD39neg T cell filled with CD73+ (red) cytoplasmic granules. Next to it is a CD4+CD73+CD39± T cell which contains many red granules plus some CD39+ (green) elements. In row 3, a CD39+CD73+ B cell and a rare double-positive CD4+CD39+ Treg cell. These cells contain CD73 in cytoplasmic granules (red) and strong surface-associated CD39 (green). This series of representative images was assembled after microscopic evaluations of at least five different slides. Control cells were stained with isotype control primary antibodies. (c) A representative Western blot of CD4+CD39+, CD4+CD73+ and CD19+ B cells isolated from PBMC of a normal control (NC) and evaluated for expression of CD39 and CD73. Note that isolated CD4+CD39+ cells are negative for CD73. Data are from one of three independent experiments performed with isolated cell subsets. (d) The relative ratio of CD39 and CD73 mRNA expression levels in the isolated CD4+ T cell subsets and B cells of NCs. The subsets were tested for CD39 and CD73 mRNA expression by reverse transcription–polymerase chain reaction (RT–PCR). The data are presented as relative ratios of Ct values for CD39/CD73 mRNA levels and CD73/CD39 mRNA levels normalized to CD39+CD73+ Treg as 1·0. Representative data from one of three independent mRNA measurements performed with cell subsets obtained from different NCs are shown.
In contrast to the above data, CD73 expression was undetectable in isolated CD4+CD39+ pTreg by Western blots (Fig. 2c), although CD39 was always strongly positive in pTreg and B cells (data not shown). Conversely, and more in line with the results of confocal microscopy performed with permeabilized cells, semi-quantitative reverse transcription–polymerase chain reaction (RT–PCR) results confirmed a very high relative ratio of CD39/CD73 mRNA species in CD4+CD39+CD73neg nTreg, and also an elevated relative ratio of C73/CD39 mRNA species in CD4+CD73+CD39neg T cells (Fig. 2d), emphasizing their mutual but unequal co-expression in nTreg and the CD4+CD73+ T cell subsets, respectively.
CD73 expression on the surface of nTreg is activation-dependent
PBMC obtained from NC were activated in vitro with anti-CD3/CD28 beads and cultured as described in Methods for 16 h. Up-regulation of surface CD73 expression was observed by flow cytometry gating on CD4+CD39+ Treg. In a representative experiment of three performed, 5% of CD4+CD39+ Treg were CD73+ [their mean fluorescence intensity (MFI) was 55] prior to activation. Following activation, the percentage of CD73+ Treg increased to 16% and the MFI to 62. These activated Treg were able to produce ADO (Table 1), and in the presence of ARL67156 (a pan ectonucleotidase inhibitor) or α, β methylene ADP (a CD73 inhibitor), the levels of ADO produced by activated nTreg were drastically reduced. In aggregate, these data indicate that expression levels of surface CD73 on activated CD4+CD39+ Treg and their ability to produce ADO are elevated relative to resting Treg.
Table 1.
5′-AMP and adenosine (ADO) production by various human lymphocyte subsets incubated in the presence of exogenous ATP†
| Cell Type | Product | ng/ml/45 min |
|---|---|---|
| B cells (control) | 5′-AMP | 100 ± 10 |
| ADO | 125 ± 15 | |
| CD4+CD39+ pTreg (resting) | 5′-AMP | 60 ± 12 |
| ADO | 5 ± 1 | |
| CD4+CD39+ pTreg (activated)‡ | 5′-AMP | n.d. |
| ADO | 131 ± 40 | |
| + ARL67156 (inhibitor) | ADO | 35 ± 12 |
| + αβ methylene ADP (inhibitor) | ADO | 10 ± 6 |
| CD4+CD39+CD73+ Tr1 cells§ | 5′-AMP | 100 ± 6 |
| ADO | 4000 ± 100 | |
| CD4+CD39+ pTreg | 5′-AMP | 64 ± 15 |
| CD4+CD73+ T cell co-culture | ADO | 100 ± 10 |
Isolated B cells or T cell subsets were incubated in the presence of exogenous adenosine triphosphate (ATP) (20 μM) for 45 min except as indicated in ‡. 5′-AMP and ADO levels in supernatants were measured by mass spectrometry. The data are means ± standard deviation from three independent experiments, except for the in-vitro-generated regulatory T cells (Treg) data acquired in two independent experiments.
ADO production was measured at 60 min. Ectonucleotidase inhibitors, ARL67156 at 250 μmol/ml and αβ methylene ADP at 100 μmol/ml, were added to some wells.
5′-AMP was measured at 15 min, because its level dropped to 0 after 15 min incubation; n.d.: not done.
CD39 and CD73 co-expression by Tr1 cells
Tr1 were generated in autologous co-cultures of CD4+CD25neg T cells obtained from patients with HNSCC with dendritic cells (DC) pulsed with irradiated tumour cells, as described previously [29]. These Tr1 were harvested and tested for co-expression of the two ectoenzymes by flow cytometry. A substantial proportion (more than 16% in Fig. 3a) of Tr1 co-expressed both enzymes on the cell surface. In fact, in Fig. 3a, the entire Tr1 population seems to be shifting towards a double-positive phenotype. The frequency of double-positive Tr1 varied from 12% to more than 40% in Tr1 generated in five independent cultures. Fluorescence microscopy also showed CD39 and CD73 co-expression in a substantial proportion of Tr1 (Fig. 3b). Furthermore, surface expression of CD39 and CD73 was up-regulated in Tr1 (Fig. 3c) relative to their limited surface expression in CD4+CD25neg T cells obtained from the same NC. Strikingly, Tr1 produced very high levels of ADO when incubated in the presence of eATP (Fig. 3d, Table 1). Tr1 cultured with eATP also produced high levels of 5′-AMP within the first 15 min of culture but then, due perhaps to a rapid substrate utilization, 5′-AMP levels dropped (Fig. 3d). Tr1 produced ADO between 5 and 60 min of culture, at which time ADO levels began to decline. The data show that iTreg can hydrolyze eATP rapidly and efficiently to 5′-AMP and ADO.
Fig. 3.
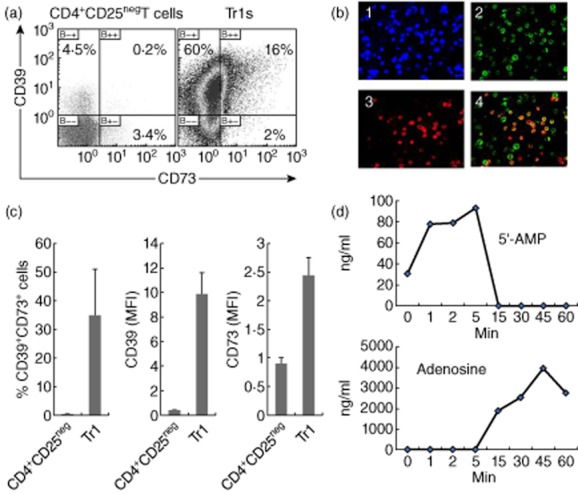
Co-expression of CD39 and CD73 and adenosine (ADO) production by in-vitro-generated regulatory T cells (Treg) (Tr1) cells. (a) Representative density plots show surface co-expression of the two ectoenzymes in Tr1 cells. Autologous CD4+CD25neg cells cultured in parallel with Tr1 served as controls and were not co-incubated with dendritic cells (DC), tumour cells or a mix of cytokines used for Tr1 generation. (b) Fluorescence microscopy of freshly harvested Tr1 stained for surface CD39 and CD73. The merged image shows co-expression of the two enzymes on a large proportion of Tr1. Representative data from one of five Tr1 cultures established with CD4+ T cells obtained from different normal controls (NC). Magnification ×200. (c) The frequency and mean fluorescence intensity (MFI) for CD39 and CD73 in Tr1. Data are means ± standard deviation (s.d.) from three independent experiments. (d) Aliquots of inucible Treg (iTreg) expressing CD39 and CD73 were incubated in the presence of exogenous adenosine triphosphate (eATP) and concentrations of 5′-AMP or immunosuppressive adenosine (ADO) in supernatants were measured by mass spectrometry. Data are from one of two independent iTreg cultures tested for ADO and 5′-AMP production.
Next, based on the rationale that pTreg are a major regulatory subset in cancer, we examined PBMC isolated from the peripheral blood of patients with HNSCC (n = 5) for co-expression of CD39 and CD73 in CD4+ T cell subsets and in CD19+ B cells used as positive controls. However, these flow cytometry results showed no difference in the frequency of CD4+ T cells co-expressing surface CD39 and CD73 on patients' Treg relative to Treg of NC (data not shown). Nevertheless, the MFI of CD39 was higher on CD4+ T cells in patients than in controls, as reported previously [10]. Importantly, when ADO production was measured upon incubating HNSCC patients' CD4+CD39+pTreg with eATP for 60 min, we detected high levels of ADO in the supernatants (754 ± 147 ng/ml; mean ± s.d. from three experiments). The addition of inhibitors, ARL67156 or α,β methylene ADP, reduced ADO levels to 102 ± 21 and 16 ± 8 ng/ml, respectively.
Exosomes isolated from plasma of NC and cancer patients carry enzymatically active CD39 and CD73
Exosomes present in plasma could originate from various immune or non-immune cells [27]. In supernatants of tumour cell lines all exosomes are tumour-derived, while in the plasma of cancer patients, especially those with advanced disease, most are likely to be derived from tumour cells [30,31]. Exosomes isolated from plasma of NC or patients with HNSCC floated at the density of 1·15-1·20 g/ml on continuous sucrose density gradients and their buoyancy was comparable to that of exosomes isolated from supernatants of tumour cell lines (data not shown). Exosomes had a typical ‘doughnut-like’ appearance by transmission electron microscopy (Fig. 4a,b). By NanoSight-based measurements, exosomes were 30–150 nM in diameter and carried typical exosomal markers, e.g. CD81, as confirmed by Western blots. Further, these exosomes were consistently positive for CD39 and CD73 (Fig. 5a). Semi-quantitative density analyses of Western blots indicated a higher ectonucleotidase expression/μg protein in exosomes obtained from plasma of HNSCC patients than in those isolated from plasma of NC (Fig. 5a). When CD39+CD73+ exosomes fractionated from plasma of NC or patients with HNSCC were incubated with eATP, they were able to hydrolyze it to 5′-AMP and to ADO, as measured by mass spectrometry (Fig. 5b). Exosomes obtained from plasma of HNSCC patients or NC plasma produced 5′-AMP and ADO. B cells, which co-express CD39 and CD73, served as positive controls. Mean levels of 5′-AMP and ADO production by CD39+CD73+ exosomes were not different for patients and NC (Fig. 5c). However, large individual differences were observed within exosomal fractions tested in both groups, suggesting a need for further examination of larger sample numbers.
Fig. 4.
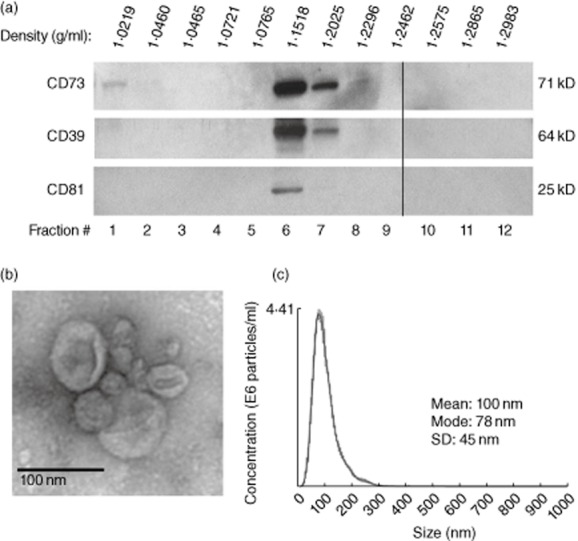
Characteristics of exosomes isolated from human plasma. (a) A representative Western blot of exosomes isolated from plasma of a normal control (NC) and floated on a continuous sucrose gradient following ultracentrifugation. Individual 1-ml fractions were collected and loaded on gels for electrophoresis. Exosomes expressing CD81 are located in fractions 6 and 7. (b) Transmission electron microscopy of exosomes collected from the sucrose gradient shown in (a) as fraction 6. Negative stain with uranyl acetate. (c) Exosomes in fraction 6 were examined in a NanoSight instrument to determine their size and particle concentration.
Fig. 5.
Characteristics of exosomes fractionated from plasma of normal control (NC) or head and neck squamous cell carcinoma (HNSCC) patients. (a) Western blots of isolated exosomes demonstrating the presence of CD39 and CD73. CD81 is a tetraspanin used as an exosome marker. Note that exosomes obtained from HNSCC patients' plasma carry higher levels of both ectoenzymes than those obtained from NCs' plasma. (b) 5′-AMP and adenosine (ADO) production by isolated CD39+CD73+ exosomes (10ug protein) incubated with exogenous adenosine triphosphate (eATP) (20 μm). Exosomes produced more 5′-AMP and ADO than B cells or CD4+CD39+ Treg (25 000 cells/well). Representative data from three experiments performed with exosomes and cells of different NC. (c) Box-plots show levels of 5′-AMP and ADO produced by exosomes obtained from NC (n = 6) or patients with HNSCC (n = 6). B cells (25 000/well) were used as a positive control. Cells were incubated in the presence of eATP (20uM) for 60 min. The baseline values are for exosomes incubated in the absence of ATP.
Adenosine and 5′-AMP production by cultured T-cell subsets
To evaluate functional consequences of the ectoenzyme expression on lymphocyte subsets, we isolated CD4+CD39+ and CD4+CD73+ cells from PBMC of NC and, upon incubation in the presence of eATP, measured nucleoside production by mass spectrometry. As shown in Fig. 6 and Table 1, resting CD4+CD39+ Treg cultured alone largely produced 5′-AMP but no ADO, and CD4+CD73+ T cells cultured alone produced little ADO or 5′-AMP (Fig. 6). Activated pTreg produced ADO and ADO production was blocked by ARL67156 or by α,β methylene ADP (Table 1). Both cell subsets were shown to produce very small quantities of inosine early in co-cultures (data not shown). As indicated earlier, Tr1 cells were strong ADO producers (Table 1). When we co-incubated CD4+CD39+ Treg with autologous CD4+CD73+ T cells in the presence of (e)ATP (Fig. 6 and Table 1), the production of ADO was significantly higher than that observed with each of these subsets cultured alone (P = 0·03). In aggregate, these data show that hydrolysis of eATP to ADO by pTreg does not require simultaneous co-expression of CD39 and CD73 on the pTreg cell surface and is readily accomplished when CD73+ T cells are added.
Fig. 6.
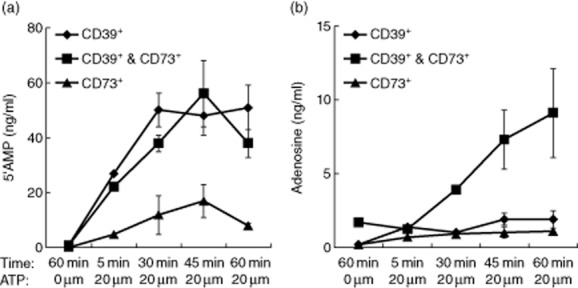
Production of 5′-AMP and adenosine (ADO) by CD4+ T cell subsets. Production of exogenous 5′-AMP (a), and ADO (b) was measured by mass spectrometry after incubation of CD4+CD39+, CD4+CD73+ or CD4+CD39+ Treg and CD4+CD73+ T cells mixed at a 1:1 ratio in the presence of eATP. (a) Only CD4+CD39+ T cells alone or CD4+CD39+ T cells supplemented with CD4+CD73+ T cells hydrolyzed eATP to 5′-AMP. (b). Only CD4+CD39+ Treg and CD4+CD73+ T cells combined together hydrolyzed eATP to ADO. The data are means ± standard deviation (s.d.) from independent experiments performed with lymphocyte subsets obtained from three different normal controls (NC).
Adenosine, 5′-AMP and inosine production by pTreg co-incubated with exosomes
Isolated pTreg (CD4+CD39+) or conventional CD4+CD39neg T cells were co-incubated with plasma-derived exosomes of NC. As the incubation time was 16 h in these experiments, inosine (the product of ADO hydrolysis) served as a measure of ADO production and its rapid hydrolysis. The data show that CD4+CD39+ pTreg co-incubated with exosomes known to carry CD39 and CD73 produce ADO, which is rapidly converted to INO in these co-cultures (Table 2).
Table 2.
5′-AMP, adenosine (ADO) and inosine (INO) production by human peripheral regulatory T cells (pTreg) incubated in the presence of exogenous adenosine triphosphate (eATP) ± plasma-derived exosomes†
| Cell type | ±EXO | 5′-AMP | ADO | INO |
|---|---|---|---|---|
| CD4+CD39+ pTreg | No | 252 ± 73 | 6 ± 2 | 167 ± 78 |
| Yes | 110 ± 57 | 17 ± 1 | 330 ± 51‡ | |
| CD4+CD39NEG Tconv | No | 43 ± 10 | 13 ± 7 | 12 ± 9 |
| Yes | 11 ± 2 | 18 ± 2 | 935 ± 27‡ | |
| ATP alone | No | 1 ± 1 | 0·1 ± 0·1 | 13 ± 2 |
| EXO alone | No | 36 ± 10 | 19 ± 2 | 22 ± 4 |
CD4+CD39+ pTreg and CD4+CD39neg Tconv were isolated from peripheral blood of normal controls (NC) and incubated in the presence of eATP (20 μM) alone or with exosomes (10 μg protein) for 16 h. 5′-AMP, ADO and INO levels were measured in cell supernatants by mass spectrometry. The data in ng/ml are mean values ± standard deviation from three independent experiments. Exosomes were isolated from plasma of different NC.
The elevated INO levels at 16 h of culture resulted presumably from ADO hydrolysis.
Discussion
The immune system is a tightly regulated network patrolling tissues for signs of injury or infections. In an attempt to preserve homeostasis, it initiates defensive inflammatory responses involving purinergic mediators such as ATP and ADO [1]. Because ADO represents one of the mechanisms responsible for immunosuppression [2,35,36], the ability of human Treg to produce ADO has been of great interest. The dominant metabolic pathway leading to ADO production is the extracellular de-phosphorylation of ATP requiring two ectonucleotidases. CD39 hydrolyzes ATP to ADP and 5′-AMP; CD73 hydrolyzes 5′-AMP to ADO. CD73 is a dimer of two identical 70 kD subunits, which are anchored to the plasma membrane via a C-terminal serine residue linked to glycophosphatidyl inositol (GPI); CD73 can be shed from the membrane by hydrolysis of its GPI anchor or by proteolytic cleavage [37].
Co-expression of CD39 and CD73 on human pTreg has been a subject of considerable controversy. In mice, Treg were reported to be CD39+CD73+ [6], while pTreg in the human blood are CD39+ and express little surface CD73 by flow cytometry. In some reports, however, CD73 protein was found to be expressed and detectable on the surface of Tr1 cells, suggesting that its expression on the cell surface can be up-regulated. Cryopreserved/thawed Treg did not express surface CD73, possibly because freezing disrupted the GPI anchor. Also, CD73 could be more readily removed from the cell surface than CD39 by enzymatic cleavage, because of the well-known sensitivity of its GPI linkage to enzymatic cleavage [37].
CD73 has a wide distribution among cells and tissues and participates in a variety of biologically critical functions, including tumour progression [38,39]. In the tumour microenvironment, CD73 expression is known to be regulated by hypoxia [40], which supports the hypothesis that CD73 gene expression is under a strict epigenetic control. Capping of CD73 seen by microscopy on the surface of T cells suggested that CD73 was being aggregated for elimination from the surface by shedding or internalization. Conversely, the fine granular pattern seen on the surface of some CD4+ T cells suggested that CD73 could be associated with the immune synapses or enzyme–receptor complexes. Based on these observations, it seems reasonable to suggest that up- or down-regulation of CD73 surface expression by Treg may be a mechanism for coupling of ADO production to the state of cellular activation. Our data showed that pTreg activation in vitro resulted in up-regulation of CD73 surface expression level and higher levels of ADO production versus non-activated pTreg. Also, the finding that Tr1 acquire high expression levels of surface CD73 produce high levels of ADO and mediate strong immune suppression of Teff functions supports the role of cellular activation in CD73 surface expression.
Production of ADO by CD4+CD39+ Treg has been linked to high levels of suppressor functions mediated by these cells [8]. However, their ability to produce ADO requires an explanation, as CD73 surface co-expression is rare in these cells. A potential explanation presented itself when a recent report showed that 5′-AMP is an A1R agonist which is independent of ectonucleotidases and can modulate cellular functions as competently as ADO [41]. Thus, CD4+CD39+ Treg signalling via A1R could directly modulate activities of Teff. At the same time, we observed that other subsets of peripheral blood lymphocytes expressed CD73 and produced ADO. In fact, CD4+CD39negCD73+ T cells and CD19+ B cells were shown to be ADO producers in the presence of 5′-AMP [23,24]. Further, exosomes isolated from the plasma of NC or cancer patients carried enzymatically competent CD39 and CD73, as also reported previously [25,26]. As T cells, B cells and exosomes are ubiquitous components in the blood, body fluids and tissues, we hypothesized that they could co-operate with CD4+CD39+ Treg by delivering membrane-tethered CD73 to enable pTreg to produce ADO. Co-culture experiments, in which CD4+CD39+ were incubated in the presence of CD4+CD73+ T cells, B cells or exosomes carrying CD39 and CD73, confirmed the validity of this co-operation mechanism for human ADO production from eATP.
The role of exosomes in the regulation of ADO production by delivering CD39 and CD73 proteins to Treg, and potentially to other immune and non-immune cells, is a new and intriguing aspect of immunosuppression operating in health and disease. Biologically active CD39+CD73+ exosomes were isolated from NC's and HNSCC patients' plasma. Their universal presence in the circulation might explain some of the early results from studies investigating the instability of 5′-AMP in rodent plasma (E. K. Jackson, unpublished data). The almost immediate decline (within seconds after a 5′-AMP spike) in the 5′-AMP plasma concentration was associated with an immediate spike in ADO and inosine and was followed by their slow decline overtime (data not shown). This rapid utilization of 5′-AMP suggests that exosome-based ectonucleotidases might be involved in this process.
Exosomes, which accumulate in body fluids of patients with cancer [33], could serve as a rich source of membrane-tethered CD73 enabling CD4+CD39+ Treg human pTreg to produce ADO. We believe that membrane-linked CD73 is necessary for co-operative interactions with CD4+CD39+ Treg. Exosomes, carrying membrane-bound ectonucleotidases, are both ubiquitous and optimally equipped to deliver enzymatically active CD73 to pTreg and thus support their immunosuppressive functions. The possibility of cellular and subcellular regulation of ADO production by subsets of human CD4+ T cells introduces a novel and intriguing aspect of immune suppression mechanisms operating in health and disease.
Author contributions
For conception and design, P. S., T. W. and E. J.; for administrative and financial support, S. L., T. W. and E. J.; for provision of study materials and patients, T. W. For collection and assembly of data, P. S., M. H., Z. S., L. M., M. M., C.-S. H., D. G. and D. C. For data analysis and interpretation, P. S., E. J. and T. W. For writing the manuscript, T. W. and P. S. All authors gave final approval of the manuscript.
Grant support
This study was supported in part by the NIH grants PO-1 CA109688 and R0-1 CA168628 to T. L. W. and HL-109002, DK-091190, DK-068575 and DK-079307 to E. K. J. P. J. S. was supported by the Pittsburgh-Essen Partnership Program (PEPP). L. M. was supported by a grant from the Swiss National Foundation. This study utilized the Flow Cytometry Core and the Cell Imaging Facility Core, both supported in part by the NIH CCSG 5P30 CA047904.
Dicslosures
All the co-authors state they have no conflicts of interest.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web-site:
Fig. S1. Flow cytometry for co-expression of CD39 and CD73 on CD4+forkhead box protein 3 (FoxP3+) regulatory T cells (Treg). Representative data for one of 10 experiments performed with peripheral blood mononuclear cells (PBMC) obtained from normal donors. Percentages of positive cells in each quadrant are indicated.
References
- 1.Antonioli L, Blandizzi C, Pacher P, Hasko G. Immunity, inflammation and cancer: a leading role for adenosine. Nat Rev Cancer. 2013;13:842–857. doi: 10.1038/nrc3613. [DOI] [PubMed] [Google Scholar]
- 2.Ghiringhelli F, Bruchard M, Chalmin F, Rebe C. Production of adenosine by ectonucleotidases: a key factor in tumor immunoescape. J Biomed Biotechnol. 2012;2012 doi: 10.1155/2012/473712. article ID 473712, 9 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007;14:1315–1323. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- 4.Bastid J, Cottalorda-Regairaz A, Alberici G, Bonnefoy N, Eliaou JF, Bensussan A. ENTPD1/CD39 is a promising therapeutic target in oncology. Oncogene. 2013;32:1743–1751. doi: 10.1038/onc.2012.269. [DOI] [PubMed] [Google Scholar]
- 5.Wang L, Fan J, Thompson LF, et al. CD73 has distinct roles in nonhematopoietic and hematopoietic cells to promote tumor growth in mice. J Clin Invest. 2011;121:2371–2382. doi: 10.1172/JCI45559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borsellino G, Kleinewietfeld M, Di Mitri D, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 8.Mandapathil M, Hilldorfer B, Szczepanski MJ, et al. Generation and accumulation of immunosuppressive adenosine by human CD4+CD25highFOXP3+ regulatory T cells. J Biol Chem. 2010;285:7176–7186. doi: 10.1074/jbc.M109.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moncrieffe H, Nistala K, Kamhieh Y, et al. High expression of the ectonucleotidase CD39 on T cells from the inflamed site identifies two distinct populations, one regulatory and one memory T cell population. J Immunol. 2010;185:134–143. doi: 10.4049/jimmunol.0803474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuler PJ, Schilling B, Harasymczuk M, et al. Phenotypic and functional characteristics of CD4+ CD39+ FOXP3+ and CD4+ CD39+ FOXP3neg T-cell subsets in cancer patients. Eur J Immunol. 2012;42:1876–1885. doi: 10.1002/eji.201142347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dwyer KM, Hanidziar D, Putheti P, et al. Expression of CD39 by human peripheral blood CD4+ CD25+ T cells denotes a regulatory memory phenotype. Am J Transplant. 2010;10:2410–2420. doi: 10.1111/j.1600-6143.2010.03291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buckley RH. Molecular defects in human severe combined immunodeficiency and approaches to immune reconstitution. Annu Rev Immunol. 2004;22:625–655. doi: 10.1146/annurev.immunol.22.012703.104614. [DOI] [PubMed] [Google Scholar]
- 13.Kameoka J, Tanaka T, Nojima Y, Schlossman SF, Morimoto C. Direct association of adenosine deaminase with a T cell activation antigen, CD26. Science. 1993;261:466–469. doi: 10.1126/science.8101391. [DOI] [PubMed] [Google Scholar]
- 14.Mandapathil M, Szczepanski M, Harasymczuk M, et al. CD26 expression and adenosine deaminase activity in regulatory T cells (Treg) and CD4(+) T effector cells in patients with head and neck squamous cell carcinoma. Oncoimmunology. 2012;1:659–669. doi: 10.4161/onci.20387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duhen T, Duhen R, Lanzavecchia A, Sallusto F, Campbell DJ. Functionally distinct subsets of human FOXP3+ Treg cells that phenotypically mirror effector Th cells. Blood. 2012;119:4430–4440. doi: 10.1182/blood-2011-11-392324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbas AK, Benoist C, Bluestone JA, et al. Regulatory T cells: recommendations to simplify the nomenclature. Nat Immunol. 2013;14:307–308. doi: 10.1038/ni.2554. [DOI] [PubMed] [Google Scholar]
- 17.Schreiber TH, Wolf D, Bodero M, Podack E. Tumor antigen specific iTreg accumulate in the tumor microenvironment and suppress therapeutic vaccination. Oncoimmunology. 2012;1:642–648. doi: 10.4161/onci.20298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whiteside TL, Schuler P, Schilling B. Induced and natural regulatory T cells in human cancer. Expert Opin Biol Ther. 2012;12:1383–1397. doi: 10.1517/14712598.2012.707184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergmann C, Strauss L, Zeidler R, Lang S, Whiteside TL. Expansion of human T regulatory type 1 cells in the microenvironment of cyclooxygenase 2 overexpressing head and neck squamous cell carcinoma. Cancer Res. 2007;67:8865–8873. doi: 10.1158/0008-5472.CAN-07-0767. [DOI] [PubMed] [Google Scholar]
- 20.Mandapathil M, Szczepanski MJ, Szajnik M, et al. Adenosine and prostaglandin E2 cooperate in the suppression of immune responses mediated by adaptive regulatory T cells. J Biol Chem. 2010;285:27571–27580. doi: 10.1074/jbc.M110.127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pulte ED, Broekman MJ, Olson KE, et al. CD39/NTPDase-1 activity and expression in normal leukocytes. Thromb Res. 2007;121:309–317. doi: 10.1016/j.thromres.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomson LF, Ruedi JM, Glass A, et al. Production and characterization of monoclonal antibodies to the glycosyl phosphatidylinositol-anchored lymphocyte differentiation antigen ecto-5′-nucleotidase (CD73) Tissue Antigens. 1990;35:9–19. doi: 10.1111/j.1399-0039.1990.tb01750.x. [DOI] [PubMed] [Google Scholar]
- 23.Saze Z, Schuler PJ, Hong CS, Cheng D, Jackson EK, Whiteside TL. Adenosine production by human B cells and B cell-mediated suppression of activated T cells. Blood. 2013;122:9–18. doi: 10.1182/blood-2013-02-482406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schuler PJ, Macatangay BJ, Saze Z, et al. CD4(+)CD73(+) T cells are associated with lower T-cell activation and C reactive protein levels and are depleted in HIV-1 infection regardless of viral suppression. AIDS. 2013;27:1545–1555. doi: 10.1097/QAD.0b013e328360c7f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clayton A, Al-Taei S, Webber J, Mason MD, Tabi Z. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J Immunol. 2011;187:676–683. doi: 10.4049/jimmunol.1003884. [DOI] [PubMed] [Google Scholar]
- 26.Smyth LA, Ratnasothy K, Tsang JY, et al. CD73 expression on extracellular vesicles derived from CD4+ CD25+ Foxp3+ T cells contributes to their regulatory function. Eur J Immunol. 2013;43:2430–2440. doi: 10.1002/eji.201242909. [DOI] [PubMed] [Google Scholar]
- 27.Zhang HG, Grizzle WE. Exosomes and cancer: a newly described pathway of immune suppression. Clin Cancer Res. 2011;17:959–964. doi: 10.1158/1078-0432.CCR-10-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuler PJ, Harasymczuk M, Schilling B, Lang S, Whiteside TL. Separation of human CD4+CD39+ T cells by magnetic beads reveals two phenotypically and functionally different subsets. J Immunol Methods. 2011;369:59–68. doi: 10.1016/j.jim.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bergmann C, Strauss L, Zeidler R, Lang S, Whiteside TL. Expansion and characteristics of human T regulatory type 1 cells in co-cultures simulating tumor microenvironment. Cancer Immunol Immunother. 2007;56:1429–1442. doi: 10.1007/s00262-007-0280-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor DD, Gercel-Taylor C, Lyons KS, Stanson J, Whiteside TL. T-cell apoptosis and suppression of T-cell receptor/CD3-zeta by Fas ligand-containing membrane vesicles shed from ovarian tumors. Clin Cancer Res. 2003;9:5113–5119. [PubMed] [Google Scholar]
- 31.Kim JW, Wieckowski E, Taylor DD, Reichert TE, Watkins S, Whiteside TL. Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Clin Cancer Res. 2005;11:1010–1020. [PubMed] [Google Scholar]
- 32.Montecalvo A, Laregina AT, Morelli AE. Methods of analysis of dendric cell-derived exosome-shuttle micro RNA and its horizontal propagation between dendritic cells. Meth. Mol Biol. 2013;1024:19–40. doi: 10.1007/978-1-62703-453-1_3. [DOI] [PubMed] [Google Scholar]
- 33.Szczepanski MJ, Szajnik M, Welsh A, Whiteside TL, Boyiadzis M. Blast-derived microvesicles in sera from patients with acute myeloid leukemia suppress natural killer cell function via membrane-associated transforming growth factor-beta1. Haematologica. 2011;96:1302–1309. doi: 10.3324/haematol.2010.039743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mandapathil M, Lang S, Gorelik E, Whiteside TL. Isolation of functional human regulatory T cells (Treg) from the peripheral blood based on the CD39 expression. J Immunol Methods. 2009;346:55–63. doi: 10.1016/j.jim.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zarek PE, Huang CT, Lutz ER, et al. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood. 2008;111:251–259. doi: 10.1182/blood-2007-03-081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohta A, Kini R, Ohta A, Subramanian M, Madasu M, Sitkovsky M. The development and immunosuppressive functions of CD4(+) CD25(+) FoxP3(+) regulatory T cells are under influence of the adenosine-A2A adenosine receptor pathway. Front Immunol. 2012;3:1–12. doi: 10.3389/fimmu.2012.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strater N. Ecto-5′-nucleotidase: structure function relationships. Purinergic Signal. 2006;2:343–350. doi: 10.1007/s11302-006-9000-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stagg J, Divisekera U, McLaughlin N, et al. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci USA. 2010;107:1547–1552. doi: 10.1073/pnas.0908801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allard B, Turcotte M, Stagg J. CD73-generated adenosine: orchestrating the tumor-stroma interplay to promote cancer growth. J Biomed Biotechnol. 2012;2012 doi: 10.1155/2012/485156. article ID 485156, 8 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X, Zhou T, Zhi X, Zhao F, Yin L, Zhou P. Effect of hypoxia/reoxygenation on CD73 (ecto-5′-nucleotidase) in mouse microvessel endothelial cell lines. Microvasc Res. 2006;72:48–53. doi: 10.1016/j.mvr.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 41.Rittiner JE, Korboukh I, Hull-Ryde EA, et al. AMP is an adenosine A1 receptor agonist. J Biol Chem. 2012;287:5301–5309. doi: 10.1074/jbc.M111.291666. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Flow cytometry for co-expression of CD39 and CD73 on CD4+forkhead box protein 3 (FoxP3+) regulatory T cells (Treg). Representative data for one of 10 experiments performed with peripheral blood mononuclear cells (PBMC) obtained from normal donors. Percentages of positive cells in each quadrant are indicated.